Abstract
Bacteria were isolated from the crop and midgut of field collected Bactrocera cacuminata (Hering) and Bactrocera tryoni (Froggatt) (Diptera: Tephritidae). Two methods were used, firstly isolation onto two types of bacteriological culture media (PYEA and TSA) and identification using the API-20E diagnostic kit, and secondly, analysis of samples using the 16S rRNA gene molecular diagnostic method. Using the API-20E method, 10 genera and 17 species of bacteria in the family Enterobacteriaceae were identified from cultures growing on the nutrient agar. The dominant species in both the crop and midgut were Citrobacter freundii, Enterobacter cloacae and Klebsiella oxytoca. Providencia rettgeri, Klebsiella pneumoniae ssp ozaenae and Serratia marcescens were isolated from B. tryoni only. Using the molecular cloning technique that is based on 16S rRNA gene sequences, five bacteria classes were dignosed — Alpha-, Beta-, Gamma- and Delta- Proteobacteria and Firmicutes — including five families, Leuconostocaceae, Enterococcaceae, Acetobacteriaceae, Comamonadaceae and Enterobacteriaceae. The bacteria affiliated with Firmicutes were found mainly in the crop while the Gammaproteobacteria, especially the family Enterobacteriaceae, was dominant in the midgut. This paper presents results from the first known application of molecular cloning techniques to study bacteria within tephritid species and the first record of Firmicutes bacteria in these flies.
Keywords: Bactrocera cacuminata, Bactrocera tryoni, PCR, 16S rRNA gene, Enterobacteriaceae, lactic acid bacteria
Introduction
The bacteria associated with tephritid fruit flies have been widely studied using traditional microbiological methods where the gut microflora is cultured on specific nutrient agar media and subsequently identified by phenotypic characterization of isolates using the API-20E system (Drew et al. 1983; Kuzina et al. 2001; Marchini et al. 2002). The API-20E system is suitable for the identification of enteric bacteria and provides a convenient method to inoculate and read tests relevant to members of the family Enterobacteriaceae and associated organisms. Consequently, a large number of studies have been conducted on bacteria isolated from the alimentary tract of fruit flies using the API-20E system resulting in identification of bacteria mainly belonging to the family Enterobacteriaceae with the species most commonly identified as Citrobacter freundii, Enterobacter agglomerans, Enterobacter cloacae, Providencia rettgeri and Klebsiella oxytoca (Capuzzo et al. 2005; Drew et al. 1983; Drew and Lloyd 1987; Fitt and O'Brien 1985; Howard et al. 1985; Kuzina et al. 2001; Lloyd et al. 1986; Murphy et al. 1994).
However, besides the Enterobacteriaceae, little is known of other species of bacteria in other families that may also inhabit the alimentary tract of fruit flies. Molecular approaches for the detection and characterization of microbes in other insect species have revealed considerable bacterial diversity (Friedrich et al. 2001; Haynes et al. 2003; Schmitt-Wagner et al. 2003). In particular, nucleic acid sequence approaches of 16S rRNA genes are now revealing considerable new data on the microbial community of insects (Brauman et al. 2001). For example, considerable research using both culture-dependent and culture-independent techniques has been conducted on diagnosing the gut bacteria of Coleoptera (Delalibera et al. 2007; Vasanthakumar et al. 2006, 2008). Studies on the adult southern pine beetle, Dendroctonus frontalis, and the adult pine engraver, Ips pini, revealed that their bacterial gut communities have a relatively low species richness. In the adult emerald ash borer, a Agrilus planipennis, more diverse bacterial community was detected and, in all three cases, a higher diversity of bacteria was detected by the analysis of 16S rRNA gene sequences of gut isolates. To date, recent molecular diagnostic techniques have not been employed to identify the alimentary tract bacteria of fruit flies.
This paper presents results of research carried out on alimentary tract bacteria of two Australian fruit fly species, Bactrocera cacuminata (Hering) and Bactrocera tryoni (Froggatt) (Diptera: Tephritidae), identifying the bacteria using both the API-20E system and 16S rRNA gene molecular analyses, and based on these results, an analysis of bacteria species diversity and community similarity in these two species of fruit flies.
Methods and Materials
Fruit fly collecting and handling
Adult flies of B. cacuminata and B. tryoni were hand collected from fruiting host plants in Brisbane, Queensland, Australia during the months of February and March, 2007. Specimens of B. cacuminata were collected from wild tobacco, Solanum mauritianum Scopoli and B. tryoni from custard apple (Annona reticulata L.), guava (Psidium guajava L.) and loquat (Eriobotrya japonica (Thunberg) (Lindl.). Captured flies were held individually in clear plastic vials to prevent cross-contamination of bacteria between flies. The vials containing flies were plugged with cotton wool for ventilation and placed in a cool ice box in the field to immobilize them and to prevent flies from regurgitating their crop contents within the tubes.
Dissection and isolation of bacteria from the alimentary tract of fruit flies
Five males and five females of each species of the field-collected fruit flies were killed immediately on return to the laboratory by freezing at -20° C for 3 min. Flies were then surface sterilized by immersing in 70% ethanol for 1 min, 0.5% sodium hypochlorite for 1 min and then washed twice in sterile distilled water (modified from Lloyd 1991). The surface-sterilized flies were individually dissected under sterile distilled water in a sterile glass cavity block. Before dissecting, the water in each glass cavity box was sampled and spread onto tryptone soya agar (TSA) (Oxoid) and peptone yeast extract agar (PYEA) (Oxoid, www.oxiod.com) and incubated at 35° C for 24–48 h to determine whether any contaminant bacteria were present. The fruit fly sample was discarded if contamination occurred on the media. Although PYEA and TSA are recognised as media that grow similar groups of microorganisms, it was decided to use both in this study to maximise the chance of isolating most of the bacteria species in the fly.
