Abstract
A strategy has been developed to deliver selectively chemotherapeutic drugs to B-cells by employing doxorubicin loaded liposomes modified by a ligand for the B cell-specific cell-surface protein CD22 also known as Siglec-2. The liposomes bound rapid and saturable to the human Burkitt lymphoma Daudi B-cell line and exhibited significantly higher cytotoxicity in vitro and in vivo compared to similar untargeted liposomes. The CD22-targeted liposome bound to B cells isolated from lymphoma patients and although binding was proportional to CD22 expression on the cell surface, low levels of expression on CLL cells were sufficient to effect cell neutralization. The glycan-based strategy for delivery chemotherapeutic agents may provide a new strategy for the treatment of B-cell lymphomas.
Keywords: B-cell lymphomas, Siglec, CD22, liposomes, carbohydrate-based targeting
Rituximab was the first monoclonal antibody (MAb) to be approved for the treatment of patients with cancer and has greatly improved survival of patients with follicular and diffuse large B-cell lymphomas.[1,2] The antibody targets cluster differentiation (CD) 20, which is resistant to internalization and mainly expressed by B-cells. Rituximab depletes both normal and malignant B-cells by cellular cytotoxicity (ADCC),[3-5] complement-dependent cellular cytotoxicity (CDC),[6] and CD20 induced apoptosis.[7] In addition, it has been proposed that rituximab can neutralize malignant B cells by promoting cross-presentation of lymphoma antigens by antigen-presenting cells (APCs) thereby priming of lymphoma antigen-specific T cells.[8]
Despite its effectiveness, approximately 50% of relapsed B-cell lymphomas patients with refractory CD20+ follicular lymphomas do not respond to initial therapy with rituximab.[9] In addition, almost 60% of initial responding patients will not longer benefit with retreatment with the monoclonal antibody.[10] It is unclear whether these forms of rituximab-resistance are due to adaptive property of the malignant B cell or to an impaired host’s immune effector mechanisms.
Several approaches to overcome antibody resistance are under evaluation such as drugs that interfere with intrinsic tumor-related mechanisms of resistance and the development of a newer generation of MAbs.[1] In particular, anti-CD20 antibodies that have a glycoengineered Fc fragment (Fc) that enhances binding to Fc gamma receptors to improve ADCC is showing promise.[11,12] Another approach that is being pursued to treat B-cell diseases is to block the interaction of B cell survival or growth factors with their receptors on B cells and the monoclonal antibodies Belimumab and Atacicept are examples of such an approach. In addition, antibodies that target CD22 and are linked to a toxin are being examined for the depletion of B-cells. In contrast to CD20, CD22 undergoes constitutive endocytosis and is well suited for efficient delivery of the toxin into the cell.[13-17]
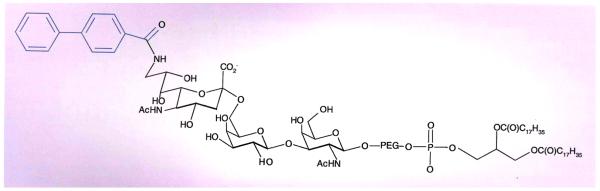
Chen et al. have developed an elegant alternative approach to deliver selectively chemotherapeutic drugs to B-cells by employing doxorubicin-loaded liposomes modified by a ligand for the B cell-specific cell-surface protein CD22[18] also known as Siglec-2 (Figure 1). [19,20] CD22 is a carbohydrate binding protein that belongs to the family of Siglecs, which recognizes sialylated glycans.[20] Sialic acids are a structurally diverse class of monosaccharides that are typically found at the outermost ends of complex glycans of glycoproteins and glycosphingolipids. In N-linked glycoproteins, sialic acids are usually linked to α(2-3)- or α(2-6)- to galactosides or α(2-6)-linked to N-acetyl-galactosamine, whereas in O-linked glycoproteins they are often found as terminal Neu5Aca(2-6)GalNAc moieties. Sialic acids can be modified by for example acetyl esters or sulfates. CD22 specifically recognizes sialic acid (2,6)-linkage to galactose.[21] Importantly, CD22 displays highly efficient, constitutive endocytosis, which is accelerated by ligation.[16] Thus, binding to CD22 by a cognate ligand was be expected to lead to rapid internalization of the relevant structure.[22]
Figure 1. The chemical structure of the BPCNeuAc modified with a lipid for incorporation into doxorubicin-modified micelles.
