Abstract
The F-box protein Skp2 (S-phase kinase-associated protein 2) positively regulates the G1-S transition by controlling the stability of several G1 regulators, such as the cell cycle inhibitor p27. We show here that Skp2 expression correlates directly with grade of malignancy and inversely with p27 levels in human lymphomas. To directly evaluate the potential of Skp2 to deregulate growth in vivo, we generated transgenic mice expressing Skp2 targeted to the T-lymphoid lineage as well as double transgenic mice coexpressing Skp2 and activated N-Ras. A strong cooperative effect between these two transgenes induced T cell lymphomas with shorter latency and higher penetrance, leading to significantly decreased survival when compared with control and single transgenic animals. Furthermore, lymphomas of Nras single transgenic animals often expressed higher levels of endogenous Skp2 than tumors of double transgenic mice. This study provides evidence of a role for an F-box protein in oncogenesis and establishes SKP2 as a protooncogene causally involved in the pathogenesis of lymphomas.
Keywords: ubiquitin, ubiquitin-ligases, cancer, cell cycle
F-box proteins (Fbps) form an expanding family of eukaryotic proteins characterized by an approximately 40-aa motif referred to as the F-box because it was first identified in cyclin F (1). Fbps are components of ubiquitin protein ligases called SCFs because they contain the following basic subunits: Skp1 (S-phase kinase-associated protein 1); a cullin subunit (called Cul1 in metazoans); Roc1 (also called Hrt1 or Rbx1) and one of many Fbps. Because Fbps act as substrate recognition factors of the SCF ligases, a large number of Fbps ensure high substrate specificity (2–4).
The human Fbp Skp2 and human Skp1 were originally identified as two proteins interacting with the cyclin A–cyclin-dependent kinase 2 (Cdk2) complex and thus were designated as Skps (5). Interestingly, this tetrameric protein complex was found in fibroblasts immortalized with simian virus 40 large T antigen and in many transformed cells but could not be detected in diploid fibroblasts (5). Skp2 is required for the G1-S transition in both transformed cells and diploid fibroblasts (5), and Skp2 overexpression induces quiescent fibroblasts to replicate their DNA in low serum (6). Recent biochemical and somatic cell genetic experiments have started to elucidate the mechanisms through which Skp2 controls the entry into S-phase. In fact, we and others have demonstrated that Skp2 is required for the ubiquitination and consequent degradation of the cell cycle inhibitor p27 both in vivo (6, 7) and in vitro (7, 8). Skp2 only binds to and allows the ubiquitination of p27 when the latter is phosphorylated on Thr-187 by Cdk2. In quiescent cells levels of p27 are high, but in response to mitogenic stimuli, levels of cyclin E, cyclin A, and Skp2 increase, resulting in the Thr-187 phosphorylation of p27 and its subsequent ubiquitin-mediated degradation. Interestingly, p27 degradation is enhanced in many aggressive human tumors (reviewed in ref. 9). Skp2 also is required for the ubiquitination of cyclin E, but only in its free, non-Cdk2 bound form, whereas cyclin E complexed to Cdk2 is not affected by Skp2 (10). Skp2-deficient cells show high levels of p27 and free cyclin E, polyploidy, and centrosome overduplication. In addition, Skp2-deficient mice grow more slowly and have smaller organs than littermate controls (10). This phenotype underscores the importance of Skp2 in positively regulating cell proliferation.
Given the role of Skp2 in inducing S-phase entry and the overexpression of Skp2 in many tumor cell lines, we designed experiments to determine whether Skp2 has a role in oncogenesis. The results of these studies are herein presented.
Materials and Methods
Antibodies.
Mouse mAbs to Skp2 were produced in collaboration with Zymed and affinity-purified as described (11) with the use of purified bacterial Skp2 protein (12). mAb to human Cul1 (13), rabbit polyclonal antibodies to human Skp2 (7), Cul1 (13), p27 (11), cyclin A (7), and phospho-T187-site specific p27 antibody (11) have been described. mAb to cyclin D3 was kindly provided by Jiri Lukas (Danish Cancer Society, Copenhagen). Rabbit antibodies to anti-E2F-1 (sc-193) and anti-cyclin E (sc-481) were from Santa Cruz Biotechnology.
Pathological Samples.
A panel of 58 well-characterized lymphomas was selected from among the cases processed in the surgical pathology laboratories of the New York University School of Medicine. The lymphomas were classified according to the international lymphoma study group, based on hematoxylin-eosin stain and immunoperoxidase stains as described (14). The lymphoproliferative disorders characterized in this study included low-grade lymphomas (small lymphocytic lymphomas, SLLs), and high-grade lymphomas (diffuse large cell lymphomas, DLCLs).
Immunohistochemical Staining, Score, and Statistical Analysis.
