Abstract
Introduction
Human adipose-derived stromal cells (hASCs) possess a great potential for tissue engineering purposes. Our laboratory is interested in harnessing hASCs for skeletal tissue regeneration and identifying those factors that enhance hASC osteogenic differentiation. We hypothesized that Insulin-Like Growth Factor (IGF) and Platelet Derived Growth Factor (PDGF) would stimulate hASC osteogenesis and that IGF would stimulate adipogenesis.
Materials and Methods
ASCs were harvested from human lipoaspirate. Previously, a microarray analysis examined gene expression throughout osteogenic differentiation. In a candidate fashion we added recombinant Insulin-Like Growth Factor (IGF-1) and Platelet-Derived Growth Factor (PDGF)-α individually as well as in combination. Osteogenesis and adipogenesis were assessed by alkaline phosphatase, Alizarin red, and Oil red O staining, as well as qRT-PCR (RUNX2, ALP, OCN, IGF1, PPARG, LPL, AP2, GCP1). Finally, intersection between IGF and PDGF signaling pathways was evaluated.
Results
IGF-1 was observed to increase osteogenic differentiation by all markers (*p<0.01). However, PDGF-α when added alone primarily did not effect osteogenic markers. PDGF-α positively regulated transcription of IGF1. Addition of PDGF-α in combination with or prior to IGF-1 enhanced osteogenesis more than either alone. IGF-1 increased while PDGF-α diminished hASC adipogenesis.
Conclusion
IGF signaling significantly increased osteogenesis in human ASCs and may be used for tissue engineering purposes. The combination of PDGF and IGF may be more beneficial than either alone in driving ASC osteogenesis. Future in vivo applications will focus on the combination of ASCs, biomimetic scaffolds and recombinant IGF.
Keywords: Adipose derived stromal cells, Skeletal tissue engineering, Tissue regeneration, Multipotent stromal cells, IGF, PDGF
Introduction
Skeletal reconstruction remains a significant challenge in patients after trauma, surgery, degenerative bone diseases and congenital anomalies. Furthermore, with an aging population, and the challenges of arthritis and osteopenia increasing, we will face a growing need to improve our treatment of skeletal diseases. Though autogenous (1) and alloplastic materials can be used for skeletal reconstruction, we believe that skeletal tissue engineering using a mulitpotent cell on a biomimetic scaffold would greatly improve our treatment options. Human adipose-derived stromal cells (hASCs) represent an easily accessible, multipotent cell source with great potential for tissue engineering purposes (2-4). Our laboratory is particularly interested in harnessing ASCs for skeletal tissue regeneration to heal long bone and calvarial defects (5, 6). Improved usage of ASCs for tissue engineering hinges on a more thorough understanding of how cytokines can influence the lineage-specific differentiation of ASCs.
In an effort to more thoroughly understand the molecular underpinnings of ASC osteogenesis, we previously performed a microarray analysis of hASCs at stratified timepoints throughout in vitro osteogenesis (7). Among other observations, we found that individual components of the Insulin-like Growth Factor (IGF) and Platelet-Derived Growth Factor (PDGF) signaling pathways to be up-regulated during the process of ASC osteogenic differentiation, including IGFBP-3 and PDGFRB. We hypothesized that not only were IGF and PDGF signaling associated with hASC osteogenesis, but that their up-regulation via exogenous recombinant protein may in fact drive ASC osteogenic differentiation.
Previous in vitro studies utilizing calvarial and long bone-derived osteoblasts have demonstrated key proteins and signaling pathways that enhance osteogenic differentiation (8-10). Among these, the effects of both IGF and PDGF signaling pathways have been studied in the context of osteoblasts and bone marrow mesenchymal cells; in both, they have been shown to stimulate osteogenesis (11, 12). Similarly, IGF-1 is a well-known promoter of adipogenic differentiation, however, its effect on hASCs is not well documented. Human ASCs represent a more available and easily harvested source of cells than either osteoblasts or bone marrow mesenchymal cells, and thus offer an attractive alternative for autologous skeletal regeneration (3). This study sought to determine the effects of these two growth factors on the differentiation of hASCs toward osteogenic and adipogenic lineages.
Materials and Methods
Institutional review board approval was obtained for human specimen collection. Primary hASCs were isolated from liposuction specimens from five female patients under the age of 50 as previously described (13). ASCs were pooled from all patients in equal numbers for all assays. All liposuction was derived from the flank, abdomen and thigh regions. Passage one and two cells only were used for all experiments.
Osteogenic Induction and Assessments
Cells were seeded onto 6-well plates at a density of 100,000 cells per well. After attachment, cells were treated with osteogenic differentiation media (ODM) (Dulbecco’s Modified Eagle Medium, 10% fetal bovine serum, 100 μg/ml ascorbic acid, 10 mM β-glycerophosphate). ODM was supplemented with rIGF-1 (10 or 20 ng/ml), rPDGFα (10 or 20 ng/ml), or rBMP-2 (200 ng/ml, R&D Systems, Minneapolis, MN). Our control groups were supplemented with vehicle control (0.1% PBS). In addition, a positive control for osteogenic differentiation was included (ODM with recombinant human BMP-2 at 200 ng/ml). Medium was changed every 3 days. Alkaline phosphatase staining was performed at 3 days to assess early osteogenic differentiation. Alizarin red staining was performed at 1 week to assay bone nodule formation. RNA was harvested after 3 and 7 days of osteogenic differentiation to examine specific gene expression. In further experiments, cells were treated in growth media with or withour rPDGFα for 48 hours, prior to osteogenic differentiation with or without rIGF-1.