The terminal monosaccharide is a modified sialic acid moiety. The biphenyl of the sialic acid moiety (highlighted in blue) significantly enhances the affinity for binding to CD22.
Methods and Results
CD22-targeted liposomes were prepared by coupling a high-affinity glycan ligand of CD22 (BPCNeuAc)[23] to a commercially available N-hydroxysuccinimide (NHS)-activated pegylated lipid, and the resulting BPCNeuAc-pegylated lipid was then incorporated into a liposomal doxorubicin formulation similar to that in current clinical use. Fluorescent-labeled BPCNeuAc liposomes bound robustly to CD22-expressing but not to wild-type CHO cells, whereas the unmodified liposomes did not exhibit binding to either cell type, demonstrating that the saccharide promotes cellular uptake. Fluorescence microscopy confirmed the results and indicated that internalization was mediated by endocytosis.
As expected, the BPCNeuAc liposomes bound rapid and saturable the human Burkitt lymphoma Daudi B-cell line, which is commonly used for evaluating drugs for treatment of B-cell lymphomas.[24,25] Furthermore the interaction could be competitively inhibited by free glycan ligands of CD22, demonstrating that specific interactions had been established. Doxorubicin, which is a chemotherapeutic agent that interacts with DNA and is being used for the treatment of lymphomas, was loaded into BPCNeuAc and naked liposomes. The BPCNeuAc modified liposomes exhibited a 33-fold higher cytotoxicity (IC50 = 1.6μM) compared to the naked liposomes (IC50 = 53μM).[26] Similar observations were made when the experiment was performed in mouse whole blood indicating that BPCNeuAc liposomes can efficiently target human B lymphoma cells both in vitro and in vivo.
Preliminary pharmacokinetics studies showed that in tumor-free mice BPCNeuAc liposomes cleared faster from the blood stream than unmodified liposomes. It was expected that this observation was due to a known cross-reactivity of the BPCNeuAc ligand with Siglec-1,[27,28] which is exclusively expressed by tissue macrophages.[29] Indeed, a dramatically reduced clearance of the carbohydrate modified liposomes was observed in mice depleted of macrophages using liposomal clodronate. To address the possibility that the carbohydrate ligand abrogates the “stealth” character of the liposomes resulting in nonspecific uptake by macrophages, liposomes were prepared that contain a related carbohydrate ligand that does not bind to Siglec 1.[27,28] As expected, such liposomes exhibited a clearance similar to that of the naked liposomes.
The efficacy of Doxorubicin-loaded CD22 targeting liposomes was examined in a standard Daudi lymphoma model in severe combined immunodeficiency mice.[30] The BPCNeuAc liposomes were administered in two doses and it was assumed that the first dose would eliminate macrophages ensuring that the second dose would have a sufficiently long circulation time to eliminate the cancer cells. Thus, Daudi cells were injected intravenously and allowed to disseminate for 24 h, and on day one and three, PBS, Doxorubicin-loaded naked liposomes, and BPCNeuAc liposomes having different densities of the carbohydrate ligand, were administered. The untreated mice had a mean time of survival (MTS) of 28.5 days, whereas the Doxorubicin-loaded naked liposomes increased the MTS to 50 days. In contrast, liposomes displaying 2% BPCNeuAc ligands exhibited a MTS of 73 days, with 3 of 8 long-term survivors being healthy at the end of the study (day 100), and liposomes with 5% ligands demonstrated a MTS greater than 100 days with 5 of 8 long-term survivors. Both treatments with drug-loaded BPCNeuAc liposomes comprising 2% or 5% ligands significantly improved survival rates compared to treatment with nontargeted liposomes. Further analysis showed that human CD19+ Daudi cells constituted most bone marrow cells in paralyzed untreated or naked liposome–treated animals. In contrast, residual tumor cells were not detectable above background (Ig isotype control) in the bone marrow of the long-term survivors at day 100, further demonstrating the efficacy of the CD22-targeted liposomal regimen.