For immunohistochemical stainings, either an anti-p27 mAb (1:1000; Transduction Laboratories, Lexington, KY; catalog no. K25020) or a mix of four different affinity-purified mAbs to Skp2 (shown in Fig. 1) (approximately 10 μg/ml) were used. Although all four mAbs to Skp2 showed a similar staining in immunohistochemistry, the signal was stronger with a mix containing the four antibodies. Slides were subjected to microwaving for 20 min in 10 mM citrate buffer (pH 8.0 for Skp2 and pH 6.0 for p27). Immunostainings were performed on formalin-fixed paraffin-embedded tissues with the avidin biotin peroxidase complex method and a semiautomated immunostainer (Dako or Ventana System, Tucson, AZ) as described (14). Immunostainings were scored independently for degree of expression by two pathologists (G.I. and R.C.). At least 20 high-power fields were chosen at random, and 2,000 cells were counted. Twenty-five percent of the cases, chosen at random, were rescored by each pathologist. There was >98% interobserver and intraobserver concordance. The neoplasms were considered positive when nuclear staining was detected in at least 25% of the tumor cells. The statistical significance of Skp2 and p27 expression vs. grade of malignancy was calculated with the Fisher's test. The statistical significance of Skp2 vs. p27 expression was calculated with the χ2 test. P ≤ 0.05 was required for significance.
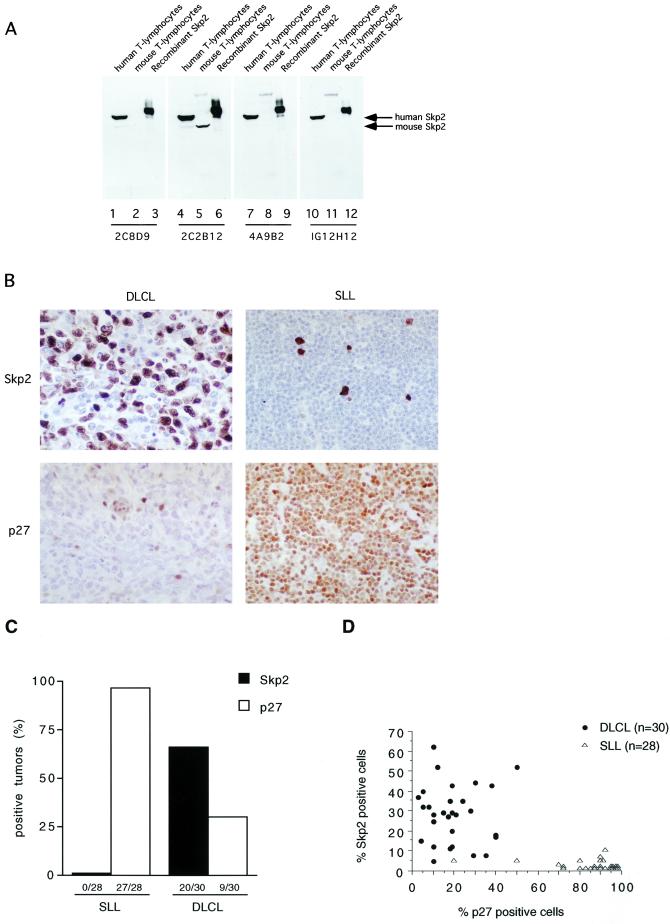
Figure 1.
Skp2 expression correlates directly with grade of malignancy and inversely with p27 levels in human lymphomas. (A) Characterization of novel mAbs to Skp2 by straight immunoblot. Lanes 1, 4, 7, and 10: 25 μg of extract from human transformed T lymphocytes (Jurkat); lanes 2, 5, 8, and 11: 25 μg of extract from mouse diploid T lymphocytes; lanes 3, 6, 9, and 12: 10 ng recombinant histidine-tagged Skp2. Each panel corresponds to a different membrane immunoblotted with the indicated affinity-purified anti-Skp2 mAb. Only clone 2C2B12 recognized both human and mouse Skp2 (which migrates faster than human Skp2). (B) Immunohistochemistry of two representative lymphomas, with the use of a mix of the four novel affinity-purified anti-Skp2 mAbs (Upper) or an anti-p27 mAb (Lower). (Left) A high-grade lymphoma (DLCL). (Right) A low-grade lymphoma (SLL). All photos were taken at the same microscope magnification (×40). Small p27-positive cells in DLCL represent normal lymphocytes infiltrating the tumor, which, in contrast, has larger mostly negative cells. (C) The expression of Skp2 and p27 was analyzed by immunohistochemistry in 30 cases of high-grade (DLCL) and 28 cases of low-grade (SLL) human lymphomas. Tumors were divided into positive (>25% of positive cells) and negative (<25% of positive cells). (D) Scatter diagram showing the distribution of Skp2 and p27 fractions in DLCL and SLL.
Constructs and Generation of Transgenic Mice.
