Abstract
Objective:
Laparoscopic procedures utilize a pneumoperitoneum to distend and separate the abdominal wall from the intra-abdominal structures. Carbon dioxide is commonly used for this purpose, although this study is inclusive of any gas used for abdominal distention. The gas is delivered from cylinders through a gas insufflation delivery system. The purpose of this study is to determine if laparoscopic gas delivery systems composed of gas cylinders and insufflators used for laparoscopy have microbes present.
Methods:
Gas delivery systems were evaluated for the presence of microbial growth using standard techniques. External connection sites, gas cylinders and the internal conduit tubing of insufflators were cultured. Fifty two (52) insufflators and sixty (60) gas cylinders were evaluated.
Results:
Twelve (12) of the sixty cylinders (20%) and fifty four (54) of the sixty insufflators (92.3%) were culture positive. The organisms identified are significant and a varied spectrum.
Conclusions:
Recognition that gas cylinders, insufflation attachments and internal components of insufflators quantitatively contain microbes is demonstrated. Reduction of microbial exposure from insufflation apparatus is achieved by cleansing external ports and use of a 0.3 micron filter for abdominal pneumoperitoneum.
Keywords: Pneumoperitoneum, Microbial colonization
INTRODUCTION
Inorganic particulate contamination by carbon dioxide (CO2) insufflation apparatus and gas delivery systems for laparoscopy was first identified in 1989.1 Creation and maintenance of a pressurized flow of gas to establish and preserve abdominal wall separation for safe endoscopic observation and manipulation is routinely accomplished by CO2 through a gas delivery system. The gas is produced by vapor pressure changes of liquid CO2 contained in chromium-molybdenum-steel alloy cylinders. A pressure-reducing insufflation (throttling) system delivers the gas to the abdomen. This study examines the CO2 laparoscopic insufflation gas delivery system and qualitatively evaluates it for the presence of bacteria and fungi.
MATERIALS AND METHODS
Carbon dioxide approved for medical procedures was obtained from medical commercial sources. The gas met US Pharmacopoeia standards2 and FDA criteria for commercial production and intra-abdominal medical use. The cylinders are made of materials meeting the Department of Transportation standards for hydrostatic pressure and safe intrastate transport.
Gas flow from cylinders was directed into sterilized insufflators, non-sterile insufflators, with and without 0.1 or 0.3 micron filters. Filter evaluation was performed using a sterile ten foot section of polyvinyl chloride tubing and a 0.1 or 0.3 micron filter connected to the exit port of the pressure regulator device. Under a laminar flow hood, gas flow was directed into a sterile flask of thioglycolate broth media at flow rates of 500 cc, one liter and three liters per minute for a total volume of 50 liters. The media was cultured, plated, and evaluated for colonization and species identification by MicroScan panels, Walkaway-40 system with manual backup. Culture media samples were simultaneously plated, incubated and assessed as a control.
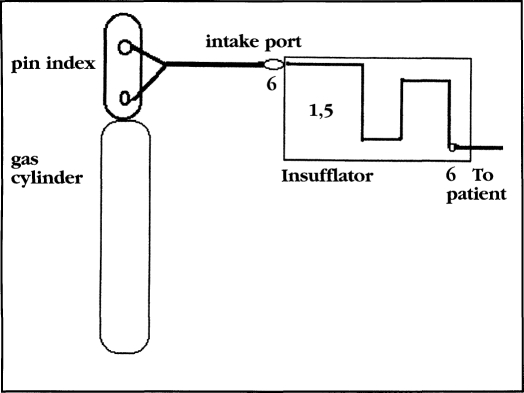
Various laparoscopy CO2 insufflators (EDER, Olympus, Solos, Storz, Weiss, Wisap, Wolf) were used to deliver CO2 gas. Insufflator history, extent, type or volume use was unknown. Fifty-two individual insufflators and sixty gas cylinders were evaluated. Different delivery circumstances and equipment sites were evaluated. The following numbers correspond to culture sites and results listed in Table 1: 1) Cylinders connected to insufflators using a sterile high pressure hose and a sterile ten foot polyvinyl chloride tube at the outflow port delivering gas into sterile growth media. Fifty-two insufflators and 60 gas cylinders, a total of 60 circumstances. 2) Gas delivery as in 1, using a 0.3 micron filter between the growth media and the insufflator for 60 samples. 3) Gas delivery as in 1, using a 0.1 micron filter, for 60 evaluations. 4) Gas delivery as in 1 through insufflators sterilized by ethylene oxide for 60 samples. 5) Internal tubing and conduits of the 52 insufflators at two separate sites for 104 samples. 6) External ports of the insufflator, front and rear making 52 samples and the pin index portion of the 60 gas cylinders for a total of 164 samples. Therefore, each gas insufflation system had cultures taken at the pin index site, intake port, two separate internal conduit sites and exit ports in 52 separate insufflators and 60 CO2 cylinders (Figure 1).
