Abstract
The pathogenicity of four native strains of Bacillus thuringiensis against Rhipicephalus (Boophilus) microplus (Canestrine) (Acari: Ixodidae) was evaluated. A R. microplus strain that is resistant to organophosphates, pyrethroids, and amidines, was used in this study. Adult R. microplus females were bioassayed using the immersion test of Drummond against 60 B. thuringiensis strains. Four strains, GP123, GP138, GP130, and GP140, were found to be toxic. For the immersion test, the total protein concentration for each bacterial strain was 1.25 mg/ml. Mortality, oviposition, and egg hatch were recorded. All of the bacterial strains had significant effects compared to the controls, but no significant differences were seen between the 4 strains. It is evident that these B. thuringiensis strains have a considerable detrimental effect on the R. microplus strain that is resistant to pesticides.
Key words: biological control, ticks, oviposition inhibition, immersion, feeding
Introduction
In tropical and sub-tropical regions of Mexico, where cattle are raised, the main ectoparasite of economic importance is Rhipicephalus (Boophilus) microplus (Canestrini) (Acari: Ixodidae) (Murrell and Barker 2003), as it causes direct damage by blood feeding and transmitting babesiosis and anaplasmosis (Bram et al. 2002). The control of this tick parasite is based on chemical products. However, R. microplus has developed resistance to almost all pesticides used including organophosphates, pyrethroids, and amidines, requiring higher doses or a mixture of several products for their effective control. These practices result in increased production costs and contamination of the environment (Li et al. 2004; Miller et al. 2005).
An alternative is the use of biological control such as the use of predators, parasitoids, and entomopathogens, including fungi, bacteria, viruses, and nematodes. Within the bacterial group, the microorganism most widely used worldwide with the highest success in the control of several insect pests is the bacterium, Bacillus thuringiensis Berliner (Bacillales: Bacillaceae). B. thuringiensis has been shown to be useful for the control of different insect pests that affect plant crops, forest trees, or that are vectors of human diseases such as dengue and malaria (Crickmore 2005; de Maagd et al. 2003; Schnepf et al. 1998). B. thuringiensis represents an important portion of the biopesticides market (Porter et al. 1993), with annual sales around 140 million US dollars and with more than 40% of the sales in the United States (National Academy of Sciences 2003). The use of B. thuringiensis is increasing rapidly because it is highly specific, significantly lowering the damage to other organisms compared to use of chemical insecticides, and also because it is biodegradable and is therefore accepted as an environmentally friendly alternative. In addition, B. thuringiensis has no adverse effects on humans. B. thuringiensis products can be combined with other pest control techniques and it is an essential component in Integrated Pest Management (IPM).
The use of B. thuringiensis for cattle tick control has been previously reported (Ostfeld et al. 2006). Hassanain et al. (1997) evaluated the activity of three subspecies of B. thuringiensis (kurstaki, israeliensis, and thuringiensis), spraying spore/crystal mixtures on the soft tick Argas persicus and the hard tick Hyalomma dromedario. In another report, Samish and Rehacek (1999) mentioned 100% mortality using mixtures of B. thuringiensis spores and blood to feed Ornithodoros erraticus through an artificial membrane. Zhioua et al. (1999) evaluated a B. thuringiensis kurstaki strain against engorged larvae of Ixodes scapularis, achieving 96% mortality with a dose of 108 spores/ml.
In this work, the pathogenicity of some native strains of B. thuringiensis against a tick R. microplus population that is resistant to chemical pesticides was evaluated.
Materials and Methods
The B. thuringiensis strains used in this study belong to the collection of the Vegetal Parasitology Laboratory at the Center of Biological Research at the University of Morelos, Mexico. The GP123, GP138, GP139, GP140 B. thuringiensis strains were grown at the University of Morelos's facilities using solid medium Luria-Bertani (LB), until complete sporulation (72 h). Crystal inclusions were observed through an optical phase-contrast microscope. Spores and crystals produced by the B. thuringiensis strains were recovered using a bacteriological loop and suspended in 20 ml of sterile water. Finally, the 0.1 mM protease inhibitor (PMSF) was added to avoid protein degradation. Total protein was quantified by the Bradford technique (Bradford 1976).