The crop and midgut of each fly were aseptically removed following the method described by Drew et al. (1983) and Lloyd (1991), and placed in a sterile 1.5 ml microcentrifuge tube and homogenized with a sterile inoculation loop. These contents were spread onto TSA and PYEA and incubated at 35° C for 24–48 h. All steps in the isolation procedure were performed in a laminar flow hood to avoid aerial contamination. To avoid cross-contamination between the different gut regions, the crop was removed first by pinching the narrow entry tube and lifting it out, and then removing the midgut. If either organ broke open before removal, that fly was discarded. A qualitative assessment of the numbers and types of colonies growing on each plate was made after 24 and 48 hours, and the predominant types were purified through repeated subculturing. The method of purification was as follows: At the end of the incubation period, each bacterial colony was aseptically removed by using an inoculation loop, spread onto TSA and PYEA and incubated aerobically at 35° C for 24–48 h. Each colony was isolated on the basis of morphological appearance and sub-cultured twice to ensure purity.
Identification of bacteria isolates with API-20E
All bacteria isolates were initially Gram-stained for Gram-positive and Gram-negative identification and tested for oxidase/catalase activity. Gram negative and rod shaped bacteria were chosen for identification with the API-20E system (bioMerieux sa 62980, www.biomerieux.com). Analytical Profile Indexes from the API-20E system were used for diagnosing species within the family Enterobacteriaceae only. The ID profiles were rated from excellent to good, based on the API codes.
Molecular cloning study of the 16S rRNA gene of gut bacteria community in the fruit fly alimentary canal.
Steps in obtaining the crops and midguts of the flies for the molecular cloning study were the same as those described above.
Three flies of each sex were dissected under sterile distilled water. The crop and midgut were removed separately and placed into sterile 1.5 ml centrifuge tubes. DNA was extracted using a modification of the CTAB/phenol-chloroform DNA extraction protocol (Doyle and Doyle 1987).
Extracted DNA from the crop and midgut were combined and the fragments were amplified in a polymerase chain reaction (PCR) using the universal primers for bacteria, forward primer Y1 (5′-TGGCTCAGAACGAACGCTGGCGGC-3′) (Sigma, www.sigmaaldrich.com) and reverse primer Y2 (5′-CCCACTGCTGCCTCCCGTAGGAG T-3′) (Sigma) (Young, Downer & Eardly, 1991). The reactions were carried out in a 100 µl volume containing 2 µl of template DNA solution, 2 µM of the primer, 200 µM of deoxynucleosidetriphosphate (Astral Scientific, www.astralscientific.com; Bioline, www.bioline.com) and 2 U of Tag DNA polymerase (Astral scientific, Bioline). The amplifications were performed using the following protocol: initial denaturation at 94° C for 5 min; 30 cycles of 45 s at 94° C, 40 s at 62° C and 2 min at 72° C, and final extension at 72° C for 10 min. After the reaction, 5 µl aliquots of PCR products were examined by electrophoresis in 1% agarose gel. The PCR products were extracted with QIA quick gel extraction kit (Qiagen, www.qiagen) for ligation.
The ligations were performed in 10 µl containing 1 µl pDrive cloning vector (50 ng/µl), 2.5 µl mix DNA, 1.5 µl distilled water, and 5 µl 2x ligation master mix (Qiagen) and introduced into Escherichia coli (strain JM109, Promega, www.promega.com) by transformation. The recombinants were selected and verified at the correct insert size by vector-targeted PCR with primer M13 F (5′-GTAAAACGACGGCCAGT-3′) (Sigma) and M13 R (5′CAGGAAACAGCTATGAC-3′) (Sigma) by the following PCR protocol: an initial denaturation at 94° C for 4 min; 35 cycles of 30 sec at 94° C, 40 sec at 53° C and 60 sec at 72° C. Finally, samples were subjected to 72° C for 4 min and then held for an indefinite period at 4° C. From each clone library, 30 clones were randomly selected and sequenced.
Classification of sequences and phylogenetic analyses
The bacteria were diagnosed by uploading the sequences obtained from the 16S rRNA analyses onto Ribosonal Database Project II (RDP-II database) (Wang et al. 2007), and classified using the classifier tool. Further, a phylogenetic tree was constructed incorporating the bacteria diagnosed from the fruit flies and related type strains on the RDP database. This was achieved by using the Molecular Evolutionary Genetics Analysis (MEGA) version 4 (Kumar et al. 2008) to align the sequences and construct a neighbour — joining bootstrap tree utilizing Kumar's two-parameter model (Nei and Kumar 2000).
Morista index and rarefaction analyses
Morista index values of isolates diagnosed by both the API-20E and molecular cloning methods were calculated after the method of Krebs (1998). In addition, rarefaction curves were drawn for isolates with a 95% or more similarity, based on the 16S rRNA sequence data. For this, analytical rarefaction software was used (version 1.2; S.M. Holland, University of Georgia, Athens, Ga; http://www.uga.edu/strata/software.html) (Schmitt-Wagner et al. 2003).