Table 1.
Organisms cultured from 52 insufflators and 60 cylinders delivering CO2 for laparoscopy pneumoperitoneum. (+) indicates species identification and colonization.
| column | 1 (60) | 6 (164) | 5(104) | 4(60) | |
|---|---|---|---|---|---|
| gas through insufflators insufflators (52) from gas tanks (60) | external fittings intake (52) output (52) pin index (60) | internal apparatus at two sites (52 x 2) | through sterile gas insufflators (52 ) tank (60) | ||
| Staphylococcus | |||||
| aureus | + 12/60 | + 26/164 | + 17/104 | + 12/60 | |
| albus | + 11/60 | + 18/164 | + 16/104 | + 9/60 | |
| saprophyticus | + 7/164 | ||||
| epidermidis | + 11/60 | + 20/164 | + 13/104 | ||
| Streptococcus | |||||
| pyogenes | + 9/60 | + 12/164 | + 8/104 | + 9/60 | |
| agalactiae | + 4/164 | ||||
| faecalis | + 3/60 | + 7/104 | |||
| intermedius | + 2/60 | + 3/164 | |||
| Listeria monocytogenes | + 3/60 | ||||
| Escherichia coli | + 5/60 | + 8/164 | + 4/104 | ||
| Enterobacter | + 6/60 | + 7/104 | + 7/60 | ||
| Erwinia | + 1/60 | + 5/164 | + 3/104 | + 3/60 | |
| Klebsiella species | + 2/60 | + 6/164 | |||
| Proteus species | + 3/60 | + 6/164 | + 6/104 | + 2/60 | |
| Serratia species | + 3/60 | + 5/164 | + 3/104 | + 3/60 | |
| Yersinia | + 4/60 | + 3/164 | + 2/104 | ||
| Pseudomonas aeruginosa | + 6/60 | + 7/164 | + 4/104 | + 5/60 | |
| Candida albicans | + 2/60 | + 8/52 | |||
| Trichophyton rubrum | + 2/52 | ||||
Figure 1.
Culture sites.
Growth media consisted of thioglycolate, trypticase soy broth, Sabourauds, Mycosel (DBL), blood agar plates, EMB plates and Legionella selective agar.
RESULTS
The various circumstances of evaluation showed that fourteen of the sixty (14/60, 23.3%) gas tanks had microbial colonization. External connection sites (52 insufflators and 60 gas cylinders or 112 sites, 27.6%) showed microbes in 31 instances. Gas cylinders had microbial growth in twelve of the sixty cylinders (12/60, 20%). No growth occurred in the sixty evaluations for each of the 0.1 or 0.3 micron filter group.
1) Gas delivered through standard laparoscopic insufflators using sterile connectors and sterile tubing grew organisms as noted in Table 1, column number 1. This represents growth from both the insufflator delivery system and the cylinder gas supply (14/60, 23.3% growth).
2) Gas delivered through standard laparoscopic insufflators with sterile connectors, sterile tubing and a 0.3 micron filter showed no growth. This represents gas delivered with a 0.3 micron filter before the culture media (0/60, 0% colonization).
3) Gas delivered through standard laparoscopic insufflators with sterile connectors, sterile tubing and a 0.1 micron filter showed no growth. This represents gas delivered with a 0.1 micron filter before the culture media (0/60, 0% colonization).
4) Gas delivered through pre-sterilized insufflators showed microbial growth as noted in Table 1, column number 4. This represents gas cylinder growth (12/60, 20% growth).
5) Cultures of the internal mechanisms, tubing and pressure-reducing apparatus grew organisms as noted in Table 1, column number 5. This represents growth of the insufflator internal apparatus only (26/164, 15.9% growth).
6) Cultures of the external fittings for inflow and egress grew organisms as noted in Table 1, column number 6. This represents external surfaces of the insufflator apparatus.
Microbial colonization was shown for the inside of gas cylinders, the external connection sites and inside the insufflation apparatus. Microbial contamination at the gas delivery site was eliminated by either a 0.1 or 0.3 micron sterile filter.
DISCUSSION
It is not surprising that the external surfaces of the insufflation apparatus, pin index system, intake and exit ports showed the presence of microbes (column 6). These surfaces are contacted by many people, inside and outside the operating room, of varying levels of skill and understanding regarding clean and aseptic technique. Using a protective sheath over the pin index portion of the cylinder during handling and transport to the operating room would reduce contamination from handling. Wiping the pin index connecting portion of the stem of the gas cylinder with a germicidal cloth before placement to the insufflator intake port is recommended to quantitatively reduce contaminants.