Alkaline Phosphatase and Alizarin Red Staining and Quantification
After 3 days of differentiation, cells were fixed with a 60% acetone and 40% citrate solution. After a brief wash with water, cells were stained with a diazonium salt solution composed of fast violet blue salt and 4% naphthol AS-MX phosphate alkaline solution. Alkaline phosphatase–positive cells were stained purple/red. Alkaline phosphatase enzymatic quantification was also performed as previously described (14), normalized to total protein content.
After 7 days of differentiation, alizarin red staining was performed to detect extracellular mineralization (15). Briefly, cells were fixed in 100% ethanol and stained with a 0.2% Alizarin red S solution (pH 6.4). The red staining represents calcium deposits on terminal differentiated cells. Matrix mineralization was quantified by extracting the alizarin red staining with a 0.1 M cetylpyridinium chloride solution. The absorbance was measured at 570 nm. Experiments were performed in triplicate wells.
Adipogenic Differentiation and Assessments
ASCs were next seeded in 12 plates at a density of 50,000 cells/well for assessment of adipogenesis (16). Adipogenic differentiation medium (ADM) containing 10 μg/ml insulin, 1 μM dexamethasone, 0.5 mM methylxanthine, and 200 μM indomethacin, with or without rIGF-1 (10 and 20 ng/ml), or rPDGFα (10 and 20 ng/ml), or vehicle as a control. At four days, medium was exchanged for 10 μg/ml insulin with or without recombinant proteins. Oil red O staining was performed after seven days of differentiation (16). Specific gene expression was examined after one week by quantitative real-time PCR, primers are given in Table 1.
TABLE 1.
Polymerase Chain Reaction Genes and Primer Sequences
| Gene Name | Forward primer sequence (5′ to 3′) | Reverse primer sequence (5′ to 3′) |
|---|---|---|
| ALP | ATGGGATGGGTGTCTCCACA | CCACGAAGGGGAACTTGTC |
| AP2 | CCAGGGACTTTGGGTACGTG | GGTTGAGAAATTCAGCTACTGCT |
| GAPDH | ATGGGGAAGGTGAAGGTCG | GGGGTCATTGATGGCAACAATA |
| GCP1 | GCTTTCTGGGTGGACTCAAGT | TCTAGTGTCTCTGTGAGGACTG |
| IGF1 | GGAGCTGTGATCTAAGGAGGC | GGGCTGATACTTCTGGGTCTT |
| LPL | TCATTCCCGGAGTAGCAGAGT | GGCCACAAGTTTTGGCACC |
| OCN | CTCCATTGACTCGAACGACTC | CAGGTCTGCGAAACTTCTTAGAT |
| PPARG | CCTATTGACCCAGAAAGCGATT | CATTACGGAGAGATCCACGGA |
| RUNX2 | ATTCCTGTAGATCCGAGCACC | GCTCACGTCGCTCATTTTGC |
RNA Isolation and Polymerase Chain Reaction
Total RNA was isolated from hASCs after 3 and 7 days of differentiation (17). Samples were treated with DNAse I (Ambion, Austin, Texas), and quantified using spectrophotometry. One microgram of total RNA was reverse transcribed (Applied Biosystems). Quantitative real-time polymerase chain reaction was carried out using the Applied Biosystems Prism 7900HT Sequence Detection System (Applied Biosystems, Foster City, Calif.). Specific primers for the genes examined were designed based on their PrimerBank (http://pga.mgh.harvard.edu/primerbank) sequence (Table 1). Quantitative RT-PCR was performed to examine the levels of gene expression, normalized to GAPDH expression levels.
Western blot
Protein detection was performed with primary antibodies against Runx2 (1:200 dilution, Santa Cruz Antibodies, Santa Cruz, CA), and β-actin (1:5000 dilution; Lab Vision, Fremont, CA) in 5% milk/Tris-buffered saline-T overnight at 4°C. Blots were then incubated with the corresponding horseradish peroxidase-linked secondary antibodies (1:10,000 dilution; BD Pharmingen, San Jose, CA) for 1 hr at room temperature. Blots were developed with ECL detection reagent (Amersham, United Kingdom) and exposed for 1 to 10 minutes using Biomax-MS film (Eastman Kodak, Rochester, NY).
Statistical Analysis
Statistical analysis was performed using the two-factor analysis of variance with replication and the two-tailed t test assuming unequal variances. Values of *p<0.01 were considered to be significant. No corrections were made for multiple testing.
Results
Effects of rIGF-1 and rPDGF-α on osteogenic differentiation of hASCs
Results from our previous microarray analysis revealed various soluble factors that increased during the osteogenic differentiation of human ASCs (7). These included components of the Transforming Growth Factor (TGF)-beta, IGF, PDGF, and Connective Tissue Growth Factor (CTGF) signaling pathways. Here, we chose to investigate the effects of two of these related signaling pathways in the osteogenic differentiation of hASCs in a candidate fashion: IGF and PDGF. To this end, rIGF-1 and rPDGF-α were added to ODM; standard osteogenic assays were performed.
Results showed that culture with rIGF-1 led to an increase in alkaline phosphatase activity at three days (blue bars, Fig. 1A). This was in contrast to rPDGF-α, which non-significantly diminished alkaline phosphatase activity (red bars, Fig. 1A). The osteogenic effect of IGF-1 was next compared to the well-studied growth factor BMP-2 (black bar, Fig. 1A). A single concentration of BMP-2 was utilized (200 ng/ml) which has been previously observed in our laboratory to maximally induce hASC osteogenesis. A similar induction of ALP activity was noted with either IGF-1 or rBMP-2 (compare blue and black bars, Fig. 1A). Next, alizarin red staining and quantification was performed at 7d differentiation (Fig. 1B,C). Human recombinant IGF-1 led to a significant increase in bone nodule formation after 7d in culture both by gross visualization and photometric quantification (blue bars and photographs, Fig. 1B,C). Recombinant PDGF-α alone had very little effect on terminal osteodifferentiation (i.e. bone nodule formation) (red bars, Fig. 1B,C). Finally, rBMP-2 was again utilized as a positive control. A similar induction of mineralization was observed with either rIGF-1 or rBMP-2 at concentrations used (compare blue and black bars, Fig. 1B).
