Abstract
Familial Hypertrophic Cardiomyopathy, FHC, is a clinically heterogeneous, autosomal-dominant disease of the cardiac sarcomere leading to extensive remodeling at both the whole heart and molecular levels. The remodeling patterns are mutation-specific, a finding that extends to the level of single amino acid substitutions at the same peptide residue. Here we utilize two well-characterized transgenic FHC mouse models carrying independent amino acid substitutions in the TM-binding region of cardiac troponin T (cTnT) at residue 92. R92Q and R92L cTnT domains have mutation-specific average peptide conformation and dynamics sufficient to alter thin filament flexibility and cross-bridge formation and R92 mutant myocytes demonstrate mutation-specific temporal molecular remodeling of Ca2+ kinetics and impaired cardiac contractility and relaxation. To determine if a greater economy of contraction at the crossbridge level would rescue the mechanical defects caused by the R92 cTnT mutations, we replaced the endogenous murine α-myosin heavy chain (MyHC) with the β-MyHC isoform. While β-MyHC replacement rescued the systolic dysfunction in R92Q mice, it failed to rescue the defects in diastolic function common to FHC-associated R92 mutations. Surprisingly, a significant component of the whole heart and molecular contractile improvement in the R92Q mice was due to improvements in Ca2+ homeostasis including SR uptake, [Ca2+]i amplitude and phospholamban phosphorylation. Our data demonstrate that while genetically altering the myosin composition of the heart bearing a thin filament FHC mutation is sufficient to improve contractility, diastolic performance is refractory despite improved Ca2+ kinetics. These data reveal a previously unrecognized role for MyHC isoforms with respect to Ca2+ homeostasis in the setting of cardiomyopathic remodeling and demonstrate the overall dominance of the thin filament mutation in determining the degree of diastolic impairment at the myofilament level.
Keywords: Familial Hypertrophic Cardiomyopathy, cardiac Troponin T, myosin heavy chain Isoforms, Ca2+ kinetics, contractile performance, cardiac relaxation
Introduction
Hypertrophic cardiomyopathy is a disorder characterized by the presence of a non-dilated, hypertrophied left ventricle (LV) and greater susceptibility to arrhythmias and sudden death. A common clinical feature of these patients is abnormal diastolic function due to impaired relaxation and reduced LV compliance despite preserved or even hyperdynamic LV systolic function [1]. In a majority of patients, the disease is familial, inherited as an autosomal-dominant, single-gene trait (Familial Hypertrophic Cardiomyopathy, FHC). Most of the FHC mutations are found in proteins that comprise the cardiac sarcomere. A number of the FHC-causing mutations in the thin filament protein cardiac Troponin T (cTnT) form a distinct subset as they are associated with mild or no ventricular hypertrophy but a relatively high frequency of sudden cardiac death [2, 3]. cTnT residue 92, an arginine, is a mutational FHC-associated hotspot leading to diverse clinical phenotypes [4]. Replacing the arginine with glutamine (Arg92Glu, R92Q) leads to sudden death at an early age with little overt hypertrophy while exchanging the arginine with leucine (Arg92Leu, R92L) usually leads to significant hypertrophy with a lower frequency of sudden death. This diversity in phenotypic expression complicates both diagnosis and treatment of patients with FHC. Understanding how changes in the structure and function of the cTnT domain containing this mutational hotspot leads to such diverse clinical phenotypes remains elusive.
cTnT plays a critical dynamic role in the regulation of the contractile cycle. Residue 92 is found in the cTnT domain that binds to the tropomyosin (TM) head-to-tail overlap, affecting the flexibility of the TM filament and stabilizing the multi-protein structure. This, in turn, changes the affinity of the TM-TN complex for actin and hence the availability of myosin-binding domains on actin for cross-bridge formation. Molecular dynamics studies of the cTnT domain containing different FHC-associated missense mutations at R92 showed that each mutation led to unique average conformations, flexibility and dynamics of the TM-binding cTnT domain [5, 6]. Mouse models of cTnT bearing R92 missense mutations have been developed and used to define the consequences of these dynamic changes within the sarcomere on whole heart and myocyte function [5, 7]. Each mutation leads to unique whole-heart and cellular phenotypes [8]. R92 cTnT mutant hearts have also revealed mutation-specific, temporal molecular remodeling of proteins in the sarcoplasmic reticulum (SR) and subsequent alterations in the Ca2+ transient [9]. Such information may eventually lead to targeted therapeutic approaches to this currently untreatable disorder.
Temporal alterations in the myosin isoform composition of cardiac sarcomeres have long been noted in pathogenic cardiac remodeling [10]. While obtaining absolute values for the percentages of α-MyHC vs β-MyHC in human cardiomyopathies is difficult, available evidence suggests that the normal human left ventricle contains ~90–92% β-MyHC and that during the development of heart failure, the loss of the remaining 8–10% α-MyHC is nearly complete [11, 12]. In cardiac ventricles of small vertebrate animals, however, the dominant myosin isoform contains the α-myosin heavy chain (α-MyHC, >95% abundant in mouse hearts). Despite high homology between these isoforms (93% amino acid identity), which MyHC isoform is present determines the velocity of sarcomere contraction and the force of contraction. The β-MyHC isoform, the so-called slow myosin isoform, has greater economy of contraction [13]. To determine if a greater economy of contraction at the crossbridge level would rescue the mechanical defects caused by the R92 cTnT mutations, we replaced the endogenous murine α-myosin heavy chain (MyHC) with the β-MyHC isoform in the hearts of our transgenic mouse models using a genetic approach. In view of the recently identified consequences of the remodeling of the sarcomere in R92 cTnT mutant hearts on Ca2+ homeostasis, we also define the consequences of replacing the MyHC isoform on intracellular Ca2+ transients, sarcoplasmic reticulum (SR) Ca2+ load and SR Ca2+ uptake, and the composition of key SR proteins.
2. Materials and Methods
2.1. Transgenic mouse models
Four to six-month-old C57Bl/6 mice bearing c-myc tagged murine cTnT with R92Q and R92L mutations were generated as described (all animals at F8 or above) [5, 7]. The R92Q and R92L lines express 67% and 60% of total cTnT as the mutant form, respectively, and were driven by −2,996 bp of a 5′ upstream sequence derived from the rat α-MyHC promoter [14]. β-MyHC expression was increased in the cardiac ventricles of these animals genetically by crossing them with a transgenic line expressing 80% β-MyHC in the left ventricle (LV) on the C57bl/6 background (provided by J. Robbins). This line expressed the full-length β-MyHC mouse cDNA driven by the 5.3 kb mouse α-MyHC promoter. All relevant genotypes were isolated and viable: wild type (WT) cTnT/α-MyHC (Non-Tg), WT cTnT/80% β-MyHC (β-Tg), the R92 mutations/α-MyHC (α-R92Q and α-R92L) and the R92 mutations/80% β-MyHC (β-R92Q and β-R92L). The genotype of each animal was identified via PCR. Histological analyses were performed as previously described [15].
The experimental protocols were approved by the Institute of Animal Studies at the Albert Einstein College of Medicine and the Standing Committee on Animals of Harvard Medical Area, and followed the recommendations of current NIH and APS guidelines.
2.2. Cardiac protein and Western blot analysis
Cardiac ventricular homogenates were used for protein isolation and subsequent SDS/PAGE analysis as previously described [7]. Quantitation was performed using BCA Protein Assay (PIERCE #23227). Separation of the cardiac MyHC isoforms was performed electrophoretically as described [14, 16]. Briefly, ventricles were homogenized in a high salt buffer (mM) (500 NaCl, 10 KPO4 (pH 7.0), 2 MgCl2, 0.5 EDTA (pH 8.0), 2 DTT and 0.1 PMSF) and centrifuged. Protein (10 μg) in the supernatant was loaded onto 7.5% acrylamide Bis-Tris 16×18cm gels in buffer (150 nM Tris pH 7.0, 4.5% SDS, 12% 2-mercaptoethanol, 30% glycerol, and 0.01% bromophenol blue). Gels were run at constant current of 60 mA for approximately 3–4 hrs at 4°C. Ventricular homogenates from mice fed an iodine deficient diet containing 0.15% propylthiouracil (Harlan Teklad Research Diets) for 8 weeks served as a β-MyHC control. Coomassie stain was used to identify protein bands.
