Abstract
This study investigated the egg-laying behaviour of ectoparsitoid, Dinarmus basalis Rondani (Hymenoptera: Pteromalidae), females when faced with a prolonged deprivation of suitable hosts leading to extreme ‘oviposition pressure’. The egg-laying behaviour of virgin D. basalis females was tested with Callosobruchus maculatus (F.) (Coleoptera: Bruchidae) hosts previously parasitized by the conspecific females in which the developing larvae had reached the last larval instar (L5) or pupae. The hyperparasitism did not prevent the occurrence of superparasitism, but only one D. basalis egg from a hyperparasitized D. basalis L5 larvae reached the adult stage due to the solitary behaviour of the D. basalis larvae. Under these experimental conditions, 60.78% of the D. basalis adults emerging from larvae were miniaturized due to the depletion of host resources.
Key words: Callosobruchus maculatus, Eupelmus vuilleti, Eupelmus orientalis, Vigna unguiculata, Bruchidae, intraspecific, interspecific discrimination, intraspecific competition, cleptoparasitic behaviour
Introduction
In West Africa (Niger, Burkina Faso, Benin), the solitary ectoparasitoid, Dinarmus basalis Rondani (Hymenoptera: Pteromalidae), and its sympatric species Eupelmus vuilleti Crawford and E. orientalis Crawford (Hymenoptera: Eupelmidae) parasitize the larvae and pupae of Callosobruchus maculatus (F.) and Bruchidius atrolineatus (Pic) (Coleoptera: Bruchidae) which develop inside the seeds of the cowpea, Vigna unguiculata (L) Walp, (Fabales: Fabaceae). After harvesting, these seeds are stocked in granaries where successive generations of bruchids develop, fluctuating in space and time. A survey of the hymenoptera population shows that the most abundant species at the beginning of storage is E. orientalis (72%), while E. vuilleti and D. basalis account for 12% and 16% respectively (Ndoutoume-Ndong and Rojas-Rousse 2007). The E. orientalis population decreases gradually during storage, disappearing completely within two months, the majority of which escape directly from the storage structures (Ndoutoume-Ndong and Rojas-Rousse 2008). However, D. basalis and E. vuilleti have been found coexisting for several months in these structures which form uniform and relatively closed habitats, resulting in inter and/or intraspecific competition (Lammers and van Huis 1989; Monge and Huignard 1991).
The coexistence of D. basalis and E. vuilleti is based on a counter-balanced competition, i.e. on two opposing behaviours (Zwölfer 1979; van Alebeek et al. 1993). This strategy implies that the females of the competitive species have interspecific discrimination capacities. In fact, D. basalis females lay fewer eggs in the presence of E. vuilleti females or in hosts parasitized by them (van Alebeek et al. 1993). In contrast, E. vuilleti females have developed an aggressive strategy, concentrating their ovipositions on hosts already parasitized by D. basalis, killing the D. basalis eggs and/or neonatal larvae by stinging them (van Alebeek et al., 1993). Host discrimination involves the detection of external and/or internal cues at the host site. External cues can be detected more rapidly than internal ones and over a longer period since they can be picked up continuously while foraging (Hoffmeister and Roitberg, 1998).
This competitive strategy of taking advantage of resources foraged by heterospecific individuals is a characteristic feature of cleptoparasitism (Sivinski et al. 1999; Jaloux and Monge 2006). The increased encounter rate with parasitized hosts and cleptoparasitic efficiency appears to be based on the detection of two independent signals (Jaloux et al. 2004; Jaloux and Monge 2005, 2006). First, olfactory detection of Dufour gland hydrocarbons, left by the D. basalis female on the cuticle on the surface of the seed, allows a seed which has been visited or exploited by D. basalis to be recognized. Secondly, detection of the proteinaceous substance produced by the D. basalis venom gland and deposited on the edge of the hole drilled through the cotyledon to reach the host indicates that the host has probably been exploited, triggering the cleptoparasitic behaviour. Because this protein is not volatile, it is probably detected through antennal or oral contact chemoreceptors (Jaloux and Monge 2006).
Internal stimuli have poor accessibility but are the most reliable indicators of previous parasitism. The study of intraspecific competition between D. basalis females shows that host discrimination is achieved through the perception of cues inside the seed, because it is only when the females have probed the host chamber with their ovipositor that they decide to accept or reject a host for oviposition (Gauthier 1996, Gauthier et al. 2002). In this species, host discrimination is expressed through two mechanisms acting independently (Gauthier et al. 1996). First, a time-dependent process: the deterring oviposition factor(s) can be perceived by the wasp after the first oviposition, reaching a maximum activity with a 24-h-old embryo; secondly, a hostquality indicator process comes into play after a host has been parasitized for 48h (Gauthier et al. 1996). During this time, a transfer of chemical information from the egg to the surface of the host occurs (Gauthier et al. 1996; Gauthier et al. 2002). The gradual deterrent effect supports the hypothesis of substances released by the egg in the course of its embryonic development. One question arising from these observations concerns the means by which the oviposition deterring effect is transferred from the egg to the host (Gauthier and Monge 1999 a).
