Abstract
In the body, cells encounter a complex milieu of signals, including topographical cues. Imposed topography can affect cells on surfaces by promoting adhesion, spreading, alignment, morphological changes, and changes in gene expression. Neural response to topography is complex, and depends on the dimensions and shapes of physical features. Looking toward repair of nerve injuries, strategies are being explored to engineer guidance conduits with precise surface topographies. How neurons and other cell types sense and interpret topography remains to be fully elucidated. Studies reviewed here include those of topography on cellular organization and function as well as potential cellular mechanisms of response.
Keywords: axon, neuron, contact guidance, cell tension, lithography
1 Introduction
1.1 Historical Introduction to Contact Guidance
That cells respond to the topography, or physical features of their environment, is intuitive, and has been observed before. In 1912, Harrison pointed out that the solid material which supports cells has a great deal of influence on their morphogenesis and movement, and described the way in which cells, when placed on a spider web, adapt their shape and movement to the web fibers (1). However, one hundred years later a number of basic questions about how cells respond to the topography of their environment remain. Early observations of contact guidance include those by Paul Weiss, who described in 1945 how contact guidance is one of the basic methods by which cells can migrate from their source to their destination (2), and Curtis and Varde in 1964 who used topography to control cell behavior (3). Around 20 years ago, microfabrication techniques gained popularity for creating cell culture platforms with which researchers could probe the behavior of cells on well defined surfaces with micro- and nanoscale geometries (4). This led to studies in which cells were studied on surfaces with anisotropic groove-ridge topographies, or isotropic topographies, as reviewed previously (5, 6).
In this article we review in vitro and in vivo studies of cellular responses to topography. We focus on neuronal cells and their distinct functions both as revealed in cell culture studies and in animal models of nerve regeneration. We also cover research involving other cell types where these serve to illuminate the complexities of cellular functions. Finally, we discuss potential cellular mechanisms that underlie this complex phenomenon.
1.2 Contact Guidance in the Developing and Regenerating Nervous System
There are a number of instances in the developing nervous system in which cells migrate along tracts of glial cells or oriented extracellular matrix (ECM) fibers. For example, during the histogenesis of the cerebral cortex, cortical neurons are guided along radial glial cells (7–9). The migrating neurons wrap around the radial glia, which act as both the scaffold and the source of new neurons (10). Similarly, migrating neuroblasts of the external granule layer of the cerebellum follow axon tracts that have already been laid (11), and axons in the developing Drosophila nervous system follow the path laid out by longitudinal glia (12). Interestingly, when granule neurons from the cerebellum were cultured with astroglial fibers from the hippocampus, and hippocampal neurons with astroglial fibers from the cerebellum, migration of each type of neuron was identical to that in the homotypic culture, indicating that the glial fibers may provide a generic pathway for neuronal migration in vivo (13). In addition, neurons in the medulla oblongata were found to migrate parallel to oriented fibers (14). Pre-formed glial tracts along which axons migrate have been described as “slings”, and it has been hypothesized that such structures can play a crucial role in establishing proper connectivity within the developing nervous system (15). Though parallel, or tangential, contact guidance has been most commonly reported, perpendicular contact guidance has also been observed, primarily in CNS rather than PNS neurons (16). Though it is clear that glia play an active role in the developing nervous system, rather than simply serving as a scaffold for neuronal development and axon pathfinding, it also appears that neurons are quite adept at following topological cues in vivo, and that the glial tracts may provide both biochemical and physical cues which are vital to ensuring the establishment of proper connectivity.
Another phenomenon observed in the developing nervous system is that of guidance by guidepost cells, specific cells in the embryonic environment which serve as intermediate locations during axon pathfinding. Though guidepost cells have been most well-documented in invertebrate systems such as the grasshopper(17) and the fruit fly (18–20), it has been suggested by a number of researchers that the mechanism is well conserved in vertebrates and even mammals (21, 22). Though this may not be a case of contact guidance in the sense that is typically meant, it illustrates the way in which pathfinding axons may grow in intervals, responding to a local cue with a structural change before stopping to seek out the next permissive local cue. As will be discussed in the next section, axons can be guided in vitro by disjoint raised structures on the order of the size of a single cell, indicating that the idea of guidance by contact with guidepost structures may be broadly applicable for navigating axons.
Contact guidance likely plays a role in the response to injury in both the peripheral and central nervous systems (PNS and CNS, respectively). Regeneration in both the PNS (23) and the CNS (24) have been reviewed extensively elsewhere. Following a nerve transection in the peripherial nervous system, the distal portion of the nerve undergoes Wallerian degeneration, in which the cytoskeleton and cell membrane of the disconnected axon begin to break down (25). It has been recognized for some time that Schwann cells (SC) aid in the repair of the injury environment (26). In response to axon transection, the myelin sheath undergoes longitudinal segmentation. SCs proliferate, forming a Büngner band, in addition to producing growth factors in response to denervation, cleaning up the debris of Wallerian degeneration, and laying down tracks which will then retract when reinnervation occurs (27). Regenerating axons and SCs have a complex interrelationship (28), that includes direction and provision of multiple guidance cues by SCs (29), and nerve regeneration occurs at a rate of 2-4 mm/day after PNS injuries (30). The bands of Büngner play an important role in this process, with oriented SCs and ECM guiding regenerating peripheral nerves.
In stark contrast to the PNS, injuries in the CNS have long been thought to be incapable of spontaneous repair. CNS axons do not regenerate in their native environment, which contains reactive astrocytes, inhibitory myelin-based glycoproteins and proteoglycans which form a glial scar (31) (32). After a spinal cord injury (SCI), macrophages are slow to infiltrate the wound site (33), which contrasts to the prompt removal of inhibitory debris by macrophages and SCs after PNS injuries. Together, the combination of physical and chemical barriers along with the lack of a prompt immune response results in very poor outcomes for patients with SCI. However, a number of neural tissue engineering strategies are being explored for both CNS injuries and PNS injuries which are too extreme to heal without intervention (34).
1.3 Axon Growth and Guidance
The motile apparatus of neurons is the growth cone, a highly sensitive structure at the tip of growing axons. Growth cone structure and pathfinding are important research topics which have been extensively reviewed elsewhere (35). Axon pathfinding is responsible both for establishing neuronal circuitry in the developing brain (36), and for the re-wiring of pathways that are damaged from injury or disease (37). Growth cone behaviors such as advancing, retracting, turning and branching are regulated by dynamic reoganization of actin filaments and microtubules, which in turn are linked to the receptors for molecules such as Netrins, Slits, Semaphorins, and Ephrins (38). Growth cones integrate complex cues which vary in both time and space, and may also be synergistic or competitive. Recent progress indicates that membrane microdomains may be responsible for generating the localized signaling that produces such specific responses to guidance cues, that calcium signaling is vital to controlling growth cone steering, and that focal adhesions may function as the biomolecular integration point of growth cone signaling and response (39). Growth cone and axon guidance are important in investigating neuronal response to topography, as it is control over axon guidance which will lead to therapeutic solutions to damaged nerves (40).
1.4 Assessment of Neuronal Response to Topography
A range of techniques have been used in the literature to assess cellular, and specifically neuronal, response to topography. Neurites have been quantified in many ways, including: the number of cells forming neurites (41) or axons (42); the average number of neurites per cell(43); the average neurite length per cell (44); the length of the longest neurite or axon (42); the total neurite extension length (45, 46); and the degree of neurite branching (43). Studies with whole explants have evaluated the distances which migratory cells and individual neurites are located relative to the explant edge (47). Because of the importance of directed growth in repair strategies for damaged spinal cord or peripheral nerves, one important metric for the ability of a surface to guide neuronal growth is alignment. Alignment can be measured for the cell soma, the cellular extensions, or both. Neurites have been subjectively quantified as either aligned or unaligned in a number of studies (48, 49). However, because this method is potentially subject to bias, and because the question of whether or not a particular neurite is aligned can be rather difficult to answer, a number of more quantitative methods have been developed. Somal or nuclear alignment can be used to indicate alignment relative to any directive cue (50). This method is especially useful for cell types which may undergo a whole-cell response to topography, such as many glial cell types. The angle of neurite outgrowth can be assessed by grouping the data into angular bins, and showing that topography increases the frequency of neurites which fall into bins close to the angle of the topographical cue (51–53). Nuclear or neurite angular data can be further quantified by circular statistical methods (54). Rather than evaluating the length of each neurite or neurite segement, the overall alignment of an entire field of view can be quantified using a fourier-transform method, which will indicate the angle at which the most activity occurs (55, 56). These methods to assess cellular and neurite outgrowth are capable of showing in a quantitative manner how neurons respond to topography. Because morphology is so closely linked to function, determining the effect of cellular growth and morphology on a tissue engineered surface gives an indication of the efficacy of that material and geometry for promoting a specific, desired response.
2 Neuronal Response to Imposed Topography: In Vitro Studies
In the past 20 years, many studies of contact guidance have been performed, owing in large part to the relative ease of fabrication of surfaces with myriad types and shapes of molecular and cellular scale topographical features. As of 1999, many studies had documented the effects of substrates with microgrooved topographies (5, 57), noting the effects of scale and surface coating. Many studies note the response of cell types which do not tend to form processes, such as fibroblasts and osteoblasts, but rather undergo a cell-wide response, generally alignment to longitudinal features. As discussed in this section, neuronal response to topography appears to be somewhat distinct. Because neurons have extensive networks of axons and dendrites (referred to non-specifically as neurites) which extend from a cell body which does not necessarily undergo the same behavior, neurons have been shown to respond to topography in particular ways, including perpendicular contact guidance, and the formation of contact-guided networks on globally isotropic topographies. Neurons have also been shown to respond strongly to the topography of glial cells, an observation which may prove useful for tissue engineered strategies of nerve repair. Most studies discussed in this section evaluate the alignment of the neuronal soma or of the extending neurites, as a primary goal of neural tissue engineering is to guide neurite extension in a desired direction (58). However, a range of other effects has been observed on surfaces with distinct topographical features, including: decreased neurite architecture/branching (43, 45), reduced number of neurites (59, 60), increased neurite length (60–62), and increased polarization (41, 42). Because neurons have such an incredible ability to explore and respond to their surroundings, in both the developing and mature nervous systems, it is not unexpected that they should have such complex responses to their extracellular environment. What remains to be done is to determine the precise features that result in a particular desired response, and to harness this ability to treat damaged nerves in the clinical setting.
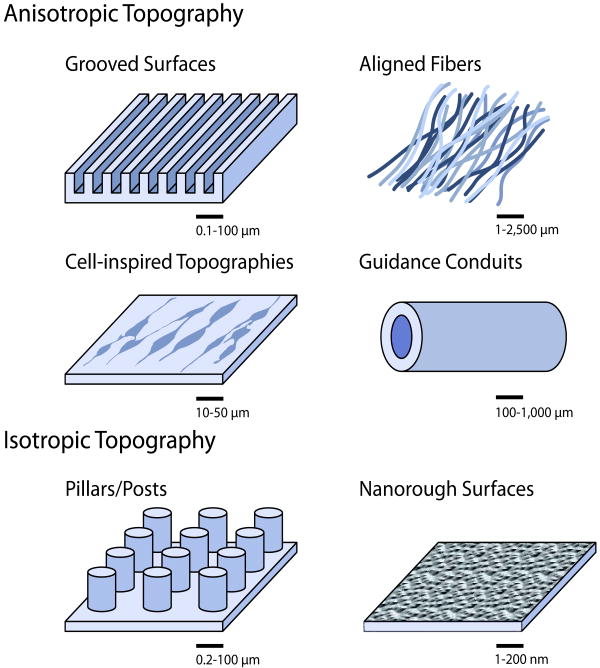
In this section, we focus on both anisotropic and isotropic topographical cues, which are illustrated schematically in Figure 1.
Figure 1.
Topographies presented to neurons in vitro.
2.1 Anisotropic Grooved Substrates
A number of studies on microgrooved topography have indicated that neurite response to topography in terms of alignment or outgrowth increases with increasing groove depth from around 0.2 to 4 μm (48, 52, 63, 64), where no effect is observed on feature sizes less than 200 nm. In studies with grooved surfaces with heights in the sub-micron to micron range, dorsal root ganglia (DRG) neurons interacted with both grooves and plateaus. Increasing groove height resulted in both increased alignment and increased restriction of cell somata and neurites onto either the groove or the plateau, reducing crossing between features separated by a step (48). Interestingly, the combination of the feature depth, along with the angle at which an approaching neurite contacts the feature (a wall or edge) have been shown to influence whether the neurite turns to follow the cue, or crosses the step to preserve its original direction. When murine embryonic cortical neurons were cultured on microgrooved platforms with a depth of 2.5 to 69 μm, axons were found to cross over the shallow grooves of 2.5 or 4.6 μm, whereas grooves 22 or 69 μm caused the axons to turn after contact with the feature (65). However, at an intermediate depth of 11 μm, about half of the axons crossed the step while the others turned in respose to the cue, and this behavior was correlated highly with the angle at which the axon approached the feature: when axons approached the edge of the platform or groove from a perpendicular angle, they tended to stay straight and cross the feature, whereas axons approaching the feature from a more parallel angle tending to turn through the relatively small angle required to align to the feature. While the specific dimensions at which cells overcome barriers may vary between cell types, these studies suggest that topographical cues can be tailored to control behaviors such as turning, branching, and alignment.
Distance between anisotropic topographical cues has also been shown to play a role in directed neurite guidance. Both groove floors and plateaus present distinct topographical cues to cells, and can vary in width from the subcellular (nanometer and single-micron) to the cellular (tens of microns) to the supracellular (up to hundreds of microns) scales. For 200 nm deep grooves, increasing the width of the plateaus and grooves from 500 to 750 nm reduced PC12 neurite alignment from 90% to 75%, though neurites were longer on flat and 750 nm grooved surfaces than the 500 nm surfaces. In addition, the 500 nm width significantly increased the elongation of the cell body (60). Both sympathetic and sensory neurons have aligned neurites to grooves of 300 nm depth, with groove widths varying from 100-400 nm and plateaus varying from 100-1600 nm, and tended to grow on the ridge edges and plateaus rather than in the grooves. Of note, it was found here that the smallest patterns with 100 nm features did not guide as well as the larger width patterns (55). PC12 cells on microgrooves with 5 and 10 μm features (2-3 μm deep) exhibited oriented neurites, though these neurites were straighter and more aligned on the smaller features. In both cases, cells were too large to sit inside the grooves, but the neurites tended to grow in the grooves (59).