The ability of the BPCNeuAc liposomes to bind to neoplastic B cells from patients with leukemia or lymphoma was examined. Blood samples were obtained from four healthy donors and thirty patients with hairy cell leukemia (HCL), chronic lymphocytic leukemia (CLL), or splenic marginal zone lymphoma (MZL). Although variations in CD22 expressions were observed, malignant B cells from patients with HCL, MZL, and CLL were sensitive to the cytotoxicity of the Doxorubicin-loaded BPCNeuAc liposomes. Whereas HCL and MZL were susceptible to both low (10μM) and high (40μM) concentrations of the targeted liposomal Doxorubicin, CLL cells were efficiently killed only by the higher Doxorubicin concentration. Collectively, these results indicate that while binding of the CD22-targeted liposome to malignant B cells is proportional to CD22 expression on the cell surface, even low levels of expression on CLL cells are sufficient to effect cell neutralization.
Discussion, Expert Commentary & Five-Year View
The term ‘magic bullet’, was coined by bacteriologist Paul Ehrlich in the late 1800s, and originally referred to a chemical that can specifically target a microorganism.[31-33] The concept of specific targeting has expanded to include cancer treatments, and has for example been successfully applied for the development of cancer therapies. In particular, selective targeting of tumor-associated cell-surface antigens with a monoclonal antibody has found clinical use[31-33] and for example, the monoclonal antibody Rituximab has greatly improved survival of patients with follicular and diffuse large B-cell lymphomas.[1,2] Despite the efficacy of rituximab, it is not a cure, especially for indolent lymphoid malignancies, and 22,000 patients die annually in the USA.[19-21,34] To address this deficiency, a number of antibodies for B-cell depletion therapy are in clinical development,[13,16,19,22] and two immunotoxins (BL22 and CMC-544) target CD22, a B-lymphocyte–specific receptor.
An alternative approach to selectively target cancer cells is based on liposomal particles that carry an encapsulated chemotherapeutic cargo.[35] Such delivery systems have reduced adverse effects typically associated with the chemotherapeutic agent due to the observation that the vascular system of tumor tissue is more permeable and allows selective penetration of liposomes in tumor tissue. Nanoparticle drugs can, however, only be employed for solid tumors. Antibody-covered liposomes (immuno-liposomes) incorporating relevant chemotherapeutic agents have been developed to further improve selectivity and, hence, the therapeutic index of encapsulated agents.[34]
Carbohydrate-protein recognition events can also be exploited for selective cell or tissue targeting. In this respect, almost all cell surface and secreted proteins are modified by covalently-linked carbohydrate moieties and the glycan structures on these glycoproteins have been implicated as essential mediators in processes such as protein folding, cell signaling, fertilization, embryogenesis, neuronal development, hormone activity, and the proliferation of cells and their organization into specific tissues.[36] In addition, overwhelming data supports the relevance of glycosylation in pathogen recognition, inflammation, innate immune responses, and the development of autoimmune diseases and cancer.[37-41]
Chen et al. have described a unique approach for depleting B-cells by employing doxorubicin-loaded liposomal nanoparticles decorated with a carbohydrate composed of (2,6)-linked sialosides. This glycan binds to the carbohydrate binding protein CD22 (Siglec-2), which is uniquely expressed by B-cells, and hence might offer an approach to selectively target this cell type. However, in general carbohydrate-protein binding is relatively weak, complicating their use for tissue targeting. In the case of CD22, this problem is compounded by the well documented fact that endogenous cis carbohydrate ligands on B-cells mask binding of synthetic ligands to CD22 in trans.[42,43] The authors have addressed these difficulties by employing a sialoside modified with a biphenylcarboxyl moiety that has a higher affinity for CD22 than the cognate ligand. Furthermore, the liposomes present multiple copies of the carbohydrate ligand that can make multiple interactions with CD22 thereby generating sufficient avidity to target B cells in a complex biologic milieu.[11,12,44]
It has been shown that such a delivery system exhibit significantly higher cytotoxicity in vitro and in vivo compared to similar untargeted liposomes. It has also been shown that Doxorubicin-loaded liposomes modified with sialosides bind to B-cells isolated from patients with chronic lymphocytic leukemia, hairy cell leukemia, and marginal zone lymphoma, with binding efficiency correlating with expression of CD22. Importantly, in vitro studies showed that the capacity of sialoside-bearing drug-loaded liposomes to kill B cells is not proportional to expression of CD22, suggesting that the high efficiency of CD22-mediated drug internalization is sufficient to induce cell death even when surface CD22 levels are relatively low.