Results
Overview of bacteria colonies isolated by dissection and culturing of alimentary parts
Of the two bacterial culture media used, more bacteria colonies grew on TSA than PYEA, while on both media more bacteria species were recovered from the fly midgut than the crop. A total of 125 bacteria colonies were isolated (Table 1), 49 from B. cacuminata (29 of these on TSA and 20 on PYEA) and 76 from B. tryoni (44 isolates on TSA and 32 on PYEA). The physical characteristics of the colonies were similar when growing on both culture media, most being cream with a few red and yellow colonies. No fungi and yeasts were recovered. Gram negative and rod shaped bacteria were the major microorganisms found in both species of fruit flies.
Table 1.
Summary of the number of bacteria colonies isolated from the crop and midguts of field collected adults of Bactrocera cacuminata (Hering) and Bactrocera tryoni (Froggatt) on two different culture media, TSA and PYEA.

Bacteria identification with API-20E
Identification using the API-20E system diagnosed one family, Enterobacteriaceae, with 10 genera and 17 known species of bacteria. The genera were Citrobacter, Enterobacter, Escherichia, Hafnia, Klebsiella, Pantoea, Providencia, Pseudomonas, Raoultella and Serratia (Table 2).
Table 2.
Genera and species of bacteria in the family Enterobacteriaceae, isolated from field collected adults of Bactrocera cacuminata (Hering) and Bactrocera tryoni (Froggatt) and identified with API-20 E.
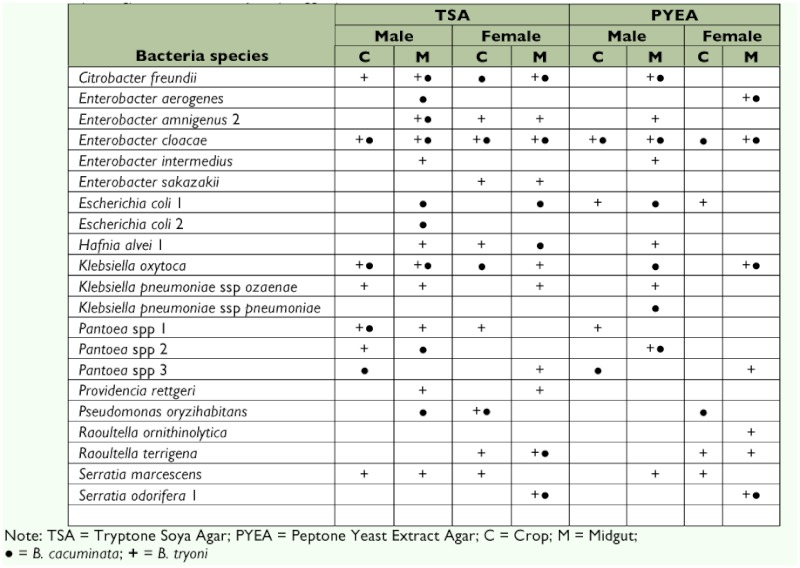
Citrobacter freundii, Enterobacter cloacae and Klebsiella oxytoca were the most commonly occurring bacteria isolated from both B. cacuminata and B. tryoni, with Escherichia coli, Pantoea spp. and Klebsiella pneumoniae ssp ozaenae being less frequent.
Enterobacter cloacae grew on TSA and PYEA from the crop and midgut of B. cacuminata but was not detected on PYEA from the crop of female B. tryoni. Enterobacter intermedius, Enterobacter sakazakii, K. pneumoniae ssp ozaenae, Providencia rettgeri, Raoultella ornithinolytica and Serratia marcescens were isolated only from B. tryoni. Providencia rettgeri was found in the midgut of both male and female B. tryoni on TSA only. Klebsiella pneumoniae ssp ozaenae and S. marcescens were frequently observed in crop and midgut isolates of either male or female B. tryoni on both culture media (Table 2).
Identification of bacteria by molecular cloning of the 16S rRNA gene
The molecular cloning of the 16S rRNA gene, from bacteria present in the crops and midguts of both fruit fly species, identified the presence of some bacteria species that were not detected through the culturing of the crop and midgut contents on TSA and PYEA and subsequently identified using the API-20E method (Table 3).
Table 3.
Classes and families of bacteria diagnosed by analysing the sequences of the 16S rRNA gene, obtained from the crops and midguts of field collected adults of Bactrocera cacuminata (Hering) and Bacterocera tryoni
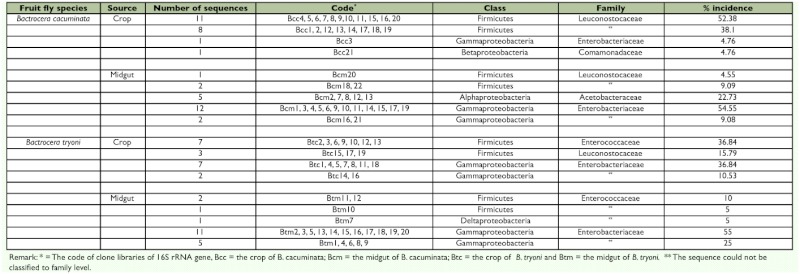
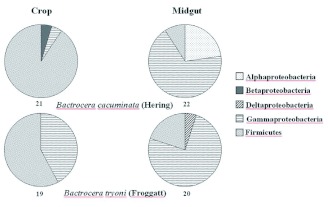
Four clone libraries of 16S rRNA were assembled, each representing either the crop or midgut of the two fly species (Table 3). There were 35 clones affiliated with Firmicutes, 40 affiliated with Gammaproteobacteria, 5 affiliated with Alphaproteobacteria and one each of Betaproteobacteria and Deltaproteobacteria (Table 3). The percentage incidence of each clone in each bacterial family is presented in Table 3.