Quantitative immunoblotting of LV homogenates was carried out to assess the levels of phospholamban (PLB), SERCA2a, Ser 16 phosphoryated PLB (P-PLB) as recently described [9]. Briefly, hearts were homogenized and sonicated at 4°C in buffer containing (mM) 10 imidazole pH 7.0, 300 sucrose, 10 NaF, 1 EDTA, 0.3 phenyl methylsulfonyl fluoride, and 0.5 dithiothreitol and Protease Inhibitor Cocktail (Sigma, P8340). Preliminary experiments were performed to obtain the correct linear ranges for quantitation in Western blot analyses. The homogenate proteins were separated on 10–20% SDS-polyacrylamide gels under reducing conditions. Separated proteins were transferred to nitrocellulose membranes (Millipore) and incubated with appropriate primary antibodies (PLB, SERCA2a; Affinity Bioreagents; Ser-16 P-PLB: Upstate). The blots were subsequently incubated with their respective peroxidase-labeled secondary antibodies (Pierce). Immunoreactivity was visualized by incubation with Amersham ECL™ Western Blotting Detection Reagents and exposure to x-ray film (CL-XPosure™ Film, Pierce). Protein levels were quantified by calibrated densitometry using NIH ImageJ analysis software (available at: http://rsb.info.nih.gov/ij/).
2.3. Isolated perfused mouse heart preparation
Hearts were isolated and perfused in the Langendorff isovolumic mode (balloon-in-LV) as described [17]. The coronary perfusate was phosphate-free Krebs-Henseleit buffer containing (mM) 118 NaCl, 5.3 KCl, 2.0 CaCl2, 25 NaHCO3, 1.2 MgSO4, 0.5 EDTA, 10 glucose and 0.5 pyruvate and equilibrated with 95% O2+5% CO2 to maintain a pH of 7.4. A custom-made water-filled balloon inserted into the LV was connected to a pressure transducer (Statham P23Db, Gould, Oxnard, CA) for recording of LV developed pressure and heart rate. The balloon volume was adjusted to set LV end-diastolic pressure at ~8 mmHg, and then held constant. Heart rate was 420 (± 9%) beats per min. Data for systolic pressure and ±dP/dt were collected on-line at a sampling rate of 200Hz using data acquisition system (MacLab, AD Instruments).
2.4. Measurement of myocyte mechanics, intracellular Ca2+ transients and SR Ca2+ load
Myocytes were isolated for measurement of mechanics and Ca2+ kinetics as described [18, 19]. Hearts were rapidly excised from anesthetized animals, cannulated via the aorta and perfused with modified Krebs solution at a constant flow of 3 ml/min. Blendzyme 4 (Roche) and trypsin (Sigma) was used to dissociate the ventricular myocytes from the extracellular matrix. Sarcomeric edge detection algorithms were used to measure contraction kinetics in real time with a video edge detector and specialized data acquisition software (SarcLen Acquisition System and IonWizard, IonOptix Inc., Milton, MA). Cells were continuously perfused at room temperature with 1.2 mM Ca2+ Tyrode solution containing 500 μM probenecid to prevent leakage of Fura-2 from the cells; cells were paced at 1 Hz and field stimulated to just above threshold (10 V). Measures of contractile function and relaxation were obtained from a minimum of six consecutive transients per cell using a minimum of four animals for each experimental group.
In order to also measure the [Ca2+]i transients, separate aliquots of freshly dissociated ventricular myocytes were incubated in 1.2 mM Ca2+ Tyrode solution containing 1 μM of membrane-permeant Fura-2 AM for 15 min at room temperature. Fura-2-loaded myocytes were alternately excited with a xenon lamp at wavelengths of 340 and 380 nm. The emission fluorescence was collected by the objective and reflected through barrier filter (510/40 nm) to a photomultiplier tube (IonOptix Corp, Milton, MA). [Ca2+]i transients were generated via measurement of the emission as a function of excitation wavelength. The peak excitation wavelength in the presence of Ca2+ is 340 nm while the peak excitation wavelength in the absence of Ca2+ is 380 nm. [Ca2+]i transients were reported as Fura-2 ratios after background fluorescence was subtracted. [Ca2+]i measurements were reported as fluorescence ratios. Indices of the [Ca2+]i transient were measured from a minimum of six consecutive Ca2+i transients per cell.
For measurement of SR Ca2+ load, fura-2-loaded cells were stimulated at least 20 times at 1 Hz in 1.22 mM Ca2+ normal Tyrode containing probenecid to bring the cellular Ca2+ content to steady state (25 °C). Caffeine was supplied as described [20]. As increasing frequency of contraction in murine cells caused little or no change in SR Ca2+ content and field stimulation at high frequencies often damages the cells, 1 Hz was used as the baseline frequency. Stimulation was paused and following a 1-sec rest, the perfusate was rapidly switched to Tyrode containing probenecid and 20 mM caffeine. Caffeine was administered without field stimulation and resulted in a [Ca2+]i transient that corresponds to total Ca2+ release from the SR. The difference between the basal and peak total cytosolic [Ca2+]i in the presence of caffeine corresponds to available SR Ca2+.
2.5. Measurement of SR Ca2+ uptake
Oxalate-supported SR Ca2+ uptake was determined as recently described [19]. Briefly, ventricular tissue from euthanized animals was homogenized in buffer containing (mM) 50 KH2PO4, 10 NaF, 1 EDTA, 300 sucrose, 0.3 PMSF, 0.5 DTT at pH of 7.0. Ca2+ uptake was measured over a range of pCa values (pCa 8 to 5). Procaine (1.13 mg/ml) and ruthenium red (1.18 mg/ml) were included in the uptake buffer to inhibit Ca2+ release from the SR. After a two min pre-incubation of the reaction containing 150 μCi/ml 45Ca2+ at 37 °C, 75 μl of ventricular homogenate was added. After 2 mins of constant stirring, the reaction was stopped by filtration through a 25 mm 0.45 micron filter. The filter was washed with cold buffer (20 Tris pH 7.0 and 2 EGTA, pH 7 and placed in a scintillation counter calibrated for 45Ca. Calcium uptake (nmol × min-1×mg−1) was calculated for each pCa point and plotted as sigmoidal dose-response curve with variable slope. pCa50 and Vmax values were then determined. Each pCa assay was performed in triplicate.
2.6. Statistical analysis
Values are presented as means ± SEM. Statistical analyses were performed using One-way ANOVA followed by Bonferroni’s multiple comparison test . A level of p< 0.05 was considered significant throughout.
3. Results
3.1. Genetically driven β-MyHC expression in cardiac ventricles of adult α-R92Q and α-R92L cTnT mice
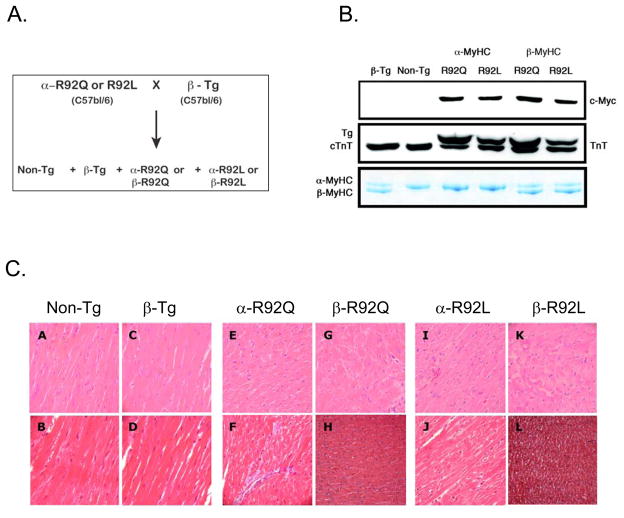
β-MyHC expression in mouse hearts bearing R92 cTnT mutations was increased using a genetic approach [21, 22]. Our well-characterized α-R92Q and α-R92L transgenic mice were crossed with a transgenic line expressing 80% β-MyHC (β-Tg) [23] (Figure 1A). The breeding scheme yielded all relevant genotypes in the expected Mendelian ratios: α-R92Q and β-R92Q, α-R92L and β-R92L as well as Non-Tg and β-Tg. Both double transgenics expressed the same MyHC isoform content, namely 80% β-MyHC and 20% α-MyHC (Fig. 1B). The percentage of cTnT transgene of the total cTnT present for each line (α-R92Q-67% and α-R92L-60%) was not altered by changing the MyHC isoform composition (Fig. 1B). Thus, mutant cTnT and β-MyHC expression resulted in replacement of endogenous cTnT and α-MyHC proteins and not over-expression, consistent with inherent regulation of contractile protein stoichiometry in murine cardiac transgenic models [15, 24]
Figure 1. β-R92Q and β-R92L double transgenic mouse heart models.
