Abstract
The effects of 13 soybean varieties (356, M4, M7, M9, Clark, Sahar, JK, BP, Williams, L17, Zane, Gorgan3, and DPX) on nutritional indices of the cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), were determined at 25 ± 1° C, 65 ± 5% RH and a photoperiod of 16:8 L:D. Fourth instar larvae reared on Zane showed the highest efficiency of conversion of digested food (ECD) and approximate digestibility (AD) values (0.299 and 0.867, respectively) compared with other varieties. The lowest value of ECD and food consumed (FC) was on 356 (0.133 and 53.82 mg, respectively). The highest and lowest efficiency of conversion of ingested food (ECI) of fifth instar larvae (0.235 and 0.156, respectively) were on Zane and M4, respectively. The ECI and ECD values of whole larval instars were the highest on M7 (0.524 and 0.820, respectively) and lowest on Sahar (0.279 and 0.353, respectively). However, the highest and lowest value of consumption index (CI) was on M7 (7.351) and BP (3.462). Among the different varieties of soybean, the highest AD value was on M9 (0.858), and the lowest was on Zane (0.597). The results indicated that M4, Sahar, and JK were partially resistant to H. armigera.
Keywords: antibiosis, food consumption, insect weight, Noctuidae, soybean resistance
Introduction
The cotton bollworm, Helicoverpa armigera (Hübner) (Lepidoptera: Noctuidae), is a highly destructive polyphagous pest causing severe loss to many economically important crops, including soybean, in Iran (Farid 1986) and elsewhere in the world (Reddy et al. 2004; Subramanian and Mohankumar 2006; Mironidis and Savopoulou-Soultani 2008). It is a major pest for 181 cultivated and uncultivated plant species, distributed in 45 families in India (Manjunath et al. 1989), and it creates serious problems in tomato (Moral Garcia 2006), leguminous (Singh and Mullick 1997), cotton (Kranthi et al. 2002), and pigeonpea (Kumari et al. 2006). Every year, the larvae of this species cause substantial economic losses to cotton, corn, tomato, legumes, and vegetable crops (Liu et al. 2004). The outbreak of this pest has been attributed to the development of insecticide resistance and the use of broad spectrum insecticides, which are known to have an detrimental effect on populations of its natural enemies and nutritional and bioclimatic factors in host plants (Fitt et al. 1995; Naseri et al. 2009). Therefore, the present research has increasingly been carried out to identify alternative measures to chemical control.
The chemical composition of host plants significantly affects survival, growth, and reproduction of phytophagous insects (Bernys and Chapman 1994). Food consumption and utilization link plant attributes with insect performance (Slansky 1990). For polyphagous insects, the availability of different host plants plays an important role in triggering population outbreaks (Singh and Parihar 1988). Growth, development, and reproduction of insects are strongly dependent on the quality and quantity of food consumed (Scriber and Slansky 1981).
Of the tools of pest management, host plant resistance is important in terms of being both economically and environmentally acceptable. Therefore, as a method of controlling pest insects, host plant resistance is not only favorable to the environment, but also reduces expenses for growers (Li et al. 2004). The factors determining nutrient availability for growth and maintenance over a given period of development are the amount and type of food consumed and the efficiency with which is utilized (Barton Browne and Raubenheimer 2003).
Previously Naseri et al. (2009) examined life history and fecundity of H. armigera on different varieties of soybean. The data obtained in that study allowed for an estimate of two of the major factors determining the susceptibility of soybean varieties, the developmental time and fecundity of H. armigera. In this research, this work was extended, and the effects of different soybean varieties on nutritional indices of H. armigera were elucidated as other factors determining the susceptibility of the examined varieties to this pest. By combining the data from the earlier study and the findings of the current research, a comprehensive scheme for an integrated pest management program for H. armigera on soybean could be designed.
In spite of the economic importance of H. armigera, no information exists on the nutritional indices of this pest on different soybean varieties, although some related studies have been conducted on the effects of host plants, apart from soybean varieties, on nutritional indices of H. armigera (Ashfaq et al. 2003) and on growth and food consumption of Heliothis zea (Farrar and Kennedy 1987). Therefore, the present study provides new information on the nutritional indices of H. armigera on different soybean varieties.
Materials and Methods
Plant sources
Seeds of the 13 soybean (Glycine max (L.) Merrill) varieties, including 356 (Delsoy4210), M4, M7, M9, Clark, Sahar, JK, BP, Williams, L17, Zane, Gorgan3, and DPX, were acquired from the Plant and Seed Modification Research Institute, Karaj, Iran. They were grown in the research field of Tarbiat Modares University in the suburbs of Tehran, Iran in May 2008. For this study, the leaves and pods of different soybean varieties were transferred to a growth chamber at 25 ± 1° C, 65 ± 5% RH, and a photoperiod of 16:8 L:D and used for feeding of first larval instars (leaves) and second to fifth larval instars (pods).