Studies on grooved features with depths greater than the height of a cell, and on the order with a cell diameter, have also been conducted. PC12 cells on grooved substrates 11 μm in depth, with walls 20-60 μm apart, exhibited oriented neurites, with the strongest orientation occuring in thinner grooves (43). However, the turning behavior of neurites encountering microgrooves of 2.5 to 69 μm depth did not appear to depend on channel width, from 50-350 μm (65). Neurons in co-culture with nerve growth factor (NGF) secreting HEK cells aligned to channels with relatively large widths (100, 150, and 250 μm wide, 150 μm deep) with the neurons in the smaller grooves exhibiting less branching, greater alignment, and longer growth (45). Taken together, these studies indicate that the distance between anisotropic cues of linear geometry may affect neurite alignment, though it is clear that the complex results merit further study.
Though the vast majority of studies using grooved substrates report parallel alignment of neuronal cell bodies, neurites, glial cells, in addition to other cell types, other types of guidance have been reported as well. In particular, it has been demonstrated that submicron grooves can elicit perpendicular contact guidance in CNS neuroblasts (66) and rat hippocampal cells (42, 67), in a manner dependent on surface feature sizes. Neurites undergoing this form of contact guidance extended at an angle perpendicular to the features, crossing over adjacent grooves and ridges rather than extending parallel to a single feature. Generally speaking, cells and cellular processes tend to conform to wide and shallow grooves, while they span narrow and deep grooves (68), which points at perpendicular contact guidance as a particularly interesting phenomenon. When a cellular extension reaches a linear topographical discontinuity, it can either cross it or follow it. It appears that this choice depends both on substrate dimension and cell type, and it may be that cellular processes of a certain type resist conforming to features which are too closely spaced and too deep, and instead cross between adjacent features, resulting in perpendicular contact guidance. This particular phenomenon has also been reported in vivo (69), and in two-dimensional (2D), three-dimensional (3D), microexplant and organotypic cerebellar cultures (70).
In addition to perpendicular contact guidance, a number of neuronal and glial cell types including DRG neurons, B104 neuroblastoma cells, hippocampal neurons, and Schwann cells, have been shown to span grooves of a dimension larger than the cell body, from 30 to 200 μm, anchored to the plateaus only by tension bearing cellular extensions, with no underlying support (71) in a climbing phenomenon termed “cellular bridging”. Both behaviors, perpendicular contact guidance and cellular bridging, depend on the dimensions of the feature, and require the cell to span adjacent regions rather than align parallel to features. Though these phenomena have been reported more rarely than alignment parallel to topographical features, it is important to keep in mind these non-canonical examples of contactact guidance on grooved surfaces, especially when we are considering questions of mechanism.
2.2 Aligned Fibers and Channels
Polymeric fibers with sizes in the nanometer and micron ranges have recently become very popular materials for tissue engineering applications (72). Like the microgrooved surfaces discussed above, aligned fibers present an anisotropic topography to cells in both in vitro and in vivo environments. Because alignment is such an important goal for neural tissue engineering, a number of in vitro studies have been performed in recent years to assess the reaction of neural stem cells, primary neurons, and supportive glial cells on aligned fibers.
Some in vitro studies of novel nerve guidance channel geometries have used aligned channels and fibers, which mimic the structure and alignment of structures which occur in the mature nervous system, such as CNS white matter tracts in the spinal cord. Yu and Shoichet fabricated nerve guidance channels with longitudinally oriented tracks (73), similar to the multi-channel scaffolds discussed below, in the section on in vivo guidance channel studies. In vitro, the adhesion and outgrowth of DRG neurons was shown to be increased on these scaffolds, especially in the presence of LN-derived peptides. In another in vitro guidance channel study, DRG explants were cultured on polypropylene filamets inside a PVC hollow fiber membrane, and the effect of filament diameter on neurite outgrowth and SC migration was measured (74). Fibers ranged from supracellular (100 to 500 μm) to cellular (30 μm) to subcellular (5 μm) in diameter, and an inverse relationship between filament diameter and both SC migration and neurite outgrowth was found. In addition, when fibers were uncoated, SCs migrated ahead of neurite outgrowth, indicating that SCs may be establishing a pathway for neurite growth, but when fibers were coated with LN or FN, the neurites actually grew past the SCs, indicating that with permissive ECM support, neurite growth may be independent of SC migation. These results agree with previous work, in which PLLA microfilaments with a relatively large diameter of 375 μm guided neurite outgrowth and SC migration, and neurite outgrowth was significantly increased in the presense of LN, past the SC leading edge (47). Wen and Tresco (74) have noted that when DRGs were cultured on aligned fibers, there was likely an optimal size range for the filament diameter, in which filaments that were very large or very small relative to the size of the growth cone did not present discernable energy differences in different growth directions.
Electrospinning is a particularly effective technique for creating matrices of oriented fibers (75). The resulting nano- or micro-scale fibers can be composed of a variety of polymeric materials, and electrospun scaffolds have similarities to natural ECM, including thin and continuous fibers, high surface-to-volume ratio, and a large number of variable-sized pores (76). Aligned electrospun fibers 400-600 nm in diameter have been shown to orient DRG alignment and SC migration in vitro (77) (Figure 2). The size of electrospun fibers can influence alignment, growth potential, and differentiation. For example, electrospun micro- and nanoscale PLLA fibers were equally capable of orienting NSC soma and processes, though the nanoscale fibers regardless of orientation increased NSC differentiation over the microscale fibers (average diameters 250nm and 1.25 μm) (78). Interestingly, neurite length increased only on the nanoscale aligned fibers. In another interesting study DRG explants were grown on poly-L-lactate nanofibers of high, intermediate, and random alignment (56). On aligned fibers, neurites sprouted radially but turned to align to fibers upon contact, and neurite length increased on aligned fibers relative to random and intermediate fibers.
Figure 2.
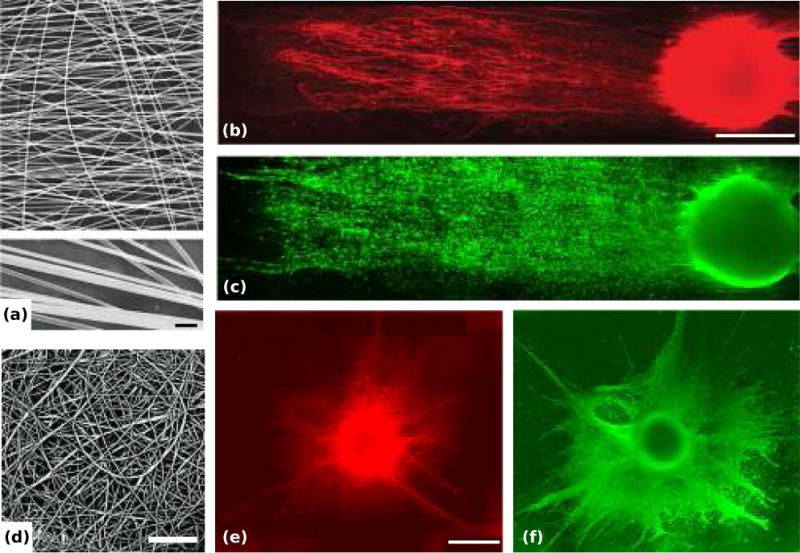
Dorsal root ganglia (DRGs) on aligned and random electrospun nanoscale fiber film in vitro. (a, d) Representative scanning electron microscopy (SEM) images of the aligned poly(acrylonitrile-comethylacrylate) fibers (a, with magnified fibers below, scale bar = 1 μm) and the random fibers (d, scale bar = 30 μm). (b, c) Double immunostained DRG on the aligned fiber film: representative montage of NF160 (a marker for axons) immunostained DRG neurons on the film (b) and montage of S-100 (a marker for Schwann cells) immunostained Schwann cells on the film (c), scale bar = 500 μm. (e, f) Double immunostained DRG on the random fiber film: representative montage of NF160+ neurons (e) and S-100+ Schwann cells (f), scale bar = 500 μm. Adapted with permission from Kim et al. (2008).
2.3 Isotropic Topography and Nanorough Surfaces
Globally isotropic topographies have been presented to neurons in the form of nano- or micron-scale pillars and holes and nano-roughness (reviewed in (79)). It has been suggested that regardless of cell type, cells respond to isotropic features with dimensions less than 5μm with a smaller, rounded morphology with less organized cytoskeletons (68). In addition, neurons exhibit distinct and fascinating behavior on substrates which have repeating topography that is the same in every direction and which provides guideposts at discrete locations (44). Two examples of neuronal response to globally isotropic topographies are shown in Figure 3.
Figure 3.
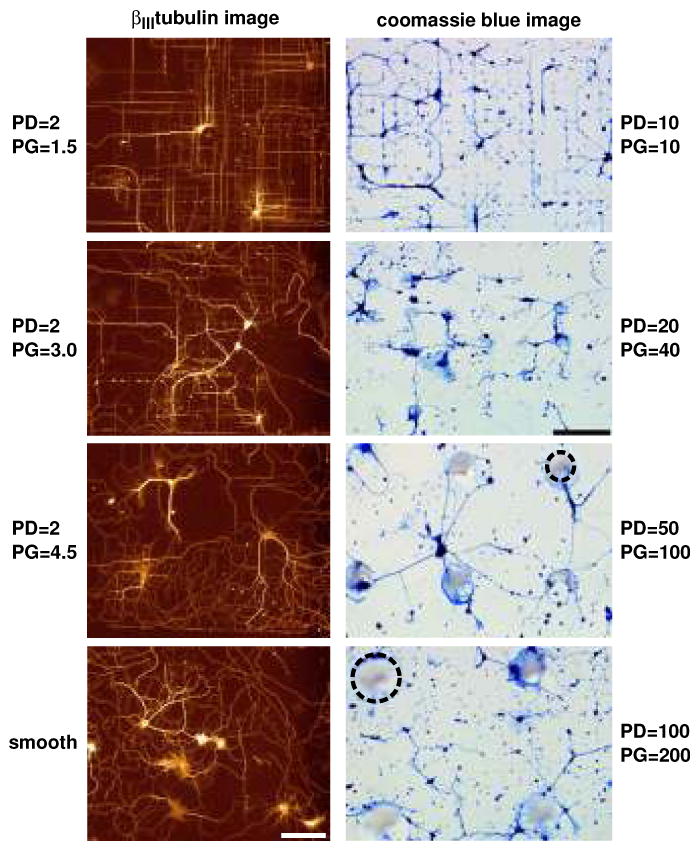
Effects of surface topography on the orientation of embryonic rat hippocampal neurons cultured on surfaces with varying pillar diameter (PD) and pillar gap (PG). Left column: Fluorescence images demonstrate the effects of poly-l-lysine-coated silicon pillars of diameter 2.0 μm at increasing pillar gap sizes. The greatest orientation was demonstrated for β III-tubulin-labeled processes on pillar gaps of 1.5 μm. Effects of orientation decreased as the gap size increased to 3.0 and 4.5 μm. Processes on smooth regions demonstrated random growth. Adapted with permission from Dowell-Mesfin et al. 2004. Right column: Optical micrographs, at the same magnification and light settings, of Coomassie blue-stained hippocampal neurons plated on glass substrates patterned with conical posts of polydimethylsiloxane (PDMS) of 10 μm diameter, 10 μm gap; 20 μm diameter, 40 μm gap; 50 μm diameter, 100 μm gap; and 100 μm diameter, 200 μm gap. Dotted circles outline representative pillars. Adapted with permission from Hanson et al. (2009).
A number of studies have shown that neurites follow interupted guidance cues with a high degree of fidelity. Hippocampal neurons plated on surfaces with 1 μm tall pillars either 0.5 or 2 μm in diameter and arranged in a grid 1.5 to 4.5 μm apart aligned to pillar geometries, moving between adjacent pillars (51) (Figure 3, left column). The neurites tended to span the smallest distance between pillars, aligning either at 0° or 90°, with the highest alignment with the larger pillars at the smallest spacing. As the spacing between pillars increased, the fidelity of alignment decreased, and at 4.5 μm spacing, the distribution of neuronal arbor was similar to that found on a flat surface. In addition, at an early time in culture (1 day), the neurons on the pillar topographies, unlike the flat surfaces, had a single presumed axon forming. This indicates that guidepost-like topography may affect the functional state of neurons developing in culture. In a similar study, hippocampal neurons were plated on surfaces with posts 10-100 μm in diameter and edge to edge spacing of 10-200 μm (53) (Figure 3, right column). The height of each post was approximately one tenth of the diameter. On surfaces with smaller features and smaller spacings, processes aligned and connected in straight lines between adjacent pillars, as above, and mostly followed a single direction by occasionally branching in the perpendicular direction. However, as feature diameters started to increase, neurites would wrap around the post they were already on, especially as the spacing increased past 40 μm. Spacing of 200 μm promoted both random outgrowth and wrapping, and flat surfaces promote random outgrowth. Therefore, as the feature size and spacing was increased, a transition from aligned outgrowth to process wrapping to random growth occured in parallel.
In these studies, we see an interesting trend where neurites tended to follow what contact they already had, but if a new contact was in close enough proximity, they jumped to that new location, resulting in highly aligned and branched neuronal arbors even on isotropic surfaces – whereas other cell types tended to undergo a whole-cell, symmetrical response resulting in rounded morphologies. This guidepost-like method of extension is remniscent of the perpendicular contact guidance discussed above, and there may be an important mechanistic or functional connection between the response to the seemingly disparate topographies of disjoint raised pillars and continuous anisotropic grooves.
In contrast to the studies discussed above, surfaces with non-periodic micron-scale pillars have also been used. These surfaces have been shown to increase adhesion of embryonic mouse brain cells, promoting the sprouting of long neurites over the pillar surfaces (80). Here, the pillars were spaced closely together and did not allow contact of the cell soma with the underlying surface, although the growing neurites were found to engulf the spikes and send additional, smaller neurite extensions down the pillars in the z direction. Similar to the studies discussed above, neurites spanned the shortest intraspike distance. In another study with pillar-like features that were flexible, gallium phosphode “nanowires” with a 50 nm diameter and 2.5 μm height supported adhesion and outgrowth of DRG neurons (81). Neuronal survival relative to control surfaces with no topography was increased, and the neurons underwent complex interactions with the nanowires, such as pulling on and bending them. A number of adhesion geometries were possible, including neuronal cell bodies lying on top of the wires or on the surface and being penetrated by the wires. In addition, the neurites could either grow on top of, be penetrated by and grow at the bottom of, or around and in between the nanowires. In both cases, non-periodic discontinous topographies were capable of supporting neuronal adhesion and extension, though the growth was not as strongly guided as in studies which incorporated more periodic topographies with a slightly greater spacing between features.