A R. microplus strain resistant to organophosphates, pyrethroids, and amidines was maintained in Holstein steers (250 kg weight) at the facilities of INIFAP-CENID-Veterinary Parasitology, at Jiutepec, Morelos, Mexico, where the bioassays were performed. Two steers were artificially infested with 1 g of R. microplus larvae. Twenty-one days after infestation, fully engorged female ticks began to drop. Females, weighing 0.2 to 0.4 g, were collected to be used in the bioassay. The adult immersion test developed by Drummond (1969) was used to determine the effect of the B. thuringiensis bacterium against R. microplus ticks. Engorged adult female ticks were immersed for 60 seconds in a 1.25 mg/ml suspension in water of B. thuringiensis. Ticks were then placed individually in 24-well polystyrene plates (Cell Wells, Corning Glass Works, http://www.corning.com/lifesciences). Inhibition of the individual amount of oviposition and egg hatch were recorded during the bioassay. Tick controls were treated with distilled water. Incubation was performed in a humidity chamber (90–95% relative humidity) at 28° C. For each B. thuringiensis strain tested, 48 female R. microplus ticks were used. Ticks were analyzed under a stereoscope to confirm female tick mortality after 5, 10, 15, and 20 days after inoculation. To measure the effects of bacterial infection on tick fertility and fecundity, an efficiency index was quantified (egg weight/engorged female tick weight) (Drummond 1969). At 10 days after innoculation, egg masses were separated from the female and weighed. The oviposition capacity of control ticks and those surviving the bacterial infection was determined by the efficiency index.
Mortality and egg hatch data were transformed (arcsine) in order to normalize and perform variance analysis (α = 0.05) and mean estimation by using Tukey's test (α = 0.05) and the statistics package SAS 2001. Data obtained from egg weight assessments were not transformed.
Results
A R. microplus strain that is resistant to organophosphates, pyrethroids, and amidines has been used for the assays. As an alternative for the control of this pest, the effectivity of some B. thuringiensis strains that were isolated from different insect and arthropod bodies collected from different regions of Mexico were analyzed. Sixty different native B. thuringiensis strains were tested, which were only characterized by the presence of a crystal inclusion during bacterial sporulation under phase-contrast optical microscope observations. Among these strains were four native strains that caused mortality in the adult immersion test assay. The mortality induced by strains GP123, GP138, GP139, GP140 on the R. microplus adult female was assayed by immersion assay. The immersion assay was first used to determine the toxicity of these four B. thuringiensis strains. All of these B. thuringiensis strains showed high mortality values statistically different (P< 0.0001) from the controls at all tested times after innoculation. None of the strains were significantly different from one another. The data suggest that GP138 strain had an earlier effect than the other strains (Table 1). The causal agent (the B. thuringiensis strains) was recovered from all dead ticks, confirming that B. thuringiensis bacteria were responsible for killing the R. microplus resistant strain.
Table 1.
Percentage of Rhipicephalus microplus adult female mortality caused by four Bacillus thurigiensis strains at different times during immersion trials.
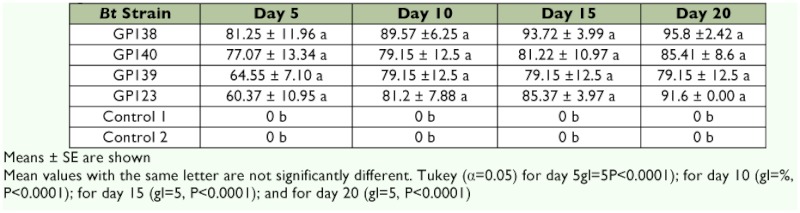
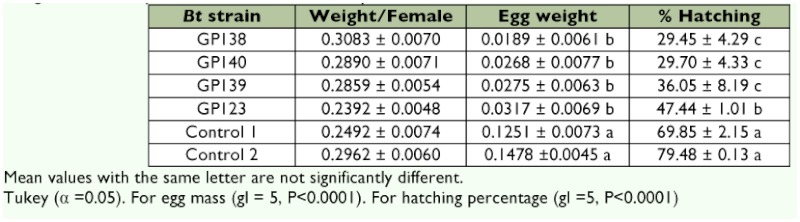
The effect of the R. microplus strain on oviposition and egg hatch was also analyzed during the immersion trials. Strains GP138, GP139 and GP140 showed similar inhibitory effects without statistically significant differences among them (Tukey's test, α = 0.05) (Table 2), but they were significantly different from the controls.