Laboratory colony
Originally, H. armigera specimens were collected from cotton fields in the Moghan region located in northwest Iran in July 2007. Stock culture was initiated on an artificial diet (Twine 1971; Naseri et al. 2009) in a growth chamber at 25 ± 1°, 65 ± 5% RH, and a photoperiod of 16:8 L:D.
Experiments
Newly hatched larvae were collected from the stock culture and divided into four replicates (10 larvae in each) and transferred into plastic containers (diameter 16.5 cm, depth 7.5 cm) with a hole covered by a fine mesh net for ventilation, containing the fresh leaves of each examined plant. The petioles of detached leaves were inserted in water-soaked cotton to maintain freshness. Nutritional indices were determined using second to fifth instars as they were more easily measurable than the first instar. A fine camel's hair brush was used to transfer the younger larvae. First instar larvae were reared in groups until the third instar, after which they were separated into individual plastic tubes (diameter 3 cm, depth 5 cm) to prevent cannibalism. Fifth instar larvae were kept in the above-described tubes for pre-pupation and pupation.
A gravimetric technique was used to determine weight gain, food consumption, and feces produced. Nutritional indices were measured on the dry weight basis. After measuring the weight of the second instar larvae, they were introduced on the pods of different soybean varieties, and the weights of the larvae were recorded daily before and after feeding until they finished feeding and reached the pre-pupal stage. The pre-pupa, pupa, and adults from the larvae reared on each variety were weighed as well. The initial fresh pods and the pods and feces remaining at the end of each experiment were weighed daily. The quantity of food ingested was determined by subtracting the diet remaining at the end of each experiment from the total weight of diet provided. The weight of feces produced by the larvae fed on each soybean variety was recorded daily. To find the dry weights of the pods, feces, and larval to adult stages, extra specimens (20 specimens for each) were weighed, oven-dried (48 hours at 60° C), and then re-weighed to establish a percentage of their dry weight. The forewing area of H. armigera adults reared on each soybean variety during its immature stages was also measured.
The following formulae were used according to Waldbauer (1968) to calculate CI (consumption index), AD (approximate digestibility), ECI (efficiency of conversion of ingested food) and ECD (efficiency of conversion of digested food):
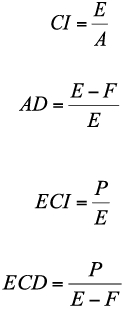 |
where, A = mean dry weight of insect over unit time, E = dry weight of food consumed, F = dry weight of feces produced, and P = insect dry weight gain.
Data analysis
Nutritional indices of H. armigera reared on different soybean varieties were analyzed with one way ANOVA using the statistical software Minitab 14 to determine the similarities or significant differences. Statistical differences among the means were evaluated using the least significant differences (LSD) test at α = 0.05. Data were checked for normality prior to analysis.
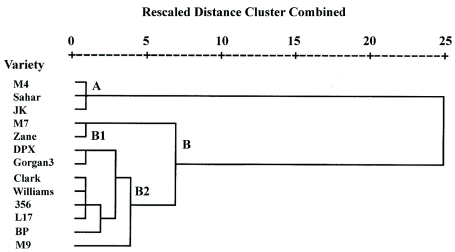
A dendrogram of soybean varieties based on nutritional indices of H. armigera overall 2nd to 5th instars (second instar + third instar + fourth instar + fifth instar larvae), herein whole larval instars, reared on different varieties of soybean was constructed after cluster analysis by Ward's method using SPSS 16.0 statistical software.
Results
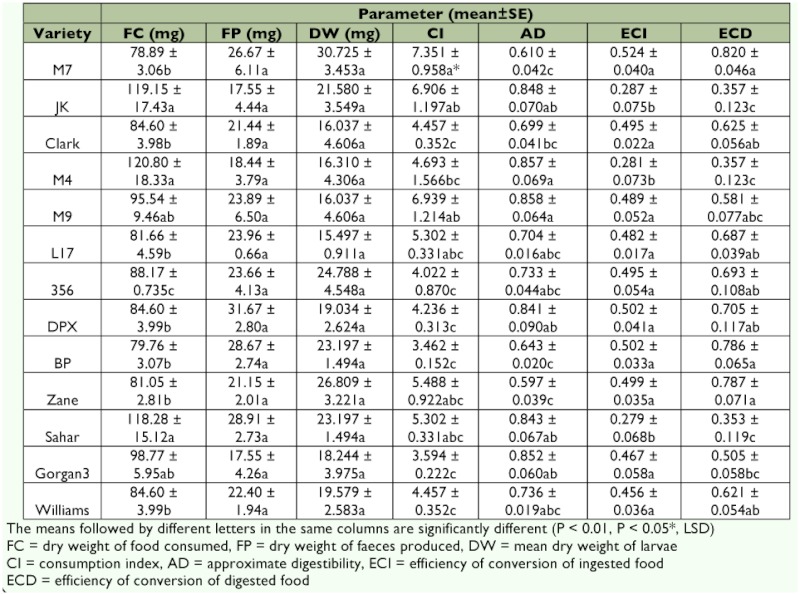
The results of the nutritional indices of fourth instar, fifth instar, and whole larval instars of H. armigera are provided in Tables 1, 2, and 3. Nutritional indices of fourth instar larvae of H. armigera were significantly different on soybean varieties (p < 0.05). The larvae reared on Zane showed the highest value of ECD (0.299 ± 0.049) (F = 2.42; df = 12, 150; p < 0.01) and AD (0.867 ± 0.019) (F = 4.06; df = 12,158; p < 0.01) compared with those reared on the other varieties. The lowest value of ECD and food consumed (F = 1.94; df = 12, 179; p < 0.05) was on 356 (0.133 ± 0.023 and 53.82 ± 4.83 mg, respectively). The larvae fed on DPX had the lowest AD value (0.532 ± 0.071). The CI of larvae reared on M7 showed the highest value (5.309 ± 0.466). However, the lowest value of this parameter (2.431 ± 0.216) was observed on variety Zane (F = 5.29; df = 12, 164; p < 0.01). Data in Table 1 indicates that there were no significant differences between larval weight (F = 0.58; df = 12, 152; p = 0.858) and ECI (F = 1.00; df = 12, 152; p = 0.448) of H. armigera on soybean varieties.