A. Breeding scheme B. Protein expression levels Both R92Q and R92L mutant proteins were myc tagged at their amino termini and identified by anti-myc Western blot analysis of ventricular homogenates. Anti-Troponin T (cTnT) identified two bands, the upper band corresponding to mutant R92Q or R92L cTnT and a lower band representing endogenous cTnT. Percent expression of mutant cTnT was 67% in both α-R92Q and β-R92Q and 50% in both α-R92L andβ-R92L. Myosin heavy chain (MyHC) isoform composition was determined by glycerol containing SDS-PAGE followed by Coomassie staining. The upper band is α-MyHC while the lower band is β-MyHC. β-Tg, β-R92Q and β-R92L ventricular homogenates exhibited the same 80% β-MyHC/20% α-MyHC composition. C. Histology of Non-Tg and transgenic left ventricular sections. Hematoxylin and Eosin prepared sections are shown in upper panels and Masson’s trichrome sections are shown in lower panels. Panels A and B: Non-Tg ventricles demonstrate normal histology. Panels C and D: β-Tg ventricles also exhibited normal histology, and were free of fibrosis. Panels E and F: α-R92Q ventricles displayed myocyte disarray and mild fibrosis. Panels G and H: β-R92Q ventricles had similar histopathology to α-R92Q ventricles. Panels I and J: α-R92L ventricular sections showed myocyte disarray, but were free of fibrosis. Panels K and L: β-R92L ventricles showed similar histopathology to α-R92L ventricles. A small region of fibrosis localized to the ventricular base was observed for both β-R92Q and β-R92L hearts. Magnification = 400X for all sections.
3.2. Increased β-MyHC expression in intact hearts bearing R92 cTnT mutations has no effect on morphology, histopathology or sarcomere lengths
A characteristic of many mouse hearts bearing cTnT-related FHC mutations is the lack of overt left ventricular hypertrophy, recapitulating the human FHC phenotypes [7, 15, 25, 26]. This is the case for the hearts studied here. LV weights for α-R92Q and α-R92L cTnT hearts were not different from Non-Tg hearts, and LV/BW were only slightly lower than for Non-Tg hearts (9 and 15%, respectively, Table 1). Increasing β-MyHC in the ventricles of WT-cTnT C57Bl/6 mice caused no change in cardiac mass, LV/BW or LV/tibia lengths (not shown). Increasing β-MyHC in the ventricles of both β-R92Q and β-R92L cTnT transgenic mice, however, led to smaller LVs; hence LV/BW (Table 1) and LV/tibia lengths (not shown) were lower compared to both Non-Tg and β-Tg hearts. Thus increasing the proportion of β-MyHC in the ventricles of WT-cTnT C57Bl/6 mice did not alter the gross morphologic phenotype of Non-Tg hearts, Moreover, increasing the proportion of β-MyHC in the ventricles of R92 cTnT mutant hearts did not lead to cardiac hypertrophy; instead, the hearts were smaller.
Table 1.
Cardiac Mass
| Genotype | n | BW (g) | LV (mg) | LV/BW (mg/g) |
|---|---|---|---|---|
| Non-Tg | 22 | 23.9 ± 0.7 | 76.6 ± 2.7 | 3.21 ± 0.07 |
| β-Tg | 22 | 24.8 ± 0.6 | 83.1 ± 2.4 | 3.37 ± 0.07 |
| α-R92Q | 12 | 25.5 ± 1.8 | 73.7 ± 10.1 | 2.93 ± 0.21# |
| β-R92Q | 12 | 23.9 ± 0.7 | 58.9 ± 2.3**##+ | 2.47 ± 0.06**##+ |
| α-R92L | 11 | 25.5 ± 0.9 | 69.6 ± 2.3 | 2.75 ± 0.08*## |
| β-R92L | 11 | 23.4 ± 0.7 | 63.8 ± 2.4*## | 2.73 ± 0.09**## |
P<0.05,
P<0.01 vs Non-Tg;
P<0.05,
P<0.01 vs β-Tg;
P<0.05 vs α-R92Q
Body weight (BW), left ventricular weight (LV) and LV/BW for non-transgenic mice (Non-Tg), for mice with cardiac α-MyHC replaced with β-MyHC (β-Tg) and mice bearing either the R92L or R92Q mutation in cTnT with either predominately α- or β-MyHC. Replacing α-MyHC with β-MyHC in hearts with normal cTnT did not change these morphologic parameters. LV/BW were lower for mouse hearts bearing the R92Q and R92L cTnT mutations with normal α-MyHC; both LV and LV/BW were lower when α-MyHC was replaced with β-MyHC. The same results are obtained when the LV weights are normalized to tibia lengths (not shown). Means ± SEM.
Our previous studies of α-R92Q and α-R92L cTnT transgenic mouse hearts demonstrated ventricular myocyte disarray and degeneration, with mild fibrosis observed for α-R92Q mouse hearts [5, 7]. This was also observed for the R92 mutant mice studied here (Fig. 1C). β-Tg ventricles were free of fibrosis and disarray, showing that replacing α-MyHC with β-MyHC in a non-cardiomyopathic heart did not result in abnormal histology. Histopathology of β-R92Q and β-R92L cTnT ventricular sections was similar to their respective α-R92Q and α-R92L littermates, showing that replacing α-MyHC with β-MyHC neither rescued nor worsened the abnormal histopathology of cTnT mutant hearts. A small region of localized fibrosis within the ventricular base was observed for both β-R92Q and β-R92L cTnT hearts.
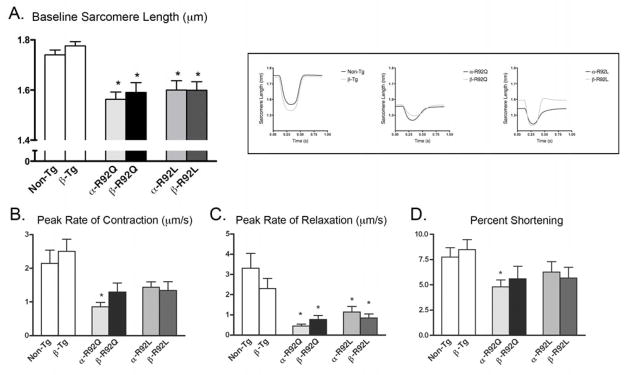
In agreement with our previous reports, sarcomere lengths (μm) for both α-R92Q and α-R92L myocytes are shorter than for Non-Tg myocytes (1.56 for α-R92Q, 1.60 for α-R92L vs. 1.74 for Non-Tg, p<0.001, Fig. 2A). Increasing β-MyHC expression in the presence of wild type cTnT (β-Tg) did not alter sarcomere length (1.72 for Non-Tg vs. 1.73 for β-Tg). Sarcomere lengths for both double transgenic mouse hearts were similar to their α-MyHC littermates. Shortened sarcomere length, a direct consequence of the cTnT mutations, was therefore refractory to increased β-MyHC expression.
Figure 2. Cardiac ventricular myocyte contraction and relaxation patterns.