Table 2.
Percentage of female weight, egg weight, and hatching of Rhipicephalus microplus females treataed with four Bacillus thurieiensis strains by immersion trials after 20 days.
Discussion
B. thuringiensis are Gram-positive bacteria that are able to produce proteins such as Cry, Cyt, Vip, and S-layer, which have insecticidal properties with different modes of action. These proteins are toxic to insect species belonging to the orders Lepidoptera, Diptera, Coleoptera, Hymenoptera, as well as for acari and nematodes (Bravo et al. 2007; Schnepf et al. 1998; Peña et al. 2006). However, there is a great diversity of arthropod species, such as ticks, for which no specific insecticidal B. thuringiensis proteins have been found.
Previous reports about the toxicity of different B. thuringiensis strains against ticks are limited. Hassanain et al. (1997) reported that B. thuringiensis kurstaki produced 100% mortality against A. persicus engorged females after five days at a dose of 1 mg/ml. B. thuringiensis israelensis caused 100% mortality at a dose of 2.5 mg/ml, and B. thuringiensis thuringiensis at a 5 mg/ml dose induced 93.3% mortality. With H. dromedarii, none of the B. thuringiensis strains produced 100% mortality, even at doses as high as 10 mg/ml. In another report, it was shown that B. thuringiensis kurstaki spores (106/ml) were toxic to engorged I. scapularis larvae. However, an LC50 has been reported with 107 spores (Zhioua et al. 1999). In this work, one dose (1.25 mg/ml) was used for immersion assays to characterize the B. thuringiensis strain collection (60 strains). The four selected B. thuringiensis strains GP123, GP138, GP139, and GP140 produced 62.5, 81.25, 64.58, and 77.08% mortality, respectively, by the fifth day. These data indicated that the GP138 strain was the most pathogenic. Analysis of the effect of B. thuringiensis strains on R. microplus with the immersion aassay led us to infer that the B. thuringiensis strains can affect R. microplus through approaches other than ingestion, probably by means of the spiracles or genital pore as was previously proposed (Zhioua et al. 1999).
It can be concluded that some B. thuringiensis strains had a toxic effect on R. microplus using the adult immersion assay. The R. microplus acaraside-resistant strain could be controlled with pathogenic B. thuringiensis strains, however, more studies are necessary to optimize the application of the B. thuringiensis. The results indicate that immersion trials are effective to control R. microplus.