Table 1.
Nutritional indices of fourth instar larvae of Helicoverpa armigero on different soybean varieties
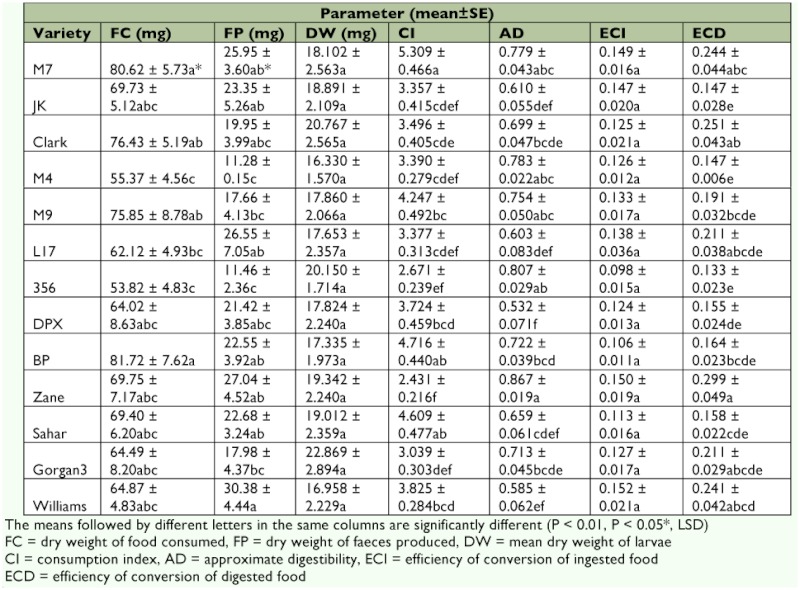
Table 2.
Nutritional indices of fifth instar larvae of Helicoverpa armigera on different soybean varieties
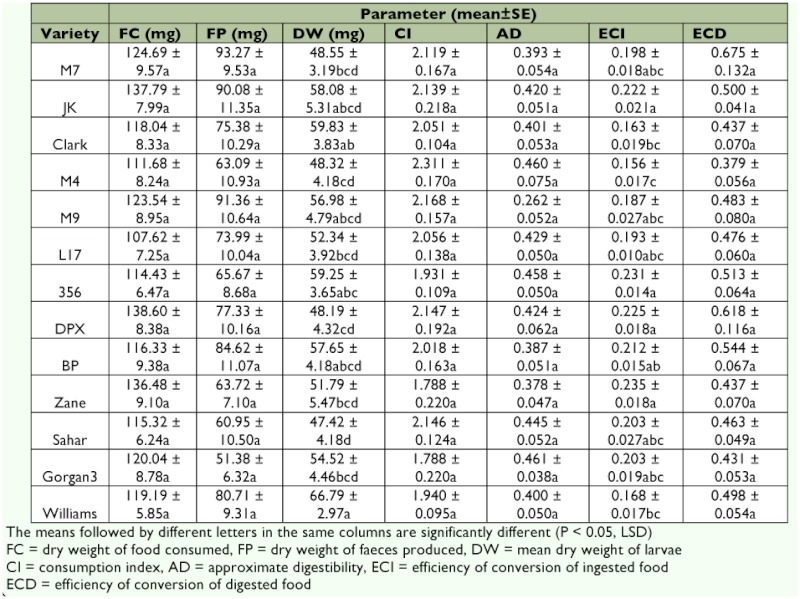
Table 3.