The crop of B. cacuminata had 21 clones which belonged to three bacterial classes, Firmicutes, Gammaproteobacteria and Betaproteobacteria and three bacterial families, Leuconostocaceae, Enterobacteriaceae and Comamonadaceae, while the midgut had 22 clones from three classes, Firmicutes, Alphaproteobacteria and Gammaproteobacteria, and three families, Leuconostocaceae, Acetobacteriaceae and Enterobacteriaceae. The crop of B. tryoni had 19 clones from two classes, Firmicutes and Gammaproteobacteria, and three families, Enterococcaceae, Leuconostocaceae and Enterobacteriaceae, while the midgut had 20 clones from three classes, Firmicutes, Gammaproteobacteria and Deltaproteobacteria, and two families, Enterococcaceae and Enterobacteriaceae (Table 3).
The relative clone frequencies are illustrated in Figure 1. In the crop of both fruit fly species, Firmicutes was the dominant bacterial class. The crop of B. cacuminata contained more bacterial classes than the crop of B. tryoni but Betaproteobacteria was found only in the crop of B. cacuminata. In the midgut of both fruit fly species, Gammaproteobacteria was the predominant bacterial class. Deltaproteobacteria was found only in the midgut of B. tryoni.
Figure 1.
Percent incidence of Alpha, Beta, Delta and Gamma Proteobacteria and Firmicutes in the crop and midgut of Bactrocera cacuminata (Hering) and Bacterocera tryoni (Froggatt). The total number of clones in each clone library is given under each pie chart. High quality figures are available online.
Lactic acid bacteria in the alimentary tract of adult fruit flies
Clones obtained from the crop of each species of fruit fly were mainly gram-positive bacteria and these were less frequently found in the midgut. Most clones belonged to the division Firmicutes, class Bacilli, order Lactobacillales, families Enterococcaceae and Leuconostocaceae. These bacteria families, called lactic acid bacteria (Holzapfel and Wood 1998), were particularly common in the crops of B. cacuminata (90.48%) and B. tryoni (52.63%) (Table 3).
Phylogenetic analysis
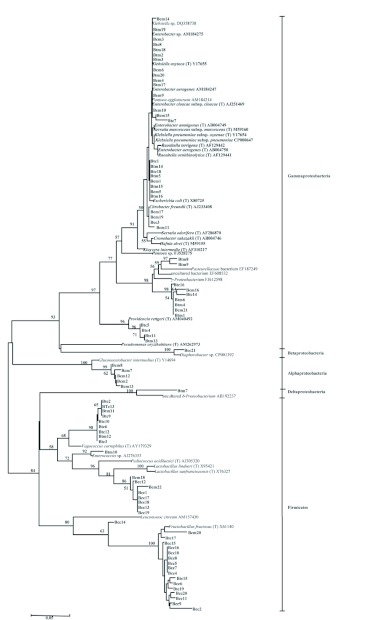
The phylogenetic tree based on the 16S rRNA sequences of bacteria species from both B. cacuminata and B. tryoni is presented in Figure 2. Sequences aggregated into five clusters in conformity with the bacterial classes Gammaproteobacteria, Alphaproteobacteria, Betaproteobacteria, Deltaproteobacteria and Firmicutes. In Gammaproteobacteria, many clones were affiliated with known species such as Enterobacter sp., Enterobacter aerogenes, Klebsiella oxytoca, Escherichia coli, Citrobacter freundii, Providencia rettgeri and unclassified Gammaproteobacterium. In Alphaproteobacteria, a few clones were affiliated with Gluconacetobacter intermedius. Furthermore, in Betaproteobacteria and Deltaproteobacteria clusters, one clone of each was affiliated with Diaphorobacter sp. and an uncultured Deltaproteobacterium, respectively. Finally, in the last cluster Firmicutes, many clones were affiliated with Vagococcus carniphilus, Lactobacillus sp. and Fructobacillus fructosus, while one clone was affiliated with Enterococcus sp.
Figure 2.
Phylogenetic tree based on 16S rRNA gene sequences from bacteria obtained from crops and midguts of two fruit flies species, Bactrocera cacuminata (Hering) and Bactrocera tryoni (Froggatt). Code: Bcc, the crop of B. cacuminata, Bcm, the midgut of B. cacuminata, Btc, the crop of B. tryoni and Bcm, the midgut of B. tryoni. The species identified by the API-20E strips and the RDP-II database are in bold type. Other codes refer to the species identified through other clone libraries. The scale bar indicates evolutionary distance (5 substitutions per 100 nucleotides). High quality figures are available online.