Figure 1. Osteogenic differentiation with rIGF-1 or rPDGF-α.
(A) Quantification of alkaline phosphatase activity normalized to total protein content after 3d with rIGF-1, rPDGF-α, or rBMP-2. (B) Photometric quantification of Alizarin red staining after 7d with rIGF-1, rPDGF-α, or rBMP-2. (C) Alizarin red staining after 7d differentiation with rIGF-1, rPDGF-α, or rBMP-2, pictures shown at 10× magnification. N=3, *P<0.01.
Effects of rIGF-1 and rPDGF-α independently on osteogenic markers in hASCs
To verify the effects of these two cytokines on hASC osteogenic differentiation, osteogenic markers were assayed by quantitative RT-PCR and western blot (Fig. 2). We first evaluated the expression of the master transcription factor Runt-related transcription factor 2 (RUNX2) which has been shown to be necessary for in vitro osteogenic differentiation (18). Results showed that rIGF-1 increased RUNX2 expression in a dose-dependent manner after 7d differentiation, approximately 8- and 3-fold at 20 and 10ng/ml, respectively 6Fig. 2A). This was verified at the protein level with western blot (Fig. 2C). In contrast, the addition of rPDGF-α had no significant effect on RUNX2 expression (Fig. 2B). Next, we examined expression of the extracellular matrix component Osteocalcin (OCN), generally considered a marker of more terminal osteogenic differentiation. Similar to RUNX2, rIGF-1 increased OCN expression, approximately 6- and 2- fold at 20 and 10ng/ml, respectively (Fig. 2D). In contrast, rPDGF-α was observed to have slightly divergent effects based on concentration. At a concentration of 10ng/ml, OCN was decreased while at 20ng/ml OCN was significantly increased (Fig. 2E).
Figure 2. Osteogenic marker expression with rIGF-1 and rPDGF-α.
(A) RUNX2 expression after rIGF-1 addition to ODM after 7d differentiation. (B) RUNX2 expression after rPDGF-α addition to ODM after 7d differentiation. (C) RUNX2 protein expression with or without rIGF-1, bands presented correspond to RUNX2 above and β-ACTIN below. (D) OCN expression after rIGF-1 addition to ODM. (E) OCN expression after rPDGF-α addition to ODM. N=3, *P<0.01.
Effects of rIGF-1 and rPDGF-α on gene expression of the IGF pathway
Recombinant IGF-1 appeared to have a greater effect on osteogenesis than rPDGF-α in hASCs. We next sought to determine if exogenous addition of either cytokine led to a reciprocal effect on endogenous production of members of the other pathway. To this end various gene expression was examined by qRT-PCR (IGF1 and PDGFA). Results showed that both rIGF-1 or rPDGF-α significantly upregulated IGF1 gene expression when added independently at seven days (Fig. 3). Conversely, we were not able to appreciate any effect of rIGF-1 on PDGF-α expression (data not shown).
Figure 3. Potential Interaction between rIGF-1 and rPDGF-α.
(A) IGF1 expression after 7d rIGF-1 treatment, by quantitative RT-PCR. (B) IGF1 expression after 7d rPDGF-α treatment. N=3, *P<0.01.
Effects of the combination of rIGF-1 and rPDGF-α on osteogenic differentiation in hASCs
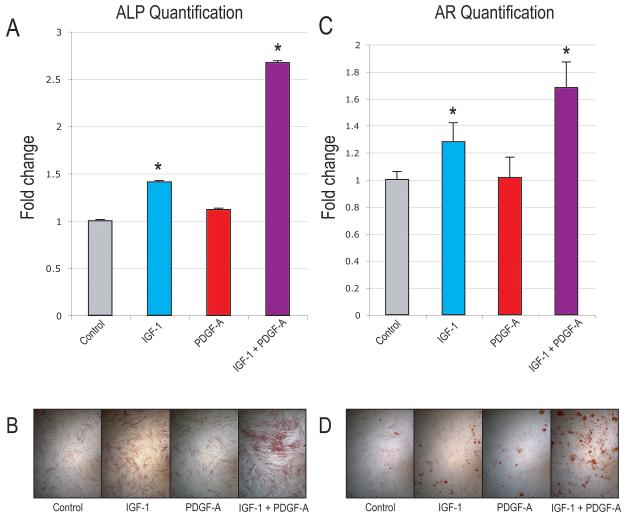
Here, we chose to investigate the additive effect of rIGF-1 and rPDGF-α on the osteogenic potential of hASCs. To assess their effect when used together, rIGF-1 and rPDGF-α were added to ODM, standard osteogenic assays were performed comparing the addition of each cytokine separately to adding each cytokine in combination. Results showed that culture of rIGF-1 alone led to significant increase in alkaline phosphatase activity as expected (Fig. 4A). However, culture of rIGF-1 with rPDGF-α led to a significantly higher increase in ALP activity than either alone. These results were also observed visually with staining for alkaline phosphatase (Fig. 4B). Similar results were observed for Alizarin red staining: the combination of rIGF-1 and rPDGF-α led to an increase in bone nodule formation over either cytokine alone (Fig. 4C,D). Thus, rPDGF-α was observed to significantly augment the pro-osteogenic effect of rIGF-1, despite it having little effect on osteogenesis when added alone.
Figure 4. Osteogenic differentiation with rIGF-1 and rPDGF-α alone and combined.