The human SKP2 cDNA was cloned in a plasmid containing the minimal CD4 enhancer (339 bp), the minimal murine CD4 promoter (487 bp), the transcriptional initiation site, and 70 bp of the untranslated first exon and part of the first intron of the murine CD4 gene (14). The transgene was released with NotI, injected into the pronucleus of fertilized eggs from B6D2F1 donors, and subsequently transferred to pseudopregnant CD-1 mice. Screening of founder animals was performed by PCR and confirmed by Southern hybridization on genomic DNA from tail biopsies. Screening of the offspring was performed by PCR amplification of tail DNA. Four founders for the SKP2 transgene were obtained, and all transmitted the transgene to their progeny. Double transgenic mice were generated by crossing CD4-SKP2 animals with truncated mouse mammary tumor virus–long terminal repeat (TMTV)-Nras mice that express the oncogenic murine Nras mutant (G61K) under a TMTV promoter (15).
Tumor Histology, Immunophenotyping, Incidence, and Latency.
Animals were monitored daily. Necropsies were performed on all animals that died spontaneously during the observation period. Histological analysis of thymus, spleen, and lymph nodes was performed as described (14). A portion of each sample was fixed in formalin, embedded in paraffin, and sectioned for staining with hematoxylin and eosin, while another portion was frozen. For immunophenotyping, fluorochrome-conjugated antibodies against CD4 (FITC), CD8 (phycoerythrin), and CD90/Thy-1 (FITC) from PharMingen were used. Survival was calculated, and the log-rank test was used to study the significance.
T Cell Preparation, Culture, and Proliferation Assay.
Thymi were dissected, washed in PBS to remove residual blood, cut into small pieces, and put in a 60-mm Petri dish containing complete medium (RPMI 1640/10% FBS/50 μM β-mercaptoethanol/2 mM l-glutamine/0.1% penicillin-streptomycin). Single-cell suspensions were mechanically prepared by crushing the pieces of thymus or lymph nodes with the bottom of a 3-ml syringe plunger. Cells were filtered through a Falcon cell strainer and subjected to hypotonic lysis of erythrocytes. Cells were then pelleted, resuspended in complete medium, seeded in a 96-well dish (0.25 × 106 cells per well), and then activated with Con A (5 μg/ml) in the presence or in the absence of IL-2 (50 ng/ml) or with phorbol 12-myristate 13-acetate (50 ng/ml) and ionomycin (1 μM). After a 48-h incubation at 37°C, [3H]thymidine (1 μCi) was added for an additional 24-h period, and proliferation was assessed by [3H]thymidine incorporation.
Extract Preparation, Immunoblotting, Immunoprecipitation, and Ubiquitination Assay.
Thymocytes were washed twice in PBS and lysed in lysis buffer (50 mM Tris, pH 8, 120 mM NaCl/0.5% Nonidet P-40/1 mM EDTA/Na3VO4/10 mM NaF, and other common protein inhibitors). Tissue samples from normal thymi and tumors were homogenized in lysis buffer with a Tissue-Tearor (Biospec Products, Bartlesville, OK). Lysates were incubated on ice for 15 min and cleared by centrifugation at 14,000 × g for 15 min at 4°C. Conditions for the ubiquitination assay have been described (7).
Results
Skp2 Expression Correlates Directly to High-Grade Malignancy and Inversely to p27 Levels in Human Lymphomas.
Skp2 expression was evaluated in a panel of high-grade (DLCLs, n = 30) and low-grade (SLLs, n = 28) human lymphoid malignancies. This series included previously analyzed samples in which we had found that aggressive lymphomas express low levels of p27 and have increased p27 degradation activity (16). Skp2 immunohistochemistry was performed with a novel series of monoclonal anti-Skp2 antibodies that specifically recognize Skp2 as a single band in immunoblots of whole-cell lysates (Fig. 1A). A distinct, mainly nuclear staining with very low background was observed, making Skp2 expression easy to evaluate (Fig. 1B). A significant direct correlation between Skp2 expression and high-grade malignancy (P < 0.0001) was observed, and both parameters inversely correlated with p27 levels (Skp2 vs. p27: P < 0.0001 in SLL and P = 0.0062 in DLCL; grade of malignancy vs. p27: P < 0.0001) (Fig. 1 C and D). Finally, levels of Skp2 directly correlated with cell proliferation, as measured by Ki-67 expression (P < 0.0001). In contrast to most human carcinomas, Ki-67 expression is an important prognostic indicator in lymphomas (17). Thus, on one hand it is possible that high levels of Skp2 in DLCLs contribute to uncontrolled proliferation; on the other, because Skp2 expression is cell cycle regulated (7, 18), these increased levels may reflect the high mitotic index of high-grade lymphomas.
Production of Transgenic Mice Carrying a CD4-SKP2 Construct.