Surfaces with varying heights that are less than 1 micron different are often considered to be nanostructured, or nanorough. These surfaces have been used with a variety of cell types and surface chemistries, and a range of responses has been observed in terms of cellular adhesion, proliferation, and morphology (68). Roughness is often measured in terms of an average feature size, called Ra. Some studies of neurons on globally isotropic surfaces with nanoscale features have been performed, with mixed results. One study showed that nanotextured titanium nitride films with Ra values from 1.3 to 5.6 nm reduced attachment of primary hippocampal neurons relative to PDL coated glass (82). Another study found that embryonic primary cortical neuron adhesion and viability was affect in a unimodal fashion by nanotextured silicon with Ra from 18 to 204 nm, where an intermediate value of Ra=64 nm promoted an optimal response, and both higher and lower roughness reduced this response (83). In addition, local nanoroughness and gradients in surface energy sized on the order of growth cones promoted PC12 extension and neuronal expression patterns in the absence of NGF (84). Though nanoscale materials have received a great deal of attention in recent years, it is not yet clear what role they will play in directing neuronal growth for tissue engineered applications.
2.4 Cellular and Biomimetic Topography
One approach to elicit neuronal guidance and reconstruct an in vivo-like environment is to co-culture neurons with other cells, with and without additional topographical guidance features. By themselves, cells provide both biochemical and topographical cues to regenerating axons. For example, aligned monolayers of Schwann cells can direct neuronal outgrowth to follow the direction of alignment even in the absence of other topographical cues (85). In addition, Schwann cells (SC) can be guided by topography themselves, and like neurites appear to be sensitive to the depth of anisotropic groove topography (86), leading to studies which indicate that there is a synergy between the cues presented by cells and cues presented by topography. When SCs were preseeded onto grooved scaffolds of 15 μm depth, over 60% of them aligned on 50 and 100 μm width grooves, while over 90% aligned on 2-20 μm grooves. These constructs which presented DRG neurons with both cellular and geometric topographical cues were able to orient the neurites in the direction of the topography and the aligned SC (62).
Cell types such as meningeal cells and astrocytes have also been used to direct neurite outgrowth in vitro, though they tend to be associated with inhibiting regrowth in the injury environment (87–89). In particular, outgrowth of both postnatal and adult DRG neurons aligned to monolayers of meningeal cells which were preplated on substrates with anisotropic nanoscale roughness (52), from 250 nm to 1 μm in depth. As expected, neurites did align to topography without the intervening cell layer, and increased topography depth in conjunction with meningeal cells elicited increased alignment. However, the meningeal cells appeared to provide some additional cue beyond their topography, as the neurites showed enhanced orientation and growth when presented with the intermediate cell layer, with the effect more pronounced with younger neurons. Similarly, DRG neurons were guided by astrocyte monolayers which had been oriented by an electric field (90). Interestingly, the same response could be seen on fixed aligned astrocytes, indicating that the astrocytes are not necessarily providing active biochemical cues. Further, astrocytes have also been shown to promote neuronal differentiation and alignment. When neural progenitor cells were plated on microgrooved surfaces (groove: 16 μm, plateau: 13 μm, depth: 4 μm) with and without an intermediate layer of astrocytes, though the progenitor cells could orient to both surfaces, only the astrocytes in conjunction with the grooves induced increased differentiation into cells expressing neuronal markers (91).
From these studies, it seems that guidance cues from cells are able to provide some information which geometric topography alone cannot, but it is not clear if this information is primarily biochemical or topographical in nature. One of our labs has replicated the micro- and nano-scale topography of adherent cells in polymer replica molds (92), which capture the topography but not the biochemistry of supportive cell layers, and thus allow for separate study of individual and multiple types of cues. Optimally aligned monolayers can be fabricated with a range of cell types, to allow future investigation into the relative ability of different cell types to guide neuronal growth (50). The topography of aligned monolayers of Schwann cells can promote neuronal adhesion and guide neurite outgrowth in the same direction as the underlying cell topography (93), as shown in Figure 4.
Figure 4.
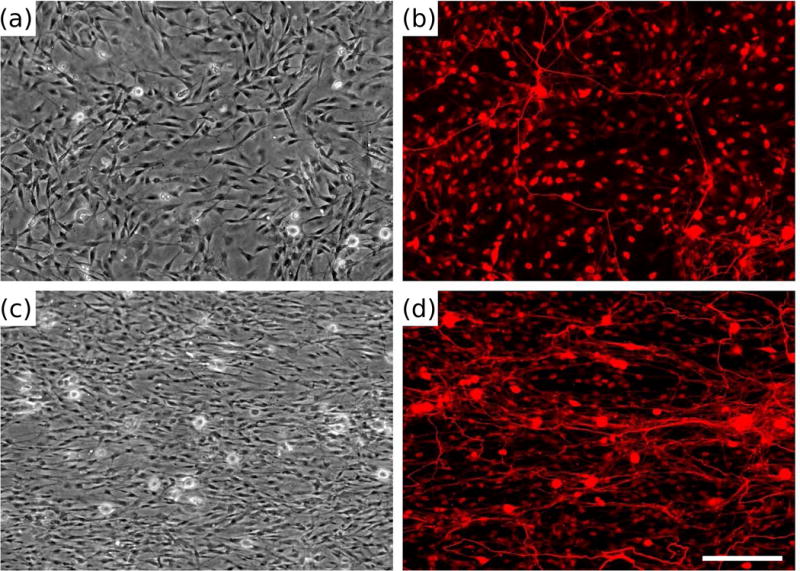
Dorsal root ganglia (DRG) morphology on biomimetic materials presenting replicated Schwann cell (SC) topography. Phase contrast (a, c) and fluorescent micrographs (b, d) of dissociated DRG cultured for 5 days on polydimethylsiloxane (PDMS) (a, b) or replicas (c, d) and stained with neurofilament immunocytochemistry (b, d). Adapted with permission from Bruder et al. (2007).
2.5 Interaction Between Topographical and Biochemical Cues
The response of cells to topography is a complex process, depending on many features of the extracellular mileu. For example, topography responses may be modulated positively by population pressure in both fibroblasts (94) and neurons (93), while corneal epithelial cells have been shown to require serum for a response to topography (95). Biochemical signals, such as nerve growth factor (NGF) and permissive laminin (LN) have been shown to enhance the effects of topography, where neurons respond more strongly to topographical cues in the presence of these molecules. For example, LN increased PC12 outgrowth on nano-scale fibers (46), DRG adhesion and outgrowth within 200 μm diameter longitudinally oriented tracts (73), DRG alignment on microgrooves (48), and PC12 alignment on microgrooves (59). In addition when PC12 cells were presented with suboptimal NGF concentrations, neuritogenesis was modulated by feature dimensions, an effect not seen at higher NGF levels (41). In particular, concentrations of 5-25 ng/ml of NGF promoted neuritogenesis in conjunction with 70-450 nm width features, while larger features of 850-1900 nm reduced neuritogenesis.
Previous studies in non-neuronal cell types have provided conflicting results about the relative strength of topographical and biochemical cues (96, 97). A handful of studies with neurons have suggested that topographical and permissive biochemical cues have distinct roles in directing neurite growth, perhaps providing a future means to engineer highly specific neuronal networks for tissue engineering applications. When embryonic hippocampal neurons were presented with biochemical cues in the form of tethered NGF and topographical cues in the form of microgrooves 1-2 μm in width and 400-800 nm in depth, the topographical cues had the most pronounced effect on polarization regardless of the simultaneous presence or absence of NGF (42). In contrast, axon length was increased by tethered NGF and not by topography, though an enhancing effect was seen when both cues were presented simultaneously, perhaps because of faster polarization due to the topography in combination with enhanced growth due to the NGF. In a study by the same group with a similar experimental setup, topographical and biochemical cues were presented competitively to hippocampal neurons (98). While each cue individually promoted polarization and growth into the cue environment, in a choice paradigm the microgrooves were preferred over both NGF and LN cues, with the effect strengthening for narrower grooves. Similar results were seen in a different experimental setup with murine embryonic cortical neurons (65). When neurons were allowed to adhere to a range of locations both in contact with PDL coated microgrooves and in a matrigel matrix suspended above the microgrooves, neurite turning behaviors were complex and depended both on where a neurite initiated and what cues it encountered during growth. In general, neurites preferred a long, straight path, which for neurons on the groove floor meant turning to follow walls, while neurites starting on plateaus grew out into the matrix rather than turning, though this meant no longer being in contact with the 2D PDL coated surface. Further studies with complex biochemical and topographical cues will have to be performed to determine the dynamic response of growing axons when they are presented with conflicting cues. Studies of neurons at the interface between 2D substrate-bound cues and a more complex 3D architecture provide further evidence that growing neurites integrate multiple cues and the response depends on both the local biochemical and structural environments (99).
3 Topography and Nerve Regeneration in Vivo: Nerve Guidance Channels
3.1 Nerve Guidance Channels
After injury, neurons face a complex environment that contains multiple inhibitory factors, and it is not surprising that efforts toward successful therapies have been correspondingly numerous. A variety of strategies have been explored, including delivery of neuroprotective agents (100), addition of exogenous growth factors (101), reducing inhibitory myelin-associated factors (102), blocking the Nogo-A signaling pathway (103), promotion of a more embryonic neuronal or ECM state (104), and cell transplantation (105,106). Repair of PNS injuries has come further clinically, as small defects may currently be treated with autografts or with polymeric nerve guidance tubes (107), but large injuries still provide a significant clinical challenge (108). Strategies from the literature for PNS injuries include the use of biodegradable scaffolds to stimulate axon growth through delivery of permissive cues such as growth factors (109, 110) and therapeutic drug delivery (111). A consensus is emerging that a combination of multiple strategies will be required for optimal recovery (105, 112), and topographical guidance will likely play a significant role in the development of these strategies.
As axon growth is highly random after injury, and rarely extends past the lesion site back into the host tissue (113), nerve guidance channels have been used to encourage injured axons to migrate back towards their targets.
Nerve guidance channels (NGC) made from a range of synthetic and natural materials (114) are currently being used clinically to bridge small (<1 cm) peripheral injuries, and they are approved to be used in gaps as large as 3 cm. Historically, guidance channels have been shown to organize the fibrin cable, promote a contained regenerative environment, and improve the outcome of peripheral and central nerve transection animal models. However, it has been concluded by a number of researchers that guidance channels will have to incorporate additional cues beyond a simple tubular structure if regrowth sufficient for regaining function is to occur (115). Though channels by themselves provide a macrotopographical cue in the form of a curved inner lumen, and DRG and cerebellar granule cell axons are sensitive to curvature (116), it does not appear that simple channels will be efficacious in repairing anything but the smallest injuries. Multifaceted biomaterial design strategies for treatment of nerve injuries include optimization of mechanical properties, cell-adhesivity, biodegradability, electrical activity, delivery of permissive molecules, and topography (reviewed in (117)). In this section we focus on optimization of topograpphical cues introduced into the luminal space of NGCs.
3.2 Multichannel Conduits
One strategy to promote increased growth is to provide cells with additional area on which to grow. This has been the aim of nerve guidance channel studies which incorporate a number of smaller channels oriented longitudinally within a larger conduit, with the rationale that a greater number of channels with increased curvature may be more likely to orient axonal growth in the longitudinal direction than a single, larger channel (116, 118). In addition, a multichannel conduit may be more likely to preserve the linear, tract-like organization found in both the spinal cord and in peripheral nerve cables. Fabrication of such conduits is not trivial, and a number of studies have focused on methods to produce multichannel conduits, which have been shown to support increased SC adhesion (119, 120) and may be bioactivated (73) to release growth factors such as NGF (121, 122). The number and diameter of the smaller channels may vary, ranging from 3 channels within a single conduit (120) to over 100 (119). Multichannel NGCS have been used in vivo in an attempt to reduce the nerve dispersion that may occur in single lumen channels (115), but not all studies have shown positive results (123).
A number of studies in rat models of SCI indicate that multichannel scaffolds, in conjunction with cellular or biomolecular therapies, may promote significant regrowth. When thin-walled scaffolds with channels of mean diameter 125 μm were used, linear organized axonal regeneration was promoted, with and without further augmentation by brain derived neurotrophic factor (BDNF) into the walls and lumen (124). In a similar study, highly porous biodegradable multichannel scaffolds seeded with SCs showed nerve regeneration after just one month in vivo following SCI (125). Furthermore, agarose multichannel guidance scaffolds supported linear regrowth of axons, whereas control animals treated only with cell suspension grafts exhibited disorganized regrowth (126). However, these scaffolds pre-injected with BNDF secreting cells and a fibrin matrix showed significant recovery over the scaffolds alone. As indicated above, CNS axons have been shown to be sensitive to curvature (116). Axonal regrowth was increased on multichannel scaffolds with smaller diameters (450 μm) as compared to larger diameters (600 μm) in a transected rat spinal cord model (118). In addition, the scarring response as measured by the fibrous rim area was decreased for the smaller diameter multichannel conduits, although both conditions contained the same number of individual channels. In this study, the biodegradable scaffolds were pre-loaded with SCs. Together these studies indicate that topography may be a powerful synergistic cue when presented in combination with cellular or biomolecular cues to promote axonal regrowth into injured areas of the spinal cord.
3.3 Channels with Intra-luminal Topographical Cues
Many fields under the umbrella of tissue engineering are concerned with fabrication of scaffolds with the goal of promoting regeneration of damaged tissues both in vitro and in vivo. A number of methods to form scaffolds with aligned topographies have been described (127), including self-assembly, phase separation, and electrospinning (75). These methods to form oriented scaffolds with fibers sized on the order of natural ECM components such as collagen have been applied to nerve guidance channels. A number of NGCs with topographical cues in the luminal space, including aligned nanoscale fibers, have been fabricated and used in vivo to promote repair of damaged nerves. As with the multichannel studies discussed above, many of these studies combined topographical cues with cellular and biomolecular cues. For example, a biodegradable foam conduit with longitudinally aligned pores was pre-coated with LN and SCs, and axon regeneration compared favorably to autografts at 6 weeks in a sciatic nerve model (128). In a similar sciatic nerve study, a guidance channel with a microgrooved lumen and pre-seeded with SCs promoted functional recovery, and though each cue alone resulted in similar overall numbers of regenerating axons as the combination of cues, functional recovery was significantly enhanced when the two cues were presented simultaneously (129).