Morisita index and rarefaction analyses
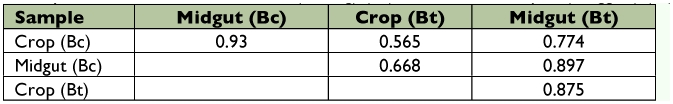
Bacteria species isolated and identified with the API-20E method showed relatively high Morisita index values indicating that most species present in the crops and midguts of both sexes of each fly species were similar. Five of the six indices were more than 0.600 and this result would be expected as the API-20E diagnostic method primarily identifies species of the family Enterobacteriaceae (Table 4).
Table 4.
Comparison of Morisita index values of community similarity of bacteria identified with the API 20 E system, isolated from the alimentary tract of Bactrocera cacuminata (Hering) (Bc) and Bacterocera tryoni (Froggatt) (Bt).
The Morisita index, based on the 16S rRNA sequence data, was categorized into three levels each with a sequence similarity ≥80%, ≥90% and ≥95% and which corresponded with the phylum, class and genus levels (Table 5). At the phylum level, the community similarity values (crop vs crop and midgut vs midgut) between the different fruit fly species had high values of 0.807 and 1.000 respectively (Table 5A). However, different parts of the alimentary tract within the same fruit fly species showed low Morisita index values, 0.262 and 0.363 (Table 5A). Comparison at class level for the same alimentary tract portion between the different fly species showed Morisita indices similar to the phylum level with 0.769 (crop vs crop) and 1.000 (midgut vs midgut) (Table 5B).
Table 5.
Morisita index values based on 16S rRNA gene sequences from the crops and midguts of Bactrocera cacuminata (Hering) (Bc) and Bactrocera tryoni (Froggatt) (Bt) at three taxonomic levels — Phylum, Class and Genus.
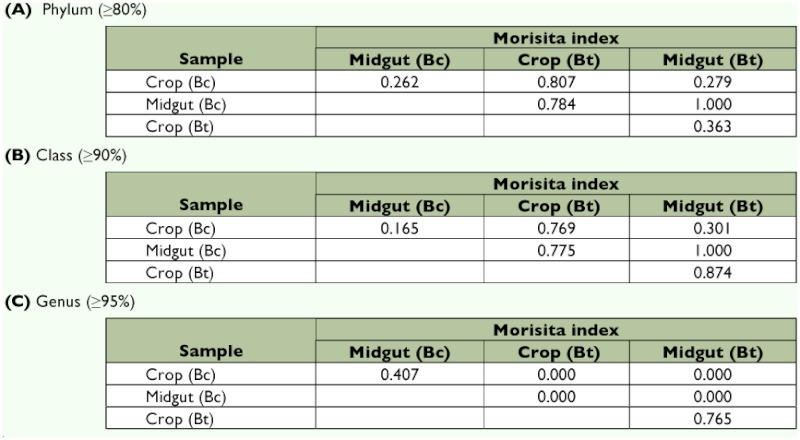
However, when comparing the different alimentary tract parts in the same fly species, the value was low in B. cacuminata (0.165) and high in B. tryoni (0.874) (Table 5B). Furthermore, when the index was assessed at the genus level, the values for the different alimentary tract portions in the same fly species were 0.407 and 0.765, while for the same alimentary tract portion between the different fruit fly species, the value was zero (Table 5C).
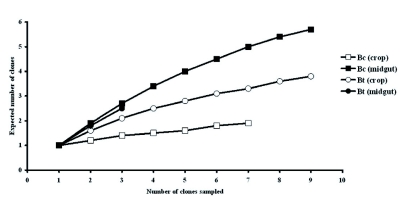
The rarefaction curves based on 95% similarity of taxonomic units (Figure 3), demonstrated steeper inclines for the microflora of the midgut than the crops of both fruit fly species. While all curves indicated a very high level of microbial diversity, the highest level was found in the midgut of B. cacuminata.
Figure 3.
Rarefaction curves based on 16S rRNA gene clones recovered from the crops and midguts of Bactrocera cacuminata (Hering) (Bc) and Bactrocera tryoni (Froggatt) (Bt). High quality figures are available online.
Discussion
There are no reports in the literature on the molecular diagnosis of bacteria associated with fruit flies. All previous studies have been based on API-20E identifications (Drew & Lloyd 1989, 1991). The two culture media used in this study, TSA and PYEA, in general produced the same species of bacteria although TSA was slightly more productive in numbers of isolates. However, the molecular analyses of crop and midgut contents demonstrated that other bacteria species, which do not grow on TSA and PYEA media, were also present. A similar study of soil bacteria revealed that TSA medium did not facilitate the isolation of some less common bacteria groups but did produce the more common ones (Davis et al. 2005).
Using the physical dissection technique, 125 bacteria colonies were isolated and identified by the API-20E identification method. This resulted in the diagnosis of bacteria belonging to a single family, the Enterobacteriaceae. Many bacterial species, including E. cloacae, C. freundii, K. oxytoca, K. pneumoniae spp. and Serratia spp., in this family, are common microflora in the alimentary canal of insects (Moreira et al. 2005; Pai, Chen and Peng 2004) and also in different species of tephritid flies (Kuzina et al. 2001; Marchini et al. 2002). Of the 17 known bacteria species diagnosed in this study, 6 occurred in B. tryoni only and two in B. cacuminata, while the remaining 9 were common to both fruit fly species. Species of bacteria that were isolated from fruit flies and studied by Lloyd et al. (1986), namely C. freundii, E. cloacae and K. oxytoca were also found in B. cacuminata and B. tryoni in this study, while P. rettgeri was only isolated from B. tryoni. In general, more bacteria species resulted from culturing on TSA medium than on PYEA. In comparing the bacteria species present in the crop and midgut of B. tryoni, there was a high level of similarity in contrast to a low level in the same comparison within B. cacuminata. Lloyd et al. (1986) reported that some genera of bacteria such as Serratia are not part of the common flora of the alimentary tract of wild adults as they were only detected from the alimentary tract of mass cultured laboratory flies. In our study Serratia was found in both field collected B. cacuminata and B. tryoni.