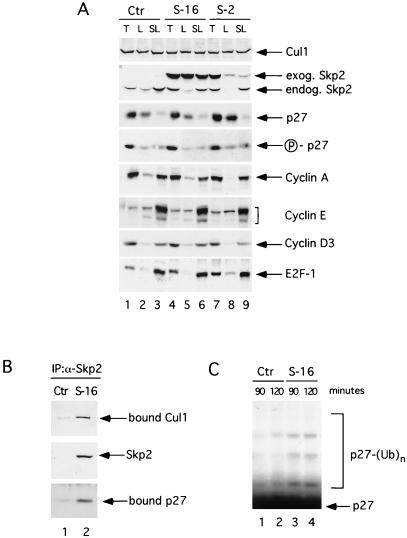
To evaluate the potential of Skp2 to deregulate growth in vivo, we generated transgenic mice expressing Skp2 targeted to the T-lymphoid lineage. Human SKP2 was placed under the control of the murine CD4 minimal promoter (lacking the CD4 silencer region) and the CD4 enhancer to drive the expression in CD4- and CD8-positive T cells (both single and double positive) (14). With the use of this construct, four independent Skp2 transgenic lines of mice were obtained that showed transgene expression in the thymus and peripheral lymphoid organs (Fig. 2A). All of the experiments described herein were performed with two CD4-SKP2 transgenic lines (lines 2 and 16) that expressed similar Skp2 levels in the thymus (Fig. 2A, second panel from the top, lanes 4 and 7). Because p27 phosphorylation on Thr-187 is necessary for its recognition by Skp2, we checked the extent of p27 phosphorylation with the use of a p27 phospho-Thr-187-specific antibody (11) and found that levels of p27 Thr-187 phosphorylation did not change in transgenic mice (Fig. 2A, fourth panel from the top). This lack of change in phosphorylation could explain why Skp2 overexpression by itself was unable to change the levels of endogenous p27 in either thymocytes or peripheral lymphocytes (Fig. 2A, third panel from the top). Alternatively, some other component required for p27 destruction could be limiting. Similarly to p27, all other cell cycle regulators analyzed were found at similar levels in control and transgenic mice. Importantly, transgenic human Skp2 was able to bind to the murine Cul1, another ubiquitin ligase subunit, as well as to murine p27 (Fig. 2B), verifying that the exogenous protein can assemble in a murine SCF complex. Furthermore, expression of transgenic Skp2 increased the in vitro p27 ubiquitination activity of thymic extracts (Fig. 2C). Once again, this difference in ubiquitination activity was detected only when purified recombinant Cdk2-cyclin E complex was added to thymic extracts, consistent with the notion that active Cdks are required for p27 ubiquitination in vitro as in vivo (8, 11, 19).
Figure 2.
Characterization of CD4-SKP2 transgenic mice. (A) Expression of Skp2 and other cell cycle regulatory proteins in a control nontransgenic mouse (Ctr, lanes 1–3) and in CD4-SKP2 transgenic mice, line 2 (S-2, lanes 4–6) and line 16 (S-16, lanes 7–9). Extracts (30 μg of protein) from thymocytes (T, lanes 1, 4, and 7), unstimulated lymphocytes from lymph nodes (L, lanes 2, 5, and 8), and lymphocytes stimulated for 48 h with phorbol 12-myristate 13-acetate + ionomycin (SL, lanes 3, 6, and 9) were immunoblotted with the antibodies to the indicated proteins. Skp2 mAb (clone 2C2B12) recognized both murine endogenous Skp2 (endog. Skp2) and human exogenous Skp2 (exog. Skp2). Human Skp2 migrates more slowly than murine Skp2 (see also Fig. 1A). The fourth panel from the top was immunoblotted with a p27 phospho-Thr-187 site-specific antibody (11) (indicated as P-p27). (B) Association of human Skp2 with endogenous murine Cul1 and p27. Extracts from thymocytes of a control nontransgenic mouse (Ctr, lane 1) and a CD4-SKP2 transgenic mouse, line 16 (S-16, lane 2) were first immunoprecipitated with a rabbit anti-Skp2 antibody that specifically recognizes human Skp2 (7) and then immunoblotted with antibodies to Skp2, Cul1, and p27. (C) p27 ubiquitinating activity in thymic extracts (30 μg of protein) from control (Ctr, lanes 1 and 2) and transgenic (S-16, lanes 3 and 4) mice. To assay for p27 ubiquitination, extracts from thymocytes were incubated for 90 min (lanes 1 and 3) or 120 min (lanes 2 and 4) in the presence of cyclin E/Cdk2. 35S-methionine-labeled in vitro-translated p27 was used as a substrate as described (7, 11). The bracket on the left side of the panels marks a ladder of bands greater than 27,000 corresponding to polyubiquitinated p27.
Cooperation Between Skp2 and N-Ras in Lymphomagenesis.