In addition, topographical cues can be combined with biochemical cues in the form of aligned collagen filaments. Spinal cord defects were successfully bridged in a rat 5mm defect model with a graft of collagen fibers parallel to the injury site (130, 131). Axons regenerated across both the proximal and distal implant interfaces at 4 weeks, and at 12 weeks functional recovery was observed. Recently, the same group has demonstrated similar results in a rabbit spinal cord injury model (132), indicating that a combination of topographical and biochemical cues shows promising results for future therapeutic treatment of both PNS and CNS injuries.
Interestingly, some in vivo studies have indicated that topography by itself, without additional support cells or growth factors, is capable of promoting nerve regeneration. Though some studies show that topography alone can promote regrowth in central nervous system injuries (133), the studies discussed below focus on the peripheral nervous system. Guidance has been provided largely in the form of fibers and filaments. Electrospun fibers can be used to fabricate conduits out of biodegradable polymers (134), or to fabricate anisotropic structures which promote SC migration and directed axonal growth, thus maximizing the innate repair abilities of the PNS. Filaments (135), fibrous layers (77), and complex 3D structures such as microbraids (136) have been shown to support PNS regeneration. PLLA microfilaments inside PLA and silicone conduits enhanced tissue cable formation and SC migration in 14 and 18 mm defects in a rat sciatic nerve model (135). Interestingly, only the filaments in combination with the biodegradable PLA conduit were able to significantly promote axonal regeneration across the longer lesions, indicating that both the inner framework structure and the outer conduit composition may be important in promoting regeneration. In addition, aligned layers of electrospun fibers promoted regeneration across a 17 mm rat sciatic nerve gap (77). A similar study used polysulfone channels modified to have 1 or 3 longitudinally placed aligned nano-fiber thin films and evaluated recovery after a 14 mm tibial nerve gap (137). Both the 1-film and the 3-film channels supported regeneration, though 1-film channels exceeded 3-film channels in terms of number of regrowing axons and nerve conduction velocity. These results suggest that minimal topographical cues with a single film can overcome the limits of endogenous repair mechanisms by facilitating efficient SC migration into the gap.
4 Mechanisms Of Cell Response to Topography
Many studies which present topographical features to cells in both in vivo and in vitro systems discuss the possible mechanisms which may be at play, but few studies have directly tested a hypothesis about how cells sense topography and then respond to it. Establishing the mechanism of cellular responses to topography is difficult, in part due to the myriad kinds of topographical cues which can be presented to cells. With rare and highly specific exceptions, all materials contain topography, whether this is imposed on the surface intentionally or unintentionally (5). As discussed above, topography is commonly presented to cells in both scaffolds designed for in vivo use, and in cell culture platforms for in vitro study (138). In addition, the functional, behavioral, and morphologic differences between cell types mean that distinct cell types may respond quite differently to topographical cues. These differences could include embryonic origin (i.e. fibroblasts are mesenchymal, while neurons are ectodermal), or they may have more to do with the differences of how these cells function in the body. Neurons are optimized to make connections, a process which requires an ability to explore the extracellular environment, rather than the sort of whole-cell response commonly observed in cells derived from epithelial, connective, or muscle tissue.
A number of ideas have been put forward and can be summarized as follows:
(1) Changes in the topography of a surface may affect the way proteins interact with that surface, thereby altering the biochemical environment presented to cells, determining how cells bind to the surface and what behaviors they undergo. (2) Topographical cues may directly orient or organize the cytoskeleton, resulting in, for example, highly aligned cells. It has been proposed that it is energetically favorable to minimize distortion to the cytoskeleton, so cells may simply take on the shape which results in the least amount of bending to stiff cytoskeletal components such as microtubules. (3) Topographical cues may result in differential localization of receptors and the components of their downstream signaling cascades, resulting in cells which are highly polarized and have different functional behaviors compared to cells without these topographical cues. (4) Topographical cues can affect the shape and morphology of cells. In turn, cell shape may be directly connected with cell function and behavior. (5) This connection between shape and function likely depends at least in part on nuclear distortion, which then results in differential gene expression and therefore behavioral and functional changes. These ideas are by no means mutually exclusive, and we suggest that cells may respond to topography in a multi-faceted manner, likely employing multiple responses described above, possibly in addition to other mechanisms which as yet remain to be elucidated.
4.1 Topography Affects Cellular Organization
When a material is implanted into the body, a layer of proteins from the interstitia and blood immediately coat the material, a response which has been shown to be important for mediating biological response to the materials (139). It is thought that cells are in fact responding to this layer of proteins, rather than the implant material itself. It has also been shown that the adsorption of proteins is influenced by surface properties (140), where low energy surfaces promote protein adsorption to a greater extent than high energy surfaces (83). Nanoscale topography has been shown to affect protein structure, activity, and stability (141). Further, surface topography in the form of nanoroughness has been correlated with increased adhesion (142). Therefore, while not yet comprehensively established, it is possible that differential protein adsorption may contribute to topographical cues in biological systems.
It has been hypothesized that alignment to anisotropic cues such as grooved surfaces occurs as (1) cells minimize the distortion to their cytoskeletons which would occur if cells ignored rather than followed guidance cues (143), and (2) that these cues create a pattern of mechanical stress, directly causing the cells to align with the grooves (144). Both hypotheses are supported by studies using topographies with subcellular geometries which indicate that actin stress fibers and microtubules align in the feature direction (48, 95, 143, 145–148). Microgrooves have also been reported to reduce the complexity of the neuronal arbor, with a stronger effect on smaller channels, which may be due to restriction of the growing cytoskeleton (43). Migrating fibroblasts on a grooved surface presented with another grooved surface with a relative perpendicular orientation have been found to send out lamellipodia into the contrasting topography, resulting in cells responding simultaneously to two perpendicular guidance cues. In this case, microtubules (MT) adapted closely to the conflicting topography, whereas actin microfilaments (MF) stayed straight, aligned to only one direction and discontinuous where the topography changed direction (143). Other studies have shown a size-dependent response in which, for certain sizes, cells following a topographical cue preferred to continue to follow it. For example, neurites wrapped around posts with relatively large (greater than 40 μm) diameters; on the other hand, if the distance between adjacent features and the size of the features themselves were both relatively small (on the order of 10 μm), cellular extensions were more likely to span the distance between features (53). The actin cytoskeleton of mesenchymal stem cells on top of non-periodic 2 μm diameter structures was punctate and disorganized, whereas stress fibers were exhibited when these cells were cultured on large, sparse 50 μm ring structures (68).
Examinations of the time course of cytoskeletal response to grooved topography in fibroblasts have revealed that cytoskeletal alignment preceeded soma alignment. Fibroblast MTs aligned first to the bottom of a groove, followed by the alignment of MFs to wall-ridge edges, followed by the alignment of the focal adhesions, which were initially present radially (147). In contrast, another study found fibroblast F-actin condensations near topography edges as soon as 5 minutes after attachment, and arranged similarly to FAs, while organization of the MT cytoskeleton occurred later (148). These studies along with others have indicated that alignment of MFs, MTS, and FAs occured along the sharp edges of features, and was followed by alignment of the entire cell body (143).
Disruption of the cytoskeleton can have a range of effects on contact guidance behaviors, and provides insight into the potential mechanisms of response to topography. It is important to note, however, that many of the cytoskeletal inhibitors discussed below may have non-specific effects on cellular processes, and these results should be interpreted in this context. An early study indicated that cytoskeletal disruption did not affect parallel and perpendicular contact guidance of neurons on subcellular microgrooves (149). This study further suggested that pharmacological inhibitors to stretch activated Ca2+ channels, G proteins, protein tyrosine kinases, and proteins kinase A and G did not affect neurite alignment, though high concentrations of protein kinase C inhibitors and calcium channel blockers did specifically attenuate perpendicular contact guidance of hippocampal neurons. On the other hand, other studies involving disruption of the cytoskeleton have indicated that it plays an important role in contact guidance. For example, actin disrupting agents cytochalasin D and latrunculin B have been shown to attenuate the alignment effects of nanoscale grooves (145). In another study, actin disruptors reduced orientation, while MT disruptors did not, suggesting that the aggregation of actin along groove/ridge boundaries is a primary driving event in determining fibroblast orientation on microgrooved substrata (148). Similarly, though cells can align to the longer feature when presented with conflicting topographies, treatment with either the inhibitor blebbestatin or Y27632 (which disrupt myosin II contractility and Rho-kinase respectively) revealed that cells may not be able to down regulate actin polymerization in minor directions without acto-myosin contractility, leaving them to follow only the local cues without the ability to coordinate cell-wide orientation (146), as shown in Figure 5. These results indicate that response to topography is an active process, and therefore may be more complex then simple cytoskeletal restriction. Further, submicron topographies which are too small to restrict growth can also orient cellular alignment. For example, growth cones of neurites on microgrooves changed direction to conform to the topographical cues presented, even though the growing neurites were located on top of, rather than within, the subcellular grooves (67). Though the response to topography appears to be active and well controlled, the cytoskeletal and signaling components at play are not yet clear.
Figure 5.
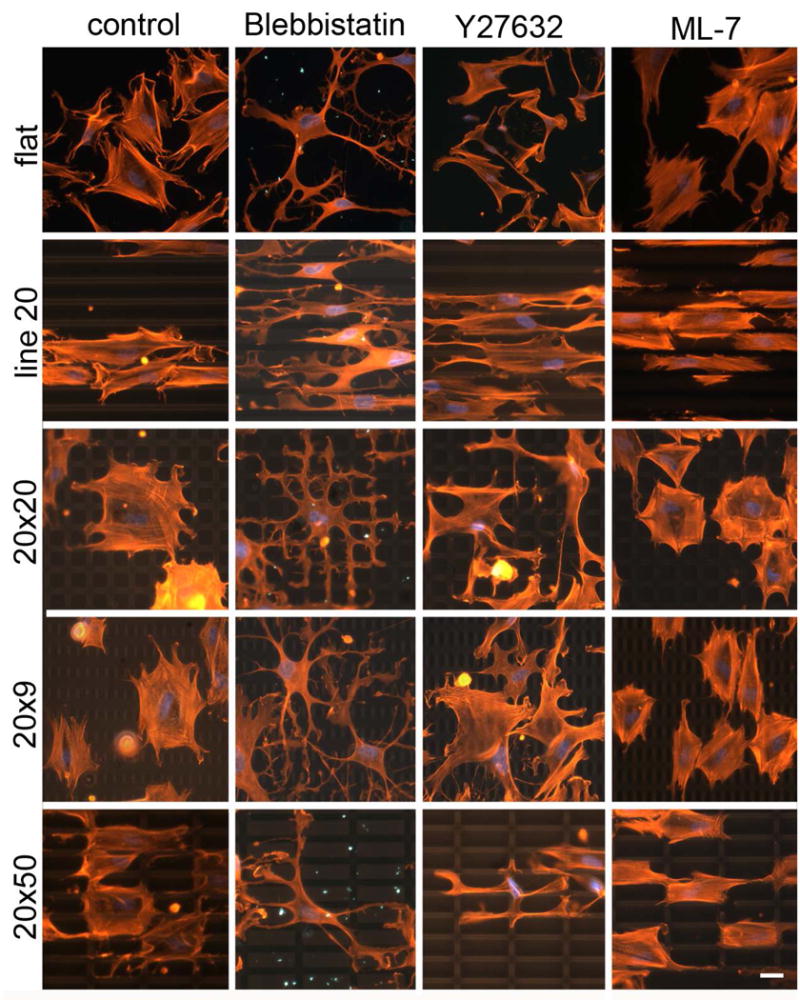
Coordination of actin cytoskeleton on grid surface during cell spreading is inhibited by Blebbistatin and Y27632, but not ML-7. Left column: F-actin of untreated cells cultured on polydimethylsiloxane (PDMS) flat, line, or grid patterns for 26 h. Other columns: F-actin of inhibitor-treated cells spreading on patterned substrates. Inhibitors were added to cell suspension during plating and cells were fixed and stained after 2 h of incubation (red: Rhodamine-Phalloidin, blue: DAPI). Adapted with permission from Mai et al. (2007).
4.2 Topography Affects Cellular Function
It is well documented that cells can respond to the mechanical properties of their environments, including stiffness (150), shear flow (151), and mechanical stretch (152); this ability is conserved throughout the living world, from bacteria (153), fungi (154) and plants (155) to animals (156). Mechanotransduction has been shown to occur through integrins, as well as through mechanosensitive ion channels (154) and g-protein coupled receptors (157). In addition, adhesion sites have the ability to strengthen when forces are applied to them, and biochemical correlations to this ability are beginning to be investigated, such as an increase in the phosphorylation of focal adhesion kinase (FAK) in response to stretch at a FA (158) and activation of the Rho-family small GTP-ase Rac in response to tension (158)(159). Mechanosensing has been hypothesized to play a role in cellular migration. For example, shear stress induces migration in endothelial cells (160), and the spatial polarization of a number of intracellular signaling molecules including Rho and Rac have been implicated in shear-induced directional migration (161). It has been hypothesized by a number of groups that the ability of cells to sense mechanical stimuli is related to their ability to response to topographical cues.
It has been hypothesized that topography may trigger a range of intracellular pathways, producing a greater response than individual chemical cues (98). It has been suggested that topographical cues impose mechanical forces on cellular membranes that are capable of rearranging membrane components such as integrin complexes. The studies which have looked at this hypothesis have mostly focused on focal adhesions (FA), which are an important mechanosensing adhesive complex (162). Though a great deal of research has been performed recently within the field of mechanotransduction (158), it remains for a direct link to be established between substrate topography, cellular response, and mechanotransducive machinery, especially FAs. These complex structures are capable of changing localization, composition and cellular behavior in response to mechanical stimuli, and are hypothesized to be the functional linker between the extracellular matrix to the intracellular cytoskeleton (163). Studies with microgrooves have shown that FAs tend to form along features (143, 147), and the actual size of the complex is influenced by the width of the ridge on which they reside (95).