Nutritional indices of whole larval instars of Heiicoverpa armigera on different soybean varieties
The larval weight (F = 2.16; df = 12, 365; p < 0.05) and ECI (F = 1.93; df = 12, 179; p < 0.05) of fifth instar H. armigera were found to be significantly different based on the soybean varieties on which individuals were reared. However, no significant difference was observed on the other estimated parameters of the pest on soybean varieties. The highest and lowest ECI values of H. armigera (0.235 ± 0.018 and 0.156 ± 0.017, respectively) were on Zane and M4, respectively. The larval weight of H. armigera showed significant difference, being heaviest on Williams (66.79 ± 2.97 mg) and lightest on Sahar (47.42 ± 4.18 mg).
The results presented in Table 3 for whole larval instars showed no significant difference for feces produced (F = 1.42; df = 12, 39; p = 0.198) and larval weight (F = 1.92; df = 12, 39; p = 0.063). The ECI (F = 3.46; df = 12, 39; p < 0.01) and ECD (F = 3.67; df = 12, 39; p < 0.01) values of the whole larval instars were the highest on M7 (0.524 ± 0.040 and 0.820 ± 0.046, respectively) and lowest on Sahar (0.279 ± 0.068 and 0.353 ± 0.119, respectively). However, the highest and lowest values of CI were on M7 (7.351 ± 0.958) and BP (3.462 ± 0.152), respectively (F = 2.52; df = 12, 39; p < 0.05). Among the different varieties of soybean, the highest value of AD was on M9 (0.858 ± 0.064), and the lowest was on Zane (0.597 ± 0.039) (F = 3.39, df = 39, p < 0.01).
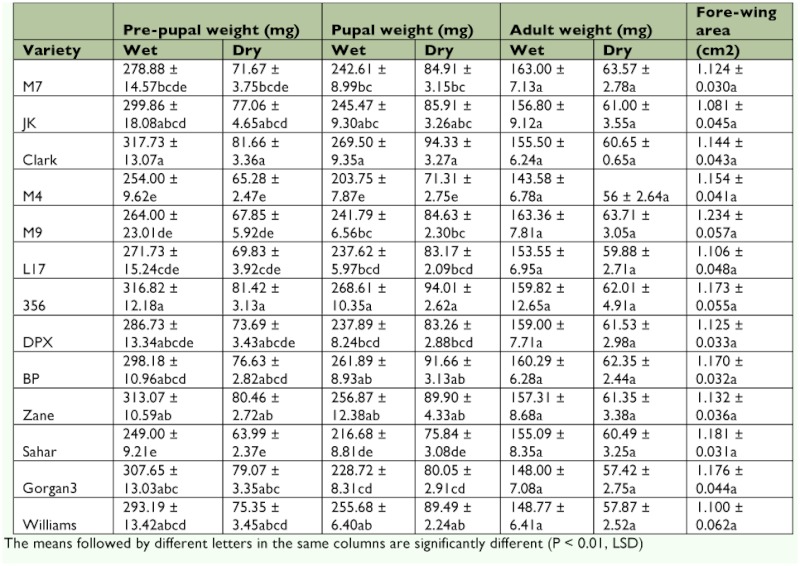
Different soybean varieties showed no significant effect on the adults' weight and forewing area of H. armigera. However, the wet and dry weights of the pre-pupa (F = 2.82; df = 12, 184; p < 0.01) and pupa (F = 5.01; df = 12, 204; p < 0.01) were affected significantly by the variety of soybean (Table 4). Pre-pupa and pupa of larvae reared on Clark were heavier than those of larvae reared on other varieties tested.
Table 4.
The mean (±SE) body weights of pre-pupa, pupa and adult stages and fore-wing area of Helicoverpa armigera on different soybean varieties
Cluster analysis
A dendrogram based on nutritional indices of H. armigera whole larval instars reared on different varieties of soybean is shown in Figure 1. The dendrogram shows two distinct clusters labelled A and B (including subclusters B1 and B2). Different varieties were grouped within each cluster based on the comparison of the nutritional indices of H. armigera reared on the varieties. Cluster A included M4, Sahar, and JK as a partially resistant group; cluster B consisted of subclusters B1 (M7 and Zane) as a susceptible group and B2 (DPX, Gorgan3, Clark, Williams, 356, L17, BP, and M9) as an intermediate group.
Figure 1.
Dendrogram of different soybean varieties based on nutritional indices of Helicoverpa armigera reared on different soybean varieties. High quality figures are available online.
Discussion
Using resistant varieties is one of the core strategies of an integrated pest management program, and secondary substances of plants or allelochemicals play a major role in plant resistance to pests (Wilson and Huffaker 1976). The use of soybean resistant to insects offers an important tool in integrated pest management (Endo et al. 2007). Differences in allelochemical concentrations between host plant varieties can affect an insect's performance as larva (Martin and Pulin 2004). The ability of an organism to convert nutrients, especially protein, will positively influence its growth and development (Sogbesan and Ugwumba 2008).
