Abstract
Juvenile hormone (JH) plays key roles in both metamorphosis and adult reproductive processes. Farnesoic acid O-methyltransferase (FAMeT) is thought to be an important enzyme in the JH biosynthetic pathway, catalyzing methylation of farnesoic acid (FA) to methyl farnesoate (MF). A full-length cDNA (NlFAMeT) encoding a 299 amino acid putative FAMeT was isolated from the brown planthopper, Nilaparvata lugens (Stal) (Hemiptera: Geometroidea), a major rice pest in many parts of Asia. NlFAMeT showed high amino acid identities (52–54%) with other insect FAMeTs. Although the NlFAMeT transcript was expressed highly in corpus allatum (CA) and brain (without CA), no correlation was found between NlFAMeT transcript and JH titers. Although only a low level of NlFAMeT transcript was detected in the ovary, a high level was found in the abdomen and should be in one or more tissues undefined in the abdomen. Also, NlFAMeT transcript had a positive change during the vitellogenesis in female adults. These data indicated that NlFAMeT might not be a key enzyme in JH synthesis in N. lugens, but that it may play an important role in the ovary development. It might also be important in some unknown process in a so-far unidentified tissue in the abdomen.
Keywords : juvenile hormone synthesis, mRNA levels, ovary development
Introduction
Juvenile hormones (JHs) are sesquiterpenoid compounds that are synthesized by the corpus allatum (CA) and are important in many pre- and postmetamorphic events in insects. JHs play major roles in the control of growth, development, metamorphosis, diapause and reproduction in insects (Riddiford et al. 2003). The regulation of JH titers is thus critical in the entire life of the insect. Neurosecretory cells in the brain release allatotropic and allatostatic factors that regulate the synthesis and secretion of JH (Stay 2000). Some important enzymes also play key roles in the synthesis and regulation of JH, such as JH methyltransferase and JH epoxidase (for a review, see Bellés et al. 2005).
The biosynthesis of JH can be divided into early and late phases: early phase being the production of farnesyl biphosphate (FBP) by the mevalonate pathway and late phase involving the conversion of FBP into JH precursors and eventually JH as the end product (Bellés et al. 2005). In the late phase in crustaceans, methyl farnesoate (MF) is the immediate precursor of JH III (Laufer et al. 1987). In crustaceans, MF may regulate juvenile characteristics in a manner similar to that of JH in insects. (Burtenshaw et al. 2008). Farnesoic acid Omethyltransferase (FAMeT) is the enzyme that catalyzes the methylation of farnesoic acid (FA) to MF with the cofactor Sadenosyl-L-methionine (SAM) in crustaceans (Holford et al. 2004). The first study of FAMeT was in the crustacean Metapenaeus ensis, the sand shrimp (Gunawardene et al. 2001; 2002). The catalytic activity of FAMeT is thought to occur at a rate-limiting step in the JH biosynthetic pathway although its exact role is yet to be defined (Williamson et al. 2001).
Recently, the first putative FAMeT (CG10527) in insects was isolated from Drosophila melanogaster, although no detectable activity was found in the recombinant FAMeT expressed in a bacterial system (Burtenshaw et al. 2008). The orthologous sequences of D. melanogaster FAMeT are also found in other insect species, such as Aedes aegypti (XP001658262.1), Anopheles gambiae (XP_318631.4), Apis mellifera (XP_ 623146.1), Melipona scutellaris (CAM 35482.1), Nasonia vitripennis (XP_00 1599775.1) and Tribolium castaneum (XP974395.1). Here, the cloning of full-length cDNA encoding a putative FAMeT gene (NlFAMeT) is reported from the brown planthopper, Nilaparvata lugens (Stal) (Hemiptera: Geometroidea), a major rice pest in many parts of Asia, which causes a big loss in rice production in recent years. The tissue and developmental expressions of NlFAMeT were also included.
Materials and Methods
Experimental insects
Insects, N. lugens, were kept in laboratory cages at 25 ± 1° C, 70–80% RH, and a 16:8 L:D photoperiod. The developmental stages were synchronized at each larval molt. Corpora allata (CA), brain (without CA), fat body, ovary, accessory glands and midgut tissues were dissected from macropterous (long-winged) females (5th instar or adults) in phosphate buffered saline (PBS) treated with 0.1% diethylpyrocarbonate (DEPC) and stored at -70° C prior to use.