(A) Quantification of alkaline phosphatase activity normalized to total protein content after 3d with rIGF-1 and rPDGF-α alone or in combination. (B) Alkaline phosphatase staining after 3d with rIGF-1 and rPDGF-α alone or in combination. (C) Photometric quantification of Alizarin red staining. Pictures are of 4× magnification, N=3, *P<0.01. (D) Alizarin red staining after 7d differentiation with rIGF-1 and rPDGF-α alone or in combination.
Effects of rPDGF-α pretreatment on rIGF-1 induced osteogenic differentiation in hASCs
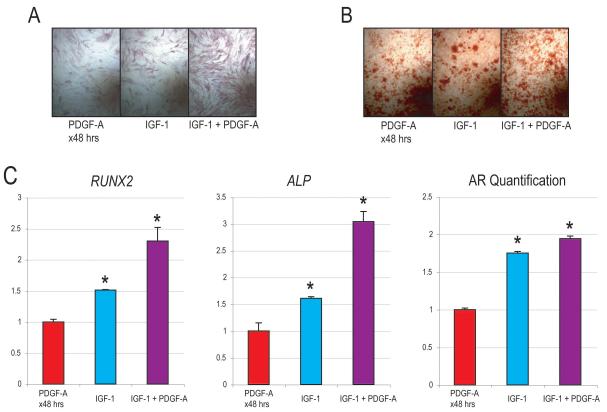
The combination of rIGF-1 and rPDGF-α seemed to induce osteogenic differentiation more than either when added to ODM alone (Fig. 4). Would pretreatment or ‘priming’ of hASCs with rPDGF-α followed by differentiation with IGF-1 have a similar effect? To answer this, hASCs were treated for 48hrs with rPDGF-α before differentiation with or without rIGF-1 (Fig. 5). Results showed as before that continuous addition of rIGF-1 (20 ng/ml) to ODM enhanced osteogenesis over baseline: observed by both alkaline phosphatase (3d) and alizarin red staining (7d) (compare Fig. 5A,B to Fig4B,D). A short pulsation of rPDGF-α (10 ng/ml × 48hrs) showed a similar enhancement of osteogenesis (compare Fig. 5A,B to Fig4B,D). Strikingly, rPDGF-α pulstation followed by continuous rIGF-1 treatment during differentiation had an optimal effect on driving hASC osteodifferentiation (Fig. 5A, B). This observation was verified by quantitative RT-PCR for RUNX2 and ALP (Fig 5C). Thus by all markers, it appeared that rPDGF-α ‘priming’ followed by rIGF-1 treatment led to a significant and appreciable inducation of hASC osteogenic differentiation.
Figure 5. Osteogenic differentiation with rIGF-1 following rPDGF-α pulsation.
Osteogenic differeniation was performed after 48 hrs treatment with or without rPDGF-α (10 ng/ml). Next, standard osteogenic assays were performed with or without rIGF-1 (20 ng/ml). (A) Alkaline phosphatase staining at 3d differentiation. (B) Alizarin red staining after 7d differentiation. (C, left) RUNX2 and ALP gene expression at 3d. (C, right) Alizarin red (AR) quantification. N=3, *P<0.01.
Effects of rIGF-1 or rPDGF-α on adipogenic differentiation in hASCs
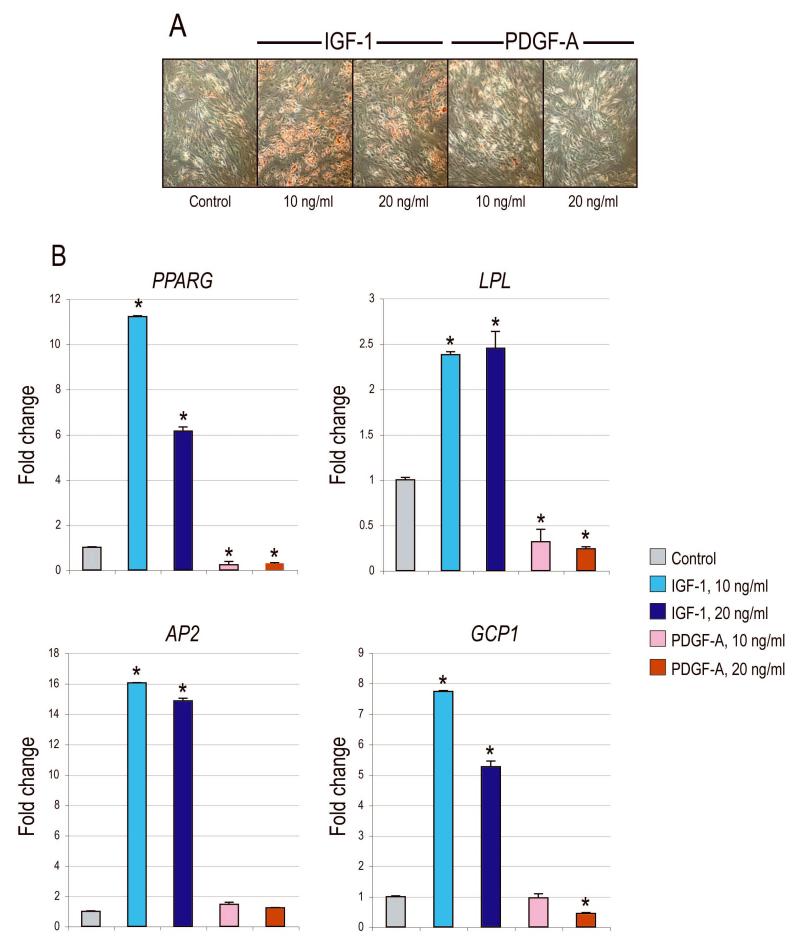
IGF-1 has been observed to be intimately involved in the adipogenic differentiation of various preadipocyte cell types (19-24); however its effects on hASC adipogenesis have not yet been described. We next evaluated the effects of rIGF-1 and rPDGF-α on hASC adipogenic differentiation (Fig. 6). Oil red O staining demonstrated significant induction lipid formation upon rIGF-1 addition to adipogenic differentation media (ADM) by 7d (Fig. 6A). In contrast, rPDGF-α addition to ADM resulted in little or no lipid induction. These fnding were quantified by real-time PCR (Fig 6B). Results showed a 2- to 16-fold increase to all markers of adipogenic differentiation, including PPAR-γ, LPL, AP2 and GCP1. In contrast, rPDGF-α either diminished or produced no change in all gene markers. Thus, rIGF-1 showed a significant, dual enhancement of both osteogenesis and adipogenesis in hASCs.