The 16S rRNA sequence data of the whole crop and midgut of the two species of fruit flies resulted in 5 classes and 5 families of bacteria being diagnosed. Within B. cacuminata there were 3 classes and 3 families in both the crop and midgut while in B. tryoni, 2 classes and 3 families in the crop and 3 classes and 2 families in the midgut. Across both fruit fly species, the class Firmicutes (family Leuconostocaceae) contained the predominant clones in the crop while the class Gammaproteobacteria clones were predominant in the midgut, particularly within the family Enterobacteriaceae. Cox and Gilmore (2007) reported that the gut microbial flora in the ferment fly, Drosophila melanogaster, consisted of 37.3% bacteria species in the division Firmicutes, particularly the genus Enterococcus. Bacteria belonging to the classes of Proteobacteria comprised 61% of the microbial flora, with the common genera being Acetobacter, Gluconobacter, Wolbachia, Enterobacter, Klebsiella, Pantoea, Citrobacter, Erwinia, Serratia, Morganella, Pesudomonas and Stenotrophomonas.
The presence of species of Firmicutes and Proteobacteria in the crop and the midgut of D. melanogaster was related to the pH within the alimentary tract (Cox and Gilmore 2007). The crop of fruit fly species was found to be acidic with a pH 3–3.5 (Drew et al. 1983) whilst the midgut was alkaline with pH 5.7–7.4, although the midgut was reported to be strongly acidic, with a pH of 1.4–2.0 in B. tryoni (Fitt and O'Brien 1985). In the case of B. tryoni, Murphy et al. (1994) showed that the main site of bacteria colonization was the midgut lumen, inside the peritrophic membrane. In other studies on Drosophila species, similar pH levels to those reported by Drew et al. (1983) for B. tryoni were found, i.e., an acidic crop, a highly alkaline ventriculus and a neutral to acidic hindgut (Clark 1999). The pH is important for the selection and growth of certain species of bacteria. Most bacteria have an optimum pH for growth of pH 6–7, but an exception is the lactic acid bacteria that can grow in acidic conditions (Dillon and Dillon 2004). The presence of Firmicutes in the crops and Proteobacteria in the midguts, in our study, is consistent with these optimum pH levels.
The presence of lactic acid bacteria in insects has been reported in several species of wood and soil-feeding termites (Bauer et al. 2000; Miyata et al. 2007) and some Lepidopteran species in which they formed the majority of carbohydrate-utilizing bacteria in the hindgut (Shannon et al., 2001).
Lactic acid bacteria have been diagnosed in fruit flies of the genus Bactrocera for the first time in this study. Four genera were present, Lactobacillus, Leuconostoc, Pediococcus and Vagococcus. These genera are important members of Firmicutes and species within this group are usually found in decomposing plants where they produce lactic acid as the major metabolic end product of carbohydrate fermentation. For example, Leuconostoc fructosum (AF360737) has been recorded in ripe fig in nature (Antunes et al. 2002).
Based on the API-20E data, the community similarity measurement (Morisita index) of the isolated gut bacteria had a percentage identity of more than 50% for each fraction of gut content and each sex of the two fruit fly species. Because this analysis was based on the Enterobacteriaceae, the primary bacteria diagnosed through the API-20E system, this community similarity measurement would be expected. The Morisita index analysis of the bacteria from the crops and midguts of the flies confirmed that, at the bacteria species level, the fruit fly species were different, but similar at the bacteria family and order level. Based on the 16S rRNA gene sequence data, the midgut of the two fruit flies species had a Morisita index value of 1.000 at both the Phylum and Class levels, confirming that both species possessed similar higher taxonomic level gut bacteria communities. However, at the genus and species level they were considerably different, with B. cacuminata having a low level of similarity between the crop and midgut (Morisita index 0.407) and B. tryoni possessing a high level of similarity (Morisita index 0.765) between these organs. When comparing B. cacuminata and B. tryoni, crop vs crop and midgut vs midgut, the Morisita index was zero at the bacteria genus level, proving a high level of dissimilarity.
The Bactrocera species possessed two major classes of bacteria in their alimentary canals, Firmicutes and Proteobacteria, the same as those reported in Drosophila (Cox and Gilmore 2007). The Firmucutes were found mainly in the crop of the flies that possess a low pH (Drew et al. 1983). In some other insects, e.g. termites and some Lepidoptera, bacteria occur in the midgut which, in these insects is strongly acidic (Bauer et al. 2000; Shannon et al. 2001). The occurrence of Proteobacteria, isolated mainly from the midgut in fruit flies has been reported in several publications (Drew and Lloyd 1991; Kuzina et al. 2001; Marchini et al. 2002; Murphy et al. 1994), and this finding contrasts with the bacterial communities of other insects, e.g. termites, which have three major groups of microorganisms, Bacteroidetes, Firmicutes and Spirochaetes (Miyata et al. 2007).