Amplification of a putative FAMeT cDNA
When this work was started, the EST database of N. lugens (http://bphest.dna.affrc.go.jp/) was not available, so the RT-PCR technique with degenerate primers was used to clone the initial fragment. Total RNA was isolated by Trizol kit (Invitrogen, www.invitrogen.com). Synthesis of firststrand cDNAs was carried out according to the reverse transcriptase XL (AMV) (Takara, www.takara-bio.co.jp) protocol with oligo dT18. The first strand cDNA (1 µl) was used as a template for PCR. Degenerate primers, BP1 (GGNGTNT GYACNGGNATGGGNGC) and BP2 (CC NCCRTANGGDATRTARCANAC), were designed from the conserved regions of insect FAMeTs, which were GVCTGWGA and VCYIPYGG, respectively. The components of PCR were PCR reaction buffer containing 0.1 mM dNTP, 5 µM each primer, and 1.0 unit of Ex-Taq DNA polymerase (Promega, www.promega.com) in a total volume of 20 µl. The thermal cycling condition was 95° C for 5 min followed by 35 cycles of 94° C for 45 s, 50° C for 1 min and 72° C for 1 min. The last cycle was followed by final extension at 72° C for 10 min. The amplified product was separated onto agarose gel and purified using the Wizard PCR Preps DAN Purification System (Promega). Purified DNA was ligated into the pGEM-T easy vector (Promega) and several independent subclones were sequenced from both directions. The sequence of the product was compared using Blast of the EST database of N. lugens (http://bphest.dna.affrc.go.jp/) and one clone (CNLHT2882) was found similar to FAMeT protein. The full-length cDNA was obtained by the RACE (rapid amplification of cDNA ends) technique according to the Smart Race kit (Clontech, www.clontech.com) protocol with genespecific primers (GSPs) for 5′-RACE (GCCACTGGAACCCAGGTAGCAG) and 3′-RACE (GGCAAAGTGGTGCCTTCGCATGG). In order to find out whether the sequence of C_NLHT2882 clone includes the complete 5′UTR (untranslated region), 5′-RACE was also carried out to identify the 5′-terminal region of the putative FAMeT gene.
Quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR)
The mRNA levels were measured by qRT-PCR using the One Step SYBR PrimeScript RT-PCR Kit (Takara). Total RNAs were treated by DNase I (Sigma-Aldrich, www.sigmaaldrich.com). qRT-PCR was performed in a 25 µl total reaction volume containing 5 ng of total RNA, 0.5 µl primer mix containing 10 µM each of forward and reverse gene specific primers, 0.5 µl of Ex TaqTM HS (5 U/µl), 0.5 µl of PrimeScript RT Enzyme Mix, 12.5 µl of 2×One Step SYBR RT-PCR Buffer and 8.5 µl of H2O. Two types of negative controls were set up: non-template reactions (replacing total RNA by H2O) and minus reverse transcriptase controls (replacing PrimeScript RT Enzyme Mix by H2O). The qRT-PCR was done with the following cycling regime: initial incubation of 42° C for 5 min and 95° C for 10 s; 40 cycles of 95° C for 5 s, 60° C for 20 s, and 72 °C for 15 s. Standard curves were obtained using a ten-fold serial dilution of pooled total RNAs from 20 individuals. β-actin (EU179846) was used as an internal control (Liu et al. 2008). The mRNA expression was quantified in relation to the expression of β-actin. The primer pair of each gene was designed to amplify about 200 bp PCR products, which were verified by nucleotide sequencing. Only data that showed good efficiency (≥85%) and correlation coefficient (≥95%) were included in the analysis. Means and standard errors for each time point were obtained from the average of three independent sample sets. Gene specific primers for FAMeT and βactin were listed as: FAMeT-F: GCAAAGTCAGCAATCCGCAAGAAC; FAMeT-R: ACACCGTAGTGGGTGACAACGAATG; β-F: TGGACTTCGAGCAGGAAATGG; β-R: ACGTCGCACTTCATGATCGAG.