Microbes can enter the insufflation apparatus, internal tubing and pressure regulation mechanisms by one of three routes or combinations of routes: 1) from connecting points (inflow and outflow); 2) the gas cylinder; or 3) a. growth of organisms from within the insufflator pressure regulation equipment because of ambient operating room contamination resulting from influx during periods of reduced or no pressure in the system or being turned off with a negative pressure generated in the insufflator allowing backflow intake, or b. from contamination via backflow of irrigation or body fluids from previous laparoscopic procedures recognized or unrecognized.3
Microbial growth requirements of organisms that are human contaminants and pathogens are varied and extensive. The growth range is from narrow, precise and extremely favorable conditions to those being able to grow or maintain the capability for growth in low temperature, reduced oxygen tension and with few nutrient requirements. Cylinders containing gas for clinical applications are not tested or regulated for any microbe or participate standard. These unclean oxidized metal containers are filled under a wide range of varying circumstances and conditions. No regulation addresses inorganic or organic contaminants in gases used for surgery (Table 2). No minimal tolerant level for particulates or microbes exists.
Table 2.
Maximum limits of impurities for carbon dioxide, USP
| Must be 99% pure | ||
|---|---|---|
| ammonia | 25 | ppm |
| carbon monoxide | 10 | ppm |
| hydrogen disulfide | 1 | ppm |
| nitric acid | 2.5 | ppm |
| odor | none | |
| sulfur dioxide | 5 | ppm |
| water | 200 | ppm |
These findings demonstrate that microbes are contained within the gas cylinders and apparatus used for laparoscopic gas delivery. This allows transmission of organisms into the abdomen of laparoscopy patients during gas delivery and throughout the procedure. Despite the fact that it appears that few “infections” occur due to this circumstance, it is wise and prudent to reduce foreign body and microbial contamination as much as possible during intra-abdominal surgery. Longer more complex procedures in patients in stressed or compromised circumstances, being very young or old and with different concomitant medical complications, requires caution and prudence to reduce contamination exposure.
The handling of gas cylinders and insufflation apparatus by personnel with little or no training and instruction contributes to the contaminated state of the apparatus, especially at points of intake and outflow attachment. Proper training regarding methods of cylinder handling and attachment to insufflation equipment needs to be addressed. Adequate knowledge and understanding of the hazards of contamination, the consequences of incorrect equipment attachment and improper handling of gas tanks' connections contribute to microbial contamination within the cylinders and associated laparoscopic gas delivery systems. This is shown by growth of microbes on the intake, egress portions of the insufflators and on the pin index systems of the gas cylinders. These areas should be cleaned by surface decontamination methods when handled and attached to any portion of the gas delivery apparatus.
The insufflation down pressure regulating throttling devices are exposed to microbes from multiple gas cylinders, contaminated intake and outflow portions of the insufflator due to improper handling and by intermittent patency to the ambient operating room environment when not in use and when gas pressures are reduced incorrectly during surgery. When not in use, the insufflator becomes a growth and culture chamber for the organisms contained within it. The insufflator then becomes a pressurized delivery vehicle of gas contaminants, inorganic debris and microbes from all portions of the gas delivery system into the patient's abdomen.
Even clean wounds from carefully performed surgery when meticulously sampled are contaminated. Therefore, the control of infection is more a quantitative than a qualitative issue.4 This concept also holds for laparoscopy. It is difficult to isolate the role of a single pathogenic factor leading to surgical infection. However, the initiating phase of bacterial infection of the surgical wound starts with microbial contamination. Contamination is not preventable even under aseptic conditions. It has been shown that 68% of 350 wounds after clean operations had bacterial growth.4 The risk of infection primarily depends on the contamination of the wound during the procedure.6
The presence of foreign material is a major pathogenic factor leading to infection.7–11 Laparoscopic gas delivery systems contain inorganic debris and foreign bodies.1 During the latent period pathogenic factors can be modified to effect the course of tissue events.12 This is the time when bacteria adhere, propagate and are protected from host defenses and antibiotics. There is less than six hours between bacterial insult and antibiotic prophylaxis for therapy to be effective. Efficient prophylaxis requires establishing a level of antibiotic concentration exceeding bactericidal resistance in the wound prior to insult or during the first six hours of surgery and maintaining these levels for an adequate period of time. Microbial multiplication in a surgical wound containing foreign material, even with a small inocula, has a latency period preceded by active microbial multiplication.11 The importance of the latency period is that microbial pathogenic effects are potentially reversible during this interval. Antibiotic prophylaxis is expensive and has potential consequences. Quantitative reduction of microbes, particulates and foreign bodies for laparoscopic gas is accomplished by a 0.3 micron filter. Its use also avoids the consequences and side-effects of antibiotics or development of resistant organisms.