Significant differences were found within the nutritional indices, especially ECI and ECD values, of H. armigera reared on different soybean varieties, suggesting that the varieties have different nutritional value. Among nutritional indices, ECI may vary with the digestibility of food and the proportional amount of the digestible portion of food which is converted to body mass and metabolized for energy needed for vital activity (Abdel-Rahman and Al-Mozini 2007). ECI is an overall measure of an insect's ability to utilize the food ingested for growth and development, and ECD is a measure of the efficiency of conversion of digested food into growth (Nathan et al. 2005). Change in ECD also indicates the overall increase or decrease of the proportion of digested food metabolized for energy. Therefore, no change in ECI and ECD values indicate that ingested secondary biochemicals do not exhibit any chronic toxicity (Koul et al. 2004).
No significant difference was observed on the nutritional indices of the fifth instar except for the larval weight and ECI. However, the nutritional indices of the fourth instar larvae of H. armigera were significantly different depending on the type of soybean variety. Therefore, the data generated for the fourth and fifth instars are not consistent with each other. This is due to the fact that the nutritional requirements of an insect change during development, and such changes are typically reflected in changes of food consumption and feeding behavior (Barton Browne 1995). In larvae, the nutritional requirements over different developmental periods are positively correlated with growth over that period, since growth is directly based on nutrient input. It is likely due to the fact that nutritional requirements would be positively correlated with the mass of the insect (Schroeder 1981; Phillipson 1981). According to Barton Browne and Raubenheimer (2003), total consumption in the fifth instar of H. armigera reared on a navy bean-based diet was about 3.5 times greater than in the fourth instar, mainly due to the greater rate of ingestion. Furthermore, the results of life table studies of H. armigera on different host plants (Liu et al. 2004) showed that the fourth instar larvae reared on corn were the heaviest, while larvae reared on tomato and tobacco were the lightest. However, the last instar larvae fed on cotton were heavier than those reared on other host plants. Another possible reason for this variation could be due to the age of larva in a particular stadium at the time of weighing. For instance, the weights of either fourth or fifth stadia are expected to be lower when the larvae are near to entering the next stadium (where the larva stops feeding before entering the next stadium) or have recently entered the next stadium (where it looses some water and the exuviae) as compared to larvae growing in the mid-part of any stadia.
Additionally, differences in physiological changes during penultimate and ultimate instar larvae are probably partially responsible for the differences in data generated for these two larval instars on soybean varieties. Juvenile hormone (JH) is one of the major controlling hormones in development changes such as molting and metamorphosis. Juvenile hormone also determines whether major changes will occur in internal organs; usually little or no changes in internal morphology occur between larval molts, but major changes occur during transformation into pupa or adult (Nation 2000). Physiological changes in the nervous system of the fifth instar cause cessation of feeding, induces wandering behavior, and metabolic changes that occur in the fat body. Because of such physiological and behavioral changes, the feeding period of the larvae was shorter in fifth instar than the fourth instar, and subsequently nutritional responses of these two larval instars were different.
The highest ECI value of H. armigera was on varieties Zane and M7, indicating that they were more efficient at the conversion of ingested food to biomass. As can be seen in Table 3, the larvae fed on the Sahar variety had the lowest value of ECD, which suggests that these larvae were apparently not as efficient in turning digested food into biomass. It is well known that the degree of food utilization depends on the digestibility of food and the efficiency with which digested food is converted into biomass (Batista Pereira et al. 2002). The reduction in dietary utilization suggests that reduction in nutritional values may be resulted from both behavioral and physiological effects (Nathan et al. 2005). The mean ECD value from this study of whole larval instars reared on different soybean varieties was higher than that reported by Wang et al. (2006) on an artificial diet (0.412 ± 0.012).
Among different varieties of soybean, the highest CI value of H. armigera was on variety M7, indicating that the rate of intake relative to the mean larval weight during the feeding period was the highest on this variety. The results for the AD value of fourth instar larvae of H. armigera fed on Clark (0.699 ± 0.047) and Sahar (0.659 ± 0.061) were nearly similar to those reported by Ashfaq et al. (2003) on Sorghum vulgaris Pers. (0.697) and Gossypium hirsutum L. var. NIAB-98 (0.662). Wang et al. (2006) noted that AD value of H. armigera was 0.214 ± 0.013 on an artificial diet.
According to the results of the cluster analysis, grouping within each cluster might be due to a high level of physiological similarity of soybean varieties, whereas the separate clusters might present significant variability in physiological characteristics between clusters. The results of the comparison of nutritional indices of H. armigera on different soybean varieties revealed that cluster A varieties were the least suitable and that subcluster B1 varieties were the most suitable host plants for H. armigera, while the varieties in subcluster B2 had an intermediate status.