Figure 6. Adipogenic differentiation with rIGF-1 or rPDGF-α.
(A) Oil red O staining at 7d adipogenic differentiation with rIGF-1 or rPDGF-α. (B) Gene expression of various adipogenic markers by qRT-PCR after 7d. From left to right: PPARG, LPL, AP2, GCP1. N=3, *P<0.01.
Discussion
With an increasing population age in the US, we anticipate that the needs for skeletal reconstruction will increase over the coming years. Furthermore, soft tissue regeneration for breast and facial reconstruction remains a significant clinical challenge. To date, translational tissue engineering efforts have been hampered by a basic lack of understanding in the biology of the stem/progenitor cells. Various signaling pathways have been shown to be of importance in ASC osteodifferentiation, including retinoic acid (25), bone morphogenetic protein signaling (26), and hedgehog signaling (manuscript in preparation). We hypothesized two cytokines, PDGF and IGF, play an important role in the osteogenic differentiation of hASCs, and that by manipulating their levels in vitro, we can up-regulate bone formation.
Insulin-like growth factors are known mediators of skeletal growth and bone formation (27-30). Specifically, IGF-1 has been shown to promote the differentiation of bone cells in an autocrine and paracrine fashion (31, 32). Prior investigators have targeted IGF-1 to stimulate osteogenesis in vitro in the treatment of marrow derived osteoblastic cells (33) and in vivo in an aged rat model (30). We have demonstrated that IGF-1 has a similar osteogenic effect on hASCs as it does on marrow derived osteoblasts. When driving hASCs down an osteogenic lineage, IGF-1 is a potential protein that can be added in vitro to a cell culture or in vivo on a cell scaffold used in bone tissue regeneration. This may be even more useful when using hASCs in elderly patients. Previous studies have demonstrated the expression of IGF-1 declines with senescence (34-36). If an elderly patient would benefit from a bone graft, perhaps hASCs with the addition of IGF would improve osteogenesis. Previous studies using rat fracture models demonstrated that IGF-1 alone as well as PDGF alone failed to stimulate OCN expression (11). We found, however, that in hASCs both of these factors with ODM significantly up regulated OCN in comparison to ODM alone.
Similar to IGF, PDGF has been shown to be involved in osteogenesis. PDGF has two different isoforms (PDGF-A and PDGF-B) (37). The degree to which PDGF supplementation stimulates osteogenesis, however, is inconsistent in the literature. Previous reports concluded that PDGF-A had little or no effect on tyrosine kinase activity in rat osteoblastic cells (38). Similarly, continuous treatment of PDGF has been shown to decrease OCN and alkaline phosphatase (38). Other studies have shown an increase bone matrix deposition in cultured calvarial cells in vitro (39) and enhanced bone formation formation in vivo (40). We found that hASCs treated with PDGF-A alone only minimally affected bone deposition and actually diminished alkaline phosphatase levels. When placed in high concentrations (20ng/ml) in hASCs, however, OCN was up-regulated though early markers such as RUNX2 remained unchanged.
The synergistic effects of IGF and PDGF have led scientists to investigate the interaction between these growth hormones. This synergism between IGF and PDGF signaling is by no means limited to the skeletal system. In fact, multiple organ systems seem to show a coordinate role of IGF/PDGF signaling including skin development and repair (41-43), cartilage repair (44), peridontium (45-47), and even the eye (48-50). Canalis et al. demonstrated that the combined application of PDGF and IGF-1 had an additive effect on DNA synthesis in rat calvaria (27) and subsequently Pfeilschifter et al (39) and Tanaka et al (51) corroborated this finding in vitro. IGF is a better stimulator of osteoblast marker expression in old bone and PDGF slightly improves this effect (11). This interaction led us to determine whether they had a synergistic effect on hASCs. We demonstrated that rPDGF-α upregulates IGF1 gene expression. Moreover, we found that the addition of rIGF-1 with rPDGF-α significantly increased osteogenesis over the addition of each one separately. This was observed when both cytokines were added simultaneously or in sequence (PDGF followed by IGF-1). Thus, it appears that rPDGF-α may act as a competence factor for IGF-1 and IGF is a progression factor for hASC osteogenesis.
The effects of rIGF-1 and rPDGF-α on hASC adipogenesis, however, were disparate. IGF-1 is a well-known promoter of adipogenic differentiation and indeed in hASCs we observed this to be true. In contrast, rPDGF-α inhibited adipogenic differentiation by the majority of markers examined. An inverse relationship has been repeatedly observed in ASCs between bone and fat formation. Thus, a potent agonist of osteogenic differentiation would be postulated to inhibit adipogenic differentiation (52). This has been observed in our laboratory in the case of retinoic acid (25), valproic acid (6), and hedgehog signaling (manuscript in preparation). Interestingly, IGF-1 does not fit this conceptual framework as we observed it to significantly enhance both osteo- and adipogenesis. Perhaps PDGF signaling functions in manner to direct IGF-1 stimulation away from an adipocyte and towards an osteoblast cell fate (see Figure 7 for a schematic of this potential relationship). More in vitro experiments must be performed to validate this conjecture.