JH titer determination
JH titers were determined as previously described (Liu et al. 2008). The GC-MS system consisted of a Hewlett Packard HP6890 series II gas Chromatograph and a mass selective detector (model 5973MS). 10 mg (about 6 individuals of the 5th instar female) N. lugens whole bodies were dried in Modulyod-230 Freeze Dryer (Thermo Electron) and were homogenized in 0.5 ml Hexane. The contents were centrifuged at 13,000 × g and 4° C for 10 min. The supernatant was dried using a stream of N2 gas and diluted to 25 µl in hexane. GC operating conditions: column HP-5, 25 m × 0.2 mm (length × diameter), film thickness 0.2 µm; column temperature programmed from 120° C (isothermal for 2 minutes) to 230° C (15° C/min); carrier gas helium, flow rate 40 ml/min; injector temperature 250° C; volume injected 1 µl. The standard JH III was purchased from Sigma-Aldrich. The overall mean for the standard JH III sample prepared at 150.0 ng/ml was 148.6 ng/ml (N = 35) with a standard deviation of 4.59 and a coefficient of variation (CV) of 2.4%. The reliability of the GC-MS methods was demonstrated by the coefficient of variation (CV ≤ 3.2%) on the standard JH III prepared at different concentrations (50–300 ng/ml).
Statistical analysis
Differences in mRNA levels or JH titers were analyzed by one-way ANOVA with at least three repeats. In all tests, 10–12 mg whole body weight was used, which included about 5–6 individuals of female adult, 6–8 individuals of the fifth instar female, or 10–12 individuals of the fourth instar larvae. For larvae under the fourth instar, larvae were pooled according to 1012 mg weight. Three repeats mean that at least three pools were used. Differences between treatment means were analysed using a least significant digit pair-wise comparison of means. The level of significance for results was set at p < 0.05.
Results
NlFAMeT cDNA
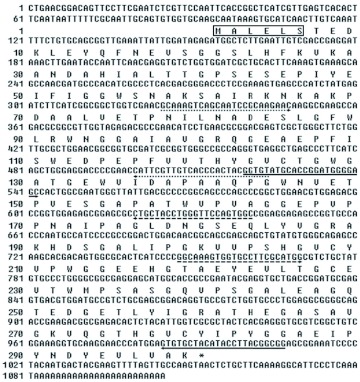
RT-PCR and RACE techniques were used to clone the full-size N. lugens FAMeT (NlFAMeT) cDNA. Figure 1 shows the fulllength cDNA sequence with the deduced amino acid sequence above the nucleotide sequence (Genbank accession number, FJ028722). The sequence has an open reading frame (ORF) of 897 bp and 299 deduced amino acids. The deduced protein sequence of NlFAMeT showed 52%–54% identities to FAMeTs from A. aegypti (XP_001658262.1), A. mellifera (XP_623146.1), and D. melanogaster (NP_611544.1). NlFAMeT also showed 32% identity at the amino acid level to M. ensis FAMeT (AAK28535.1), the first studied FAMeT from crustaceans (Figure 2).
Figure 1.
Nucleotide and deduced amino acid sequence of NlFAMeT. The positions of the primers used in the initial degenerate RT-PCR are shown by single lines with direction arrows. The primers used in rapid amplification of cDNA ends (RACE) are shown by the dashed lines with direction arrows. The primers used in quantitative real-time reverse transcriptase polymerase chain reaction (qRT-PCR) are shown by the dotted lines with direction arrows. A putative SANT domain profile is boxed. The stop codon is indicated by an asterisk (*). High quality figures are available online.
Figure 2.
The alignment of amino acid sequence of NlFAMeT with the sequences of other insect FAMeTs. Numbers on the right side of the alignment indicate the position of residues in the sequence of each protein. Identical amino acids are indicated by asterisks (*). The amino acids that are identical in four insect FAMeTs, but not in M. ensis FAMeT, are indicated by plus signs (+). A putative SANT domain profile is underlined. MeFAMeT (Metapenaeus ensis, AAK28535.1), AaFAMeT (Aedes aegypti, XP_001658262.1), AmFAMeT (Apis mellifera, XP623146.1), DmFAMeT (Drosophila melanogaster, NP_6111544.1) and NlFAMeT (Nilavarpata lugens, FJ028722) are used in the alignment. High quality figures are available online.