Furthermore, recent evidence suggests that cellular response to topography may depend on integrins and downstream signaling molecules. In one study, fibroblast alignment and directed motility on aligned collagen fibers was shown to depend on the presence of a characteristic 67 nm banding pattern on the collagen fibrils, an ultrastructual cue that is independent of the overall fiber alignment (164). On aligned fibers without the 67 nm banding pattern, or on fibers containing the band but in the presence of an RGD peptide, the fibroblasts responded as if they were on a flat surface rather than on top of aligned nanoscale topography. This may be due to a periodicity inherent in the cellular adhesion apparatus. In particular, a number of cell types have been demonstrated to require the spacing of adhesive RGD peptides to be less than 73 nm apart (165), a finding which has been supported by a physical model of receptor-ligand binding (166). It may be that cellular adhesion and response to topography act through different integrin signaling complexes (164). In addition, a particular response to topography has been shown to depend on both FAK and myosin II contractility. Fibroblasts on pillars (approximately 2 μm tall, 10 μm diameter, 16 μm spacing) showed increased speed, acceleration, and turning relative to cells on flat surfaces (167). This increased motility and “zig-zagging” motion from pillar to pillar was associated with an increase in the lifespan of focal adhesions, and was dependent on both myosin II based contractility and FAK expression. FAK has also been demonstrated to be required for cellular response to mechanical stimulation (168). Frey, et al, hypothesized that topographical cues enhance localized adhesions and contractions (167), which may be linked to the similar responses observed to topographical and mechanical signals. Lastly, Rac may be involved in the cellular response to topography. Nanoscale islands 27 nm in height have been shown to promote Rac localization to the cell periphery (169), a result remniscent of Rac localization following mechanical tension (159). As Rac has been demonstrated to be involved in lamellipodial formation and cellular extension (162, 170), it is an attractive candidate for further research into the mechanism of cellular response to topographical cues.
A number of recent studies have taken advantage of advances in the ability to selectively pattern proteins to probe the connection between cell shape and behavior. For example, cellular shape can causally determine the direction of cellular migration. When fibroblasts and endothelial cells were constrained to an asymmetrical shape with a wide end opposite a narrow end and then released from this constraint, they migrated along the axis of constraint, towards the wider end (171). Similarly, micron-scale islands of FN were used to direct motility and alignment, indicating that both the spacing between and shape of potential adhesion sites were significant, with alignment and migration occuring either in the direction of elongated adhesion sites or closely spaced adhesion sites (170). Lamellipodia preferentially formed near nascent focal adhesions, around the cell periphery, and cellular orientation subsequently governed the direction in which cells migrated. This study agreed with previous work which found that when cell shape was constrained, lamellipodia and FAs preferentially formed at the areas of highest curvature, corresponding to the location of the highest tractional forces exerted by the cells on the substrate (172–174). These studies have helped to establish that there is a causal relationship between cellular shape, cytoskeletal organization, and behavior.
It has been suggested that topographical cues can influence gene expression in a range of cell types. Microarrays have found large-scale and in some cases enigmatic results, showing relative increases followed by relative decreases in a large number of genes when fibroblasts on grooved surfaces were compared to those on flat surfaces, including those involved in translational and transcriptional machinery, the vesicular secretion of proteins, Rho family proteins, fibronectin regulators, and MT associated proteins (175). Recent studies by Dalby, et al have shown that subcellular isotropic topography can influence gene expression. Nanopit geometries reduced cellular spreading and resulted in reduced focal adhesions, reduced stress fibers, reduced nuclear area, and broad downregulation of genes (176). In addition, when nanopits and nanopillars were compared, changes in the chromosomal locations of gene regulation were observed, which correlated with differences in cell spreading on the two topographies (177). Currently, researchers are attempting to answer the question of what levels of gene regulation exist beyond the molecular mechanisms which act at the level of individual genes. One fascinating hypothesis is that gene regulation is affected by changes in cell shape and cytoskeletal organization primarily through nuclear distortion (178) which is appealing in its directness and simplicity. Though the causal relationships are not yet clear, it has been observed that there are links between nuclear architecture and gene expression (179). The hypothesis that shape changes due to surface topography alters nuclear morphology and the position of chromosomes, leading to changes in the probability of specific genes being expressed has been referred to as “self-induced mechanotransduction” (180).
5 Conclusion
Topographical cues exert powerful effects on many cell types, including neurons and glia. These cues often act in concert with biochemical cues, acting synergistically to enhance or improve the efficiency of the biochemical cues by providing directional and additional biochemical cues to guide the biological response. The mechanisms by which topography affects neuronal systems are just being elucidated, but given our ability to engineer topographies with great precision on the nano- and micron scales, dramatic gains have already been made in our ability to manipulate neurons and glia both in vitro and in vivo. Future studies that unravel the mechanisms of topographical modulation of cell responses will significantly impact neural repair. It will also be important to determine what aspects of the mechanism of cellular response to topography are shared across multiple cell types, versus mechanisms which may be unique to specific cell types such as neurons. An increased appreciation for, and understanding of, cellular responses to topogrpahical cues may have implications in fields ranging from developmental biology to regenerative medicine.
Acknowledgments
The authors would like to thank Yu-Ting Liu, Vivek Mukhatyar, and Ian Martin for their help with figures and manuscript preparation. This work was supported by funding from: NSF Career Award and Advance Career Development, VA Center for Restorative and Regenerative Medicine, and NIH #EB005722 to DHK, and a Brown University Brain Sciences Graduate Research Award to JM. RVB acknowledges funding from NIH - NS44409 & NS65109, GTEC - A Regenerative Medicine Center at Georgia Tech and Emory, The Coulter Foundation, Ian's Friends Foundation, National Science Foundation (CBET 651716) and the Georgia Cancer Coalition.
Contributor Information
Diane Hoffman-Kim, Center for Biomedical Engineering and Department of Molecular Pharmacology, Physiology, and Biotechnology, Brown University, Providence, RI 02912, USA.
Jennifer A. Mitchel, Center for Biomedical Engineering and Department of Molecular Pharmacology, Physiology, and Biotechnology, Brown University, Providence, RI 02912, USA
Ravi V. Bellamkonda, Neurological Biomaterials and Cancer Therapeutics, Laboratory for Neuroengineering, Wallace H Coulter Department of Biomedical Engineering, Georgia Institute of Technology/Emory University, Atlanta, GA 30332, USA
Literature Cited
- 1.Harrison RG. The cultivation of tissues in extraneous medium as a method of morphogenetic study. Anat Rec. 1912;6:181–93. [Google Scholar]
- 2.Weiss P. The problem of specificity in growth and development. Yale J Bio Med. 1947;19(3):235–278. [PMC free article] [PubMed] [Google Scholar]
- 3.Curtis AS, Varde M. Control of cell behavior: topological factors. J Natl Cancer Inst. 1964;33:15–26. [PubMed] [Google Scholar]
- 4.Voldman J, Gray ML, Schmidt MA. Microfabrication in biology and medicine. Annu Rev Biomed Eng. 1999;1:401–25. doi: 10.1146/annurev.bioeng.1.1.401. [DOI] [PubMed] [Google Scholar]
- 5.Curtis A, Wilkinson C. Topographical control of cells. Biomaterials. 1997;18(24):1573–83. doi: 10.1016/s0142-9612(97)00144-0. [DOI] [PubMed] [Google Scholar]
- 6.Lim JY, Donahue HJ. Cell sensing and response to micro- and nanostructured surfaces produced by chemical and topographic patterning. Tissue Eng. 2007;13(8):1879–91. doi: 10.1089/ten.2006.0154. [DOI] [PubMed] [Google Scholar]
- 7.Rakic P. Mode of cell migration to the superficial layers of fetal monkey neocortex. J Comp Neurol. 1972;145(1):61–83. doi: 10.1002/cne.901450105. [DOI] [PubMed] [Google Scholar]
- 8.O'Rourke NA, Sullivan DP, Kaznowski CE, Jacobs AA, McConnell SK. Tangential migration of neurons in the developing cerebral cortex. Development. 1995;121(7):2165–76. doi: 10.1242/dev.121.7.2165. [DOI] [PubMed] [Google Scholar]
- 9.Nadarajah B, Alifragis P, Wong RO, Parnavelas JG. Ventricle-directed migration in the developing cerebral cortex. Nat Neurosci. 2002;5(3):218–24. doi: 10.1038/nn813. [DOI] [PubMed] [Google Scholar]
- 10.Malatesta P, Appolloni I, Calzolari F. Radial glia and neural stem cells. Cell Tissue Res. 2008;331(1):165–78. doi: 10.1007/s00441-007-0481-8. [DOI] [PubMed] [Google Scholar]
- 11.Hynes RO, Patel R, Miller RH. Migration of neuroblasts along preexisting axonal tracts during prenatal cerebellar development. J Neurosci. 1986;6(3):867–76. doi: 10.1523/JNEUROSCI.06-03-00867.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jacobs JR, Goodman CS. Embryonic development of axon pathways in the Drosophila CNS. I. A glial scaffold appears before the first growth cones. J Neurosci. 1989;9(7):2402–11. doi: 10.1523/JNEUROSCI.09-07-02402.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hatten ME. Riding the glial monorail: a common mechanism for glial-guided neuronal migration in different regions of the developing mammalian brain. Trends Neurosci. 1990;13(5):179–84. doi: 10.1016/0166-2236(90)90044-b. [DOI] [PubMed] [Google Scholar]
- 14.Ono K, Kawamura K. Migration of immature neurons along tangentially oriented fibers in the subpial part of the fetal mouse medulla oblongata. Exp Brain Res. 1989;78(2):290–300. doi: 10.1007/BF00228900. [DOI] [PubMed] [Google Scholar]
- 15.Silver J, Lorenz SE, Wahlsten D, Coughlin J. Axonal guidance during development of the great cerebral commissures: descriptive and experimental studies, in vivo, on the role of preformed glial pathways. J Comp Neurol. 1982;210(1):10–29. doi: 10.1002/cne.902100103. [DOI] [PubMed] [Google Scholar]
- 16.Nagata I, Nakatsuji N. Rodent CNS neuroblasts exhibit both perpendicular and parallel contact guidance on the aligned parallel neurite bundle. Development. 1991;112(2):581–90. doi: 10.1242/dev.112.2.581. [DOI] [PubMed] [Google Scholar]
- 17.Sabry JH, O'Connor TP, Evans L, Toroian-Raymond A, Kirschner M, Bentley D. Microtubule behavior during guidance of pioneer neuron growth cones in situ. J Cell Biol. 1991;115(2):381–95. doi: 10.1083/jcb.115.2.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Auld V. Glia as mediators of growth cone guidance: studies from insect nervous systems. Cell Mol Life Sci. 1999;55(11):1377–85. doi: 10.1007/s000180050378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hidalgo A. Neuron-glia interactions during axon guidance in Drosophila. Biochem Soc Trans. 2003;31(Pt 1):50–5. doi: 10.1042/bst0310050. [DOI] [PubMed] [Google Scholar]
- 20.Whitington PM, Quilkey C, Sink H. Necessity and redundancy of guidepost cells in the embryonic Drosophila CNS. Int J Dev Neurosci. 2004;22(3):157–63. doi: 10.1016/j.ijdevneu.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 21.Palka J, Whitlock KE, Murray MA. Guidepost cells. Curr Opin Neurobiol. 1992;2(1):48–54. doi: 10.1016/0959-4388(92)90161-d. [DOI] [PubMed] [Google Scholar]
- 22.O'Conner T. Intermediate targets and segmental pathfinding. Cell Mol Life Sci. 1999;55(11):1358–64. [Google Scholar]
- 23.Dahlin L, Johansson F, Lindwall C, Kanje M. Chapter 28: Future perspective in peripheral nerve reconstruction. Int Rev Neurobiol. 2009;87:507–30. doi: 10.1016/S0074-7742(09)87028-1. [DOI] [PubMed] [Google Scholar]