Representative contraction/relaxation tracings are shown in the inset. A. α-R92Q and α-R92L mutations resulted in shorter sarcomere lengths (μm) (1.56 for α-R92Q, 1.60 for α-R92L vs. 1.74 for Non-Tg, p<0.001); double transgenic sarcomere lengths remained statistically similar to their respective MyHC littermates. B. The peak rate of contraction (μm/s) was impaired in α-R92Q (0.86 for α-R92Q vs. 1.14 for Non-Tg) and rescued in β-R92Q: peak rate of contraction was normal in the R92L myocytes. C. The peak rate of relaxation in isolated α-R92Q and α-R92L myocytes was severely impaired and did not improve with increased β-MyHC expression. D. Percent sarcomeric shortening was impaired only for α-R92Q myocytes (4.8% for α-R92Q vs. 7.5% for Non-Tg, p<0.05); percent shortening for β-MyHC myocytes was not different from Non-Tg. An n of 4–6 animals were used for each group with at least 50 cells analyzed. Data are presented as means ± SEM. * p<0.05 vs Non-Tg.
3.3. Systolic performance, but not diastolic performance, is rescued in cTnT R92 mutant hearts with increased β-MyHC expression
In order to test whether increasing β-MyHC expression rescues the whole-heart physiology of either α-R92L or α-R92Q cTnT mutant hearts, hearts from Non-Tg, single Tg and double Tg mice were isolated and perfused in the isovolumic mode (Table 2). Replacing α-MyHC with β-MyHC in hearts with wild type cTnT did not alter the indices of isovolumic systolic performance (SP, +dP/dt, +dP/dt normalized to peak developed pressure (not shown), the ratio of +dP/dt to −dP/dt (not shown)) or diastolic performance (−dP/dt). Thus replacing α-MyHC with β-MyHC in wild type cTnT C57Bl/6 mice was neither a negative or positive modulator of either systolic or diastolic contractile performance. In contrast, the physiologic phenotype of α-R92Q and α-R92L cTnT mutant hearts differed. α-R92Q hearts demonstrated both systolic and diastolic dysfunction while functional indices tended to be lower for α-R92L hearts. Replacing α-MyHC with β-MyHC in R92Q cTnT mutant hearts improved indices of systolic performance; values for −dP/dt, which were 30% lower than for Non-Tg mouse hearts, however, did not improve. Replacing α-MyHC with β-MyHC in R92L cTnT mutant hearts had little effect. Compared to Non-Tg hearts, indices of systolic performance for β-R92L hearts were unchanged; −dP/dt, which was 14% lower inα-R92L hearts was now 20% lower in β-R92Lhearts. Increasing β-MyHC expression, therefore, rescued systolic performance in the R92Q but did not correct the significant diastolic dysfunction characteristic of both R92Q and R92L hearts.
Table 2.
Contractile and Relaxation Performance
| Genotype | n | LVSP (mmHg) | +dp/dt (mmHg/sec) | −dp/dt (mmHg/sec) |
|---|---|---|---|---|
| Non-Tg | 18 | 109 ± 4 | 3237 ± 163 | 2751 ± 61 |
| β-Tg | 16 | 109 ± 3 | 3867 ± 245 | 2506 ± 81 |
| α-R92Q | 12 | 92 ± 4*# | 2857 ± 142*## | 1932 ± 165**## |
| β-R92Q | 12 | 108 ± 4+ | 3845 ± 136+ | 2261 ± 128* |
| α-R92L | 8 | 99 ± 3 | 3594 ± 178 | 2368 ± 91 |
| β-R92L | 10 | 103 ± 5 | 3777 ± 148 | 2246 ± 169* |
P<0.05,
P<0.01 vs Non-Tg;
P<0.05,
P<0.01 vs β-Tg;
P<0.05 vs α-R92Q
Peak systolic pressure (SP), rate of tension development (+dP/dt) and rate of relaxation (−dP/dt) measured in hearts perfused in the isovolumic mode isolated from Non-Tg mice, β-Tg mice and mice bearing either the R92L or R92Q cTnT mutation with either α–MyHC- or β–MyHC. Replacing α-MyHC with β-MyHC in hearts with normal cTnT had no effect on these indices of cardiac performance; SP normalized to LV weight (calculated from Table 1) and +dP/dt normalized to peak developed pressure were also unchanged (not shown). Replacing R92 cTnT with R92L cTnT in hearts with α-MyHC did not change indices of systolic performance but the rate of relaxation tended to be lower. In contrast, replacing R92 cTnT with R92Q cTnT slowed both the rate of tension development and the rate of relaxation. Comparing α-R92L and β-R92L hearts, SP and +dP/dt were the same but −dP/dt remained low. Replacing α-MyHC with β-MyHC in hearts with R92Q cTnT improved +dP/dt and +dP/dt/(SP) but not −dP/dt. Thus, replacing α-MyHC with β-MyHC in hearts bearing these R92 cTnT mutations corrected systolic but not diastolic dysfunction. Means ± SEM.
3.4. Improved contraction in myocytes isolated from β-MyHC double transgenics
To further test whether replacing α-MyHC with β-MyHC rescues systolic but not diastolic performance of R92 mutant hearts, we measured contractile properties of ventricular myocytes isolated from single and double transgenic and Non-Tg hearts (Fig. 2). Representative contraction/relaxation tracings are shown in the inset. The peak rate of contraction (μm/s) (Fig. 2B) was severely impaired in α-R92Q (0.86 for α-R92Q vs. 2.14 for Non-Tg, P<0.001). The peak rate of contraction of β-R92Q myocytes was similar to that for Non-Tg myocytes, demonstrating that replacing α-MyHC with β-MyHC enhanced contractility in R92Q myocytes. In contrast, peak rate of contraction for α-R92L myocytes was not different from Non-Tg myocytes and was unchanged for β-R92L myocytes. The peak rate of relaxation (μm/s) was impaired in both α-R92Q and α-R92L myocytes (0.447 for α-R92Q, 1.16 for α-R92L vs. 3.31 for Non-Tg, P<0.05) (Fig. 2C). In contrast to the effect of increasing β-MyHC expression on peak rate of contraction for R92Q myocytes, the peak rates of relaxation in isolated β-R92Q and β-R92L myocytes did not improve. Percent sarcomere shortening was impaired only for α-R92Q myocytes (4.8% for α-R92Q vs. 7.5% for Non-Tg, p<0.05) (Fig. 2D). Similar to the results for peak rate of contraction, percent shortening was restored to Non-Tg levels in β-R92Q myocytes; percent shortening for α-R92L and β-R92L were not different from Non-Tg myocytes. Thus the results from both myocytes and intact heart experiments show that replacing α-MyHC with β-MyHC rescues the systolic performance of R92Q while diastolic dysfunction characteristic of both R92 cTnT mutant hearts is refractory to this maneuver.