Tissue expression of NlFAMeT mRNA
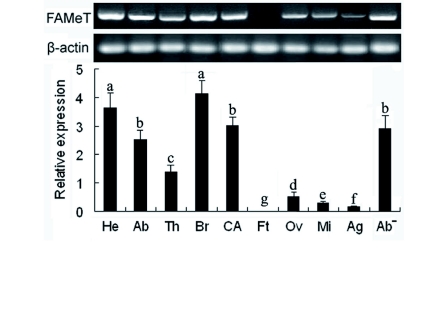
The NlFAMeT mRNA levels of different parts (head, thorax, and abdomen) and tissues (CA, brain, fatbody, ovary, accessory glands, and midgut) of macropterous females (1 day old) were determined by qRT-PCR (Figure 3). The head part showed the highest expression of NlFAMeT among three parts tested. Among all tissues tested, the brain (without CA) showed the highest expression level, and CA also showed very high expression. Low levels were detected in the ovary, midgut and accessory glands, and no trace was found in the fatbody. When the tissues, fatbody, ovary, and midgut were removed from the abdomen, the remaining parts (Ab ) also showed a high expression level of NlFAMeT, which indicated one or some tissues other than fatbody, ovary and midgut contained high levels of NlFAMeT mRNA.
Figure 3.
Tissue expression of the NlFAMeT mRNA in macropterous females detected by the quantitative real-time RT-PCR. (Above) The gel picture of qRT-PCR. The band for FAMet is 200 bp in length, and that for β-actin is 199 bp. (Below) The relative expression levels of NlFAMeT mRNA in different tissues. He, head (with CA); Ab, abdomen; Th, thorax; CA, corpus allatum; Br, brain (without CA); Ft, fatbody; Ov, ovary; Mi, midgut; Ag, accessory glands; Ab-, abdomen without Ft, Ov and Mi tissues. The data represent mean values ± SE of at least three repeats, normalized relative to β-actin transcript levels. Different lowercase letters above the columns indicate the significant differences at the p < 0.05 level. High quality figures are available online.
Developmental expression of NlFAMeT mRNA
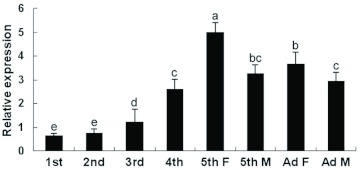
The NlFAMeT mRNA levels of the head parts from larvae and macropterous adults were also determined (Figure 4). The samples were used from 1-day-old larvae in each instar and 1-day-old adults. In larvae, from 1st instar to 5th instar, the expression levels increased gradually and reached the peak in the 5th instar. Both in the 5th instar and in adults, the expression levels in female insects were significantly higher than those in male insects. In female insects, but not male insects, the expression level in the 5th instar larvae was obviously higher than that in adults.
Figure 4.
Developmental expression of the NlFAMeT mRNA in head parts of Nilavarpata lugens. 1 st, 1 st instar larvae; 2nd, 2nd instar larvae; 3rd, 3rd instar larvae; 4th, 4th instar larvae; 5th F, 5th instar female larvae; 5th M, 5th instar male larvae; Ad F, female adults (1 day old); Ad M, male adults (1 day old). The data represent mean values ± SE of at least three repeats. Different lowercase letters above the columns indicate the significant differences at the p < 0.05 level. High quality figures are available online.
NlFAMeT mRNA levels and JH titers in 5th instar larvae
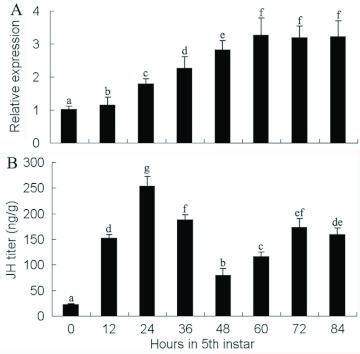
In order to evaluate the relationship between NlFAMeT mRNA levels and JH titers in 5th instar larvae (macropterous female), NlFAMeT expression levels in CA and JH titers were determined with a 12 h interval. In the 5th instar macropterous female larvae, NlFAMeT mRNA levels increased gradually from its ecdysis and reached the peak at 60 h, which kept with little variation until the adult emergence (Figure 5A). The peak of JH titers existed at 24 h after the ecdysis of the 5th instar macropterous female larvae and had two bottom expressions at 0 h and 48 h (Figure 5B). These results showed that NlFAMeT mRNA levels and JH titers had no correlation in the 5th instar macropterous female larvae.
Figure 5.
The dynamics of the relative NlFAMeT transcript levels (A) in corpus allatum (CA) and JH titers (B) in 5th instar macropterous females. The data represent mean values ± SE of at least three repeats. Different lowercase letters above the columns indicate the significant differences at the p < 0.05 level. High quality figures are available online.