The results support a strategy for developing a new use for approved drugs by converting the delivery mechanism to active targeting and uptake by a targeted cell. This concept is of direct therapeutic interest for treatment of non-Hodgkin lymphoma or other B-cell lymphomas, with more than a dozen clinical trials completed or in progress evaluating liposomal Doxorubicin in place of Doxorubicin for standard cyclophosphamide, doxorubicin, prednisone, and vincristine (CHOP) chemotherapy.[45-47] The targeted liposomes breach the cells by an endocytic mechanism, and therefore it is anticipated that the therapeutic benefits can be synergistized with anti-CD20 rituximab and other immune-mediated therapies.
Although the carbohydrate-modified liposomes provided excellent efficacy in a B-cell lymphoma model, there is a need for further improvement. In particular, the employed carbohydrate ligand is also recognized by Siglec-1, which is expressed by macrophages and as a result leads to a rapid clearance of the liposomes.[27,28] Although Chen et al. were able to achieve sustained delivery of chemotherapeutic drug by a two-stage delivery strategy, there is a need to further improve the selectivity of the carbohydrate ligand.
The results presented by Chen et al. combined with several other reports highlight that glycan-decorated liposomes may provide a robust platform for targeted delivery of therapeutic agents to cells that express a cognate glycan-binding protein.[48-53] For example, the well-known myeloid antigen CD33 (Siglec-3) also recognizes sialylated oligosaccharides and may be employed for targeted treatment of myeloid leukemia’s.
Key Issues.
CD22 also known as Siglec-2 is uniquely expressed by B-cells and recognizes sialic acids (2-6)-linked to galactosides. This carbohydrate binding protein provides unique opportunities to deplete B-cells.
Liposomes modified with a high affinity ligand for CD22 bound rapid and saturable to the human Burkitt lymphoma Daudi B cell line. A carbohydrate-modified delivery system exhibited significantly higher cytotoxicity in vitro and in vivo compared to similar untargeted liposomes.
High affinity binding was achieved by employing a sialoside modified with a biphenylcarboxyl moiety that has a higher affinity for CD22 than the cognate ligand. Furthermore, the liposomes present multiple copies of the carbohydrate ligand that can make multiple interactions with CD22 thereby generating sufficient avidity to target B cells in a complex biologic milieu.
The CD22-targeted liposome binds to B cells isolated from lymphoma patients and although binding was proportional to CD22 expression on the cell surface, low levels of expression on CLL cells were sufficient to effect cell neutralization.
Acknowledgement
This work was supported by a grant from the National Cancer Institute of the US National Institutes of Health (R01CA088986).
Footnotes
Evaluation of: Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood, 115(23), 4778-4786 (2010).