Faced with hosts offering its offspring little chance of survival, D. basalis females lay a few eggs and resorb the others; egg resorption is a transitory process which ceases after 5 days if there is a return to favourable conditions (Gauthier 1996; Gauthier and Monge 1999 b).
However, in the Sudano-Sahelian zone of Burkina Faso and in the Guinean zone of Togo, the biological control of bruchids by releasing D. basalis females in leguminosae V. unguiculata granaries leads to unfavourable conditions at the end of the storage period due to substantially reduced numbers of the bruchid C. maculatus following the development of successive generations of D. basalis (Sanon et al. 1998; Amevoin et al. 2007). In this extreme situation, E. vuilleti and E. orientalis females, living sympatrically with D. basalis, are able to express facultative hyperparasitism when confronted by hosts parasitized by conspecific or heterospecific females (Rojas-Rousse et al. 1999; Rojas-Rousse et al. 2005). Facultative hyperparasitism involves the development of the progeny as either primary or secondary parasitoids (Sullivan 1987). In fact, facultative hyperparasitism is a very aggressive behaviour of females that sting and kill the developing primary parasitoid (L5 larvae or pre-pupae or pupae) before ovipositing on it. For E. vuilleti females the facultative hyperparasitism can be considered as an extreme expression of cleptoparasitism (expressed only towards eggs and/or neonatal larvae of primary parasitoids) (van Alebeek et al. 1993; Leveque et al. 1993; Jaloux et al. 2004, 2005; Rojas-Rousse et al. 2005).
At the end of the storage period in granaries, because the development of successive generations of D. basalis leads to a reduced number of unparasitized hosts and an increased number of parasitized hosts, D. basalis females might have to forage among hosts that offer their offspring little chance of survival. To understand the consequences of these particular environmental conditions on the population of D. basalis, this study investigated the egg-laying behaviour of D. basalis females when faced with a prolonged deprivation of suitable hosts leading to extreme ‘Oviposition pressure’. The egg-laying behaviour of virgin D. basalis females was tested with hosts parasitized by conspecific females in which the developing primary parasitoid larvae had reached the last larval instar (L5) or pupae stage. By this time the phytophagous host has been almost entirely consumed by the primary developing parasitoid larva (Rojas-Rousse et al. 2005). Under these experimental conditions of low quality host patches, we investigated the egg-laying behaviour of D. basalis females and their ability to develop at the expense of their conspecific larvae, i.e to hyper-parasitize.
Materials and Methods
Insect stocks
Bruchid and parasitoid stocks were derived from C. maculatus and D. basalis adults emerging from cowpea cultures (V. unguiculata) at the end of the rainy season in the Niamey region. C. maculatus is a common pest that develops inside cowpea seeds, concealed from the parasitoid females.
In the laboratory, bruchids and the primary parasitoids were mass-reared in climatecontrolled rooms under conditions close to those of their area of origin: 12:12 L:D, 23– 33° C and 40% RH.
The strain of C. maculatus was maintained by placing males and females (50 pairs) in rearing boxes containing 300 cowpea seeds. The females laid eggs on the seeds, and the neonate larvae perforated the coat. The four larval stages and the pupal stage were completed within the seed.
For primary parasitoid rearing, hundreds of 1 or 2 day-old adults of D. basalis were placed in transparent cages (25*30*40 cm) in presence of 200 cowpea seeds containing L4 larvae or pupae of C. maculatus. Parasitoids were provided daily with a sucrose saturated cotton roll fixed in the middle of the cage. After 2 days the seeds, parasitized or not, were removed from the cages. Primary parasitoid adults emerged from the parasitized seeds after 12–15 days for D. basalis The parasitoid females used in the experiments were isolated in Petri dishes and fed with a sucrose solution.
Experimental methods
All the experiments were carried out in the laboratory. Translucent gelatine capsules were used that mimic the bruchid pupal chamber, the size and shape being replicated by using both parts of the capsule (Cortesero and Monge 1994; Damiens et al. 2001; Jaloux et al. 2004). This system allows development to occur normally.
Activation of oogenesis of D. basalis virgin females during their first four days of life, production of paralysed hosts, D. basalis L5 larvae and pupae males
Immediately after emergence, the D. basalis virgin females were put into groups of eight in small cylindrical Plexiglass boxes (250 cm3) and provided daily with 16 cowpea seeds each containing one C. maculatus L4 larva or pupa primary host until the evening of the fourth day. Egg production reaches a peak on the fourth day of egg-laying and remains constant until the females are 8 days old (Gauthier 1996; Gauthier and Monge 1999 a).