NlFAMeT mRNA levels at different stages of ovarian development
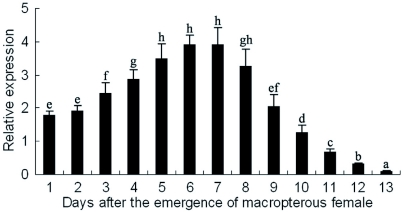
In macropterous females of N. lugens, the vitellogenesis begins at the 2nd day of the emergency, peaks at the 7th day and terminates at the 14th day (Yi 2005). During this period, NlFAMeT mRNA levels of Ab- samples from macropterous females were determined each day. As shown in Figure 6, NlFAMeT mRNA levels increased gradually from the emergence of macropterous females and peaked at the 6th and 7th days, after which the expression levels decreased quickly and reached at a very low level at the 13th day. These results showed that the changes of NlFAMeT mRNA levels were in accordance with the vitellogenesis period.
Figure 6.
The dynamics of the relative NlFAMeT transcript levels in Ab- samples from macropterous female adults. The data represent mean values ± SE of at least three repeats. Different lowercase letters above the columns indicate the significant differences at the p < 0.05 level. High quality figures are available online.
Discussion
Farnesoic acid O-methyltransferase (FAMeT) is the enzyme that catalyses the final step in the MF biosynthetic pathway in crustaceans (Wainwright et al. 1998; Wang et al. 1994). FAMeT was also present in the insect corpora allata. Variations in activity of the O-methyltransferase during development appear to be an important component in the regulation of JH biosynthesis in insects (Gunawardene et al. 2002). FAMeT is thought to catalyze the conversion of FA to MF using SAM as a cofactor. While this enzyme has been extensively studied in crustaceans, there is little known about its role in insects.
By RT-PCR and RACE techniques, a fulllength cDNA coding a putative FAMeT (NlFAMeT) was cloned from N. lugens. The deduced protein sequence showed high identities to other insect FAMeTs, which suggested that the NlFAMeT cDNA encodes a FAMeT or FAMeT-like protein. Although FAMeT is thought to catalyze the reaction using a SAM cofactor, the sequence in all organisms examined lacked the typical SAM binding motif, including M. ensis FAMeT (Figure 2), which is currently the only FAMeT that displays the activity in vitro (Gunawardene et al. 2002), suggesting FAMeT from insects, as well as from crustaceans, may be a novel SAM independent methyltransferase (Burtenshaw et al. 2008). A putative SANT (switching-defective protein 3, adaptor 2, nuclear receptor corepressor, and transcription factor IIIB) profile is present in the N-terminal region of D. melanogaster FAMeT, suggesting that protein-protein interactions might be required for functional structure and activity of insect FAMeT proteins (Figure 2). The recombinant FAMeT (rFAMeT) from D. melanogaster was expressed and purified in a bacterial system, but no activity was detected either with the rFAMeT alone or when added to a CA extract (Burtenshaw et al. 2008). In the laboratory, the heterologous expression of NlFAMeT in a bacterial system had been tried, but no positive results were obtained; so those expression data were not included in this report.
Although the first insect FAMeT was been isolated and characterized in D. melanogaster, the tissue expression of insect FAMeT mRNA is little known. Here, NlFAMeT was shown to be highly expressed in the head and abdomen, and the peak level was detected in the brain and CA, which indicated that the head and abdomen were the main sources of NlFAMeT transcript. In D. melanogaster, CA portion of the ring gland was identified as the important tissue highly expressing FAMeT (Burtenshaw et al. 2008). In the cockroach, Diploptera punctata, the rate limiting step controlling JH III biosynthesis in CA is catalyzed by FAMeT (Yagi et al. 1991). These results indicated CA is the main tissue expressing insect FAMeT transcript. Although the relatively high level of NlFAMeT transcript was detected in the abdomen, all tissues dissected from the abdomen only showed low levels. When these tissues were removed from the abdomen, the remaining part (Ab-) still showed a high level of NlFAMeT transcript, which indicated one or some unknown tissues contain high levels of NlFAMeT mRNA. Because of the insect's size, it is difficult to dissect more tissues completely from the abdomen, and the tissues leading to high levels of NlFAMeT transcript in the abdomen have not been found at present.