References
- 1.Bello C, Sotomayor EM. Monoclonal antibodies for B-cell lymphomas: rituximab and beyond. Hematology. 2007:233–242. doi: 10.1182/asheducation-2007.1.233. [DOI] [PubMed] [Google Scholar]
- 2.Ratanatharathorn V, Pavletic S, Uberti JP. Clinical applications of rituximab in allogeneic stem cell transplantation: anti-tumor and immunomodulatory effects. Cancer Treat Rev. 2009;35(8):653–661. doi: 10.1016/j.ctrv.2009.07.004. [DOI] [PubMed] [Google Scholar]
- 3.Cartron G, Dacheux L, Salles G, et al. Therapeutic activity of humanized anti-CD20 monoclonal antibody and polymorphism in IgG Fc receptor FcgammaRIIIa gene. Blood. 2002;99(3):754–758. doi: 10.1182/blood.v99.3.754. [DOI] [PubMed] [Google Scholar]
- 4.Weng WK, Levy R. Two immunoglobulin G fragment C receptor polymorphisms independently predict response to rituximab in patients with follicular lymphoma. J. Clin. Oncol. 2003;21(21):3940–3947. doi: 10.1200/JCO.2003.05.013. [DOI] [PubMed] [Google Scholar]
- 5.Ghielmini M, Rufibach K, Salles G, et al. Single agent rituximab in patients with follicular or mantle cell lymphoma: clinical and biological factors that are predictive of response and event-free survival as well as the effect of rituximab on the immune system: a study of the Swiss Group for Clinical Cancer Research (SAKK) Ann. Oncol. 2005;16(10):1675–1682. doi: 10.1093/annonc/mdi320. [DOI] [PubMed] [Google Scholar]
- 6.Smith MR. Rituximab (monoclonal anti-CD20 antibody): mechanisms of action and resistance. Oncogene. 2003;22(47):7359–7368. doi: 10.1038/sj.onc.1206939. [DOI] [PubMed] [Google Scholar]
- 7.Alas S, Emmanouilides C, Bonavida B. Inhibition of interleukin 10 by rituximab results in down-regulation of bcl-2 and sensitization of B-cell non-Hodgkin’s lymphoma to apoptosis. Clin. Cancer Res. 2001;7(3):709–723. [PubMed] [Google Scholar]
- 8.Selenko N, Maidic O, Draxier S, et al. CD20 antibody (C2B8)-induced apoptosis of lymphoma cells promotes phagocytosis by dendritic cells and cross-priming of CD8+ cytotoxic T cells. Leukemia. 2001;15(10):1619–1626. doi: 10.1038/sj.leu.2402226. [DOI] [PubMed] [Google Scholar]
- 9.McLaughlin P, Grillo-Lopez AJ, Link BK, et al. Rituximab chimeric anti-CD20 monoclonal antibody therapy for relapsed indolent lymphoma: half of patients respond to a four-dose treatment program. J. Clin. Oncol. 1998;16(8):2825–2833. doi: 10.1200/JCO.1998.16.8.2825. [DOI] [PubMed] [Google Scholar]
- 10.Davis TA, Grillo-Lopez AJ, White CA, et al. Rituximab anti-CD20 monoclonal antibody therapy in non-Hodgkin’s lymphoma: safety and efficacy of re-treatment. J. Clin. Oncol. 2000;18(17):3135–3143. doi: 10.1200/JCO.2000.18.17.3135. [DOI] [PubMed] [Google Scholar]
- 11.Weiner GJ, Bowles JA, Link BK, Campbell MA, Wooldridge JE, Breitmeyer JB. An anti-CD20 monoclonal antibody (mAb) with enhanced affinity for CD16 activates NK cells at a lower concentrations and more effectively than rituximab [abstract] Blood. 2005;106 doi: 10.1182/blood-2006-04-020057. Abstract no. 348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Mossner E, Brunker P, Moser S, et al. Increasing the efficacy of CD20 antibody therapy through the engineering of a new type II anti-CD20 antibody with enhanced direct and immune effector cell-mediated B-cell cytotoxicity. Blood. 2010;115(22):4393–4402. doi: 10.1182/blood-2009-06-225979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kreitman RJ, Margulies I, Stetler-Stevenson M, Wang QC, FitzGerald DJ, Pastan I. Cytotoxic activity of disulfide-stabilized recombinant immunotoxin RFB4(dsFv)-PE38 (BL22) toward fresh malignant cells from patients with B-cell leukemias. Clin. Cancer Res. 2000;6(4):1476–1487. [PubMed] [Google Scholar]