The body weight is an important fitness indicator of insect population dynamics (Liu et al. 2004). Pupal weight can be an indirect, but easily measured, indicator of lepidopteran fitness (Leuck and Perkins 1972). The pupae produced by larvae reared on Sahar and M4 were lighter than that of pupae produced by larvae reared on the other varieties. This reinforces the suggestion that Sahar and M4 are more unsuitable host plants for H. armigera larvae than the others. Liu et al. (2004) showed that the pupal weight of H. armigera, which ranged from 167.1 ± 3.9 mg on tomato to 285.2 ± 4.2 mg on corn, was affected by different host plants. The present findings on the pupal weight of H. armigera reared on variety Zane (256.87 ± 12.38 mg) were similar to those reported by Liu et al. (2004) on common bean (257.1 ± 5.1 mg). Furthermore, the heaviest pupal weight of H. armigera was on variety Clark. According to an earlier study (Naseri et al. 2009), the larval period of H. armigera was the shortest on variety Clark, and also, the cluster analysis of that study revealed that the variety Clark was grouped within a susceptible cluster. In spite of the fact that a significant difference was found between the pupal weights of H. armigera on 13 soybean varieties, no significant differences were observed for adult weights.
The quality of larval food may affect the pupal and adult phenotypic characteristics. Obvious effects of larval diets are pupal distortions and wing malformations in the imago (Rosenthal and Dahlman 1975). The fecundity (number of eggs laid per female), longevity, and forewing area of lepidopteran adults are the most commonly used parameters for determining the effect of larval diet on the adult stage. In this study, adult weight and forewing area of adults reared on different soybean varieties was examined. Because no significant effects were found for the larval host plants (soybean varieties) on the adult size (forewing area), these effects likely have disappeared in the weight of adults. However, previous research (Naseri et al. 2009) showed significant effects on fecundity of H. armigera fed on different soybean varieties. Additionally, the insect's ability to store energy (e.g., pupal weight and lipids and glycogen levels) varied depending on the larval host plants (Liu et al. 2007). However, the effects of host plants on pupal weight, adult weight, and larval growth are independent of each other (Hwang et al. 2008).
The results of the present study suggested that M7 and Zane were more nutritive, and M4, Sahar and JK were less nutritive for H. armigera larvae than the others. The results related to M7 and Sahar (as suitable and unsuitable host plants, respectively) are in agreement with previous findings (Naseri et al. 2009). The results of that study on the life history and fecundity of H. armigera reared on the 13 soybean varieties indicated that the shortest development time, the lowest percentage mortality of immature stages, highest daily fecundity (eggs per reproduction day), and the total fecundity (eggs during reproduction period) were on variety M7, which is consistent with the current research regarding ECI and ECD values of whole larval instars on this variety. Cluster analysis of the previous study and the present experiment strongly demonstrate the susceptibility of M7 to H. armigera compared with the other varieties. Additionally, the dendrogram of soybean varieties of that study showed that variety Sahar was partially resistant due to longer development time, higher mortality, and lower development index of the immature stages on this variety, which is consistent with the results of cluster analysis of the present research on nutritional indices of H. armigera on 13 soybean varieties.
Analysis of nutritional indices can lead to the understanding of the behavioral and physiological basis of an insect response to host plants (Lazarevic and Peric-Mataruga 2003). Variation in the nutritional indices of the pest on different soybean varieties could be due to the result of differences in plant quality, either reflected by a difference in nutrients required by the pest or differences in the level of secondary biochemicals. The least suitability of some varieties as a host plant of H. armigera may be due to the presence of some secondary phytochemicals in these varieties acting as antixenotic and/or antibiotic agents or absence of primary nutrients essential for growth and development of H. armigera.
There are many factors affecting host suitability including nutrient content and secondary substances of the host and the capability of digestion and assimilation by an insect. For a better understanding of the insect-plant interaction, basic biochemical studies for the extraction and identification of phytochemicals, which adversly influence the build up of H. armigera populations on soybean are required. Through this research, the population dynamics of the pest may be determined on different host varieties and the information could be used to manage the pest population to below the economic injury level. Meanwhile, these results provide data for establishing suitable conditions for rearing H. armigera. For instance, mass culture methods could be improved by selecting host plants for rapid development, maximum survival, or high fecundity in order to use these individuals for mass rearing of natural enemies.
Acknowledgements
This research was partly supported by a grant from the Center of Excellence for Integrated Pests and Diseases Management of Oil Crops of Iran and partly from Tarbiat Modares University, which is greatly appreciated.