The seeds were removed every day and stored until the terminal developmental phase of D. basalis (last L5 larval stage and /or young pupae) on the 7th ± 1 day after egg-laying (Damiens et al. 2001). The seeds were opened to isolate stung and paralysed C. maculatus hosts, the L5 larvae and young pupae of which were presented to the D. basalis virgin females during the choice tests.
Hyperparasitism choice between parasitized D. basalis L5 larvae and young pupae
To produce extreme oviposition pressure, the D. basalis virgin females received no hosts from the fourth to eighth day of life. These virgin females, ‘conditioned’ by deprivation of suitable hosts for 4 days, were used in the hyperparasitism tests.
The D. basalis L5 larva or pupa was confined in a transparent cell, the same size and shape as the lodge of a bruchid larva in a seed, and with holes drilled on the surface to simulate the bruchid larval gallery providing access to the host.
Eight ‘conditioned’ virgin D. basalis females were kept in a small cylindrical Plexiglass box (250 cm3) with eight D. basalis hosts put singly into gelatine capsules containing alternately one D. basalis L5 larva or one young pupa (total per box: four L5 larvae and four young pupae). Egg-laying was observed every day for 4 days (total in 4 days: 16 L5 larvae and 16 young pupae). Six sets were completed (total: 16x 6 L5 larvae and 16x 6 young pupae). These experiments were carried out under the same climatic conditions as those used for rearing bruchids and parasitoids.
Choice between non-stung (healthy) and stung-paralysed C. maculatus hosts
D. basalis can distinguish pupae from L5 larvae by their physiology and by the texture of their integument: soft in larvae and chitinised in pupae. Before egg-laying, D. basalis females inflict a sting which has a paralysing action on the host. However, as the females' decision to accept or reject a host is based on the perception of cues inside the seed when they probe the host chamber with their ovipositor, we hypothesized that D. basalis females could be deluded about the quality of the host stage (Gauthier et al. 2002). To test this hypothesis that hosts can be rejected due to their immobility after the paralysing sting, the egg-laying behaviour of the ‘conditioned’ virgin females exposed to primary healthy and stung-paralysed C. maculatus hosts is examined. The same experimental method described above were used, since the C. maculatus stungparalysed L4 larvae hosts would be easy to identify due to their immobility and melanized scars (Rojas-Rousse et al. 1995).
These experiments were carried out under the same conditions as those used for choice between D. basalis L5 larvae and young pupae. Egg-laying of virgin D. basalis females (4 days old) was observed for 2 consecutive days (total in 2 days: 8 stung C. maculatus and 8 non-stung C. maculatus L4). Seven sets were studied (total: 8 × 7 stung L4 larvae, and 8 × 7 healthy C. maculatus L4 larvae). The experiments were carried out under the same climatic conditions as those used for rearing bruchids and parasitoids.
Development of eggs laid on hyperparasitized hosts
The number of eggs laid by virgin D. basalis females on D. basalis hosts was noted, and the hyperparasitized host + eggs were placed in a cell in a Plexiglass sheet closed by a Plexiglass cover-slide until emergence of the hyperparasitoid adult male. This developmental chamber has already been used successfully for developing parasitoids (Darrouzet et al. 2003). As only virgin D. basalis females were used, when more than one egg was laid per host, the larval competition that occurred did not affect the final sex-ratio because reproduction was by arrhenokotous parthenogenesis and consequently only male eggs were involved.
Statistical analysis
The chi-square test was used to test the homogeneity of the egg-laying behaviour of D. basalis females between the data sets. If homogeneity was accepted, all the data sets could be pooled.
To evaluate the hyperparasitism behaviour of D. basalis females, the observed numbers of hyperparasitized and non-hyperparasitized L5 larvae were compared with those theoretically expected under the null hypothesis, whereby no preference would be shown by the egg-laying females. According to this null hypothesis, the theoretical probability of hyperparasitism was 1/2. The same method was used to demonstrate the behaviour of the females exposed to non-stung (i.e. healthy) and stung-paralysed C. maculatus hosts. The eggs laid on each type of hyperparasitized host were counted and compared using Student's t-test.
Results
Choice between D. basalis L5 and pupae hosts
In each data set studied, some D. basalis L5 hosts presented at the beginning of the test reached the pupal stage, and the egg-laying distribution indicated that the D. basalis pupae were not hyperparasitized (Table 1).
Table 1.
Comparison of hyperparasitism behaviour of virgin D. basalis females exposed to D. basalis L5 larvae hosts and D. basalis pupae hosts
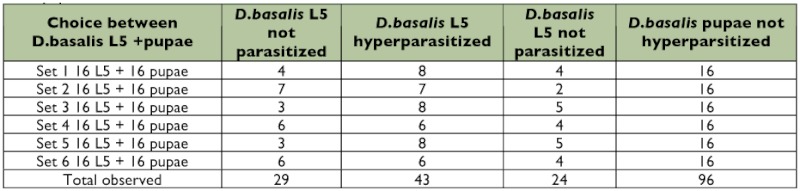
Some of the D. basalis L5 hosts were hyperparasitized (Table 1). The null hypothesis was tested that there would be no difference in the proportion of hyperparasitized and non-hyperparasitized D. basalis L5 hosts between the six data sets. Since this hypothesis of homogeneity was confirmed, the six data sets were pooled (χ2 calculated = 3.65: α = 0.05, χ2ddl 5 = 11.07).