All CD4-SKP2 mice were healthy (n = 74, mean/median follow-up 12 months) and did not display alterations of thymus, spleen, or lymph nodes or develop tumors, as shown by gross and histological examinations, as well as by flow cytometric analysis (n = 14, mean/median 9 months). To test whether deregulated Skp2 expression could cooperate in tumorigenesis with a known oncogene, we crossed CD4-SKP2 mice with TMTV-Nras mice (line A) (15). Our choice of this particular Nras transgenic line was made for the following three reasons: first, these transgenic mice are suitable for cooperation studies because they express very low levels of activated N-Ras in thymus, and only a fraction of them (approximately 35%) develop high-grade T cell lymphomas within the first 12 months of life (15). Second, of the three RAS genes, only NRAS is mutated in a substantial number of human lymphoproliferative neoplasms (reviewed in ref. 20). Third, SKP2 cooperates with activated Nras in a colony formation assay using mouse fibroblasts (unpublished results). We found that 75% of the mice (n = 20) inheriting both SKP2 and Nras transgenes developed T cell lymphomas and died by 110 days of age, at which time only 29% of TMTV-Nras mice (n = 31) had died (Fig. 3A). This marked acceleration in tumor onset, increased penetrance of lymphomagenesis, and decreased survival rate are highly significant (P < 0.0001 by the log-rank test). Immunophenotyping and histological examination revealed that tumors of the thymus in both double and single transgenic mice were indistinguishable and were classified as high-grade T cell lymphomas. As described for the TMTV-Nras tumors (15), these lymphomas were characterized by large atypical cells with high mitotic rates and frequent apoptotic bodies, which often infiltrated surrounding perilymphoid tissues and peripheral lymphoid organs (not shown).
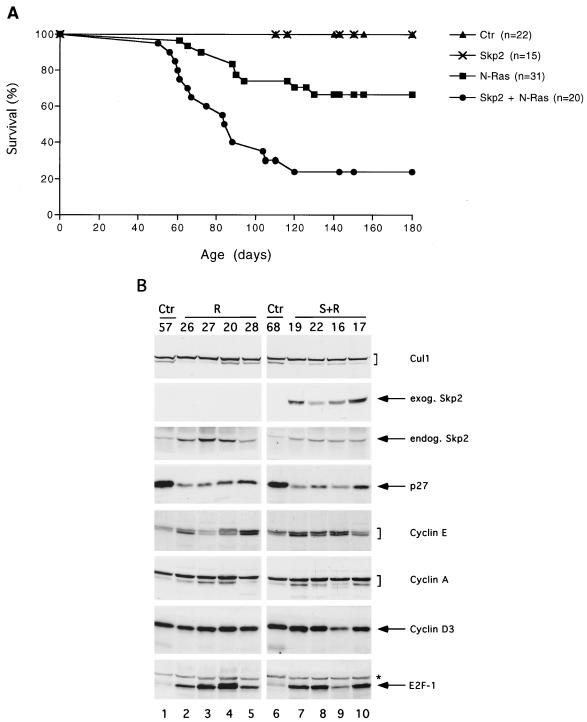
Figure 3.
Skp2 cooperates with N-Ras to induce T cell lymphomas in mice. (A) Percentage of survival in progeny of transgenic crosses of CD4-SKP2 mice (lines 2 and 16) and TMTV-Nras mice (line A). Nontransgenic control mice and single transgenic mice are indicated as Ctr, Skp2, and N-Ras, respectively; double transgenic mice are indicated as Skp2 + N-Ras. The percentage of survivors is given against the age in days. No significant difference in survival was observed when lines 2 and 16 were analyzed independently (not shown). (B) Immunoblotting of tissue extracts (30 μg of protein) from control thymi (Ctr) and tumors developed in TMTV-Nras mice (R) or in double transgenic mice (S + R). Numbers represent different animals from which tissues were obtained. Extracts were immunoblotted with the antibodies to the indicated proteins. The asterisk in the E2F-1 panel indicates a nonspecific band.
To characterize these T cell lymphomas at the molecular level, tumor extracts from both single (n = 15) and double (n = 15) transgenic mice were analyzed by immunoblotting. Fig. 3B shows a representative analysis of these tissue extracts. Overall, compared with control thymi, tumor cells expressed lower levels of p27 and higher levels of E2F-1 and cyclin E but not of cyclin A and cyclin D3. Significantly, levels of endogenous Skp2 in lymphomas of TMTV-Nras mice (Fig. 3B, lanes 2–5, third panel from the top) were 2- to 5-fold higher than in controls (15 of 15 tumors analyzed) and even higher than in tumors of double transgenic animals (9 of 15). This higher level of endogenous Skp2 suggests that in the absence of the exogenous SKP2 transgene, cells with high Skp2 expression are selected during Ras-mediated lymphomagenesis.