One key event is the clearing of JH that generally precedes the moult from the last larval stage to the pupal stage of holometabolous insects (Campbell et al. 2001). The very low JH titer at this time is generally achieved by the combined effect of reduced JH synthesis and scavenging by JH degrading enzymes (Roe and Venkatesh 1990). In N. lugens, with incomplete metamorphosis, the low JH titer is identified in the late stage of final instar (5th instar) (Dai et al. 2001). Some important enzymes are found to play key roles in the synthesis and regulation of JH (Bellés et al. 2005). To achieve the low JH titer in final instar, the key enzymes in the synthesis of JH will be lowly expressed and enzymes in the degradation of JH highly expressed, such as JH methyltransferase and JH esterase (Bellés et al. 2005; Dai et al. 2001). In insect species from Lepidoptera, the last steps include farnesoic acid synthesis by an aldehyde dehydrogenase, the conversion of FA to active JH (JH III) by C-10,11 epoxidation by a P450 monooxygenase and methylation of the carboxyl group by an S-adenosyl-L-methionine (SAM)-dependent methyltransferase (MTase). In species from Orthoptera and Dictyoptera, the last key steps include the methylation of FA to MF and the epoxidation of MF to active JH (Bellés et al. 2005; Shinoda and Itoyama 2003). Here, NlFAMeT transcript was highly expressed in the 5th instar larvae of N. lugens, which gradually increased from its ecdysis and kept at peak at its later stages (60–84 h). However, JH titers had the peak at 24 h and showed different changes during the 5th instar, compared to that of NlFAMeT mRNA levels. These results indicated FAMeT might not be a key enzyme in the synthesis of JH in this insect species, at least not in the 5th instar larvae.
Although NlFAMeT transcript level was low in the ovary, a high level was detected in some undefined tissues in the abdomen. And NlFAMeT transcript levels increased when then vitellogenesis began at the 2nd day and peaked at 6th–7th day after the emergence of macropterous females, which also was the peak of vitellogenesis. After the peak of vitellogenesis, NlFAMeT transcript levels decreased and reached a very low level when vitellogenesis terminated on the 13th day. These data indicated NlFAMeT might play important roles in the development of the insect ovary. In the shrimp, M. ensis, FAMeT mRNA is expressed throughout ovarian maturation in the nerve and eyestalk, suggesting a possible role of FAMeT in the regulation of reproduction (Gunawardene et al. 2002).
Acknowledgments
This work was supported by Special Fund for Basic Expenditure for Scientific & Research of Central Non-profit Scientific Research Institutions (2009RG004-2) and National S&T Major Project (2009 ZX08011-009B).
Abbreviations
- FAMeT
farnesoic acid O-methyltransferase;
- FA
farnesoic acid;
- MF
methyl farnesoate;
- CA
corpus allatum;
- JH
juvenile hormone;
- SAM
Sadenosyl-L-methionine;
- FBP
farnesyl biphosphate;
- PCR
polymerase chain reaction;
- qRT-PCR
Quantitative real-time reverse transcriptase polymerase chain reaction;
- RACE
rapid amplification of cDNA ends
References
- Bellés X, Martin D, Piulachs MD. The mevalonate pathway and the synthesis of juvenile hormone in insects. Annual Review of Entomology. 2005;50:181–99. doi: 10.1146/annurev.ento.50.071803.130356. [DOI] [PubMed] [Google Scholar]
- Burtenshaw SM, Su PP, Zhang JR, Tobe SS, Dayton L, Bendena WG. A putative farnesoic acid O-methyltransferase (FAMeT) orthologue in Drosophila melanogaster (CG10527): Relationship to juvenile hormone biosynthesis? Peptides. 2008;29:242–251. doi: 10.1016/j.peptides.2007.10.030. [DOI] [PubMed] [Google Scholar]
- Campbell PM, Harcourt RL, Crone EJ, Claudianos C, Hammock BD, Russell RJ, Oakeshott JG. Identification of a juvenile hormone esterase gene by matching its peptide mass fingerprint with a sequence from the Drosophila genome project. Insect Biochemistry and Molecular Biology. 2001;31:513–520. doi: 10.1016/s0965-1748(01)00035-2. [DOI] [PubMed] [Google Scholar]