Abbreviations
- AD
approximate digestibility;
- Cl
consumption index;
- ECD
efficiency of conversion of digested food;
- ECI
efficiency of conversion of ingested food
References
- Abdel-Rahman HR, Al-Mozini RN. Antifeedant and toxic activity of some plant extracts against larvae of cotton leafworm Spodoptera littoralis (Lepidoptera: Noctuidae). Pakistan Journal of Biological Science. 2007;10:4467–4472. doi: 10.3923/pjbs.2007.4467.4472. [DOI] [PubMed] [Google Scholar]
- Ashfaq M, Ahmad KJ, Ali A. Morphophysical factors affecting consumption and coefficient of utilization of Helicoverpa armigera (Hubner). Pakistan Journal of Applied Sciences. 2003;3:225–230. [Google Scholar]
- Barton Browne L. Ontogenetic changes in feeding behavior. In: Chapman RF, Boer Gde, editors. Regulatory Mechanisms in Insect Feeding. Chapman & Hall; 1995. pp. 307–342. [Google Scholar]
- Barton Browne LB, Raubenheimer D. Ontogenetic changes in the rate of ingestion and estimates of food consumption in fourth and fifth instar Helicoverpa armigera caterpillars. Journal of Insect Physiology. 2003;49:63–71. doi: 10.1016/s0022-1910(02)00247-0. [DOI] [PubMed] [Google Scholar]
- Batista Pereira GL, Petacci F, Fernandes BJ, Correa AG, Vieira PC, Fatima da Silva M, Malaspina O. Biological activity of astilbin from Dimorphandra mollis against Anticarsia gemmatalis and Spodoptera frugiperda. Pest Management Science. 2002;58:503–507. doi: 10.1002/ps.478. [DOI] [PubMed] [Google Scholar]
- Bernys EA, Chapman RF. Host-Plant Selection by Phytophagous Insects. Chapman and Hall; 1994. [Google Scholar]
- Endo N, Hirakawa I, Wada T, Tojo S. Induced resistance to the common cutworm, Spodoptera litura (Lepidoptera: Noctuidae) in three soybean cultivars. Applied Entomology and Zoology. 2007;42:199–204. [Google Scholar]
- Farid A. Study of bollworm Heliothis armigera (Hub.) on tomato in Jyroft and Kahnuj. Applied Entomology and Phytopathology. 1986;54:15–24. [Google Scholar]
- Farrar RR, Kennedy GG. Growth, food consumption and mortality of Heliothis zea larvae on foliage of the wild tomato Lycopersicon hirsutum f. glabratum and the cultivated tomato, L. esculentum. Entomologia Experimentalis et Applicata. 1987;44:213–219. [Google Scholar]
- Fitt GP. The ecology of Heliothis in relation to agroecosystems. Annual Review of Entomology. 1989;34:17–52. [Google Scholar]
- Fitt GP, Dillon ML, Hamilton JG. Spatial dynamics of Helicoverpa populations in Australia: simulation modeling and empirical studies of adult movement. Computers and Electronics in Agriculture. 1995;13:177–192. [Google Scholar]
- Hwang SY, Liu CH, Shen TC. Effects of plant nutrient availability and host plant species on the performance of two Pieris butterflies (Lepidoptera: Pieridae). Biochemical Systematics and Ecology. 2008;36:505–513. [Google Scholar]
- Koul O, Singh G, Sing R, Singh J. Bioefficacy and mode-of-action some limonoids of salanin group from Azadirachta indica A. Juss and their role in a multicomponent system against lepidopteran larvae. Journal of Bioscience. 2004;29:409–416. doi: 10.1007/BF02712112. [DOI] [PubMed] [Google Scholar]
- Kranthi S, Kranthi KR, Wanjari RR. Wound inducible defense related proteins in cotton against Helicoverpa armigera. Indian Journal of Entomology. 2002;64:73–79. [Google Scholar]
- Kumari DA, Reddy DJ, Sharma HC. Antixenosis mechanism of resistance in pigeonpea to the pod borer, Helicoverpa armigera. Journal of Applied Entomology. 2006;130:10–14. [Google Scholar]
- Lazarevic J, Peric-Mataruga V. Nutritive stress effects on growth and digestive physiology of Lymantria dispar larvae. Yugoslav Medical Biochemistry. 2003;22:53–59. [Google Scholar]
- Leuck DB, Perkins WD. A method of evaluating fall armyworm progeny reduction when evaluating control achieved by hostplant resistance. Journal of Economic Entomology. 1972;65:482–483. [Google Scholar]
- Li Y, Hill CB, Hartman GL. Effect of three resistant soybean genotypes on the fecundity, mortality and maturation of soybean aphid (Homoptera, Aphididae). Journal of Economic Entomology. 2004;97:1106–1111. doi: 10.1093/jee/97.3.1106. [DOI] [PubMed] [Google Scholar]
- Liu Z, Gong P, Wu K, Wei W, Sun J, Li D. Effects of larval host plants on overwintering preparedness and survival of cotton bollworm, Helicoverpa armigera (Hubner) (Lepidoptera: Noctuidae). Journal of Insect Physiology. 2007;53:1016–1026. doi: 10.1016/j.jinsphys.2007.05.005. [DOI] [PubMed] [Google Scholar]