To evaluate the behaviour of D. basalis females exposed to L5 hosts, the numbers of hyperparasitized (N = 43) and nonhyperparasitized (N = 29) hosts observed were compared with those theoretically expected under the null hypothesis. Under these conditions, D. basalis females hyperparasitized as many L5 hosts as they avoided (χ2 calculated = 2.72: α = 0.05, χ2ddl 1 = 3.84). These results indicate only that the D. basalis females were able to lay on their last stage larvae.
Choice between primary non-stung (healthy) and stung-paralysed C. maculatus hosts
To test whether D. basalis pupae hosts were avoided due to their immobility, D. basalis females were presented alternately with stung-paralysed and non-stung (i.e., healthy) primary C. maculatus L4 hosts. They were able to lay eggs on both categories of hosts (Table 2).
Table 2.
Comparison of egg-laying behaviour of virgin D. basalis females exposed to C. maculatus primary paralysed hosts (i.e., immobile hosts), and healthy moving hosts.
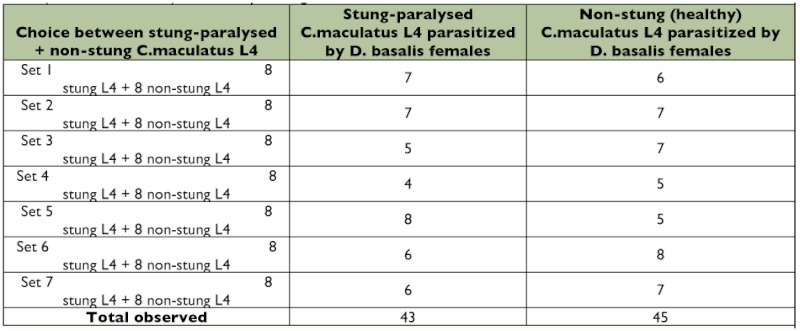
The null hypothesis was tested whereby no difference of parasitism would be observed between the seven data sets. As this hypothesis of homogeneity was confirmed, the seven data sets were pooled (χ2 calculated = 1.53: α = 0.05, χ2ddl 6 = 12.59).
To evaluate whether D. basalis females showed a preference for one or other type of primary host, the numbers of parasitized and avoided hosts were compared with those theoretically expected under the null hypothesis (i.e., 44 parasitized and avoided C. maculatus L4 hosts: 43 + 45/2) (Table 2). The observed number of primary hosts (healthy or stung) parasitized by D. basalis females was not significantly different from the theoretically expected number (χ2 calculated = 0.19: α = 0.05, χ2ddl 1 = 3.84). Thus, when D. basalis females could choose between non-stung (healthy) or stungparalysed C. maculatus L4 larvae, they parasitized both categories equally.
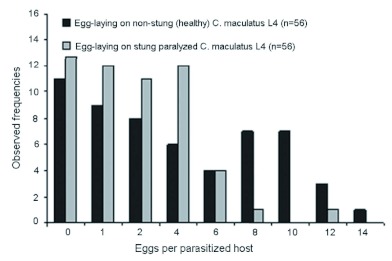
However, analysis of the eggs laid per host showed that 51.8% (29/56) of stung-paralysed primary hosts, and 64.28% (36/56) of the healthy primary hosts presented, were superparasitized (i.e., more than one egg laid per host) (Figure 1). The percentage of superparasitized hosts did not differ significantly between the two types of primary host presented alternately to D. basalis females (t-test for frequencies comparison: t = 1.02, significance level α = 0.05, t [.05] ∞ = 1.96). However, on average, D. basalis females laid significantly more eggs per healthy primary host than per stung-paralysed primary host: 4.25 ± 1.05 and 2.04 ± 0.29 respectively (mean number of eggs laid ± 95% confidence interval); (Student's test: t = 3.65 significance level α = 0.05 t [.05] ∞ = 1.96).
Figure 1.
Distribution of eggs laid by Dinarmus basalis virgin females exposed to moving, i.e., healthy Callosobruchus maculatus primary hosts, and non-moving, i.e., stung-paralyzed C. maculatus hosts (previously stung by D. basalis females). When there was more than one egg per host, the host was superparasitized. High quality figures are available online.