3.5. Enhanced Ca2+ kinetics in myocytes isolated from β-MyHC double transgenics
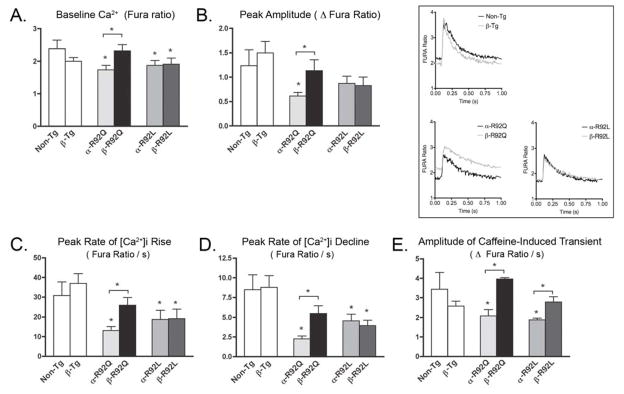
We recently showed that α-R92 cTnT mutant hearts demonstrated mutation-specific differences in Ca2+ kinetics and regulation of Ca2+ homeostasis [9]. Here, we compare these properties for myocytes isolated from α- and β-R92Q and α- and β-R92L hearts (Fig. 3). Representative Ca2+ transients are shown in the inset. Baseline Ca2+ levels (expressed as Fura ratios) were lower for both α-R92Q and α-R92L myocytes (Fig. 3A). Increasing β-MyHC expression significantly improved baseline Ca2+ levels in β-R92Q myocytes (1.74 for α-R92Q vs. 2.35 for β-R92Q and 2.39 for Non-Tg) but not for β-R92L myocytes. The peak amplitude (Δ Fura ratio) for R92L myocytes was not different from Non-Tg myocytes, but was impaired in α-R92Q myocytes (0.616 vs. 1.24 for Non-Tg, p<0.05); in β-R92Q myocytes peak amplitude was restored to Non-Tg levels (Fig. 3B). The peak rates of Ca2+ rise and decline, lower in both types of mutant myocytes, increased only for β-R92Q myocytes (Fig. 3C and 3D). Neither the rates of peak Ca2+ rise nor peak Ca2 decline improved for β R92L myocytes. The SR Ca2+ load (Δ Fura ratio), as determined by rapid caffeine administration, was reduced for both α-R92Q and α-R92L myocytes (2.33 for α-R92Q and 1.89 for α-R92L vs. 3.69 for Non-Tg, p<0.05) (Fig. 3E). Increased β-MyHC expression enhanced SR loading in myocytes isolated from both double transgenics (3.97 for β-R92Q and 2.97 for β-R92L vs. 3.69 for Non-Tg). Thus, exchanging α-MyHC with β-MyHC enhanced Ca2+ kinetics as well as contractility in R92Q myocytes but not for R92L myocytes. Elevated expression of β-MyHC increased the amplitude of the caffeine-induced transient and the SR Ca2+ load in both types of cTnT cardiomyopathic myocytes.
Figure 3. Ventricular myocyte Ca2+ kinetics.
Representative Ca2+ transients are shown in the inset. A. Baseline Ca2+ levels (Fura ratio) were lower for both α-R92Q and α-R92L isolated myocytes. Baseline Ca2+ levels were higher in β-R92Q myocytes (1.74 for α-R92Q vs. 2.35 for β-R92Q and 2.39 for Non-Tg) but baseline Ca2+ levels in β-R92L remained similar to α-R92L. B. Peak amplitude (Δ Fura ratio) was impaired in α-R92Q myocytes (0.616 vs. 1.24 for Non-Tg) and rescued in β-R92Q. Peak amplitude for R92L myocytes did not differ from controls. C and D. The peak rate of Ca2+ rise and decline, both impaired in both α-R92Q and α-R92L myocytes, increased to Non-Tg levels in β-R92Q myocytes but not in β-R92L myocytes. E. The SR Ca2+ load (Δ Fura ratio) was reduced for α-R92Q and α-R92L myocytes (2.33 for α-R92Q and 1.89 for α-R92L vs. 3.69 for Non-Tg). Increased β-MyHC restored SR loading in isolated myocytes from both double transgenics (3.97 for β-R92Q and 2.97 for β-R92L vs. 3.69 for Non-Tg). For Ca2+ transients, an n of 4–6 animals were used for each group with at least 50 cells analyzed. For caffeine-induced measurement of SR Ca2+ load, an n of 4–5 were animals used for each group with at least 10 cells analyzed. Data are presented as mean ± SEM. * p<0.05 vs Non-Tg and as indicated.
3.6. Impaired SR Ca2+ uptake in α-R92Q myocytes is restored by elevated β-MyHC expression
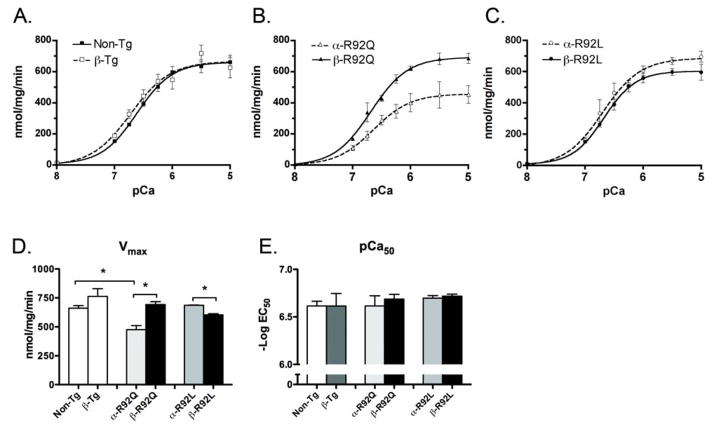
In order to determine if increased β-MyHC expression in cTnT models of FHC altered SR-mediated Ca2+ flux, oxalate-supported SR Ca2+ uptake was measured using homogenates from single and double Tg and Non-Tg ventricles (Fig. 4). Replacing α-MyHC with β-MyHC did not change SERCA2a-mediated SR Ca2+ uptake (Fig 4A). α-R92Q homogenates exhibited substantial impairment in SERCA2a-mediated SR Ca2+ uptake compared to Non-Tg homogenates (Fig. 4A and B). The maximal velocity of uptake was reduced by ~30% in α-R92Q as compared to Non-Tg (476 for α-R92Q vs 661 for Non-Tg, p<0.001) (Fig. 4D). The magnitude of this reduced SR Ca2+ uptake would likely result in impaired relaxation and is consistent with the impaired relaxation observed here for both at whole heart and myocyte levels. SERCA2a Ca2+ uptake in hearts from β-R92Q mice was higher than for Non-TG hearts (693 for β-R92Q vs 661 for Non-Tg) (Fig. 4B). This observation was unexpected since there is no known direct link between β-MyHC isoform content and SERCA2a-mediated SR Ca2+ uptake. This improved SR Ca2+ uptake would be expected to improve diastolic performance in hearts bearing the R92Q mutation; however, contrary to this hypothesis, diastolic performance was enhanced only minimally if at all in the β-R92Q double transgenic hearts or myocytes. In contrast to results for R92Q mutant hearts, Ca2+ uptake values and Vmax (603 for β-R92L vs. 687 for α-R92L, p<0.001) for β-R92L homogenates were slightly lower than values obtained from Non-Tg and α-R92L homogenates (Fig. 4A, C and D). These results suggest a mutation-specific effect on Ca2+ uptake with increased β-MyHC expression: while SERCA2a-mediated Ca2+ uptake was enhanced in β-R92Q, it was slightly decreased in β-R92L hearts.
Figure 4. SERCA2a-mediated Ca2+ uptake by the sarcoplasmic reticulum (SR) in ventricular homogenates.
A. SR uptake for β-Tg was not different from Non-Tg. B and C. α-R92Q displayed substantial impairment in SERCA2a-mediated SR Ca2+ uptake, which was rescued in β-R92Q. In contrast, SR uptake for α-R92L was not different from Non-Tg and was not altered for β-R92L. D. Vmax for α-R92Q displayed substantial impairment in SERCA2a-mediated SR Ca2+ uptake and was rescued in β-R92Q. Vmax for α-R92L and b-R92L was not different from Non-Tg or b-Tg. E. pCa50 values were indistinguishable among all groups. An n of 4–6 animals were used per experimental group, each performed in triplicate. Data are presented as mean ± SEM. * p<0.05 vs Non-Tg and as indicated.