Microbial adherence is required for multiplication and invasion of bacteria to precede wound infection. Antibiotics modify the interaction of microbes with natural and foreign surfaces. Foreign surfaces rapidly become coated with host proteins that facilitate bacterial adhesion.13 Kinetic studies show that interaction of the organisms with host proteins is rapid and irreversible. There is intense binding of microbes and ligands to fibronectin, fibrinogen, collagen, laminin and other extracellular matrix proteins.14 Reducing foreign body exposure of the peritoneal cavity from gas cylinder debris by filtration, diminishes host protein reaction with significant impairment of bacterial adhesion and reduces progression of infection.
No attempt was made in this study to relate clinical infection to the observed colonization of bacterial and yeast species found within the gas delivery system apparatus and components.
Use of sterile or filtered materials (i.e., gases, solids, or liquids) when placed in the human body is an accepted standard of medical care. This study shows that insufflation of CO2 into the peritoneal cavity that is not filtered by a 0.3 micron filter contains microbial organisms from the cylinder or insufflator or both. The insufflator device and apparatus internally and externally showed microbial colonization. Reduction of microbial exposure from gas insufflation apparatus is accomplished by a 0.3 micron bacterial filter placed before gas is delivered into the patient. Efficiency of 0.3 micron gas filtration for microbe free gas is also demonstrated.
Further studies are necessary to determine the contribution of bacterial virulence factors at the surgical site in the presence of foreign bodies (inorganic particulates) to the occurrence of infection, tissue healing and/or adhesion formation from laparoscopic surgeries.
CONCLUSIONS
Due to longer and more complex laparoscopic procedures being performed on patients compromised by age and surgical disease processes compounded by pre-existing medical conditions, surgical margins of safety can be reduced. These circumstances, coupled with these qualitative findings of bacterial and fungal colonization found within the laparoscopic gas delivery system, are fair warning and should raise our awareness to this circumstance and warrant methods to decrease or eliminate this exposure. Germicidal cleansing of external port connections and gas filtration to pre-condition all gases prior to intra-abdominal instillation are preferred methods to reduce microbe exposure from a laparoscopic gas insufflation system.
References:
- 1. Ott DE. Contamination via gynecologic endoscopy insufflation. J Gynecol Surg. 1989;5:205–208 [Google Scholar]
- 2. United States Pharmacopeia and National Formulary and Supplements, XXI-NF, 1984
- 3. ECRI Health Devices-Laparoscopic Insufflators. 1992;21:177–181 [PubMed] [Google Scholar]
- 4. Waldvogel FA, Vaudaux PE, Pittet D, Lew PD. Perioperative antibiotic prophylaxis of wound and foreign body infections. Rev Inf Dis. 1991;13(suppl 10):S782–789 [DOI] [PubMed] [Google Scholar]
- 5. Howe CW. Bacterial flora of clean wounds and its relation to subsequent sepsis. Am J Surg. 1964;107:696–700 [DOI] [PubMed] [Google Scholar]
- 6. Wiley AM, Haeri GBA. Routes of infection a study of using “tracer particles” in the orthopedic operating room. Clin Orthop. 1979;139:150–155 [PubMed] [Google Scholar]
- 7. Bisno AL, Waldvogel FA. eds. Infections associated with indwelling medical devices. Washington, DC: American Society for Microbiology; 1989 [Google Scholar]
- 8. Elek SD, Conen PE. The virulence of Staphylococcus pyogenes for man: a study on the problems of wound infection. Br J Exp Path. 1957:38:573–586 [PMC free article] [PubMed] [Google Scholar]
- 9. James RC, MacLeod CJ. Induction of staphylococcal infections in mice with small inocula introduced on sutures. Br J Exp Path. 1961;42:266–277 [PMC free article] [PubMed] [Google Scholar]
- 10. Noble WC. The production of subcutaneous staphylococcal skin lesions in mice. Br J Exp Path. 1965;46:254–262 [PMC free article] [PubMed] [Google Scholar]
- 11. Zimmerli W, Waldvogel FA, Vaudaux P, Nydegger UE. Pathogenesis of foreign body infection: description and characteristics of an animal model. J Infect Dis. 1982;146:487–497 [DOI] [PubMed] [Google Scholar]
- 12. Miles AA. Nonspecific defense reactions in bacterial infections. Ann N Y Acad Sco. 1956;66:356–369 [Google Scholar]
- 13. Vaudaux PE, Lew PD, Waldvogel FA. Host factors predisposing to foreign body infection. In: Bisno AL, Waldvogel FA.eds. Infections associated with indwelling medical devices. Washington, DC: American Society for Microbiology;1989:27–59 [Google Scholar]
- 14. Waldstrom T, Speziale P, Rozgonyi F, Ljungh A, Maxe I, Ryden C. Interactions of coagulase- negative staphylococci with fibronectin and collagen as possible first step of tissue colonization in wound and other tissue trauma. Zentralbl Bakteriol Hyg. (A) 1987;(suppl 16):83–91 [Google Scholar]