- Dai H, Wu X, Wu S. The change of juvenile hormone titer and its relation with wing dimorphism of brown planthopper, Nilaparvata lugens. Acta Entomologica Sinica. 2001;44:27–32. [Google Scholar]
- Holford KC, Edwards KA, Bendena WG, Tobe SS, Wang Z, Borst DW. Purification and characterization of a mandibular organ protein from the American lobster, Homarus americanus: A putative farnesoic acid O-methyltransferase. Insect Biochemistry and Molecular Biology. 2004;34:785–798. doi: 10.1016/j.ibmb.2004.04.003. [DOI] [PubMed] [Google Scholar]
- Laufer H, Landau M, Homola E, Borst DW. Methyl farnesoate: Its site of synthesis and regulation of secretion in a juvenile crustacean. Insect Biochemistry. 1987;17:1129–1131. [Google Scholar]
- Liu S, Yang B, Gu J, Yao X, Zhang Y, Song F, Liu Z. Molecular cloning and characterization of a juvenile hormone esterase gene from brown planthopper, Nilaparvata lugens. Journal of Insect Physiology. 2008;54:1495–1502. doi: 10.1016/j.jinsphys.2008.08.010. [DOI] [PubMed] [Google Scholar]
- Riddiford LM, Hiruma K, Zhou X, Nelson CA. Insights into the molecular basis of the hormonal control of molting and metamorphosis from Manduca sexta and Drosophila melanogaster. Insect Biochemistry and Molecular Biology. 2003;33:1327–1338. doi: 10.1016/j.ibmb.2003.06.001. [DOI] [PubMed] [Google Scholar]
- Roe RM, Venkatesh K. Metabolism of juvenile hormones: Degradation and titer regulation. In: Gupta AP, editor. Morphogenic Hormones of Arthropods. Vol. 1. Rutgers University Press; 1990. pp. 125–180. [Google Scholar]
- Shinoda T, Itoyama K. Juvenile hormone acid methyltransferase: A key regulatory enzyme for insect metamorphosis. Proceedings of the National Academy of Sciences of the United States of America. 2003;100:11986–11991. doi: 10.1073/pnas.2134232100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Silva Gunawardene YIN, Chow BKC, He JG, Chan SM. The shrimp FAMeT cDNA is encoded for a putative enzyme involved in the methylfarnesoate (MF) biosynthetic pathway and is temporally expressed in the eyestalk of different sexes. Insect Biochemistry and Molecular Biology. 2001;31:1115–1124. doi: 10.1016/s0965-1748(01)00060-1. [DOI] [PubMed] [Google Scholar]
- Silva Gunawardene YIN, Tobe SS, Bendena WG, Chow BKC, Yagi KJ, Chan SM. Function and cellular localization of farnesoic acid Omethyltransferase (FAMeT) in the shrimp, Metapenaeus ensis. European Journal of Biochemistry. 2002;269:3587–3595. doi: 10.1046/j.1432-1033.2002.03048.x. [DOI] [PubMed] [Google Scholar]
- Stay B. A review of the role of neurosecretion in the control of juvenile hormone synthesis: A tribute to Berta Scharrer. Insect Biochemistry and Molecular Biology. 2000;30:653–662. doi: 10.1016/s0965-1748(00)00036-9. [DOI] [PubMed] [Google Scholar]
- Wainwright G, Webster SG, Rees HH. Neuropeptide regulation of biosynthesis of the juvenoid, methyl farnesoate, in the edible crab, Cancer pagurus. Biochemical Journal. 1998;334:651–657. doi: 10.1042/bj3340651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang Z, Ding Q, Yagi KJ, Tobe SS. Terminal stages in juvenile hormone biosynthesis in Corpora Allata of Diploptera punctata: Development changes in enzyme activity and regulation by allatostatins. Journal of Insect Physiology. 1994;40:217–223. [Google Scholar]
- Williamson M, Lenz C, Winther AM, Nassel DR, Grimmelikhuijzen CJ. Molecular cloning, genomic organization, and expression of a B-type (cricket-type) allatostatin preprohormone from Drosophila melanogaster. Biochemical and Biophysical Research Communications. 2001;281:544–550. doi: 10.1006/bbrc.2001.4402. [DOI] [PubMed] [Google Scholar]
- Yagi KJ, Konz KG, Stay B, Tobe SS. Production and utilization of farnesoic acid in the juvenile hormone biosynthetic pathway by corpora allata of larval Diploptera punctata. General and Comparative Endocrinology. 1991;81:284–294. doi: 10.1016/0016-6480(91)90013-v. [DOI] [PubMed] [Google Scholar]
- Yi W. Characterization of vitellin and effectation of high temperature on its vitellogenesis in brown planthopper, Nilaparvata lugens (Stål). MS thesis, Nanjing Agricultural University; Nanjing, China: 2005. [Google Scholar]