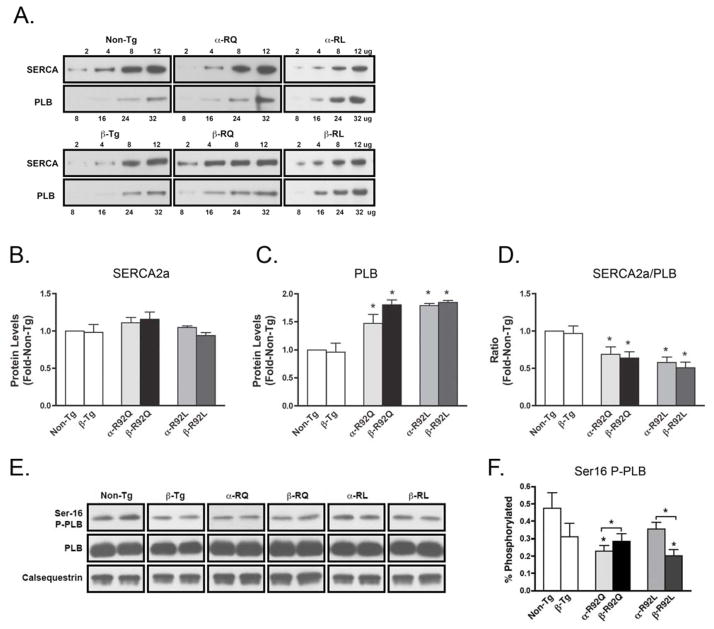
3.7 Increased β-MyHC expression leads to changes in the phosphorylation of PLB
To identify potential mechanisms for the observed mutation-specific responses to changes in MyHC isoform composition in the cTnT FHC mouse hearts, semi-quantitative Western blot analysis was performed to determine the levels of known Ca2+ homeostasis proteins. Analysis was performed using ventricular homogenates for SERCA2a; the endogenous inhibitor of SERCA2a, PLB; and Ser-16 phosphorylated-PLB (P-PLB), which functions to relieve PLB inhibition of SERCA2a (Fig. 5). Figure 5A and 5E show representative semiquantitative Western blots of SERCA2a and PLB and Ser-16 PLB phosphorylation status performed on ventricular homogenates of 4- to 6-month-old Non-Tg, β-Tg, α-R92Q, α-R92L, β-R92Q, and β-R92L mice. No significant differences between Non-Tg and β-Tg hearts were found for the expression levels of SERCA2a, PLB or P-PLB. SERCA2a levels in all genotypes studied were similar to Non-Tg (Fig. 5B). PLB protein levels, on the other hand, were significantly increased in α-R92Q and α-R92L ventricles (1.4 fold and 1.8, respectively, vs. Non-Tg, P<0.05) (Fig. 5C), leading to decreased SERCA2a/PLB (Fig. 5D). Ser16 P-PLB for α-R92Q was lower than for Non-Tg hearts; however, for α-R92L, it was within the range of Non-Tg P-PLB levels (Fig. 5F).
Figure 5. Quantitation of SERCA2a/PLB and PKA-mediated PLB phosphorylation.
A. Representative semiquantitative Western blots of SERCA2a and PLB performed on ventricular homogenates of 4- to 6-month-old Non-Tg, β-Tg, α-R92Q, α-R92L, β-R92Q, and β-R92L mice. B. Sarcoplasmic reticulum ATPase (SERCA2a) levels were indistinguishable among the groups. C and D. Phospholamban (PLB) levels were increased in all transgenic genotypes leading to a decrease in SERCA2a/PLB for all transgenic genotypes. E. Representative Western blots of Ser16 phosphorylation status of PLB, total PLB, and calsequestrin control, performed on ventricular homogenates of 4- to 6-month-old Non-Tg, β-Tg, α-R92Q, α-R92L, β-R92Q, and β-R92L mice. F. Ser16 phosphorylated PLB (P-PLB) for α-R92Q was lower than for Non-Tg and increased for β-R92Q. In contrast, P-PLB for α-R92L was not different from Non-Tg and decreased for β-R92L. An n of 4–5 animals were used per experimental group. Data are presented as mean ± SEM. * p<0.05 vs Non-Tg and as indicated.
SERCA2a/PLB did not change for the β-MyHC expressing transgenics compared to their α-MyHC expressing littermates (Fig. 5D). Importantly, compared to their α-MyHC expressing littermate hearts, P-PLB increased for β-R92Q while it decreased for β-R92L (Fig. 5F).
4. Discussion
Transgenic mouse models expressing FHC-associated mutations in both thick and thin filament proteins have recapitulated many of the diverse clinical phenotypes observed in patients with this complex disorder. The R92 hotspot in the TM-binding region of cTnT exemplifies this scenario, with independent amino acid replacements leading to mutation-specific phenotypes manifest at all levels, ranging from peptides to intact hearts. The R92Q and R92L cTnT mutant hearts are representative of this diversity. The R92L mutation in the TM-binding region of cTnT leads to a phenotype that is overall more benign than the R92Q cTnT mutation. While both mutant cTnT hearts display some myocyte disarray, shorter sarcomere lengths and diastolic dysfunction, indices for whole heart and myocyte systolic performance for R92L hearts are indistinguishable from Non-Tg hearts. In contrast, R92Q hearts contain regions of fibrosis and demonstrate systolic dysfunction at both the whole heart and myocyte levels. Here, using these mouse models of FHC, we test a novel hypothesis: Replacing the endogenous fast (α) myosin in the thick filament of murine cardiac sarcomeres with a more efficient (β) myosin will rescue the mechanical defects characteristic of the R92 cTnT mutant phenotypes. While the α to β myosin replacement rescued the systolic dysfunction characteristic of the R92Q mutant heart, in part, due to a significant improvement in Ca2+ homeostasis, it failed to rescue the defects in diastolic function common to these FHC-associated R92 missense mutations, showing that this strategy cannot overcome the changes in thin filament structure and function leading to slowed sarcomere relaxation.
Switching the MyHC composition in wild type C57Bl/6 mouse hearts from 95/5% α/β–MyHC to 20/80% α/β–MyHC had no significant effect on any measured parameter. Neither measures of cardiac structure nor contractile function at either the whole heart or isolated myocyte levels differed. Similarly, none of the measures of myocyte Ca2+ levels, kinetics or SR loading or composition differed. Thus, for this mouse strain at baseline, the myosin isoform composition is neither a positive nor negative modulator of contractile performance or Ca2+ homeostasis. This conclusion may differ depending on genetic background, as a study of a similar MyHC switch in hearts of FVB/N mice showed a decrease in the rate of relaxation with unchanged systolic performance [27]. As we have done here, matching genetic background is a necessary requirement for the studies described here. The absence of any consequences of the myosin switch in wild type hearts also means that changes caused by switching myosin isoforms in hearts with mutant thin filaments can be ascribed to differences in thick-thin filament interaction due to the mutation in cTnT and to any downstream consequences of this interaction.
Measures of myocellular Ca2+ kinetics reveal changes that can explain some, but not all, of the mutant-specific changes in contractile performance. Indices of Ca2+ homeostasis for R92L myocytes are either the same as for Non-Tg myocytes (peak amplitude, SR mediated Ca2+ uptake) or quantitatively less severely depressed than observed for R92Q myocytes. Baseline Ca2+ levels were lower in both mutant hearts, possibly due to changes in the Ca2+-buffering capacity of cTnT R92 mutant cardiac myocytes, though the precise mechanism remains unclear [19, 28]. Lower SERCA2a/PLB ratios were observed in both mutant hearts due to higher PLB levels, not to changes in SERCA2a, and are concordant with alterations in SR Ca2+ uptake activity. Thus, while the lower SERCA2a/PLB ratio is consistent with the impaired SERCA2a SR Ca2+ uptake rates for α-R92Q, the decrease in SERCA2a/PLB for α-R92L myocytes does not correlate with our observation of normal SR Ca2+ uptake rate for α-R92L myocytes. Other mechanisms must contribute.
Altered SR uptake capacity can also be mediated by the phosphorylation state of PLB. Phosphorylation of Ser 16 in PLB relieves inhibition of SERCA2a levels and promotes Ca2+ uptake by the SR. Ser16 P-PLB was near normal for α-R92L myocytes but lower for α-R92Q myocytes. Based on these findings, we suggest that decreased SERCA2a-mediated Ca2+ uptake in the α-R92L myocytes may be compensated for by normal levels of Ser-16 PLB phosphorylation. In contrast, P-PLB levels in α-R92Q myocytes were decreased compared to Non-Tg myocytes, resulting in no functional compensation. Importantly, none of these changes in Ca2+ homeostasis are sufficient to compensate for the structural changes in the thin filament that lead to impaired relaxation in R92 mutant myocytes. Thus, analysis of Ca2+ kinetics revealed unexpected mutation-specific salutary effects of changing myosin isoform composition, suggesting that changes in Ca2+ homeostasis contribute to rescuing systolic but not diastolic dysfunction in these FHC mutant hearts. These results demonstrate a previously unrecognized modulatory role for MyHC isoforms with respect to Ca2+ homeostasis in the setting of cardiomyopathic remodeling.