Development of eggs laid on hyperparasitized D. basalis L5 hosts
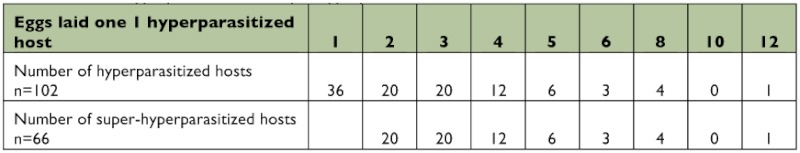
The individual development of 102 hyperparisitized D. basalis L5 hosts was observed in the Plexiglass cells. Since one to twelve eggs were laid per hyperparasitized D. basalis L5 host, hyperparasitism did not prevent the occurrence of superparasitism (Table 3). Although only one egg per host reached the adult stage due to the solitary behaviour of the D. basalis larvae, under our experimental conditions we observed that 64.7% of D. basalis L5 hosts were superhyperparasitized (66/102) (Table 3). Under these experimental conditions, egg development occurred in three possible ways. First, 60.78% of the hyperparasitized D. basalis L5 hosts (62/102) reached the miniaturized hyperparasitoid adult male stage. Second, the eggs hatched but the neonatal hyperparasitoid larvae died allowing the D. basalis L5 host to reach the adult stage (i.e., the primary parasitoid adult); this was observed in nine hyperparasitized hosts (9/102). And finally, the eggs laid on 31 hyperparasitized D. basalis L5 hosts died during embryonic development (31/102).
Table 3.
Number of hyperparasitized and super-hyperparasitized hosts
Discussion
Parasitoids use a large number of physical and chemical cues when they forage for hosts, and many of them mark the host patch, the host substrate, and/or the host itself (Godfray 1994; Quicke 1997). These marks may enable females to discriminate between unexploited and previously exploited resources, and also inform other females, conspecifics or heterospecifics, about the presence of a possibly superior competitor (Roiteberg and Mangel 1988). In this way, D. basalis parasitoid females are able to discriminate the quality of their host, but detailed behavioural observations show that this host discrimination is based on internal cues, and the lack of evidence of external marks is unexpected (Gauthier et al. 2002). For D. basalis females, host quality (healthy or parasitized 24h or 48h beforehand) does not affect the attractiveness of seeds in which hosts are concealed (Gauthier et al. 2002). It is only when the D. basalis female has drilled the cotyledons of the seed to reach the host chamber and has probed this chamber with her ovipositor that the decision is made whether to accept or reject the host for oviposition (Gauthier et al. 2002). These internal cues have low accessibility but are the most reliable indicators of previous parasitism and of the age of parasitic instars (Gauthier 1996). However, D. basalis females exhibit a wide range of oviposition behavioural plasticity, and host discrimination ability does not always involve avoidance of superparasitism (Gauthier et al. 1996). Under unfavourable conditions, D. basalis females are able to resorb the unlaid eggs with no effect on future oviposition because they have a relatively large daily oviposition window compared to the potential number of eggs laid (Gauthier 1996; Gauthier et al. 1996; Gauthier and Monge 1999a).
Superparasitism may sometimes be advantageous when unparasitized hosts are scarce, and/or the neonatal larvae born from eggs of the last oviposition have a better chance of competiting successively against older competitors, and/or the risk of parasitism by conspecific or heterospecific females is high (Visser et al. 1992; Godfray 1994). In this way, when D. basalis females superparasitize hosts, the survival probability of the second egg laid has been shown to vary with the age of the first parasite already on the host (Gauthier 1996). In fact, the survival rate of the second parasitoid increases with the interval between ovipositions (Visser et al. 1992; Mackauer 1990).
The use of D. basalis in granaries as a biological bruchid control agent leads to an increased number of parasitized hosts creating unfavourable conditions during the storage period (Sanon et al. 1998; Amevoin et al. 2007). These conditions were recreated in the laboratory using D. basalis virgin females that did not receive suitable hosts, i.e. primary bruchid L4 or pupae, for four consecutive days. After this extreme oviposition pressure caused by host deprivation, the females were presented with D. basalis L5 larvae hosts. Only virgin D. basalis were used in these experiments to eliminate all consequences of parthenogenetic reproduction, i.e. number of matings, sex ratio at egg-laying, etc. The D. basalis L5 larvae hosts had molted to a passive stage corresponding to the end of the feeding period on the primary host, i.e. bruchid L4 or pupae. Under these experimental conditions, the D. basalis virgin females were able to hyperparasitize the D. basalis L5 larvae hosts However, future research should investigate the behaviour of inseminated D. basalis females under unfavourable conditions, particularly in view of the fact that the progeny of hyperparasitized inseminated Eupelmus vuilleti females are largely male (Rojas-Rousse et al. 2005). Since D. basalis hyperparasitoid larvae feed externally, no special adaptations may be needed to attack the primary parasitoid (Godfray 1994). Under our experimental conditions, 60.78% of hyperparasitized D. basalis L5 larvae hosts became hyperparasitoid miniaturized adult males. Approximately a third of the hyperparasitized D. basalis L5 larvae hosts died after being stung by adult females during egg-laying, although the venomous sting generally only induces permanent paralysis and developmental arrest of the host during the development stages of first larvae (Doury et al. 1995). This hyperparasitism corresponds to the facultative hyperparasitism arising from competition between parasitoids for host resources, which is considered as one possible evolutionary pathway leading to obligatory hyperparasitism (Godfray 1994).