- 14.Kreitman RJ, Wilson WH, Bergeron K, et al. Efficacy of the anti-CD22 recombinant immunotoxin BL22 in chemotherapy-resistant hairy-cell leukemia. N. Engl. J. Med. 2001;345(4):241–247. doi: 10.1056/NEJM200107263450402. [DOI] [PubMed] [Google Scholar]
- 15.DiJoseph JF, Dougher MM, Kalyandrug LB, et al. Antitumor efficacy of a combination of CMC-544 (inotuzumab ozogamicin), a CD22-targeted cytotoxic immunoconjugate of calicheamicin, and rituximab against non-Hodgkin’s B-cell lymphoma. Clin. Cancer Res. 2006;12(1):242–249. doi: 10.1158/1078-0432.CCR-05-1905. [DOI] [PubMed] [Google Scholar]
- 16.Dijoseph JF, Dougher MM, Armellino DC, Evans DY, Damle NK. Therapeutic potential of CD22-specific antibody-targeted chemotherapy using inotuzumab ozogamicin (CMC-544) for the treatment of acute lymphoblastic leukemia. Leukemia. 2007;21(11):2240–2245. doi: 10.1038/sj.leu.2404866. [DOI] [PubMed] [Google Scholar]
- 17.Du X, Beers R, Fitzgerald DJ, Pastan I. Differential cellular internalization of anti-CD19 and -CD22 immunotoxins results in different cytotoxic activity. Cancer Res. 2008;68(15):6300–6305. doi: 10.1158/0008-5472.CAN-08-0461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen WC, Completo GC, Sigal DS, Crocker PR, Saven A, Paulson JC. In vivo targeting of B-cell lymphoma with glycan ligands of CD22. Blood. 2010;115(23):4778–4786. doi: 10.1182/blood-2009-12-257386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Castillo J, Winer E, Quesenberry P. Newer monoclonal antibodies for hematological malignancies. Exp. Hematol. 2008;36(7):755–768. doi: 10.1016/j.exphem.2008.04.018. [DOI] [PubMed] [Google Scholar]
- 20.Molina A. A decade of rituximab: improving survival outcomes in non-Hodgkin’s lymphoma. Annu. Rev. Med. 2008;59:237–250. doi: 10.1146/annurev.med.59.060906.220345. [DOI] [PubMed] [Google Scholar]
- 21.Evans LS, Hancock BW. Non-Hodgkin lymphoma. Lancet. 2003;362(9378):139–146. doi: 10.1016/S0140-6736(03)13868-8. [DOI] [PubMed] [Google Scholar]
- 22.Haas KM, Sen S, Sanford IG, Miller AS, Poe JC, Tedder TF. CD22 ligand binding regulates normal and malignant B lymphocyte survival in vivo. J. Immunol. 2006;177(5):3063–3073. doi: 10.4049/jimmunol.177.5.3063. [DOI] [PubMed] [Google Scholar]
- 23.Collins BE, Blixt O, Han S, et al. High-affinity ligand probes of CD22 overcome the threshold set by cis ligands to allow for binding, endocytosis, and killing of B cells. J Immunol. 2006;177(5):2994–3003. doi: 10.4049/jimmunol.177.5.2994. [DOI] [PubMed] [Google Scholar]
- 24.Ghetie MA, Tucker K, Richardson J, Uhr JW, Vitetta ES. Eradication of minimal disease in severe combined immunodeficient mice with disseminated Daudi lymphoma using chemotherapy and an immunotoxin cocktail. Blood. 1994;84(3):702–707. [PubMed] [Google Scholar]
- 25.Newton DL, Hansen HJ, Mikulski SM, Goldenberg DM, Rybak SM. Potent and specific antitumor effects of an anti-CD22-targeted cytotoxic ribonuclease: potential for the treatment of non-Hodgkin lymphoma. Blood. 2001;97(2):528–535. doi: 10.1182/blood.v97.2.528. [DOI] [PubMed] [Google Scholar]