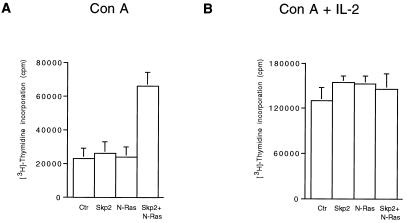
To investigate the presence of a preneoplastic phenotype, we examined healthy young mice at 6 weeks of age, bearing either transgene alone or both together. No obvious differences from nontransgenic littermates were detected by histological examination, immunoblotting of cell cycle regulators, or flow cytometric analysis of the thymi from these mice (n = 24). The lack of gross differences between single and double transgenic mice might be due, at least partially, to the fact that N-Ras is expressed at very low levels in these young mice. Later in life, demethylation of the TMTV promoter and the subsequent increase in N-Ras expression correlate with tumor development (21). However, in vitro studies provide a sensitive method of detecting proliferation, and, despite low N-Ras expression and the small percentage of thymocytes responding to mitogens (10–20%), we were able to observe that thymocytes of double transgenic mice proliferated significantly more than thymocytes of control and single transgenic mice. This difference was appreciable in response to a suboptimal mitogenic stimulus (Con A only) but not to strong mitogenic stimuli (Con A + IL-2 or phorbol 12-myristate 13-acetate + ionomycin) (Fig. 4 and data not shown). This increased sensitivity to a suboptimal mitogenic stimulus, together with the IL-2-independent proliferation, provides an insight into the preneoplastic phenotype of these transgenic mice. Thus, the molecular basis for the oncogenic cooperation between Skp2 and N-Ras may lie in their ability to increase proliferation in the target cell population of healthy mice.
Figure 4.
Increased sensitivity to a suboptimal mitogenic stimulus in thymocytes from young double transgenic mice. [3H]thymidine incorporation in thymocytes from control nontransgenic mice (Ctr) and mice expressing Skp2 and N-Ras transgenic proteins. Thymocytes were treated with Con A (A) or with Con A and IL-2 (B) for 72 h. [3H]thymidine was added to the medium during the last 24 h. The results are shown as the mean of [3H]thymidine incorporation obtained in several independent experiments with seven nontransgenic mice, three CD4-SKP2 mice, five TMTV-Nras mice, and eight double transgenic mice.
Discussion
Mammalian Fbps form an expanding family of proteins that target a large number of cellular regulators for ubiquitin-mediated degradation (1, 22, 23). The data presented here provide a demonstration that an Fbp can be the product of a bona fide oncogene. In fact, we have shown with a mouse model that Skp2 cooperates with activated N-Ras in tumorigenesis.
Although Skp2 overexpression has been observed in transformed cell lines (5) and human cancers (Fig. 1), the overexpression of Skp2 alone is insufficient to induce neoplasia unless N-Ras is coexpressed (Figs. 2 and 3). Similarly, another well-established cell cycle regulator and protooncoprotein, cyclin D1, induces the generation of lymphomas in mice only in cooperation with Myc (24, 25). Furthermore, although p27-deficient mice do not spontaneously develop lymphomas, a synergism between Myc and p27 loss has been observed in lymphomagenesis. This effect is probably linked to the impressive thymic hyperplasia observed in p27-deficient mice (reviewed in ref. 9).
The cooperation between Skp2 and activated Ras also is observed in in vitro transformation assays with the use of mouse fibroblasts (unpublished results). In addition, cells overexpressing Skp2 and activated Ras induce tumors when injected into athymic nude mice (W. Krek, personal communication). The fact that TMTV-Nras tumors express higher levels of endogenous Skp2 than tumors of double transgenic mice (Fig. 3B) might explain why they contain low levels of p27 and further supports the view that elevated Skp2 levels provide a growth advantage to cells expressing N-Ras. Because most oncogenes have multiple targets and often activate more than one pathway, the molecular mechanisms of their cooperation are very complex. Similarly, the mechanisms of cooperation between Skp2 and N-Ras are not immediately evident. We hypothesize that one likely route of cooperation is that the well-established Ras-mediated activation of G1 Cdks leads to the phosphorylation of Skp2 substrates, allowing their recognition by Skp2. This hypothesis is based on and supported by the following observations: (i) Targets of SCF ligases need to be phosphorylated before they can be recognized by the Fbp subunit (reviewed in refs. 2 and 3), and all of the SCFSkp2 targets identified so far are Cdk substrates. (ii) The best characterized Skp2 substrate, p27, interacts with Skp2 only when phosphorylated on Thr-187 by Cdk2 (7, 8). (iii) Under conditions of growth inhibition, Skp2 expression induces a decrease in p27 levels only in the presence of active Cdks (A. Carrano and M.P., unpublished results). (iv) Cotransfection of cultured cells with Skp2 and cyclin E synergistically enhances cell cycle progression at levels of expression that had no effects individually (C. Nelson and J. Albrecht, personal communication).