The hyperparasitism behaviour did not modify the reproductive behaviour of the egg-laying females because stinging always occurred before egg-laying, although the eggs were laid externally. However, this behaviour did not exclude superparasitism because more than one egg per host could be laid. In fact, 64.7% of the hyperparasitized D. basalis L5 larvae hosts were super-hyperparasitized. However, 8.82% of the hosts without stings reached the primary parasitoid male adult stage after all the neonatal hyperparasitoid larvae died during fights.
Under our experimental conditions, the D. basalis virgin females could choose between D. basalis L5 larvae and pupal hosts. Egg distribution showed that no D. basalis pupa host was ever hyperparasitized. Besides their physiological differences, D. basalis L5 larvae and pupae can be distinguished by the texture of their integument (soft in the L5 larvae and chitinised in the pupae), which is the basis of mechanical and/or chemical cues perceived by the females' ovipositor at egglaying. These cues could underlie the variability of responses to host quality. Another hypothesis is that it is more difficult for a young hyperparasitoid neonatal larva to become implanted on the chitinised integument of a pupa than on the soft integument of an L5 larva, especially if it has not been immobilised, i.e. paralysed, by the adult female at egg-laying. This behaviour, induced by notable physical differences between the L5 and the pupae, was also observed during the primary parasitism of the C. maculatus host. In fact, egg-laying is greater on the larval stages than on the younger non-chitinised and older chitinised pupae (Terrasse and RojasRousse 1986) The immobility of the D. basalis pupae alone did not seem to induce the cues favouring their rejection, because when the D. basalis virgin females could choose between healthy (i.e. non-paralysed) or paralysed C. maculatus L4 larvae, they laid eggs on both categories, although significantly more on the healthy host.
The responses of D. basalis females when faced with host deprivation leading to extreme oviposition pressure revealed that they were able to parasitize their own developing primary last instar larvae. Some species of primary parasitoids can be facultative hyperparasitoids, but the hyperparasitism is always interspecific (Strand 1986). The first recorded observation of a facultative hyperparasitoid developing within its own species was Anaphes victus (Hymenoptera: Mymaridae), and no other hyperparasitoids are known in this family (Sullivan 1987; van Baaren et al. 1995).
Facultative hyperparasitism is functionally similar to conspecific superparasitism. In solitary parasitoids, conspecific superparasitism can be advantageous if there are high levels of competition, long inter-patch travel times, low quality patches and timelimited resources (van Alphen and Visser 1990). The D. basalis females hyperparasitized their own species when they were confronted with a low-quality patch (D. basalis L5 larvae or D. basalis pupae) and when there was a high level of competition with females who were or had been present in their habitat. With an average success rate of 60%, the facultative hyperparasitism of D. basalis females can be seen as highly adaptive when faced with low quality patches. In fact, we observed that the D. basalis females were also able to hyperparasitize other species, such as Eupelmus vuilleti and Monoksa dorsiplana Boucek (Hymenoptera: Pteromalidae) (primary parasitoids of bruchids), but secondary parasitoid adults never emerged (personal observations). However, there are fitness costs associated with this mode of development, because a significant decrease in the size of secondary parasitoids has been observed following the depletion of host resources (Brodeur, 2000).
Acknowledgements
I would like to pay my last respects to Professor Vincent Labeyrie, who died 8th September 2008. With the support of the CNRS, he created the first Experimental Ecology laboratory in Tours (France) and taught me about the ecological problems arising from the population dynamics of phytophagous and entomophagous insects in agrosystems. I would like to thank Elizabeth Yates (Inter-connect) for correcting the English text.