- 26.Cheng WW, Allen TM. Targeted delivery of anti-CD19 liposomal doxorubicin in B-cell lymphoma: a comparison of whole monoclonal antibody, Fab’ fragments and single chain Fv. J. Controlled Release. 2008;126(1):50–58. doi: 10.1016/j.jconrel.2007.11.005. [DOI] [PubMed] [Google Scholar]
- 27.Zaccai NR, Maenaka K, Maenaka T, et al. Structure-guided design of sialic acid-based Siglec inhibitors and crystallographic analysis in complex with sialoadhesin. Structure. 2003;11(5):557–567. doi: 10.1016/s0969-2126(03)00073-x. [DOI] [PubMed] [Google Scholar]
- 28.Blixt O, Han S, Liao L, et al. Sialoside analogue arrays for rapid identification of high affinity siglec ligands. J. Am. Chem. Soc. 2008;130(21):6680–6681. doi: 10.1021/ja801052g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hartnell A, Steel J, Turley H, Jones M, Jackson DG, Crocker PR. Characterization of human sialoadhesin, a sialic acid binding receptor expressed by resident and inflammatory macrophage populations. Blood. 2001;97(1):288–296. doi: 10.1182/blood.v97.1.288. [DOI] [PubMed] [Google Scholar]
- 30.Ghetie MA, Richardson J, Tucker T, Jones D, Uhr JW, Vitetta ES. Disseminated or localized growth of a human B-cell tumor (Daudi) in SCID mice. Int. J. Cancer. 1990;45(3):481–485. doi: 10.1002/ijc.2910450318. [DOI] [PubMed] [Google Scholar]
- 31.Eccles SA. Monoclonal antibodies targeting cancer: ‘magic bullets’ or just the trigger? Breast Cancer Res. 2001;3(2):86–90. doi: 10.1186/bcr276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Morphy R, Kay C, Rankovic Z. From magic bullets to designed multiple ligands. Drug Discov. Today. 2004;9(15):641–651. doi: 10.1016/S1359-6446(04)03163-0. [DOI] [PubMed] [Google Scholar]
- 33.Imai K, Takaoka A. Comparing antibody and small-molecule therapies for cancer. Nat. Rev. Cancer. 2006;6(9):714–727. doi: 10.1038/nrc1913. [DOI] [PubMed] [Google Scholar]
- 34.Bello C, Sotomayor EM. Monoclonal antibodies for B-cell lymphomas: rituximab and beyond. Hematology. 2007:233–242. doi: 10.1182/asheducation-2007.1.233. [DOI] [PubMed] [Google Scholar]
- 35.Torchilin VP. Recent advances with liposomes as pharmaceutical carriers. Nat. Rev. Drug Discov. 2005;4(2):145–160. doi: 10.1038/nrd1632. [DOI] [PubMed] [Google Scholar]
- 36.Taylor ME, Drickamer K. Paradigms for glycan-binding receptors in cell adhesion. Curr. Opin. Cell. Biol. 2007;19(5):572–577. doi: 10.1016/j.ceb.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Ohtsubo K, Marth JD. Glycosylation in cellular mechanisms of health and disease. Cell. 2006;126(5):855–867. doi: 10.1016/j.cell.2006.08.019. [DOI] [PubMed] [Google Scholar]
- 38.Brockhausen I. Mucin-type O-glycans in human colon and breast cancer: glycodynamics and functions. EMBO Rep. 2006;7(6):599–604. doi: 10.1038/sj.embor.7400705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JR, Crawford BE, Esko JD. Glycan antagonists and inhibitors: a fount for drug discovery. Crit. Rev. Biochem. Mol. Biol. 2007;42(6):481–515. doi: 10.1080/10409230701751611. [DOI] [PubMed] [Google Scholar]
- 40.Crocker PR, Paulson JC, Varki A. Siglecs and their roles in the immune system. Nat. Rev. Immunol. 2007;7(4):255–266. doi: 10.1038/nri2056. [DOI] [PubMed] [Google Scholar]