Figure 7. Schematic of IGF and PDGF effects on hASC differentiation.
ASCs derived from liposuction aspirate (middle) may be differentiated down adipogenic (top) and osteogenic (bottom) lineages. Based on our experiments IGF positively regulated adipogenesis (green), while PDGF inhbited it (red). Conversely, IGF enhanced bone formation (green), while PDGF appeared to enhance IGF-induced osteogenesis (green line).
Conclusion
Recombinant IGF-1 significantly increased the osteogenic and adipogenic potential hASCs, whereas rPDGF-α alone inhibited adipogenesis and affected ASC osteogenesis to a lesser degree. Recombinant PDGF-α appears to up-regulate IGF1 expression, and when used together or in sequence, these growth factors stimulate osteogenesis to a greater degree than either alone. Thus, there may exist potential synergy between these two signaling pathways in hASC osteogenesis. Future in vivo applications will focus on the combination of ASCs, biomimetic scaffolds and recombinant IGF.
Acknowledgments
Sources of Support: This study was supported by National Institutes of Health, National Institute of Dental and Craniofacial Research grant R01 DE-13194, the Oak Foundation and Hagey Foundation to M.T.L.
B.L was supported by the National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases grant 1F32AR057302-01.
Footnotes
Disclosure Statement: The authors above have no financial interest in any of the products, devices, procedures or anything else connected with the article. There was no internal or external funding received to complete this study.
University of Stanford IRB approval was obtained prior to commencement of the study (IRB # 2188).
References
- 1.Ahlmann E, Patzakis M, Roidis N, et al. Comparison of anterior and posterior iliac crest bone grafts in terms of harvest-site morbidity and functional outcomes. J Bone Joint Surg Am. 2002;84-A:716–720. doi: 10.2106/00004623-200205000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Wan DC, Nacamuli RP, Longaker MT. Craniofacial bone tissue engineering. Dental clinics of North America. 2006;50:175–190. vii. doi: 10.1016/j.cden.2005.11.003. [DOI] [PubMed] [Google Scholar]
- 3.Xu Y, Malladi P, Wagner DR, et al. Adipose-derived mesenchymal cells as a potential cell source for skeletal regeneration. Current opinion in molecular therapeutics. 2005;7:300–305. [PubMed] [Google Scholar]
- 4.Stosich MS, Mao JJ. Adipose tissue engineering from human adult stem cells: clinical implications in plastic and reconstructive surgery. Plast Reconstr Surg. 2007;119:71–83. doi: 10.1097/01.prs.0000244840.80661.e7. discussion 84-75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Gupta DM, Kwan MD, Slater BJ, et al. Applications of an athymic nude mouse model of nonhealing critical-sized calvarial defects. J Craniofac Surg. 2008;19:192–197. doi: 10.1097/scs.0b013e31815c93b7. [DOI] [PubMed] [Google Scholar]
- 6.Xu Y, Hammerick KE, James AW, et al. Inhibition of histone deacetylase activity in reduced oxygen environment enhances the osteogenesis of mouse adipose-derived stromal cells. Tissue Eng Part A. 2009;15:3697–3707. doi: 10.1089/ten.tea.2009.0213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lee J, G. D, Pannetta NJ, Levi B, James AW, Wan D, Longaker MTL. Elucidating Mechanisms of Osteogenesis in Human Adipose-derived Stromal Cells via Microarray Analysis. J Craniofac Surg. 2009 doi: 10.1097/SCS.0b013e3181e488d6. Accepted for publication. [DOI] [PubMed] [Google Scholar]
- 8.Hughes FJ, Turner W, Belibasakis G, et al. Effects of growth factors and cytokines on osteoblast differentiation. Periodontology 2000. 2006;41:48–72. doi: 10.1111/j.1600-0757.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- 9.Lian JB, Javed A, Zaidi SK, et al. Regulatory controls for osteoblast growth and differentiation: role of Runx/Cbfa/AML factors. Crit Rev Eukaryot Gene Expr. 2004;14:1–41. [PubMed] [Google Scholar]
- 10.Mott DA, Mailhot J, Cuenin MF, et al. Enhancement of osteoblast proliferation in vitro by selective enrichment of demineralized freeze-dried bone allograft with specific growth factors. The Journal of oral implantology. 2002;28:57–66. doi: 10.1563/1548-1336(2002)028<0057:EOOPIV>2.3.CO;2. [DOI] [PubMed] [Google Scholar]
- 11.Tanaka H, Wakisaka A, Ogasa H, et al. Effect of IGF-I and PDGF administered in vivo on the expression of osteoblast-related genes in old rats. J Endocrinol. 2002;174:63–70. doi: 10.1677/joe.0.1740063. [DOI] [PubMed] [Google Scholar]
- 12.Tanaka H, Wakisaka A, Ogasa H, et al. Local and systemic expression of insulin-like growth factor-I (IGF-I) mRNAs in rat after bone marrow ablation. Biochem Biophys Res Commun. 2001;287:1157–1162. doi: 10.1006/bbrc.2001.5711. [DOI] [PubMed] [Google Scholar]
- 13.Levi B, A.W. J, Xu Y, et al. Divergent modulation of adipose-derived stromal cell differentiation by TGF-beta1 based on species of derivation. Plast Reconstr Surg. 2009 doi: 10.1097/PRS.0b013e3181df64dc. Under Review. [DOI] [PubMed] [Google Scholar]