References
- Amevoin K, Sanon A, Apossaba M, Glitho IA. Biological control of bruchids infesting cowpea by the introduction of Dinarmus basalis Rondani (Hymenoptera: Pteromalidae) adults into farmers'stores in West Africa. Journal of Stored Products Research. 2007;43:240–247. [Google Scholar]
- van Alebeek FAN, Rojas-Rousse D, Leveque L. Interspecific competition between Eupelmus vuilleti and Dinarmus basalis, two solitary ectoparasitoids of Bruchidae larvae and pupae. Entomology Experimentalis and Applicata. 1993;69:21–31. [Google Scholar]
- van Alphen JJM, Visser ME. Superparasitism as an adaptative strategy for insects parasitoids. Annual Review of Entomology. 1990;35:59–79. doi: 10.1146/annurev.en.35.010190.000423. [DOI] [PubMed] [Google Scholar]
- van Baarem J, Boivin G, Nenon JP. Intraspecific hyperparasitism in a primary hymenopteran parasitoid. Behavioural Ecology and Sociobiology. 1995;36:237–242. [Google Scholar]
- Brodeur J. Host specificity and trophic relationships of hyperparasitoids. In: Hochberg ME, Ives AR, editors. Parasitoid Population Biology. Vol. 11. Princeton University Press; 2000. pp. 163–183. [Google Scholar]
- Cortesero AM, Monge JP. Influence of pre-emergence experience on response to host plant odors in the larval parasitoid Eupelmus vuilleti. Entomology Experimentalis and Applicata. 1994;72:281–288. [Google Scholar]
- Damiens D, Imbert E, Bressac Ch, Thibeaudeau Ch, Chevrier C. Egglaying, pre-imaginal growth dynamics, and mortality in Eupelmus orientalis and Dinarmus basalis: Two solitary ectoparasitoids of Callosobruchus maculatus. Entomologia Experimentalis and Applicata. 2001;99:97–105. [Google Scholar]
- Darrouzet E, Imbert E, Chevrier C. Self-superparasitism consequences for offspring sex ratio in the solitary ectoparasitoid Eupelmus vuilleti. Entomologia Experimentalis and Applicata. 2003;109:167–171. [Google Scholar]
- Doury G, Rojas-Rousse D, Periquet G. Ability of Eupelmus orientalis ectoparasioid larvae to develop on an unparalysed host in the absence of female stinging behaviour. Journal of Insect Physiology. 1995;41:287–296. [Google Scholar]
- Godfray HCJ. Parasitoids. Behavioural and Evolutionary Ecology. Princeton University Press; 1994. [Google Scholar]
- Gauthier N. Etude d'un ectoparasitoïde solitaire Dinarmus basalis (Hym. Pteromalidae) en situation de compétition intra- et interspécifique: activité reproductrice et réponses comportementales. Thèse, Université de Tours; France: 1996. [Google Scholar]
- Gauthier N, Monge JP, Huignard J. Superparasitism and host discrimination in the solitary ectoparasitoid Dinarmus basalis. Entomology Experimentalis and Applicata. 1996;79:91–99. [Google Scholar]
- Gauthier N, Monge JP. Could the egg itself be the source of the oviposition deterrent marker in the ectoparasitoid Dinarmus basalis? Journal of Insect Physiology. 1999 a;45:393–400. doi: 10.1016/s0022-1910(98)00138-3. [DOI] [PubMed] [Google Scholar]
- Gauthier N, Monge JP. Behavioural and physiological responses to conflicting oviposition stimuli in a synovigenic parasitoid. Physiological Entomology. 1999 b;24:303–310. [Google Scholar]
- Gauthier N, Bénédet F, Tricault Y, Monge JP, Huignard J. Marking behavior and discrimination of concealed hosts by the ectoparasitoid, Dinarmus basalis Rond. (Hym. Pteromalidae). Journal of Insect Behavior. 2002;15:589–606. [Google Scholar]
- Hoffmeister TS, Roitberg BD. To mark the host or the patch: decisions of a parasitoid searching for concealed host larvae. Evolutionnary Ecology. 1998;11:145–168. [Google Scholar]
- Jaloux B, Sanon A, Huignard J, Monge JP. Interspecific relationships between the solitary ectoparasitoid, Eupelmus vuilleti Craw. (Eupelmidae). Journal of Insect Behavior. 2004;17:793–808. [Google Scholar]
- Jaloux B, Monge JP. Sources of chemical signals which enhance multiparasitism preference by a cleptoparasitoid. Journal of chemical Ecology. 2005;31:1325–1337. doi: 10.1007/s10886-005-5289-y. [DOI] [PubMed] [Google Scholar]
- Jaloux B, Monge JP. Kairomone stimulates increased probes and host stings in a cleptoparasitoid. Physiological Entomology. 2006;31:197–200. [Google Scholar]
- Lammers P. M., van Huis A. Uscana lariophaga Steffan (Hym. Trichogrammatidae), egg parasitoid of the stored insect pest Callosobruchus maculatus (F.) and Bruchidius atrolineatus Pic (Col. Bruchidae): Population studies in the fiels and storage in Niger. Proceedings of the International Conference on Integrated Pest Management in Tropical and Subtropical Ecosystems, Feb. 8–15, Bad Dürkheim. 1989;3:1013–1022. [Google Scholar]