- 41.van Kooyk Y, Rabinovich GA. Protein-glycan interactions in the control of innate and adaptive immune responses. Nat. Immunol. 2008;9(6):593–601. doi: 10.1038/ni.f.203. [DOI] [PubMed] [Google Scholar]
- 42.Collins BE, Paulson JC. Cell surface biology mediated by low affinity multivalent protein-glycan interactions. Curr. Opin. Chem. Biol. 2004;8(6):617–625. doi: 10.1016/j.cbpa.2004.10.004. [DOI] [PubMed] [Google Scholar]
- 43.Collins BE, Blixt O, DeSieno AR, Bovin N, Marth JD, Paulson JC. Masking of CD22 by cis ligands does not prevent redistribution of CD22 to sites of cell contact. Proc. Natl. Acad. Sci. U. S. A. 2004;101(16):6104–6109. doi: 10.1073/pnas.0400851101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lundquist JJ, Toone EJ. The cluster glycoside effect. Chem. Rev. 2002;102(2):555–578. doi: 10.1021/cr000418f. [DOI] [PubMed] [Google Scholar]
- 45.Aviles A, Neri N, Castaneda C, Talavera A, Huerta-Guzman J, Gonzalez M. Pegylated liposomal doxorubicin in combination chemotherapy in the treatment of previously untreated aggressive diffuse large-B-cell lymphoma. Med. Oncol. 2002;19(1):55–58. doi: 10.1385/MO:19:1:55. [DOI] [PubMed] [Google Scholar]
- 46.Tsavaris N, Kosmas C, Vadiaka M, et al. Pegylated liposomal doxorubicin in the CHOP regimen for older patients with aggressive (stages III/IV) non-Hodgkin’s lymphoma. Anticancer Res. 2002;22(3):1845–1848. [PubMed] [Google Scholar]
- 47.Levine AM, Tulpule A, Espina B, et al. Liposome-encapsulated doxorubicin in combination with standard agents (cyclophosphamide, vincristine, prednisone) in patients with newly diagnosed AIDS-related non-Hodgkin’s lymphoma: results of therapy and correlates of response. J. Clin. Oncol. 2004;22(13):2662–2670. doi: 10.1200/JCO.2004.10.093. [DOI] [PubMed] [Google Scholar]
- 48.Bruehl RE, Dasgupta F, Katsumoto TR, et al. Polymerized liposome assemblies: bifunctional macromolecular selectin inhibitors mimicking physiological selectin ligands. Biochemistry. 2001;40(20):5964–5974. doi: 10.1021/bi002921s. [DOI] [PubMed] [Google Scholar]
- 49.Ikehara Y, Niwa T, Biao L, et al. A carbohydrate recognition-based drug delivery and controlled release system using intraperitoneal macrophages as a cellular vehicle. Cancer Res. 2006;66(17):8740–8748. doi: 10.1158/0008-5472.CAN-06-0470. [DOI] [PubMed] [Google Scholar]
- 50.Hashida N, Ohguro N, Yamazaki N, et al. High-efficacy site-directed drug delivery system using sialyl-Lewis X conjugated liposome. Exp. Eye Res. 2008;86(1):138–149. doi: 10.1016/j.exer.2007.10.004. [DOI] [PubMed] [Google Scholar]
- 51.Ikehara Y, Shiuchi N, Kabata-Ikehara S, et al. Effective induction of anti-tumor immune responses with oligomannose-coated liposome targeting to intraperitoneal phagocytic cells. Cancer Lett. 2008;260(1-2):137–145. doi: 10.1016/j.canlet.2007.10.038. [DOI] [PubMed] [Google Scholar]
- 52.O’Reilly MK, Paulson JC. Siglecs as targets for therapy in immune-cell-mediated disease. Trends Pharmacol. Sci. 2009;30(5):240–248. doi: 10.1016/j.tips.2009.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhang H, Ma Y, Sun XL. Recent developments in carbohydrate-decorated targeted drug/gene delivery. Med. Res. Rev. 2010;30(2):270–289. doi: 10.1002/med.20171. [DOI] [PubMed] [Google Scholar]