- 14.James AW, Xu Y, Wang R, et al. Proliferation, osteogenic differentiation, and fgf-2 modulation of posterofrontal/sagittal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2008;122:53–63. doi: 10.1097/PRS.0b013e31817747b5. [DOI] [PubMed] [Google Scholar]
- 15.Xu Y, James AW, Longaker MT. Transforming Growth Factor-beta1 Stimulates Chondrogenic Differentiation of Posterofrontal Suture-Derived Mesenchymal Cells In Vitro. Plast Reconstr Surg. 2008;122:1649–1659. doi: 10.1097/PRS.0b013e31818cbf44. [DOI] [PubMed] [Google Scholar]
- 16.Malladi P, Xu Y, Chiou M, et al. Hypoxia inducible factor-1alpha deficiency affects chondrogenesis of adipose-derived adult stromal cells. Tissue engineering. 2007;13:1159–1171. doi: 10.1089/ten.2006.0265. [DOI] [PubMed] [Google Scholar]
- 17.James AW, Xu Y, Lee JK, et al. Differential effects of TGF-beta1 and TGF-beta3 on chondrogenesis in posterofrontal cranial suture-derived mesenchymal cells in vitro. Plast Reconstr Surg. 2009;123:31–43. doi: 10.1097/PRS.0b013e3181904c19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Choi JY, Pratap J, Javed A, et al. Subnuclear targeting of Runx/Cbfa/AML factors is essential for tissue-specific differentiation during embryonic development. Proceedings of the National Academy of Sciences of the United States of America. 2001;98:8650–8655. doi: 10.1073/pnas.151236498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hausman GJ, Richardson RL, Simmen FA. Secretion of insulin-like growth factor (IGF)-I and -II and IGF binding proteins (IGFBPs) in fetal stromal-vascular (S-V) cell cultures obtained before and after the onset of adipogenesis in vivo. Growth Dev Aging. 2002;66:11–26. [PubMed] [Google Scholar]
- 20.Holly J, Sabin M, Perks C, et al. Adipogenesis and IGF-1. Metabolic syndrome and related disorders. 2006;4:43–50. doi: 10.1089/met.2006.4.43. [DOI] [PubMed] [Google Scholar]
- 21.Bluher S, Kratzsch J, Kiess W. Insulin-like growth factor I, growth hormone and insulin in white adipose tissue. Best practice & research. 2005;19:577–587. doi: 10.1016/j.beem.2005.07.011. [DOI] [PubMed] [Google Scholar]
- 22.Grohmann M, Sabin M, Holly J, et al. Characterization of differentiated subcutaneous and visceral adipose tissue from children: the influences of TNF-alpha and IGF-I. Journal of lipid research. 2005;46:93–103. doi: 10.1194/jlr.M400295-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Boney CM, Moats-Staats BM, Stiles AD, et al. Expression of insulin-like growth factor-I (IGF-I) and IGF-binding proteins during adipogenesis. Endocrinology. 1994;135:1863–1868. doi: 10.1210/endo.135.5.7525256. [DOI] [PubMed] [Google Scholar]
- 24.Smith PJ, Wise LS, Berkowitz R, et al. Insulin-like growth factor-I is an essential regulator of the differentiation of 3T3-L1 adipocytes. J Biol Chem. 1988;263:9402–9408. [PubMed] [Google Scholar]
- 25.Malladi P, Xu Y, Yang GP, et al. Functions of Vitamin D, Retinoic Acid, and Dexamethasone on Mouse Adipose-Derived Mesenchymal Cells (AMCs) Tissue engineering. 2005 doi: 10.1089/ten.2006.12.2031. [DOI] [PubMed] [Google Scholar]
- 26.Wan DC, Shi YY, Nacamuli RP, et al. Osteogenic differentiation of mouse adipose-derived adult stromal cells requires retinoic acid and bone morphogenetic protein receptor type IB signaling. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:12335–12340. doi: 10.1073/pnas.0604849103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Canalis E. Effect of insulinlike growth factor I on DNA and protein synthesis in cultured rat calvaria. The Journal of clinical investigation. 1980;66:709–719. doi: 10.1172/JCI109908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Schlechter NL, Russell SM, Spencer EM, et al. Evidence suggesting that the direct growth-promoting effect of growth hormone on cartilage in vivo is mediated by local production of somatomedin. Proceedings of the National Academy of Sciences of the United States of America. 1986;83:7932–7934. doi: 10.1073/pnas.83.20.7932. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schoenle E, Zapf J, Humbel RE, et al. Insulin-like growth factor I stimulates growth in hypophysectomized rats. Nature. 1982;296:252–253. doi: 10.1038/296252a0. [DOI] [PubMed] [Google Scholar]
- 30.Tanaka H, Quarto R, Williams S, et al. In vivo and in vitro effects of insulin-like growth factor-I (IGF-I) on femoral mRNA expression in old rats. Bone. 1994;15:647–653. doi: 10.1016/8756-3282(94)90313-1. [DOI] [PubMed] [Google Scholar]
- 31.Canalis E, McCarthy TL, Centrella M. Growth factors and cytokines in bone cell metabolism. Annual review of medicine. 1991;42:17–24. doi: 10.1146/annurev.me.42.020191.000313. [DOI] [PubMed] [Google Scholar]
- 32.Rodan GA. Introduction to bone biology. Bone. 1992;13(Suppl 1):S3–6. doi: 10.1016/s8756-3282(09)80003-3. [DOI] [PubMed] [Google Scholar]
- 33.Machwate M, Zerath E, Holy X, et al. Insulin-like growth factor-I increases trabecular bone formation and osteoblastic cell proliferation in unloaded rats. Endocrinology. 1994;134:1031–1038. doi: 10.1210/endo.134.3.8119139. [DOI] [PubMed] [Google Scholar]