- Liu Z, Li D, Gong PY, Wu KJ. Life table studies of the cotton bollworm, Helicoverpa armigera (Hubner) (Lepidoptera, Noctuidae), on different host plants. Environmental Entomology. 2004;33:1570–1576. [Google Scholar]
- Manjunath TM, Bhatnagar VS, Pawar CS, Sithanantham S. Economic importance of Heliothis spp. in India and an assessment of their natural enemies and host plants. Proceedings of the Workshop on Biological Control of Heliothis: Increasing the effectiveness of natural enemies. New Delhi, India: 1989. pp. 197–228. [Google Scholar]
- Martin LA, Pulin AS. Host-plant specialization and habitat restriction in an endangered insect, Lycaena dispar batavus (Lepidoptera: Lycaenidae) I. Larval feeding and oviposition preferences. European Journal of Entomology. 2004;101:51–56. [Google Scholar]
- Mironidis GK, Savopoulou-Soultani M. Development, survivorship and reproduction of Helicoverpa armigera (Lepidoptera, Noctuidae) under constant and alternating temperatures. Environmental Entomology. 2008;37:16–28. doi: 10.1603/0046-225X(2008)37[16:DSAROH]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- Moral Garcia FJ. Analysis of the spatiotemporal distribution of Helicoverpa armigera (Huber) in a tomato field using a stochastic approach. Biosystems Engineering. 2006;93:253–259. [Google Scholar]
- Naseri B, Fathipour Y, Moharramipour S, Hosseininaveh V. Comparative life history and fecundity of Helicoverpa armigera (Lepidoptera: Noctuidae) on different soybean varieties. Entomological Science. 2009;12(2):147–154. [Google Scholar]
- Nathan SS, Chung PG, Murugan K. Effect of biopesticides applied separately or together on nutritional indices of the rice leafolder Cnaphalocrocis medinalis. Phytoparasitica. 2005;33:187–195. [Google Scholar]
- Nation JL. Insect Physiology and Biochemistry. CRC Press; 2000. [Google Scholar]
- Phillipson J. Bioenergetic options and phylogeny. In: Townsend CR, Calow P, editors. Physiological Ecology: An Evolutionary Approach to Resource Use. Blackwell Scientific; 1981. [Google Scholar]
- Reddy KS, Rao GR, Rao PA, Rajasekhar P. Life table studies of the capitulum borer, Helicoverpa armigera (Hubner) infesting sunflower. Journal of the Entomological Research. 2004;28:13–18. [Google Scholar]
- Rosenthal GA, Dahlman DL. Nonprotein amino acid-insect interactions: II. Effects of canaline-urea cycle amino acids on growth and development of the tobacco hornworm, Manduca sexta L. (Sphingidae). Comparative Biochemistry and Physiology. 1975;52A:105–108. doi: 10.1016/s0300-9629(75)80138-1. [DOI] [PubMed] [Google Scholar]
- Schroeder LA. Consumer growth efficiencies: Their limits and relationships to ecological energetics. Journal of Theoretical Biology. 1981;93:805–828. [Google Scholar]
- Scriber JM, Slansky F. The nutritional ecology of immature insects. Annual Review of Entomology. 1981;26:183–211. [Google Scholar]
- Singh AK, Mullick S. Effect of leguminous plants on the growth and development of gram pod borer, Helicoverpa armigera. Indian Journal of Entomology. 1997;59:209–214. [Google Scholar]
- Singh OP, Parihar SBB. Effect of different hosts on the development of Heliothis armigera Hub. Bulletin of Entomology. 1988;29:168–172. [Google Scholar]
- Slansky F., Jr Insect nutritional ecology as a basis for studying host plant resistance. Florida Entomologist. 1990;73:359–378. [Google Scholar]
- Sogbesan AO, Ugwumba AAA. Nutritional evaluation of termite (Macrotermes subhyalinus) meal as animal protein supplements in the diets of Heterobranchus longifilis (Valenciennes, 1840) fingerlings. Turkish Journal of Fisheries and Aquatic Sciences. 2008;8:149–157. [Google Scholar]
- Subramanian S, Mohankumar S. Genetic variability of the bollworm, Helicoverpa armigera, occurring on different host plants. Journal of Insect Science. 2006;6:1–8. doi: 10.1673/2006_06_26.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Twine BH. Cannibalistic behaviour of Heliothis armigera (Hub.). Queensland Journal of Agricultural and Animal Sciences. 1971;28:153–157. [Google Scholar]
- Waldbauer GP. The consumption and utilization of food by insects. Advances in Insect Physiology. 1968;5:229–288. [Google Scholar]
- Wang Y, Cai QN, Zhang QW, Han Y. Effect of the secondary substances from wheat on the growth and digestive physiology of cotton bollworm Helicoverpa armigera (Lepidoptera: Noctuidae). European Journal of Entomology. 2006;103:255–258. [Google Scholar]
- Wilson F, Huffaker CB. The physiology, scope and importance of biological control. Huffaker CH, Messenger PS, editors. Theory and Practice of Biological Control. 1976:3–15.