- 24.Mladinic M, Muller KJ, Nicholls JG. Central nervous system regeneration: from leech to opossum. J Physiol. 2009;587(Pt 12):2775–82. doi: 10.1113/jphysiol.2009.169938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Saxena S, Caroni P. Mechanisms of axon degeneration: from development to disease. Prog Neurobiol. 2007;83(3):174–91. doi: 10.1016/j.pneurobio.2007.07.007. [DOI] [PubMed] [Google Scholar]
- 26.Bunge RP. The role of the Schwann cell in trophic support and regeneration. J Neurol. 1994;242(1 Suppl 1):S19–21. doi: 10.1007/BF00939235. [DOI] [PubMed] [Google Scholar]
- 27.Griffin JW, Thompson WJ. Biology and pathology of nonmyelinating Schwann cells. Glia. 2008;56(14):1518–31. doi: 10.1002/glia.20778. [DOI] [PubMed] [Google Scholar]
- 28.Torigoe K, Tanaka HF, Takahashi A, Awaya A, Hashimoto K. Basic behavior of migratory Schwann cells in peripheral nerve regeneration. Exp Neurol. 1996;137(2):301–8. doi: 10.1006/exnr.1996.0030. [DOI] [PubMed] [Google Scholar]
- 29.Chen YY, McDonald D, Cheng C, Magnowski B, Durand J, Zochodne DW. Axon and Schwann cell partnership during nerve regrowth. J Neuropathol Exp Neurol. 2005;64(7):613–22. doi: 10.1097/01.jnen.0000171650.94341.46. [DOI] [PubMed] [Google Scholar]
- 30.Stoll G, Muller HW. Nerve injury, axonal degeneration and neural regeneration: basic insights. Brain Pathol. 1999;9(2):313–25. doi: 10.1111/j.1750-3639.1999.tb00229.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Busch SA, Silver J. The role of extracellular matrix in CNS regeneration. Curr Opin Neurobiol. 2007;17(1):120–7. doi: 10.1016/j.conb.2006.09.004. [DOI] [PubMed] [Google Scholar]
- 32.Pekny M, Nilsson M. Astrocyte activation and reactive gliosis. Glia. 2005;50(4):427–34. doi: 10.1002/glia.20207. [DOI] [PubMed] [Google Scholar]
- 33.Avellino AM, Hart D, Dailey AT, MacKinnon M, Ellegala D, Kliot M. Differential macrophage responses in the peripheral and central nervous system during wallerian degeneration of axons. Exp Neurol. 1995;136(2):183–98. doi: 10.1006/exnr.1995.1095. [DOI] [PubMed] [Google Scholar]
- 34.Schmidt CE, Leach JB. Neural tissue engineering: strategies for repair and regeneration. Annu Rev Biomed Eng. 2003;5:293–347. doi: 10.1146/annurev.bioeng.5.011303.120731. [DOI] [PubMed] [Google Scholar]
- 35.O'Donnell M, Chance RK, Bashaw GJ. Axon growth and guidance: receptor regulation and signal transduction. Annu Rev Neurosci. 2009;32:383–412. doi: 10.1146/annurev.neuro.051508.135614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Bernhardt RR. Cellular and molecular bases of axonal pathfinding during embryogenesis of the fish central nervous system. J Neurobiol. 1999;38(1):137–60. doi: 10.1002/(sici)1097-4695(199901)38:1<137::aid-neu11>3.0.co;2-p. [DOI] [PubMed] [Google Scholar]
- 37.Becker CG, Becker T. Growth and pathfinding of regenerating axons in the optic projection of adult fish. J Neurosci Res. 2007;85(12):2793–9. doi: 10.1002/jnr.21121. [DOI] [PubMed] [Google Scholar]
- 38.Kalil K, Dent EW. Touch and go: guidance cues signal to the growth cone cytoskeleton. Curr Opin Neurobiol. 2005;15(5):521–6. doi: 10.1016/j.conb.2005.08.005. [DOI] [PubMed] [Google Scholar]
- 39.Wen Z, Zheng JQ. Directional guidance of nerve growth cones. Curr Opin Neurobiol. 2006;16(1):52–8. doi: 10.1016/j.conb.2005.12.005. [DOI] [PubMed] [Google Scholar]
- 40.Geller HM, Fawcett JW. Building a bridge: engineering spinal cord repair. Exp Neurol. 2002;174(2):125–36. doi: 10.1006/exnr.2002.7865. [DOI] [PubMed] [Google Scholar]
- 41.Foley JD, Grunwald EW, Nealey PF, Murphy CJ. Cooperative modulation of neuritogenesis by PC12 cells by topography and nerve growth factor. Biomaterials. 2005;26(17):3639–44. doi: 10.1016/j.biomaterials.2004.09.048. [DOI] [PubMed] [Google Scholar]
- 42.Gomez N, Lu Y, Chen S, Schmidt CE. Immobilized nerve growth factor and microtopography have distinct effects on polarization versus axon elongation in hippocampal cells in culture. Biomaterials. 2007;28(2):271–84. doi: 10.1016/j.biomaterials.2006.07.043. [DOI] [PubMed] [Google Scholar]
- 43.Mahoney MJ, Chen RR, Tan J, Saltzman WM. The influence of microchannels on neurite growth and architecture. Biomaterials. 2005;26(7):771–8. doi: 10.1016/j.biomaterials.2004.03.015. [DOI] [PubMed] [Google Scholar]
- 44.Haq F, Venkatramani A, Keith C, Zhang G. Neurite development in PC12 cells cultured on nanopillars and nanopores with sizes comparable with filopodia. Int J Nanomed. 2007;2(1):107–115. doi: 10.2147/nano.2007.2.1.107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Houchin-Ray T, Swift LA, Jang JH, Shea LD. Patterned PLG substrates for localized DNA delivery and directed neurite extension. Biomaterials. 2007;28(16):2603–11. doi: 10.1016/j.biomaterials.2007.01.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koh HS, Yong T, Chan CK, Ramakrishna S. Enhancement of neurite outgrowth using nano-structured scaffolds coupled with laminin. Biomaterials. 2008;29(26):3574–82. doi: 10.1016/j.biomaterials.2008.05.014. [DOI] [PubMed] [Google Scholar]
- 47.Rangappa N, Romero A, Nelson KD, Eberhart RC, Smith GM. Laminin-coated poly(L-lactide) filaments induce robust neurite growth while providing directional orientation. J Biomed Mater Res. 2000;51(4):625–34. doi: 10.1002/1097-4636(20000915)51:4<625::aid-jbm10>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 48.Miller C, Jeftinija S, Mallapragada S. Synergistic effects of physical and chemical guidance cues on neurite alignment and outgrowth on biodegradable polymer substrates. Tissue Eng. 2002;8(3):367–78. doi: 10.1089/107632702760184646. [DOI] [PubMed] [Google Scholar]
- 49.Miller C, Jeftinija S, Mallapragada S. Micropatterned Schwann cell-seeded biodegradable polymer substrates significantly enhance neurite alignment and outgrowth. Tissue Eng. 2001;7(6):705–15. doi: 10.1089/107632701753337663. [DOI] [PubMed] [Google Scholar]
- 50.Kofron C, Hoffman-Kim D. Optimization by response surface methodology of confluent aligned monolayers of glial and non-glial cells. Cellular and Molecular Engineering. 2009 doi: 10.1007/s12195-009-0087-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Dowell-Mesfin NM, Abdul-Karim MA, Turner AM, Schanz S, Craighead HG, Roysam B, Turner JN, Shain W. Topographically modified surfaces affect orientation and growth of hippocampal neurons. J Neural Eng. 2004;1(2):78–90. doi: 10.1088/1741-2560/1/2/003. [DOI] [PubMed] [Google Scholar]
- 52.Walsh JF, Manwaring ME, Tresco PA. Directional neurite outgrowth is enhanced by engineered meningeal cell-coated substrates. Tissue Eng. 2005;11(7-8):1085–94. doi: 10.1089/ten.2005.11.1085. [DOI] [PubMed] [Google Scholar]
- 53.Hanson JN, Motala MJ, Heien ML, Gillette M, Sweedler J, Nuzzo RG. Textural guidance cues for controlling process outgrowth of mammalian neurons. Lab Chip. 2009;9(1):122–31. doi: 10.1039/b803595d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Li GN, Hoffman-Kim D. Evaluation of neurite outgrowth anisotropy using a novel application of circular analysis. J Neurosci Methods. 2008 Sep;174:202–214. doi: 10.1016/j.jneumeth.2008.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Johansson F, Carlberg P, Danielsen N, Montelius L, Kanje M. Axonal outgrowth on nano-imprinted patterns. Biomaterials. 2006;27(8):1251–8. doi: 10.1016/j.biomaterials.2005.07.047. [DOI] [PubMed] [Google Scholar]
- 56.Corey JM, Lin DY, Mycek KB, Chen Q, Samuel S, Feldman EL, Martin DC. Aligned electrospun nanofibers specify the direction of dorsal root ganglia neurite growth. J Biomed Mater Res A. 2007;83(3):636–45. doi: 10.1002/jbm.a.31285. [DOI] [PubMed] [Google Scholar]
- 57.Tranquillo RT. Self-organization of tissue-equivalents: the nature and role of contact guidance. Biochem Soc Symp. 1999;65:27–42. [PubMed] [Google Scholar]
- 58.Bellamkonda RV. Peripheral nerve regeneration: an opinion on channels, scaffolds and anisotropy. Biomaterials. 2006;27(19):3515–8. doi: 10.1016/j.biomaterials.2006.02.030. [DOI] [PubMed] [Google Scholar]
- 59.Yao L, Wang S, Cui W, Sherlock R, O'Connell C, Damodaran G, Gorman A, Windebank A, Pandit A. Effect of functionalized micropatterned PLGA on guided neurite growth. Acta Biomater. 2009;5(2):580–8. doi: 10.1016/j.actbio.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 60.Cecchini M, Ferrari A, Beltram F. PC12 polarity on biopolymer nanogratings. J Phys: Conf Series. 2008;100 [Google Scholar]
- 61.Li J, McNally H, Shi R. Enhanced neurite alignment on micropatterned poly-L-lactic acid films. J Biomed Mater Res A. 2008;87(2):392–404. doi: 10.1002/jbm.a.31814. [DOI] [PubMed] [Google Scholar]
- 62.Lietz M, Dreesmann L, Hoss M, Oberhoffner S, Schlosshauer B. Neuro tissue engineering of glial nerve guides and the impact of different cell types. Biomaterials. 2006;27(8):1425–36. doi: 10.1016/j.biomaterials.2005.08.007. [DOI] [PubMed] [Google Scholar]
- 63.Hirono T, Torimitsu K, Kawana A, Fukuda J. Recognition of artificial microstructures by sensory nerve fibers in culture. Brain Res. 1988;446(1):189–94. doi: 10.1016/0006-8993(88)91314-5. [DOI] [PubMed] [Google Scholar]
- 64.Clark P, Connolly P, Curtis AS, Dow JA, Wilkinson CD. Topographical control of cell behaviour: II. Multiple grooved substrata. Development. 1990;108(4):635–44. doi: 10.1242/dev.108.4.635. [DOI] [PubMed] [Google Scholar]
- 65.Li N, Folch A. Integration of topographical and biochemical cues by axons during growth on microfabricated 3-D substrates. Exp Cell Res. 2005;311(2):307–16. doi: 10.1016/j.yexcr.2005.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Nagata I, Kawana A, Nakatsuji N. Perpendicular contact guidance of CNS neuroblasts on artificial microstructures. Development. 1993;117(1):401–8. doi: 10.1242/dev.117.1.401. [DOI] [PubMed] [Google Scholar]
- 67.Rajnicek A, Britland S, McCaig C. Contact guidance of CNS neurites on grooved quartz: influence of groove dimensions, neuronal age and cell type. J Cell Sci. 1997;110(Pt 23):2905–13. doi: 10.1242/jcs.110.23.2905. [DOI] [PubMed] [Google Scholar]
- 68.Martinez E, Engel E, Planell JA, Samitier J. Effects of artificial micro- and nano-structured surfaces on cell behaviour. Ann Anat. 2009;191(1):126–35. doi: 10.1016/j.aanat.2008.05.006. [DOI] [PubMed] [Google Scholar]
- 69.Ono K, Shokunbi T, Nagata I, Tokunaga A, Yasui Y, Nakatsuji N. Filopodia and growth cones in the vertically migrating granule cells of the postnatal mouse cerebellum. Exp Brain Res. 1997;117(1):17–29. doi: 10.1007/pl00005787. [DOI] [PubMed] [Google Scholar]
- 70.Nagata I, Ono K, Kawana A, Kimura-Kuroda J. Aligned neurite bundles of granule cells regulate orientation of Purkinje cell dendrites by perpendicular contact guidance in two-dimensional and three-dimensional mouse cerebellar cultures. J Comp Neurol. 2006;499(2):274–89. doi: 10.1002/cne.21102. [DOI] [PubMed] [Google Scholar]
- 71.Goldner JS, Bruder JM, Li G, Gazzola D, Hoffman-Kim D. Neurite bridging across micropatterned grooves. Biomaterials. 2006;27(3):460–72. doi: 10.1016/j.biomaterials.2005.06.035. [DOI] [PubMed] [Google Scholar]
- 72.Vasita R, Katti DS. Nanofibers and their applications in tissue engineering. Int J Nanomedicine. 2006;1(1):15–30. doi: 10.2147/nano.2006.1.1.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yu TT, Shoichet MS. Guided cell adhesion and outgrowth in peptide-modified channels for neural tissue engineering. Biomaterials. 2005;26(13):1507–14. doi: 10.1016/j.biomaterials.2004.05.012. [DOI] [PubMed] [Google Scholar]
- 74.Wen X, Tresco PA. Effect of filament diameter and extracellular matrix molecule precoating on neurite outgrowth and Schwann cell behavior on multifilament entubulation bridging device in vitro. J Biomed Mater Res A. 2006;76(3):626–37. doi: 10.1002/jbm.a.30520. [DOI] [PubMed] [Google Scholar]
- 75.Murugan R, Ramakrishna S. Design strategies of tissue engineering scaffolds with controlled fiber orientation. Tissue Eng. 2007;13(8):1845–66. doi: 10.1089/ten.2006.0078. [DOI] [PubMed] [Google Scholar]
- 76.Kumbar SG, James R, Nukavarapu SP, Laurencin CT. Electrospun nanofiber scaffolds: engineering soft tissues. Biomed Mater. 2008;3(3):034002. doi: 10.1088/1748-6041/3/3/034002. [DOI] [PubMed] [Google Scholar]
- 77.Kim YT, Haftel VK, Kumar S, Bellamkonda RV. The role of aligned polymer fiber-based constructs in the bridging of long peripheral nerve gaps. Biomaterials. 2008;29(21):3117–27. doi: 10.1016/j.biomaterials.2008.03.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Yang F, Murugan R, Wang S, Ramakrishna S. Electrospinning of nano/micro scale poly(L-lactic acid) aligned fibers and their potential in neural tissue engineering. Biomaterials. 2005;26(15):2603–10. doi: 10.1016/j.biomaterials.2004.06.051. [DOI] [PubMed] [Google Scholar]