But what are the targets of Skp2 in oncogenesis? Two substrates are well documented targets of Skp2: p27 and free, Cdk2-unbound cyclin E. Although it has been suggested that other cell cycle proteins (e.g., other G1 cyclins and E2F-1) are also Skp2 substrates, only limited evidence is currently available (18, 26). Furthermore, Skp2-deficient cells have elevated levels of p27 and free cyclin E but not of E2F-1 and other G1 cyclins (10). In the lymphomas of our transgenic mice, p27 is down-regulated but cyclins and E2F-1 are not (Fig. 3B), indicating that, at least in this tumor model, they are not Skp2 substrates. Although tumors of both single and double transgenic mice have decreased p27, we speculate that in tumors of double transgenic mice p27 degradation is accelerated by the presence of exogenous Skp2 (indeed these tumors have a shorter latency) and that this decrease might contribute to uncontrolled proliferation. Still, it is improbable that p27 is the sole essential target of Skp2. Rather, the accelerated degradation of several substrates (some of which are still unknown) is likely to play a role in oncogenic events mediated by Skp2. In agreement with this view, although activated Ras cooperates with Skp2 in transformation, it is not sufficient to transform mouse embryonic fibroblasts lacking p27 and p21 (27), suggesting that Skp2's role in oncogenesis involves more than just its function in p27 degradation.
A decrease in p27 levels, although not sufficient, appears to be a frequent step in the genesis of human lymphomas and carcinomas. In fact, an impressive number of studies has shown that low levels of p27 are of independent prognostic significance in these two types of cancers (reviewed in ref. 9). Furthermore, the most aggressive of these tumors contain increased p27 degradation activity (16, 28, 29). The increased Skp2 abundance and its inverse correlation with p27 levels found in TMTV-Nras tumors (Fig. 3B), human high-grade lymphomas (Fig. 1), oral squamous cell carcinomas (R. Jordan and J. Slingerland, personal communication), and breast and prostate carcinomas (M. Loda and M.P., unpublished results) strongly suggest that Skp2 may be responsible for p27 destabilization observed in these tumors. Furthermore, high levels of Skp2 are not due just to increased proliferation, inasmuch as the direct correlation between Skp2 expression and high proliferation observed in lymphomas is abrogated in a subset of carcinomas.
In summary, this study demonstrates that Skp2, an Fbp involved in the control of the G1-S transition, has oncogenic potential. Furthermore, the transgenic mice we have generated represent a unique tool for evaluating the functional interactions of Skp2 with other protooncogenes and the efficacy of potential therapeutic agents targeting the ubiquitin-proteasome pathway.
Acknowledgments
We thank R. Gimeno, A. C. Carrano, M. Loda, K. Zhou, and S. Chang for their contribution to this work; W. Krek for communication of results before publication; and J. Bloom, G. Draetta, D. Levy, and L. Yamasaki for critically reading the manuscript. M.P. is grateful to L. Yamasaki and T. H. Honir for insightful discussion and continuous support. G.I. is supported by National Institutes of Health (NIH) Grants CA76642 and CA14462; A.P. is supported by NIH Grant R01-CA36327; E.L. is supported by the Molecular Oncology Program (NIH Grant 5T32-CA09161); and M.P. is supported by an Irma T. Hirschl Scholarship, a Human Frontier Science Program Organization Grant (RG0229), NIH Grants R01-CA76584 and R01-GM57587, and Kaplan Comprehensive Cancer Center NIH Grants P30-CA16087 and R21-CA66229.
Abbreviations
- Fbp
F-box protein
- Skp
S-phase kinase-associated protein
- SLL
small lymphocytic lymphoma
- DLCL
diffuse large cell lymphoma
- TMTV
truncated mouse mammary tumor virus–long terminal repeat
- SCFs
ubiquitin protein ligases containing Skp1, a cullin subunit (called Cul1 in metazoans), Roc1 (also called Hrt1 or Rbx1), and one Fbp as basic subunits
- Cdk
cyclin-dependent kinase
Footnotes
This paper was submitted directly (Track II) to the PNAS office.