The results in this study indicated that the crop and midgut were inhabited by different groups of bacteria. However, both B. cacuminata and B. tryoni possessed similar bacteria genera and species. The Firmicutes were found in the alimentary tract of adult wild Bactrocera species for the first time, demonstrating that the gut bacteria of fruit flies do not belong primarily to the Proteobacteria, especially Enterobacteriaceae as previously thought. Indeed, the rarefaction analyses confirmed a very high level of microbial diversity, especially with regard to bacteria, in the fruit fly species studied. This study has been the first to use 16s rRNA gene sequencing on tropical tephritid species and the bacterial community in the adult fly gut proved to be more diverse than that reported in species of Coleoptera (Delalibera et al. 2007; Vasanthakumar et al. 2006, 2008) and our rarefaction analyses indicated a bottomless pit of microbial diversity.
The phylogenetic analysis, demonstrated through the phylogenetic tree, that most of the fruit fly bacteria isolated are taxonomically close to known species listed on databases. In particular, isolates within class Gammaproteobacteria, family Enterobacteriaceae, were similar to known species. In the other four classes of bacteria, some of the isolated bacteria appear to be similar to but different from known species. Further research is required on both the taxonomic identity of these fruit fly associated bacteria as well as the biological relationships between fruit flies and bacteria, especially the lactic acid bacteria in the class Firmicutes.
Acknowledgements
This study was supported by grants from the Royal Golden Jubilee PhD. Scholarship Research Foundation (RGJ), Thailand, the Center for Agricultural Biotechnology under the Higher Education Development, Commission on Higher Education, the Ministry of Education, Thailand, The International Centre for the Management of Pest Fruit Flies (ICMPFF) and Griffith School of Environment, Nathan campus, Griffith University, Queensland, Australia.
References
- Antunes A, Rainey FA, Nobre MF, Schumann P, Ferreira AM, Ramos A, Santos H, da Costa MS. Leuconostoc ficulneum sp. nov., a novel lactic acid bacterium isolated from a ripe fig, and reclassification of Lactobacillus fructosus as Leuconostoc fructosum comb, nov. International Journal of Systematic and Evolutionary Microbiology. 2002;52:647–655. doi: 10.1099/00207713-52-2-647. [DOI] [PubMed] [Google Scholar]
- Bauer S, Tholen A, Overmann J, Brune A. Characterization of abundance and diversity of lactic acid bacteria in the hindgut of wood- and soil-feeding termites by molecular and culture-dependent techniques. Archives of Microbiology. 2000;173:126–137. doi: 10.1007/s002039900120. [DOI] [PubMed] [Google Scholar]
- Brauman A, Dore J, Eggleton P, Bignell D, Breanak JA, Kane MD. Molecular phylogenetic profiling of prokaryotic communities in guts of termites with different feeding habits. FEMS Microbiology Ecology. 2001;35:27–36. doi: 10.1111/j.1574-6941.2001.tb00785.x. [DOI] [PubMed] [Google Scholar]
- Capuzzo C, Firrao G, Mazzon L, Squartini A, Girolami V. Candidatus Erwinia dacicola, a coevolved symbiotic bacterium of the olive fly Bactrocera oleae (Gmelin). International Journal of Systematic and Evolutionary Microbiology. 2005;55:1641–1647. doi: 10.1099/ijs.0.63653-0. [DOI] [PubMed] [Google Scholar]
- Clark TM. Evolution and adaptive significance of larval midgut alkalinization in the insect superoder. Journal of Chemical Ecology. 1999;25:1945–1960. [Google Scholar]
- Cox CR, Gilmore MS. Native microbial colonization of Drosophila melanogaster and its use as a model of Enterococcus faecalis pathogenesis. Infection and Immunity. 2007;75:1565–1576. doi: 10.1128/IAI.01496-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis KER, Joseph SJ, Janssen PH. Effects of growth medium, inoculum size, and incubation time on culturability and isolation of soil bacteria. Applied and Environmental Microbiology. 2005;71:826–834. doi: 10.1128/AEM.71.2.826-834.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delalibera I, Vasanthakumar A, Burwitz BJ, Schloss PD, Klepzig KD, Handelsman J, Raffa KF. Composition of the bacteria community in the gut of the pine engraver, Ips pint (Say) (Coleoptera) colonizing red pine. Symbiosis. 2007;43:93–104. [Google Scholar]
- Dillon RJ, Dillon VM. The gut bacteria of insects: Nonpathogenic interaction. Annual Review of Entomology. 2004;49:71–92. doi: 10.1146/annurev.ento.49.061802.123416. [DOI] [PubMed] [Google Scholar]
- Doyle JJ, Doyle JL. A rapid DNA isolation procedure from small quantities of fresh leaf tissue. Phytochemistry Bulletin. 1987;19:11–15. [Google Scholar]
- Drew RAI, Lloyd AC. Relationship of fruit flies (Diptera: Tephritidae) and their bacteria to host plants. Annals of the Entomological Society of America. 1987;80:629–636. [Google Scholar]