References
- Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Analytical Biochemistry. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Bram RA, George EJ, Reichard RE, Tabachnick JW. Threat of foreign arthropod-pathogens to livestock in the United States. Journal of Medical Entomology. 2002;39(3):405–416. doi: 10.1603/0022-2585-39.3.405. [DOI] [PubMed] [Google Scholar]
- Bravo A, Gill SS, Soberon M. Mode of action of Bacillus thuringiensis Cry and Cyt toxins and their potential for insect control. Toxicon. 2007;49:423–435. doi: 10.1016/j.toxicon.2006.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crickmore N. Using worms to better understand how Bacillus thuringiensis kills insects. TRENDS in Microbiology. 2005;13(8):347–350. doi: 10.1016/j.tim.2005.06.002. [DOI] [PubMed] [Google Scholar]
- de Maagd RA, Bravo A, Berry C, Crickmore N, Schnepf HE. Structure, diversity and evolution of protein toxins from spore-forming entomopathogenic bacteria. Annual Review of Genetics. 2003;37:409–433. doi: 10.1146/annurev.genet.37.110801.143042. [DOI] [PubMed] [Google Scholar]
- Drummond RO, Gladney WJ. Further evaluation of animal systemic insecticides. Journal of Medical Entomology. 1969;6(4):934–6. doi: 10.1093/jee/62.4.934. [DOI] [PubMed] [Google Scholar]
- Inokuma H, Kemp HD. Establishment of Boohilus microplus infected with Babesia bigemina by using in vitro tube feeding technique. Journal of Veterinary Science Society. 1997;60(4):509–512. doi: 10.1292/jvms.60.509. [DOI] [PubMed] [Google Scholar]
- Hassanain MA, El Garhy FM, Abdel-Ghaffar AF, El-Sharaby A, Abdel Megeed NK. Biological control studies of soft and hard ticks in Egypt. I. The effect of Bacillus thuringiensis varieties on soft and hard ticks (Ixodidade). Parasitology Reserch. 1997;83:209–213. doi: 10.1007/s004360050235. [DOI] [PubMed] [Google Scholar]
- Li AY, Davey BR, Miller JR, George EJ. Detection and characterization of amitraz resistance in the southern cattle tick, Boophilus microplus (Acari: Ixodidae). Journal of Medical Entomolology. 2004;41(2):193–200. doi: 10.1603/0022-2585-41.2.193. [DOI] [PubMed] [Google Scholar]
- Miller JR, Davey BR, George EJ. First report of Organophosphate-resistant Boophilus microplus (Acari: Ixodidae) within the United States. Journal of Medical Entomology. 2005;42(5):912–917. doi: 10.1603/0022-2585(2005)042[0912:FROOBM]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Murrell A, Barker SC. Synonymy of Boophilus Curtice, 1891 with Rhipicephalus Koch, 1844 (Acari: Ixodidae). Systematic Parasitology. 2003;56(3):169–72. doi: 10.1023/b:sypa.0000003802.36517.a0. [DOI] [PubMed] [Google Scholar]
- National Academy of Sciences. The future use of pesticides in US agriculture. National Academy Press USA; 2003. pp. 312–332. [Google Scholar]
- Ostfeld RS, Price A, Hornbostel LV, Benjamin AM, Keesing F. Controlling ticks and tick-borne zoonosis with biological and chemical agents. BioScience. 2006;56(5):383–394. [Google Scholar]
- Peña G, Miranda-Rios J, de la Riva G, Pardo-López L, Soberón M, Bravo A. A Bacillus thuringiensis S-layer protein involved in toxicity against Epilachna varivestis (Coleoptera: Coccinellidae). Applied and Environmental Microbiology. 2006;72(1):353–360. doi: 10.1128/AEM.72.1.353-360.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porter AG, Davidson WE, Liu WJ. Mosquiticidal toxins of bacilli and their genetic manipulation for effective biological control of mosquitoes. Microbiology and Molecular Biology Reviews. 1993;57(4):838–861. doi: 10.1128/mr.57.4.838-861.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samish M, Rehacek J. Pathogens and predators of ticks and their potential in biological control. Annual Review of Entomology. 1999;44:159–182. doi: 10.1146/annurev.ento.44.1.159. [DOI] [PubMed] [Google Scholar]
- Schnepf E, Crickmore N, van Rie J, Lereclus D, Baum J, Feitelson J, Zeigler RD, Dean HD. Bacillus thuringiensis and its pesticidal crystal proteins. Microbiology and Molecular Biology Reviews. 1998;62(3):775–806. doi: 10.1128/mmbr.62.3.775-806.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhioua E, Heyer H, Browning M, Ginsberg HS, LeBrun RA. Pathogenicity of Bacillus thurigiensis variety Kurstaki to Ixodes scapularis (Acari:Txodidae). Journal of Medical Entomology. 1999;36(6):90–902. doi: 10.1093/jmedent/36.6.900. [DOI] [PubMed] [Google Scholar]