- 79.Kriparamanan R, Aswath P, Zhou A, Tang L, Nguyen KT. Nanotopography: cellular responses to nanostructured materials. J Nanosci Nanotechnol. 2006;6(7):1905–19. doi: 10.1166/jnn.2006.330. [DOI] [PubMed] [Google Scholar]
- 80.Papadopoulou E, Samara A, Barberoglou M, Manousaki A, Pagakis S, Anastasiadou E, Fotakis C, Stratakis E. Silicon Scaffolds Promoting 3D Neuronal Web of Cytoplasmic Processes. Tissue Eng, Pt C. doi: 10.1089/ten.tec.2009.0216. ahead of print. [DOI] [PubMed] [Google Scholar]
- 81.Hallstrom W, Martensson T, Prinz C, Gustavsson P, Montelius L, Samuelson L, Kanje M. Gallium phosphide nanowires as a substrate for cultured neurons. Nano Lett. 2007;7(10):2960–5. doi: 10.1021/nl070728e. [DOI] [PubMed] [Google Scholar]
- 82.Cyster LA, Parker KG, Parker TL, Grant DM. The effect of surface chemistry and nanotopography of titanium nitride (TiN) films on primary hippocampal neurones. Biomaterials. 2004;25(1):97–107. doi: 10.1016/s0142-9612(03)00480-0. [DOI] [PubMed] [Google Scholar]
- 83.Khan SP, Auner GG, Newaz GM. Influence of nanoscale surface roughness on neural cell attachment on silicon. Nanomedicine. 2005;1(2):125–9. doi: 10.1016/j.nano.2005.03.007. [DOI] [PubMed] [Google Scholar]
- 84.Lamour G, Journiac N, Soues S, Bonneau S, Nassoy P, Hamraoui A. Influence of surface energy distribution on neuritogenesis. Colloids Surf B Biointerfaces. 2009;72(2):208–18. doi: 10.1016/j.colsurfb.2009.04.006. [DOI] [PubMed] [Google Scholar]
- 85.Thompson DM, Buettner HM. Neurite outgrowth is directed by schwann cell alignment in the absence of other guidance cues. Ann Biomed Eng. 2006;34(1):161–8. doi: 10.1007/s10439-005-9013-4. [DOI] [PubMed] [Google Scholar]
- 86.Hsu SH, Chen CY, Lu PS, Lai CS, Chen CJ. Oriented Schwann cell growth on microgrooved surfaces. Biotechnol Bioeng. 2005;92(5):579–88. doi: 10.1002/bit.20634. [DOI] [PubMed] [Google Scholar]
- 87.Fawcett JW, Asher RA. The glial scar and central nervous system repair. Brain Res Bull. 1999;49(6):377–91. doi: 10.1016/s0361-9230(99)00072-6. [DOI] [PubMed] [Google Scholar]
- 88.Shearer MC, Fawcett JW. The astrocyte/meningeal cell interface–a barrier to successful nerve regeneration? Cell Tissue Res. 2001;305(2):267–73. doi: 10.1007/s004410100384. [DOI] [PubMed] [Google Scholar]
- 89.Silver J, Miller JH. Regeneration beyond the glial scar. Nat Rev Neurosci. 2004;5(2):146–56. doi: 10.1038/nrn1326. [DOI] [PubMed] [Google Scholar]
- 90.Alexander JK, Fuss B, Colello RJ. Electric field-induced astrocyte alignment directs neurite outgrowth. Neuron Glia Biol. 2006;2(2):93–103. doi: 10.1017/S1740925X0600010X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Recknor JB, Sakaguchi DS, Mallapragada SK. Directed growth and selective differentiation of neural progenitor cells on micropatterned polymer substrates. Biomaterials. 2006;27(22):4098–108. doi: 10.1016/j.biomaterials.2006.03.029. [DOI] [PubMed] [Google Scholar]
- 92.Bruder JM, Monu NC, Harrison MW, Hoffman-Kim D. Fabrication of polymeric replicas of cell surfaces with nanoscale resolution. Langmuir. 2006;22(20):8266–70. doi: 10.1021/la0608563. [DOI] [PubMed] [Google Scholar]
- 93.Bruder JM, Lee AP, Hoffman-Kim D. Biomimetic materials replicating Schwann cell topography enhance neuronal adhesion and neurite alignment in vitro. J Biomater Sci Polym Ed. 2007;18(8):967–82. doi: 10.1163/156856207781494412. [DOI] [PubMed] [Google Scholar]
- 94.Grew JC, Ricci JL, Alexander H. Connective-tissue responses to defined biomaterial surfaces. II. Behavior of rat and mouse fibroblasts cultured on microgrooved substrates. J Biomed Mater Res A. 2008;85(2):326–35. doi: 10.1002/jbm.a.31378. [DOI] [PubMed] [Google Scholar]
- 95.Teixeira AI, Abrams GA, Bertics PJ, Murphy CJ, Nealey PF. Epithelial contact guidance on well-defined micro- and nanostructured substrates. J Cell Sci. 2003;116(Pt 10):1881–92. doi: 10.1242/jcs.00383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Charest JL, Eliason MT, Garcia AJ, King WP. Combined microscale mechanical topography and chemical patterns on polymer cell culture substrates. Biomaterials. 2006;27(11):2487–94. doi: 10.1016/j.biomaterials.2005.11.022. [DOI] [PubMed] [Google Scholar]
- 97.Britland S, Morgan H, Wojiak-Stodart B, Riehle M, Curtis A, Wilkinson C. Synergistic and hierarchical adhesive and topographic guidance of BHK cells. Exp Cell Res. 1996;228(2):313–25. doi: 10.1006/excr.1996.0331. [DOI] [PubMed] [Google Scholar]
- 98.Gomez N, Chen S, Schmidt CE. Polarization of hippocampal neurons with competitive surface stimuli: contact guidance cues are preferred over chemical ligands. J R Soc Interface. 2007;4(13):223–33. doi: 10.1098/rsif.2006.0171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Kofron CM, Fong VJ, Hoffman-Kim D. Neurite outgrowth at the interface of 2D and 3D growth environments. J Neural Eng. 2009;6(1):016002. doi: 10.1088/1741-2560/6/1/016002. [DOI] [PubMed] [Google Scholar]
- 100.Kang CE, Poon PC, Tator CH, Shoichet MS. A new paradigm for local and sustained release of therapeutic molecules to the injured spinal cord for neuroprotection and tissue repair. Tissue Eng Part A. 2009;15(3):595–604. doi: 10.1089/ten.tea.2007.0349. [DOI] [PubMed] [Google Scholar]
- 101.Vavrek R, Girgis J, Tetzlaff W, Hiebert GW, Fouad K. BDNF promotes connections of corticospinal neurons onto spared descending interneurons in spinal cord injured rats. Brain. 2006;129(Pt 6):1534–45. doi: 10.1093/brain/awl087. [DOI] [PubMed] [Google Scholar]
- 102.Yu P, Huang L, Zou J, Zhu H, Wang X, Yu Z, Xu XM, Lu PH. DNA vaccine against NgR promotes functional recovery after spinal cord injury in adult rats. Brain Res. 2007;1147:66–76. doi: 10.1016/j.brainres.2007.02.013. [DOI] [PubMed] [Google Scholar]
- 103.Walmsley AR, Mir AK. Targeting the Nogo-A signalling pathway to promote recovery following acute CNS injury. Curr Pharm Des. 2007;13(24):2470–84. doi: 10.2174/138161207781368611. [DOI] [PubMed] [Google Scholar]
- 104.Selzer ME. Promotion of axonal regeneration in the injured CNS. Lancet Neurol. 2003;2(3):157–66. doi: 10.1016/s1474-4422(03)00322-3. [DOI] [PubMed] [Google Scholar]
- 105.Bunge MB. Novel combination strategies to repair the injured mammalian spinal cord. J Spinal Cord Med. 2008;31(3):262–9. doi: 10.1080/10790268.2008.11760720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Calancie B, Madsen PW, Wood P, Marcillo AE, Levi AD, Bunge RP. A guidance channel seeded with autologous Schwann cells for repair of cauda equina injury in a primate model. J Spinal Cord Med. 2009;32(4):379–88. doi: 10.1080/10790268.2009.11754411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Schlosshauer B, Dreesmann L, Schaller HE, Sinis N. Synthetic nerve guide implants in humans: a comprehensive survey. Neurosurgery. 2006;59(4):740–7. doi: 10.1227/01.NEU.0000235197.36789.42. discussion 747–8. [DOI] [PubMed] [Google Scholar]
- 108.Ichihara S, Inada Y, Nakamura T. Artificial nerve tubes and their application for repair of peripheral nerve injury: an update of current concepts. Injury. 2008;39 4:29–39. doi: 10.1016/j.injury.2008.08.029. [DOI] [PubMed] [Google Scholar]
- 109.Xu X, Yee WC, Hwang PY, Yu H, Wan AC, Gao S, Boon KL, Mao HQ, Leong KW, Wang S. Peripheral nerve regeneration with sustained release of poly(phosphoester) microencapsulated nerve growth factor within nerve guide conduits. Biomaterials. 2003;24(13):2405–12. doi: 10.1016/s0142-9612(03)00109-1. [DOI] [PubMed] [Google Scholar]
- 110.Pfister LA, Papaloizos M, Merkle HP, Gander B. Nerve conduits and growth factor delivery in peripheral nerve repair. J Peripher Nerv Syst. 2007;12(2):65–82. doi: 10.1111/j.1529-8027.2007.00125.x. [DOI] [PubMed] [Google Scholar]
- 111.Kato K, Liu H, Kikuchi SI, Myers RR, Shubayev VI. Immediate antitumor necrosis factor-alpha (etanercept) therapy enhances axonal regeneration after sciatic nerve crush. J Neurosci Res. 2009 doi: 10.1002/jnr.22202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Ruff RL, McKerracher L, Selzer ME. Repair and neurorehabilitation strategies for spinal cord injury. Ann N Y Acad Sci. 2008;1142:1–20. doi: 10.1196/annals.1444.004. [DOI] [PubMed] [Google Scholar]
- 113.Blesch A, Lu P, Tuszynski MH. Neurotrophic factors, gene therapy, and neural stem cells for spinal cord repair. Brain Res Bull. 2002;57(6):833–8. doi: 10.1016/s0361-9230(01)00774-2. [DOI] [PubMed] [Google Scholar]
- 114.de Ruiter GC, Malessy MJ, Yaszemski MJ, Windebank AJ, Spinner RJ. Designing ideal conduits for peripheral nerve repair. Neurosurg Focus. 2009;26(2):E5. doi: 10.3171/FOC.2009.26.2.E5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Brushart TM, Mathur V, Sood R, Koschorke GM. Joseph H. Boyes Award. Dispersion of regenerating axons across enclosed neural gaps. J Hand Surg Am. 1995;20(4):557–64. doi: 10.1016/s0363-5023(05)80267-9. [DOI] [PubMed] [Google Scholar]
- 116.Smeal RM, Tresco PA. The influence of substrate curvature on neurite outgrowth is cell type dependent. Exp Neurol. 2008;213(2):281–92. doi: 10.1016/j.expneurol.2008.05.026. [DOI] [PubMed] [Google Scholar]
- 117.Straley KS, Wong Po Foo C, Heilshorn S. Biomaterial Design Strategies for the Treatment of Spinal Cord Injuries. J Neurotrauma. 2009 doi: 10.1089/neu.2009.0948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Krych AJ, Rooney GE, Chen B, Schermerhorn TC, Ameenuddin S, Gross L, Moore MJ, Currier BL, Spinner RJ, Friedman JA, Yaszemski MJ, Windebank AJ. Relationship between scaffold channel diameter and number of regenerating axons in the transected rat spinal cord. Acta Biomater. 2009;5(7):2551–9. doi: 10.1016/j.actbio.2009.03.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Sundback C, Hadlock T, Cheney M, Vacanti J. Manufacture of porous polymer nerve conduits by a novel low-pressure injection molding process. Biomaterials. 2003;24(5):819–30. doi: 10.1016/s0142-9612(02)00409-x. [DOI] [PubMed] [Google Scholar]
- 120.Bender MD, Bennett JM, Waddell RL, Doctor JS, Marra KG. Multi-channeled biodegradable polymer/CultiSpher composite nerve guides. Biomaterials. 2004;25(7-8):1269–78. doi: 10.1016/j.biomaterials.2003.08.046. [DOI] [PubMed] [Google Scholar]
- 121.Yang Y, De Laporte L, Rives CB, Jang JH, Lin WC, Shull KR, Shea LD. Neurotrophin releasing single and multiple lumen nerve conduits. J Control Release. 2005;104(3):433–46. doi: 10.1016/j.jconrel.2005.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Stokols S, Tuszynski MH. The fabrication and characterization of linearly oriented nerve guidance scaffolds for spinal cord injury. Biomaterials. 2004;25(27):5839–46. doi: 10.1016/j.biomaterials.2004.01.041. [DOI] [PubMed] [Google Scholar]
- 123.de Ruiter GC, Spinner RJ, Malessy MJ, Moore MJ, Sorenson EJ, Currier BL, Yaszemski MJ, Windebank AJ. Accuracy of motor axon regeneration across autograft, single-lumen, and multichannel poly(lactic-co-glycolic acid) nerve tubes. Neurosurgery. 2008;63(1):144–53. doi: 10.1227/01.NEU.0000335081.47352.78. discussion 153–5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Stokols S, Tuszynski MH. Freeze-dried agarose scaffolds with uniaxial channels stimulate and guide linear axonal growth following spinal cord injury. Biomaterials. 2006;27(3):443–51. doi: 10.1016/j.biomaterials.2005.06.039. [DOI] [PubMed] [Google Scholar]
- 125.Moore MJ, Friedman JA, Lewellyn EB, Mantila SM, Krych AJ, Ameenuddin S, Knight AM, Lu L, Currier BL, Spinner RJ, Marsh RW, Windebank AJ, Yaszemski MJ. Multiple-channel scaffolds to promote spinal cord axon regeneration. Biomaterials. 2006;27(3):419–29. doi: 10.1016/j.biomaterials.2005.07.045. [DOI] [PubMed] [Google Scholar]
- 126.Stokols S, Sakamoto J, Breckon C, Holt T, Weiss J, Tuszynski MH. Templated agarose scaffolds support linear axonal regeneration. Tissue Eng. 2006;12(10):2777–87. doi: 10.1089/ten.2006.12.2777. [DOI] [PubMed] [Google Scholar]
- 127.Smith LA, Ma PX. Nano-fibrous scaffolds for tissue engineering. Colloids Surf B Biointerfaces. 2004;39(3):125–31. doi: 10.1016/j.colsurfb.2003.12.004. [DOI] [PubMed] [Google Scholar]
- 128.Hadlock T, Sundback C, Hunter D, Cheney M, Vacanti JP. A polymer foam conduit seeded with Schwann cells promotes guided peripheral nerve regeneration. Tissue Eng. 2000;6(2):119–27. doi: 10.1089/107632700320748. [DOI] [PubMed] [Google Scholar]
- 129.Rutkowski GE, Miller CA, Jeftinija S, Mallapragada SK. Synergistic effects of micropatterned biodegradable conduits and Schwann cells on sciatic nerve regeneration. J Neural Eng. 2004;1(3):151–7. doi: 10.1088/1741-2560/1/3/004. [DOI] [PubMed] [Google Scholar]