References
- 1.Bai C, Sen P, Hofman K, Ma L, Goebel M, Harper W, Elledge S. Cell. 1996;86:263–274. doi: 10.1016/s0092-8674(00)80098-7. [DOI] [PubMed] [Google Scholar]
- 2.Patton E, Willems A, Tyers M. Trends Genet. 1998;14:6–14. doi: 10.1016/s0168-9525(98)01473-5. [DOI] [PubMed] [Google Scholar]
- 3.Deshaies R J. Annu Rev Cell Dev Biol. 1999;15:435–467. doi: 10.1146/annurev.cellbio.15.1.435. [DOI] [PubMed] [Google Scholar]
- 4.Kipreos E, Pagano M. Genome Biol. 2000;1:3002.1–3002.7. doi: 10.1186/gb-2000-1-5-reviews3002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhang H, Kobayashi R, Galaktionov K, Beach D. Cell. 1995;82:915–925. doi: 10.1016/0092-8674(95)90271-6. [DOI] [PubMed] [Google Scholar]
- 6.Sutterluty H, Chatelain E, Marti A, Wirbelauer C, Senften M, Muller U, Krek W. Nat Cell Biol. 1999;1:207–214. doi: 10.1038/12027. [DOI] [PubMed] [Google Scholar]
- 7.Carrano A C, Eytan E, Hershko A, Pagano M. Nat Cell Biol. 1999;1:193–199. doi: 10.1038/12013. [DOI] [PubMed] [Google Scholar]
- 8.Tsvetkov L M, Yeh K H, Lee S, Sun H, Zhang H. Curr Biol. 1999;9:661–664. doi: 10.1016/s0960-9822(99)80290-5. [DOI] [PubMed] [Google Scholar]
- 9.Slingerland J, Pagano M. J Cell Physiol. 2000;183:10–17. doi: 10.1002/(SICI)1097-4652(200004)183:1<10::AID-JCP2>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 10.Nakayama K, Nagahama H, Minamishima Y, Matsumoto M, Nakamichi I, Kitagawa K, Shirane M, Tsunematsu R, Tsukiyama T, Ishida N, et al. EMBO J. 2000;19:2069–2081. doi: 10.1093/emboj/19.9.2069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Montagnoli A, Fiore F, Eytan E, Carrano A C, Draetta G, Hershko A, Pagano M. Genes Dev. 1999;13:1181–1189. doi: 10.1101/gad.13.9.1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schulman B, Carrano A C, Kinnucan E, Jeffrey P, Bowen Z, Elledge S, Harper W, Pagano M, Pavletich N. Nature (London) 2000;408:381–386. doi: 10.1038/35042620. [DOI] [PubMed] [Google Scholar]
- 13.Latres E, Chiaur D S, Pagano M. Oncogene. 1999;18:849–855. doi: 10.1038/sj.onc.1202653. [DOI] [PubMed] [Google Scholar]
- 14.Chiarle R, Podda A, Prolla G, Podack E R, Thorbecke G J, Inghirami G. J Immunol. 1999;163:194–205. [PubMed] [Google Scholar]
- 15.Mangues R, Symmans W F, Lu S, Schwartz S, Pellicer A. Oncogene. 1996;13:1053–1063. [PubMed] [Google Scholar]
- 16.Chiarle R, Budel L M, Skolnik J, Frizzera G, Chilosi M, Corato A, Pizzolo G, Magidson J, Montagnoli A, Pagano M, et al. Blood. 2000;95:619–626. [PubMed] [Google Scholar]
- 17.Gerdes J. Semin Cancer Biol. 1990;1:199–206. [PubMed] [Google Scholar]
- 18.Marti A, Wirbelauer C, Scheffner M, Krek W. Nat Cell Biol. 1999;1:14–19. doi: 10.1038/8984. [DOI] [PubMed] [Google Scholar]
- 19.Nguyen H, Gitig D M, Koff A. Mol Cell Biol. 1999;19:1190–1201. doi: 10.1128/mcb.19.2.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Adams J M, Harris A W, Strasser A, Ogilvy S, Cory S. Oncogene. 1999;18:5268–5277. doi: 10.1038/sj.onc.1202997. [DOI] [PubMed] [Google Scholar]
- 21.Mangues R, Schwartz S, Seidman I, Pellicer A. Mol Carcinog. 1995;14:94–102. doi: 10.1002/mc.2940140205. [DOI] [PubMed] [Google Scholar]
- 22.Cenciarelli C, Chiaur D S, Guardavaccaro D, Parks W, Vidal M, Pagano M. Curr Biol. 1999;9:1177–1179. doi: 10.1016/S0960-9822(00)80020-2. [DOI] [PubMed] [Google Scholar]
- 23.Winston J T, Koepp D M, Zhu C, Elledge S J, Harper J W. Curr Biol. 1999;9:1180–1182. doi: 10.1016/S0960-9822(00)80021-4. [DOI] [PubMed] [Google Scholar]
- 24.Bodrug S, Warner B, Bath M, Lindeman G, Harris A, Adams J. EMBO J. 1994;13:2124–2130. doi: 10.1002/j.1460-2075.1994.tb06488.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lovec H, Grzeschiczek A, Kowalski M, Moroy T. EMBO J. 1994;13:3487–3495. doi: 10.1002/j.1460-2075.1994.tb06655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yu Z K, Gervais J, Zhang H. Proc Natl Acad Sci USA. 1998;95:11324–11329. doi: 10.1073/pnas.95.19.11324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Groth A, Weber J D, Willumsen B M, Sherr C J, Roussel M F. J Biol Chem. 2000;275:27473–27480. doi: 10.1074/jbc.M003417200. [DOI] [PubMed] [Google Scholar]
- 28.Loda M, Cukor B, Tam S, Lavin P, Fiorentino M, Draetta G, Jessup J, Pagano M. Nat Med. 1997;3:231–234. doi: 10.1038/nm0297-231. [DOI] [PubMed] [Google Scholar]
- 29.Piva R, Cancelli I, Cavalla P, Bortolotto S, Dominguez J, Draetta G F, Schiffer D. J Neuropathol Exp Neurol. 1999;58:691–696. doi: 10.1097/00005072-199907000-00002. [DOI] [PubMed] [Google Scholar]