- 34.Rudman D, Kutner MH, Rogers CM, et al. Impaired growth hormone secretion in the adult population: relation to age and adiposity. The Journal of clinical investigation. 1981;67:1361–1369. doi: 10.1172/JCI110164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Florini JR, Prinz PN, Vitiello MV, et al. Somatomedin-C levels in healthy young and old men: relationship to peak and 24-hour integrated levels of growth hormone. Journal of gerontology. 1985;40:2–7. doi: 10.1093/geronj/40.1.2. [DOI] [PubMed] [Google Scholar]
- 36.Tanaka H, Barnes J, Liang CT. Effect of age on the expression of insulin-like growth factor-I, interleukin-6, and transforming growth factor-beta mRNAs in rat femurs following marrow ablation. Bone. 1996;18:473–478. doi: 10.1016/8756-3282(96)00041-5. [DOI] [PubMed] [Google Scholar]
- 37.Heldin CH, Backstrom G, Ostman A, et al. Binding of different dimeric forms of PDGF to human fibroblasts: evidence for two separate receptor types. The EMBO journal. 1988;7:1387–1393. doi: 10.1002/j.1460-2075.1988.tb02955.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Yu X, Hsieh SC, Bao W, et al. Temporal expression of PDGF receptors and PDGF regulatory effects on osteoblastic cells in mineralizing cultures. Am J Physiol. 1997;272:C1709–1716. doi: 10.1152/ajpcell.1997.272.5.C1709. [DOI] [PubMed] [Google Scholar]
- 39.Pfeilschifter J, Oechsner M, Naumann A, et al. Stimulation of bone matrix apposition in vitro by local growth factors: a comparison between insulin-like growth factor I, platelet-derived growth factor, and transforming growth factor beta. Endocrinology. 1990;127:69–75. doi: 10.1210/endo-127-1-69. [DOI] [PubMed] [Google Scholar]
- 40.Nash TJ, Howlett CR, Martin C, et al. Effect of platelet-derived growth factor on tibial osteotomies in rabbits. Bone. 1994;15:203–208. doi: 10.1016/8756-3282(94)90709-9. [DOI] [PubMed] [Google Scholar]
- 41.Lynch SE, Colvin RB, Antoniades HN. Growth factors in wound healing. Single and synergistic effects on partial thickness porcine skin wounds. The Journal of clinical investigation. 1989;84:640–646. doi: 10.1172/JCI114210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Wagner W, Wehrmann M. Differential cytokine activity and morphology during wound healing in the neonatal and adult rat skin. Journal of cellular and molecular medicine. 2007;11:1342–1351. doi: 10.1111/j.1582-4934.2007.00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Breuing K, Andree C, Helo G, et al. Growth factors in the repair of partial thickness porcine skin wounds. Plast Reconstr Surg. 1997;100:657–664. doi: 10.1097/00006534-199709000-00018. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt MB, Chen EH, Lynch SE. A review of the effects of insulin-like growth factor and platelet derived growth factor on in vivo cartilage healing and repair. Osteoarthritis and cartilage / OARS, Osteoarthritis Research Society. 2006;14:403–412. doi: 10.1016/j.joca.2005.10.011. [DOI] [PubMed] [Google Scholar]
- 45.Parkar MH, Kuru L, Giouzeli M, et al. Expression of growth-factor receptors in normal and regenerating human periodontal cells. Archives of oral biology. 2001;46:275–284. doi: 10.1016/s0003-9969(00)00099-6. [DOI] [PubMed] [Google Scholar]
- 46.Shih SD, Rees TD, Miller EG, et al. The effects of platelet-derived growth factor-BB and insulin-like growth factor-1 on epithelial dysplasia. Journal of periodontology. 1996;67:1224–1232. doi: 10.1902/jop.1996.67.11.1224. [DOI] [PubMed] [Google Scholar]
- 47.Giannobile WV, Hernandez RA, Finkelman RD, et al. Comparative effects of platelet-derived growth factor-BB and insulin-like growth factor-I, individually and in combination, on periodontal regeneration in Macaca fascicularis. Journal of periodontal research. 1996;31:301–312. doi: 10.1111/j.1600-0765.1996.tb00497.x. [DOI] [PubMed] [Google Scholar]
- 48.Etheredge L, Kane BP, Hassell JR. The effect of growth factor signaling on keratocytes in vitro and its relationship to the phases of stromal wound repair. Investigative ophthalmology & visual science. 2009;50:3128–3136. doi: 10.1167/iovs.08-3077. [DOI] [PubMed] [Google Scholar]
- 49.Imanishi J, Kamiyama K, Iguchi I, et al. Growth factors: importance in wound healing and maintenance of transparency of the cornea. Progress in retinal and eye research. 2000;19:113–129. doi: 10.1016/s1350-9462(99)00007-5. [DOI] [PubMed] [Google Scholar]
- 50.Andresen JL, Ledet T, Ehlers N. Keratocyte migration and peptide growth factors: the effect of PDGF, bFGF, EGF, IGF-I, aFGF and TGF-beta on human keratocyte migration in a collagen gel. Current eye research. 1997;16:605–613. doi: 10.1076/ceyr.16.6.605.5081. [DOI] [PubMed] [Google Scholar]
- 51.Tanaka H, Liang CT. Effect of platelet-derived growth factor on DNA synthesis and gene expression in bone marrow stromal cells derived from adult and old rats. J Cell Physiol. 1995;164:367–375. doi: 10.1002/jcp.1041640217. [DOI] [PubMed] [Google Scholar]
- 52.Pei L, Tontonoz P. Fat’s loss is bone’s gain. The Journal of clinical investigation. 2004;113:805–806. doi: 10.1172/JCI21311. [DOI] [PMC free article] [PubMed] [Google Scholar]