Unlike the benign consequences of switching MyHC composition in hearts with normal thin filament structure and function, switching MyHC composition in mouse hearts bearing the R92 cTnT mutations in the thin filament led to mutation-specific changes in cardiac performance and Ca2+ homeostasis. Switching MyHC composition in mouse hearts bearing the R92L mutation in cTnT changed the amplitude of caffeine-induced transient and the extent of Ser 16 P-PLB. The switch did not alter systolic or diastolic performance or other measures of Ca2+ homeostasis. What was normal remained normal and what was dysfunctional remained abnormal. In contrast, for the more severe phenotype of the R92Q cTnT mutant heart, switching to β-MyHC had significant but selective salutary consequences. At both the whole heart and myocyte levels, systolic performance improved while diastolic performance remained refractory. Baseline Ca2+ levels and peak rates of Ca2+ rise and decline all improved, as did the amplitude of caffeine-induced Ca2+ transient and Vmax for SERCA2a-mediated SR Ca2+ uptake. PLB remained high but Ser 16 P-PLB also improved. These results suggest that the observed increase in SR Ca2+-mediated uptake for β-R92Q compared to α-R92Q results, at least in part, from a compensatory phosphorylation of PLB at Ser-16 that was not observed in β-R92L hearts. The opposite consequences of the MyHC switch on Ser 16 P-PLB for the R92Q and R92L hearts is unexpected and shows that remodeling of the SR is influenced not only by the type of MyHC present in the mutant sarcomere but also the structure and function of the thin filament.
A change in the binding of Ca2+ to cTnC subunit of cTn was found for the developing rabbit heart undergoing a similar change in MyHC composition as generated in the current study, suggesting that cTnC Ca2+ binding affinity can be altered by a switch in myosin isoform composition in the myofibril [29]. It is possible that this also occurred in the β-R92Q hearts in the current study, explaining the improvement in Ca2+ kinetics and the increase in Ser 16 P-PLB. However, as these improvements were not observed for β-Tg or β-R92L hearts, this mechanism is unlikely to represent a general effect of the change in myosin background. Other mutation-specific sarcomere-driven mechanisms must be dominant in determining the end phenotype.
Differences in cross-bridge cycling rates and ATPase activities for the different myosin isoforms studied in cell-free systems are well established, but still debated is whether changing myosin isoform composition in hypertrophied or failing myocardium in man has any functional consequences. In the failing human myocardium, the small amount of α-MyHC present in the ventricles is lost, and the relative amount of β-MyHC at the protein level increases from ~92% to near 100% [12, 30]. In view of the results presented here showing that no changes could be attributed solely to a change in myosin isoform composition when the relative composition of β-MyHC increased over 10-fold from 5 to 80% in wild type hearts, it seems unlikely that the small change in MyHC composition in human heart with normal thin filament composition has a functional correlate. Our results for the R92 cTnT mutant hearts suggest, however, that it does matter in the setting of an underlying cardiomyopathy. As noted in Rundell, et al., it has long been speculated that the α-MyHC to β-MyHC switch in human heart failure represents an early compensatory change [31]. It is possible that one unexpected salutary effect on contractility may be an initial improvement in Ca2+ homeostasis as observed here.
Importantly, the current studies show that the type of myosin present in the thick filament determines the degree of contractile dysfunction and integrity of Ca2+ homeostasis for hearts with FHC-associated mutations in a thin filament protein. It may also be important for FHC-associated mutations in myosin that also affect actin-myosin binding. A recent in vitro study compared the ATPase activity of myosin bearing the R403Q mutation in the α-MyHC vs. the β-MyHC-backbones [32]. This mutation is found in the actin-binding domain of myosin and, like the R92 cTnT mutations studied here, affects thick and thin filament binding. Whereas ATPase activity was 30% higher when the mutation was located in the α-MyHC, it was 10% lower for the mutant was in the β-MyHC backbone. Whether this applies to other myosin mutations located in other domains of this large molecule is altered remain to be determined, however, it is an intriguing observation. Taken together, the salutary consequences of substituting a fast myosin with a slow myosin support the notion that at least some FHC-associated mutations may be better tolerated in hearts containing predominantly β-MyHC in the sarcomeres.
It is important to emphasize that the improvements in Ca2+ homeostasis caused by changing MyHC isoform composition from fast to a slow myosin did not rescue the diastolic dysfunction that is characteristic of the two FHC-associated mutations representing divergent physiological and clinical phenotypes studied here. These results suggest that the differences in thin filament flexibility caused by the discrete R92 cTnT mutants are responsible for persistent diastolic dysfunction, even when the thick filament contains a more economical myosin. In contrast, the systolic dysfunction caused by the R92Q mutation in cTnT can be compensated for by replacing the fast myosin with a more economical myosin isoform, and that this improvement in part may be driven by an improvement in myocellular Ca2+ kinetics via a yet unknown mechanism. Our results are consistent with the growing literature that suggests that while the contractile state of the sarcomere in FHC is intricately linked with Ca2+ handling (and may eventually lead to mutation-specific, Ca2+ targeted interventions), the oft-noted and clinically challenging diastolic dysfunction in FHC will likely require more sophisticated, sarcomere-targeted therapeutics.
Acknowledgments
This work was supported in part by National Heart, Lung, andBlood Institute Grants 5F31-HL-085915-04 (to P. J. Guinto) andR01-HL-075619-06 (to J. C. Tardiff, J.S. Ingwall).
Footnotes
Disclosure Statement:
None.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Elliott P, McKenna WJ. Hypertrophic cardiomyopathy. Lancet. 2004 Jun 5;363(9424):1881–91. doi: 10.1016/S0140-6736(04)16358-7. [DOI] [PubMed] [Google Scholar]
- 2.Watkins H, McKenna WJ, Thierfelder L, Suk HJ, Anan R, O’Donoghue A, et al. Mutations in the genes for cardiac troponin T and alpha-tropomyosin in hypertrophic cardiomyopathy. N Engl J Med. 1995 Apr 20;332(16):1058–64. doi: 10.1056/NEJM199504203321603. [DOI] [PubMed] [Google Scholar]
- 3.Moolman JC, Corfield VA, Posen B, Ngumbela K, Seidman C, Brink PA, et al. Sudden death due to troponin T mutations. J Am Coll Cardiol. 1997 Mar 1;29(3):549–55. doi: 10.1016/s0735-1097(96)00530-x. [DOI] [PubMed] [Google Scholar]
- 4.Forissier JF, Carrier L, Farza H, Bonne G, Bercovici J, Richard P, et al. Codon 102 of the cardiac troponin T gene is a putative hot spot for mutations in familial hypertrophic cardiomyopathy. Circulation. 1996 Dec 15;94(12):3069–73. doi: 10.1161/01.cir.94.12.3069. [DOI] [PubMed] [Google Scholar]