- Leveque L, Monge JP, Rojas-Rousse D, van Alebeek FAN, Huignard J. Analysis of multiparasitism by Eupelmus vuilleti (Craw.) (Eupelmidae) and Dinarmus basalis (Rond.) (Pteromalidae) in the presence of one of their common hosts, Bruchidius atrolineatus (Pic) (Coleoptera: Bruchidae). Oecologia. 1993;94:272–277. doi: 10.1007/BF00341327. [DOI] [PubMed] [Google Scholar]
- Mackauer M. Host discrimination and larval competition in solitary endoparasitoids. In: Mackauer M, Ehler LE, Roland J, editors. Critical issues in biological control. Intercept/VCH; Andover: 1990. pp. 41–62. [Google Scholar]
- Monge JP, Huignard J. Population fluctuations of two bruchid species Callosobruchus maculatus (F.) and Bruchidius atrolineatus Pic (Col. Bruchidae) and their parasitoids Dinarmus basalis (Rondani) and Eupelmus vuilleti (Crawford) (Hymenoptera, Pteromalidae, Eupelmidae) in a storage situation in Niger. Journal of African Zoloogy. 1991;105:187–196. [Google Scholar]
- Ndoutoume-Ndong A, Rojas-Rousse D. Y a-t-il élimination d'Eupelmus orientalis Crawford par Eupelmus vuilleti Crawford (Hymenoptera, Eupelmidae) des systèmes de stockage du niébé (Vigna unguiculata Walp)? Annales de la Société Entomologique de France. 2007;43:139–144. [Google Scholar]
- Ndoutoume-Ndong A, Rojas-Rousse D. Rôle de l'intensité lumineuse sur les capacités parasitaires d'Eupelmus orientalis Crawford et d'Eupelmus vuilleti Crawford, parasitoïdes des Bruchidae ravageurs de graines de niébé (Vigna unguiculata Walp). Biotechnologie, Agronomie, Société, Environnement. 2008;12:3–8. [Google Scholar]
- Quicke DLJ. Parasitic wasps. Chapman and Hall: 1997. [Google Scholar]
- Roiteberg BD, Mangel M. On the evolutionary ecology of marking pheromones. Evolutionary Ecology. 1988;2:289–315. [Google Scholar]
- Rojas-Rousse D, Doury G, Terrasse C, Kahnes R. Behavioural plasticity in the stinging act of female ectoparasitoids. Physiological Entomology. 1995;20:147–154. [Google Scholar]
- Rojas-Rousse D, Ndoutoume-Ndong A, Kalmes R. Facultative hyperparasitism of developing parasitoids by the ectoparasitoids Eupelmus vuilleti and Eupelmus orientalis (Craw). Comptes Rendus de l'Académie des Sciences Paris. 1999;322:393–399. [Google Scholar]
- Rojas-Rousse D, Bressac Ch, Thibeaudeau C, Kalmes R, Darrouzet E, Chevrier C. Capacité de reproduction des femelles Eupelmus vuilleti (Eupelmidae), inséminés par des mâles développés en hyperparasitoïdes de Dinarmus basalis (Pteromalidae). Comptes Rendus de l'Académie des Sciences Paris. 2005;328:802–811. doi: 10.1016/j.crvi.2005.06.003. [DOI] [PubMed] [Google Scholar]
- Sanon A, Ouedraogo AP, Tricault Y, Credland PF, Huignard J. Biological control of bruchids in cowpeastores by release of Dinarmus basalis (Hymenoptera: Pteromalidae) adults. Biological control. 1998;27:717–725. [Google Scholar]
- Sivinski J, Marshall S, Petersson E. Kleptoparasitism and phoresy in the Diptera. The Florida Entomologist. 1999;82:179–197. [Google Scholar]
- Strand MR. The physiological interactions of parasitoids with their hosts and their influence on reproductive strategies. In: Waage JK, Greathead DJ, editors. Insects parasitoids. Academic Press; 1986. pp. 97–136. [Google Scholar]
- Sullivan DJ. Insect hyperparasitism. Annual Review of Entomology. 1987;32:49–70. doi: 10.1146/annurev.ento.44.1.291. [DOI] [PubMed] [Google Scholar]
- Terrasse Ch. Mise en évidence et hypothèses de régulation de l'influence du stade de l'hôte, Callosobruchus maculatus F. (Coléoptère: Bruchidae), et de sa taille sur le taux sexuel d'un de ses parasitoides, Bruchicida vuilleti Cwf. (Hyménoptère: Eupelmidae). Thèse de Doctorat de l'Université de Tours (France); 1986. [Google Scholar]
- Terrasse Ch, Rojas-Rousse D. Distribution de la ponte et évitement du superparsitisme chez l'hyménoptère solitaire Bruchicida vuilleti Cwf. (Hyménoptère: Eupelmidae), parasite des stades larvaires de son hôte, Callosobruchus maculatus F. (Coléoptère: Bruchidae). Journal of Applied Entomology. 1986;101:243–256. [Google Scholar]
- Visser ME, Luyckx B, Nell HW, Boskamp GJF. Adaptative superparasitism and patch time allocation in solitary parasitoids/ marking of parasitized hosts in relation to the pay-off from superparasitism. Ecological Entomology. 1992;17:76–82. [Google Scholar]
- Zwölfer H. Strategies and counterstrategies in insert population systems competing for space and food in flower heads and plant galls. Fottschritte der Zoologie. 1979;25:331–353. [Google Scholar]