- 130.Yoshii S, Oka M, Shima M, Akagi M, Taniguchi A. Bridging a spinal cord defect using collagen filament. Spine (Phila Pa 1976) 2003;28(20):2346–51. doi: 10.1097/01.BRS.0000085302.95413.16. [DOI] [PubMed] [Google Scholar]
- 131.Yoshii S, Oka M, Shima M, Taniguchi A, Taki Y, Akagi M. Restoration of function after spinal cord transection using a collagen bridge. J Biomed Mater Res A. 2004;70(4):569–75. doi: 10.1002/jbm.a.30120. [DOI] [PubMed] [Google Scholar]
- 132.Yoshii S, Ito S, Shima M, Taniguchi A, Akagi M. Functional restoration of rabbit spinal cord using collagen-filament scaffold. J Tissue Eng Regen Med. 2009;3(1):19–25. doi: 10.1002/term.130. [DOI] [PubMed] [Google Scholar]
- 133.Reynolds LF, Bren MC, Wilson BC, Gibson GD, Shoichet MS, Murphy RJ. Transplantation of porous tubes following spinal cord transection improves hindlimb function in the rat. Spinal Cord. 2008;46(1):58–64. doi: 10.1038/sj.sc.3102063. [DOI] [PubMed] [Google Scholar]
- 134.Panseri S, Cunha C, Lowery J, Del Carro U, Taraballi F, Amadio S, Vescovi A, Gelain F. Electrospun micro- and nanofiber tubes for functional nervous regeneration in sciatic nerve transections. BMC Biotechnol. 2008;8:39. doi: 10.1186/1472-6750-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Cai J, Peng X, Nelson KD, Eberhart R, Smith GM. Permeable guidance channels containing microfilament scaffolds enhance axon growth and maturation. J Biomed Mater Res A. 2005;75(2):374–86. doi: 10.1002/jbm.a.30432. [DOI] [PubMed] [Google Scholar]
- 136.Lu MC, Huang YT, Lin JH, Yao CH, Lou CW, Tsai CC, Chen YS. Evaluation of a multi-layer microbraided polylactic acid fiber-reinforced conduit for peripheral nerve regeneration. J Mater Sci Mater Med. 2009;20(5):1175–80. doi: 10.1007/s10856-008-3646-4. [DOI] [PubMed] [Google Scholar]
- 137.Clements IP, Kim YT, English AW, Lu X, Chung A, Bellamkonda RV. Thin-film enhanced nerve guidance channels for peripheral nerve repair. Biomaterials. 2009;30(23-24):3834–46. doi: 10.1016/j.biomaterials.2009.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Flemming RG, Murphy CJ, Abrams GA, Goodman SL, Nealey PF. Effects of synthetic micro- and nano-structured surfaces on cell behavior. Biomaterials. 1999;20(6):573–88. doi: 10.1016/s0142-9612(98)00209-9. [DOI] [PubMed] [Google Scholar]
- 139.Wilson CJ, Clegg RE, Leavesley DI, Pearcy MJ. Mediation of biomaterial-cell interactions by adsorbed proteins: a review. Tissue Eng. 2005;11(1-2):1–18. doi: 10.1089/ten.2005.11.1. [DOI] [PubMed] [Google Scholar]
- 140.Roach P, Farrar D, Perry CC. Surface tailoring for controlled protein adsorption: effect of topography at the nanometer scale and chemistry. J Am Chem Soc. 2006;128(12):3939–45. doi: 10.1021/ja056278e. [DOI] [PubMed] [Google Scholar]
- 141.Kane RS, Stroock AD. Nanobiotechnology: protein-nanomaterial interactions. Biotechnol Prog. 2007;23(2):316–9. doi: 10.1021/bp060388n. [DOI] [PubMed] [Google Scholar]
- 142.Lim JY, Hansen JC, Siedlecki CA, Hengstebeck RW, Cheng J, Winograd N, Donahue HJ. Osteoblast adhesion on poly(L-lactic acid)/polystyrene demixed thin film blends: effect of nanotopography, surface chemistry, and wettability. Biomacromolecules. 2005;6(6):3319–27. doi: 10.1021/bm0503423. [DOI] [PubMed] [Google Scholar]
- 143.Hamilton DW, Oakley C, Jaeger NA, Brunette DM. Directional change produced by perpendicularly-oriented microgrooves is microtubule-dependent for fibroblasts and epithelium. Cell Motil Cytoskeleton. 2009;66(5):260–71. doi: 10.1002/cm.20354. [DOI] [PubMed] [Google Scholar]
- 144.Walboomers XF, Croes HJ, Ginsel LA, Jansen JA. Growth behavior of fibroblasts on microgrooved polystyrene. Biomaterials. 1998;19(20):1861–8. doi: 10.1016/s0142-9612(98)00093-3. [DOI] [PubMed] [Google Scholar]
- 145.Gerecht S, Bettinger CJ, Zhang Z, Borenstein JT, Vunjak-Novakovic G, Langer R. The effect of actin disrupting agents on contact guidance of human embryonic stem cells. Biomaterials. 2007;28(28):4068–77. doi: 10.1016/j.biomaterials.2007.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Mai J, Sun C, Li S, Zhang X. A microfabricated platform probing cytoskeleton dynamics using multidirectional topographical cues. Biomed Microdevices. 2007;9(4):523–31. doi: 10.1007/s10544-007-9060-8. [DOI] [PubMed] [Google Scholar]
- 147.Oakley C, Brunette DM. The sequence of alignment of microtubules, focal contacts and actin filaments in fibroblasts spreading on smooth and grooved titanium substrata. J Cell Sci. 1993;106(Pt 1):343–54. doi: 10.1242/jcs.106.1.343. [DOI] [PubMed] [Google Scholar]
- 148.Wojciak-Stothard B, Curtis AS, Monaghan W, McGrath M, Sommer I, Wilkinson CD. Role of the cytoskeleton in the reaction of fibroblasts to multiple grooved substrata. Cell Motil Cytoskeleton. 1995;31(2):147–58. doi: 10.1002/cm.970310207. [DOI] [PubMed] [Google Scholar]
- 149.Rajnicek A, McCaig C. Guidance of CNS growth cones by substratum grooves and ridges: effects of inhibitors of the cytoskeleton, calcium channels and signal transduction pathways. J Cell Sci. 1997;110(Pt 23):2915–24. doi: 10.1242/jcs.110.23.2915. [DOI] [PubMed] [Google Scholar]
- 150.Oakes PW, Patel DC, Morin NA, Zitterbart DP, Fabry B, Reichner JS, Tang JX. Neutrophil morphology and migration are affected by substrate elasticity. Blood. 2009;114(7):1387–95. doi: 10.1182/blood-2008-11-191445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Ali MH, Schumacker PT. Endothelial responses to mechanical stress: where is the mechanosensor? Crit Care Med. 2002;30(5 Suppl):S198–206. doi: 10.1097/00003246-200205001-00005. [DOI] [PubMed] [Google Scholar]
- 152.Katsumi A, Naoe T, Matsushita T, Kaibuchi K, Schwartz MA. Integrin activation and matrix binding mediate cellular responses to mechanical stretch. J Biol Chem. 2005;280(17):16546–9. doi: 10.1074/jbc.C400455200. [DOI] [PubMed] [Google Scholar]
- 153.Booth IR, Edwards MD, Black S, Schumann U, Miller S. Mechanosensitive channels in bacteria: signs of closure? Nat Rev Microbiol. 2007;5(6):431–40. doi: 10.1038/nrmicro1659. [DOI] [PubMed] [Google Scholar]
- 154.Kumamoto CA. Molecular mechanisms of mechanosensing and their roles in fungal contact sensing. Nat Rev Microbiol. 2008;6(9):667–73. doi: 10.1038/nrmicro1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Monshausen GB, Gilroy S. Feeling green: mechanosensing in plants. Trends Cell Biol. 2009;19(5):228–35. doi: 10.1016/j.tcb.2009.02.005. [DOI] [PubMed] [Google Scholar]
- 156.Katsumi A, Orr AW, Tzima E, Schwartz MA. Integrins in mechanotransduction. J Biol Chem. 2004;279(13):12001–4. doi: 10.1074/jbc.R300038200. [DOI] [PubMed] [Google Scholar]
- 157.Makino A, Prossnitz ER, Bunemann M, Wang JM, Yao W, Schmid-Schonbein GW. G protein-coupled receptors serve as mechanosensors for fluid shear stress in neutrophils. Am J Physiol Cell Physiol. 2006;290(6):C1633–9. doi: 10.1152/ajpcell.00576.2005. [DOI] [PubMed] [Google Scholar]
- 158.Chen CS, Tan J, Tien J. Mechanotransduction at cell-matrix and cell-cell contacts. Annu Rev Biomed Eng. 2004;6:275–302. doi: 10.1146/annurev.bioeng.6.040803.140040. [DOI] [PubMed] [Google Scholar]
- 159.Katsumi A, Milanini J, Kiosses WB, del Pozo MA, Kaunas R, Chien S, Hahn KM, Schwartz MA. Effects of cell tension on the small GTPase Rac. J Cell Biol. 2002;158(1):153–64. doi: 10.1083/jcb.200201105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Li S, Huang NF, Hsu S. Mechanotransduction in endothelial cell migration. J Cell Biochem. 2005;96(6):1110–26. doi: 10.1002/jcb.20614. [DOI] [PubMed] [Google Scholar]
- 161.Wojciak-Stothard B, Ridley AJ. Shear stress-induced endothelial cell polarization is mediated by Rho and Rac but not Cdc42 or PI 3-kinases. J Cell Biol. 2003;161(2):429–39. doi: 10.1083/jcb.200210135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Geiger B, Bershadsky A. Assembly and mechanosensory function of focal contacts. Curr Opin Cell Biol. 2001;13(5):584–92. doi: 10.1016/s0955-0674(00)00255-6. [DOI] [PubMed] [Google Scholar]
- 163.Chiquet M, Gelman L, Lutz R, Maier S. From mechanotransduction to extracellular matrix gene expression in fibroblasts. Biochim Biophys Acta. 2009;1793(5):911–20. doi: 10.1016/j.bbamcr.2009.01.012. [DOI] [PubMed] [Google Scholar]
- 164.Poole K, Khairy K, Friedrichs J, Franz C, Cisneros DA, Howard J, Mueller D. Molecular-scale topographic cues induce the orientation and directional movement of fibroblasts on two-dimensional collagen surfaces. J Mol Biol. 2005;349(2):380–6. doi: 10.1016/j.jmb.2005.03.064. [DOI] [PubMed] [Google Scholar]
- 165.Arnold M, Cavalcanti-Adam EA, Glass R, Blummel J, Eck W, Kantlehner M, Kessler H, Spatz JP. Activation of integrin function by nanopatterned adhesive interfaces. Chemphyschem. 2004;5(3):383–8. doi: 10.1002/cphc.200301014. [DOI] [PubMed] [Google Scholar]
- 166.Wei Y. Physical interpretation of the maximum receptor-ligand bond spacing to ensure cell adhesion in ligand-coated substrates. Langmuir. 2008;24(11):5644–6. doi: 10.1021/la800048e. [DOI] [PubMed] [Google Scholar]
- 167.Frey MT, Tsai IY, Russell TP, Hanks SK, Wang YL. Cellular responses to substrate topography: role of myosin II and focal adhesion kinase. Biophys J. 2006;90(10):3774–82. doi: 10.1529/biophysj.105.074526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Wang HB, Dembo M, Hanks SK, Wang Y. Focal adhesion kinase is involved in mechanosensing during fibroblast migration. Proc Natl Acad Sci U S A. 2001;98(20):11295–300. doi: 10.1073/pnas.201201198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Dalby MJ, Giannaras D, Riehle MO, Gadegaard N, Affrossman S, Curtis AS. Rapid fibroblast adhesion to 27nm high polymer demixed nanotopography. Biomaterials. 2004;25(1):77–83. doi: 10.1016/s0142-9612(03)00475-7. [DOI] [PubMed] [Google Scholar]
- 170.Xia N, Thodeti CK, Hunt TP, Xu Q, Ho M, Whitesides GM, Westervelt R, Ingber DE. Directional control of cell motility through focal adhesion positioning and spatial control of Rac activation. FASEB J. 2008;22(6):1649–59. doi: 10.1096/fj.07-090571. [DOI] [PubMed] [Google Scholar]
- 171.Jiang X, Bruzewicz DA, Wong AP, Piel M, Whitesides GM. Directing cell migration with asymmetric micropatterns. Proc Natl Acad Sci U S A. 2005;102(4):975–8. doi: 10.1073/pnas.0408954102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Parker KK, Brock AL, Brangwynne C, Mannix RJ, Wang N, Ostuni E, Geisse NA, Adams JC, Whitesides GM, Ingber DE. Directional control of lamellipodia extension by constraining cell shape and orienting cell tractional forces. FASEB J. 2002;16(10):1195–204. doi: 10.1096/fj.02-0038com. [DOI] [PubMed] [Google Scholar]
- 173.Thery M, Pepin A, Dressaire E, Chen Y, Bornens M. Cell distribution of stress fibres in response to the geometry of the adhesive environment. Cell Motil Cytoskeleton. 2006;63(6):341–55. doi: 10.1002/cm.20126. [DOI] [PubMed] [Google Scholar]
- 174.James J, Goluch ED, Hu H, Liu C, Mrksich M. Subcellular curvature at the perimeter of micropatterned cells influences lamellipodial distribution and cell polarity. Cell Motil Cytoskeleton. 2008;65(11):841–52. doi: 10.1002/cm.20305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Dalby MJ, Riehle MO, Yarwood SJ, Wilkinson CD, Curtis AS. Nucleus alignment and cell signaling in fibroblasts: response to a micro-grooved topography. Exp Cell Res. 2003;284(2):274–82. doi: 10.1016/s0014-4827(02)00053-8. [DOI] [PubMed] [Google Scholar]
- 176.Dalby MJ, Biggs MJ, Gadegaard N, Kalna G, Wilkinson CD, Curtis AS. Nanotopographical stimulation of mechanotransduction and changes in interphase centromere positioning. J Cell Biochem. 2007;100(2):326–38. doi: 10.1002/jcb.21058. [DOI] [PubMed] [Google Scholar]
- 177.Dalby MJ, Gadegaard N, Herzyk P, Sutherland D, Agheli H, Wilkinson CD, Curtis AS. Nanomechanotransduction and interphase nuclear organization influence on genomic control. J Cell Biochem. 2007;102(5):1234–44. doi: 10.1002/jcb.21354. [DOI] [PubMed] [Google Scholar]
- 178.Dahl KN, Ribeiro AJ, Lammerding J. Nuclear shape, mechanics, and mechanotransduction. Circ Res. 2008;102(11):1307–18. doi: 10.1161/CIRCRESAHA.108.173989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Mateos-Langerak J, Goetze S, Leonhardt H, Cremer T, van Driel R, Lanctot C. Nuclear architecture: Is it important for genome function and can we prove it? J Cell Biochem. 2007;102(5):1067–75. doi: 10.1002/jcb.21521. [DOI] [PubMed] [Google Scholar]
- 180.Dalby MJ. Topographically induced direct cell mechanotransduction. Med Eng Phys. 2005;27(9):730–42. doi: 10.1016/j.medengphy.2005.04.005. [DOI] [PubMed] [Google Scholar]