- 5.Ertz-Berger BR, He H, Dowell C, Factor SM, Haim TE, Nunez S, et al. Changes in the chemical and dynamic properties of cardiac troponin T cause discrete cardiomyopathies in transgenic mice. Proc Natl Acad Sci USA. 2005 Dec 13;102(50):18219–24. doi: 10.1073/pnas.0509181102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Guinto JMP, Schwartz S, Tardiff JC. Computational characterization of mutations in cardiac troponin T known to cause familial hypertrophic cardiomyopathy. Journal of Theoretical and Computational Chemistry. 2007;6(3):413–9. doi: 10.1142/S0219633607003271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tardiff JC, Hewett TE, Palmer BM, Olsson C, Factor SM, Moore RL, et al. Cardiac troponin T mutations result in allele-specific phenotypes in a mouse model for hypertrophic cardiomyopathy. JClinInvest. 1999;104(4):469–81. doi: 10.1172/JCI6067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Javadpour MM, Tardiff JC, Pinz I, Ingwall JS. Decreased energetics in murine hearts bearing the R92Q mutation in cardiac troponin T. J Clin Invest. 2003 Sep;112(5):768–75. doi: 10.1172/JCI15967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Guinto P, Haim T, Dowell-Martino CC, Sibinga N, Tardiff JC. Temporal and Mutation-specific Alterations in Ca2+ Homeostasis Differentially Determine the Progression of cTnT-related Cardiomyopathies in Murine Models. Am J Physiol Heart Circ Physiol. 2009 Aug;297(2):H614–26. doi: 10.1152/ajpheart.01143.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Davis JO, Carroll WR, Trapasso M, Yankopoulos NA. Chemical characterization of cardiac myosin from normal dogs and from dogs with chronic congestive heart failure. J Clin Invest. 1960 Sep;39:1463–71. doi: 10.1172/JCI104165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nakao K, Minobe W, Roden R, Bristow MR, Leinwand LA. Myosin heavy chain gene expression in human heart failure. J Clin Invest. 1997 Nov 1;100(9):2362–70. doi: 10.1172/JCI119776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miyata S, Minobe W, Bristow MR, Leinwand LA. Myosin heavy chain isoform expression in the failing and nonfailing human heart. Circ Res. 2000 Mar 3;86(4):386–90. doi: 10.1161/01.res.86.4.386. [DOI] [PubMed] [Google Scholar]
- 13.Holubarsch C, Goulette RP, Litten RZ, Martin BJ, Mulieri LA, Alpert NR. The economy of isometric force development, myosin isoenzyme pattern and myofibrillar ATPase activity in normal and hypothyroid rat myocardium. Circ Res. 1985 Jan;56(1):78–86. doi: 10.1161/01.res.56.1.78. [DOI] [PubMed] [Google Scholar]
- 14.Krenz M, Sanbe A, Bouyer-Dalloz F, Gulick J, Klevitsky R, Hewett TE, et al. Analysis of myosin heavy chain functionality in the heart. J Biol Chem. 2003 May 9;278(19):17466–74. doi: 10.1074/jbc.M210804200. [DOI] [PubMed] [Google Scholar]
- 15.Tardiff JC, Factor SM, Tompkins BD, Hewett TE, Palmer BM, Moore RL, et al. A Truncated Cardiac Troponin T Molecule in Transgenic Mice Suggests Multiple Cellular Mechanisms for Familial Hypertrophic Cardiomyopathy. Journal of Clinical Investigation. 1998;101(12):2800–11. doi: 10.1172/JCI2389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Reiser PJ, Kline WO. Electrophoretic separation and quantitation of cardiac myosin heavy chain isoforms in eight mammalian species. Am J Physiol. 1998 Mar;274(3 Pt 2):H1048–53. doi: 10.1152/ajpheart.1998.274.3.H1048. [DOI] [PubMed] [Google Scholar]
- 17.Saupe KW, Spindler M, Tian R, Ingwall JS. Impaired cardiac energetics in mice lacking muscle-specific isoenzymes of creatine kinase. CircRes. 1998;82(8):898–907. doi: 10.1161/01.res.82.8.898. [DOI] [PubMed] [Google Scholar]
- 18.Wolska BM, Solaro RJ. Method for isolation of adult mouse cardiac myocytes for studies of contraction and microfluorimetry. 1996;271(3 Pt 2):H1250–H5. doi: 10.1152/ajpheart.1996.271.3.H1250. [DOI] [PubMed] [Google Scholar]
- 19.Haim TE, Dowell C, Diamanti T, Scheuer J, Tardiff JC. Independent FHC-related cardiac troponin T mutations exhibit specific alterations in myocellular contractility and calcium kinetics. J Mol Cell Cardiol. 2007 Jun 1;42(6):1098–110. doi: 10.1016/j.yjmcc.2007.03.906. [DOI] [PubMed] [Google Scholar]
- 20.Bassani RA, Bassani JW, Bers DM. Mitochondrial and sarcolemmal Ca2+ transport reduce [Ca2+]i during caffeine contractures in rabbit cardiac myocytes. J Physiol. 1992;453:591–608. doi: 10.1113/jphysiol.1992.sp019246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Lopes AC, Furlanetto R, Sasso WS, Didio LJ. Subcellular alterations of cardiac fibers in rats subjected to hypothyroidism. Journal of submicroscopic cytology and pathology. 1993 Apr;25(2):263–6. [PubMed] [Google Scholar]
- 22.Kiss E, Jakab G, Kranias EG, Edes I. Thyroid hormone-induced alterations in phospholamban protein expression. Regulatory effects on sarcoplasmic reticulum Ca2+ transport and myocardial relaxation. Circ Res. 1994 Aug;75(2):245–51. doi: 10.1161/01.res.75.2.245. [DOI] [PubMed] [Google Scholar]
- 23.Krenz M, Robbins J. Impact of beta-myosin heavy chain expression on cardiac function during stress. J Am Coll Cardiol. 2004 Dec 21;44(12):2390–7. doi: 10.1016/j.jacc.2004.09.044. [DOI] [PubMed] [Google Scholar]
- 24.Gulick J, Hewett TE, Klevitsky R, Buck SH, Moss RL, Robbins J. Transgenic Remodeling of the Regulatory Myosin Light Chains in the Mammalian Heart. CircRes. 1997;80(5):655–64. doi: 10.1161/01.res.80.5.655. [DOI] [PubMed] [Google Scholar]
- 25.Knollmann BC, Blatt SA, Horton K, de Freitas F, Miller T, Bell M, et al. Inotropic stimulation induces cardiac dysfunction in transgenic mice expressing a troponin T (I79N) mutation linked to familial hypertrophic cardiomyopathy. J Biol Chem. 2001;276(13):10039–48. doi: 10.1074/jbc.M006745200. [DOI] [PubMed] [Google Scholar]
- 26.Hernandez OM, Szczesna-Cordary D, Knollmann BC, Miller T, Bell M, Zhao J, et al. F110I and R278C troponin T mutations that cause familial hypertrophic cardiomyopathy affect muscle contraction in transgenic mice and reconstituted human cardiac fibers. J Biol Chem. 2005 Nov 4;280(44):37183–94. doi: 10.1074/jbc.M508114200. [DOI] [PubMed] [Google Scholar]
- 27.Hoyer K, Krenz M, Robbins J, Ingwall JS. Shifts in the myosin heavy chain isozymes in the mouse heart result in increased energy efficiency. J Mol Cell Cardiol. 2007 Jan 1;42(1):214–21. doi: 10.1016/j.yjmcc.2006.08.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fatkin D, McConnell BK, Mudd JO, Semsarian C, Moskowitz IG, Schoen FJ, et al. An abnormal Ca(2+) response in mutant sarcomere protein-mediated familial hypertrophic cardiomyopathy. J Clin Invest. 2000 Dec 6;106(11):1351–9. doi: 10.1172/JCI11093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McAuliffe JJ, Gao LZ, Solaro RJ. Changes in myofibrillar activation and troponin C Ca2+ binding associated with troponin T isoform switching in developing rabbit heart. Circ Res. 1990 May;66(5):1204–16. doi: 10.1161/01.res.66.5.1204. [DOI] [PubMed] [Google Scholar]
- 30.Noguchi T, Camp P, Jr, Alix SL, Gorga JA, Begin KJ, Leavitt BJ, et al. Myosin from failing and non-failing human ventricles exhibit similar contractile properties. J Mol Cell Cardiol. 2003 Jan;35(1):91–7. doi: 10.1016/s0022-2828(02)00282-1. [DOI] [PubMed] [Google Scholar]
- 31.Rundell VL, Manaves V, Martin AF, de Tombe PP. Impact of beta-myosin heavy chain isoform expression on cross-bridge cycling kinetics. Am J Physiol Heart Circ Physiol. 2005 Feb 1;288(2):H896–903. doi: 10.1152/ajpheart.00407.2004. [DOI] [PubMed] [Google Scholar]
- 32.Lowey S, Lesko LM, Rovner AS, Hodges AR, White SL, Low RB, et al. Functional effects of the hypertrophic cardiomyopathy R403Q mutation are different in an alpha- or beta-myosin heavy chain backbone. J Biol Chem. 2008 Jul 18;283(29):20579–89. doi: 10.1074/jbc.M800554200. [DOI] [PMC free article] [PubMed] [Google Scholar]