- Drew RAI, Lloyd AC. 3.1.3. Bacteria associated with fruit flies and their host plants. In: Robinson AS, Hooper G, editors. Fruit flies: Their biology, natural enemies and control. Elsevier Science Publishers; 1989. pp. 131–138. [Google Scholar]
- Drew RAI, Lloyd AC. Bacteria in the life cycle of Tephritid fruit flies. In: Baraosa V, Krischik VA, Jones CG, editors. Microbial Mediation of Plant-Herbivore Interaction. John Wiley & Sons. Inc.; 1991. pp. 441–465. [Google Scholar]
- Drew RAI, Courtice AC, Teakle DS. Bacteria as a natural source of food for adult fruit flies (Diptera: Tephritidae). Oecologia (Berlin) 1983;60:279–284. doi: 10.1007/BF00376839. [DOI] [PubMed] [Google Scholar]
- Fitt GP, O'Brien RW. Bacteria associated with four species of Dacus (Diptera: Tephritidae) and their role in the nutrition of the larvae. Oecologia. 1985;85:447–454. doi: 10.1007/BF00384954. [DOI] [PubMed] [Google Scholar]
- Friedrich MW, Schmitt-Wagner D, Lueders T, Brune A. Axial differences in community structure of Crenarchaeota and Euryrchaeota in the highly compartmentalized gut of the soil-feeding termite Cubitermes orthognathus. Applied and Environmenalt Microbiology. 2001;67:4880–4890. doi: 10.1128/AEM.67.10.4880-4890.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haynes S, Darby AC, Daniell TJ, Webster G, van Veen FJF, Godfray HCJ, Prosser JI, Douglas AE. Diversity of bacteria associated with natural aphid populations. Applied and Environmental Microbiology. 2003;69:7216–7223. doi: 10.1128/AEM.69.12.7216-7223.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzapfel WH, Wood BJB. The genera of lactic acid bacteria. 1st ed. London Blackie Academic & Professional; 1998. [Google Scholar]
- Howard DJ, Bush GL, Breznak JA. The evolutionary significance of bacteria associated with Rhagoletis. Evolution. 1985;39:405–417. doi: 10.1111/j.1558-5646.1985.tb05677.x. [DOI] [PubMed] [Google Scholar]
- Krebs CJ. Whither small rodent population studies? Researches on Population Ecology. 1998;40:123–125. [Google Scholar]
- Kuzina LV, Peloquin JJ, Vacek DC, Miller TA. Isolation and identification of bacteria associated with adult laboratory Mexican fruit flies, Anastrepha ludens (Diptera: Tephritidae). Current Microbiology. 2001;42:290–294. doi: 10.1007/s002840110219. [DOI] [PubMed] [Google Scholar]
- Lloyd AC. Bacteria associated with Bactrocera species of fruit flies (Diptera: Tephritidae) and their host trees in Queensland. Ph.D. thesis, University of Queensland; 1991. [Google Scholar]
- Lloyd AC, Drew RAI, Teakle DS, Hayward AC. Bacteria associated with some Dacus species (Diptera: Tephritidae) and their host fruit in Queensland. Australian Journal of Biological Sciences. 1986;39:361–368. [Google Scholar]
- Kumar S, Nei M, Dudley J, Tamura K. MEGA: a biologist-centric software for evolutionary analysis of DNA and protein sequences. Briefings in Bioinformatics. 2008;9:299–306. doi: 10.1093/bib/bbn017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marchini D, Rosetto M, Dallai R, Marri L. Bacteria associated with the oesophageal bulb of the medfly Ceratitis capitata (Diptera: Tephritidae). Current Microbiology. 2002;44:120–124. doi: 10.1007/s00284-001-0061-1. [DOI] [PubMed] [Google Scholar]
- Miyata R, Noda N, Tamaki H, Kinjyo K, Aoyagi H, Uchiyama H, Tanaka H. Inflience of feed components on symbiotic bacteria community structure in the gut of the wood-feeding higher termite Nasutitermes takasagoensis. Bioscience Biotechnology and Biochemistry. 2007;71:1244–1251. doi: 10.1271/bbb.60672. [DOI] [PubMed] [Google Scholar]
- Moreira DDO, de Morais V, Vieira-da-Motta O, de C Campos-Farinha AE, Tonhasca A., Jr Ants as carriers of antibiotic-resistant bacteria in hospitals. Neotropical Entomology. 2005;34:999–1006. [Google Scholar]
- Murphy KM, Teakle DS, Macrae IC. Kinetics of colonization of adult Queensland fruit flies (Bactrocera tryoni) by dinitrogen-fixing alimentary tract bacteria. Applied and Environmental Microbiology. 1994;60:2508–2517. doi: 10.1128/aem.60.7.2508-2517.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nei M, Kumar S. Molecular Evolution and Phylogenetics. Oxford University Press; 2000. [Google Scholar]
- Pai HH, Chen WC, Peng CF. Cockroaches as potential vectors of nosocomial infections. Infection Control and Hospital Epidemiology. 2004;25:979–984. doi: 10.1086/502330. [DOI] [PubMed] [Google Scholar]
- Schmitt-Wagner D, Friedrich MW, Wagner B, Brune A. Phylogenetic diversity, abundance, and axial distribution of bacteria in the intestinal tract of two soil-feeding termites (Cubitermes spp.). Applied and Environmental Microbiology. 2003;69:6007–6017. doi: 10.1128/AEM.69.10.6007-6017.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shannon AL, Attwood G, Hopcroft DH, Christeller JT. Characterization of lactic acid bacteria in the larval midgut of the keratinophagous lepidopteran, Hofmannophila pseudospretella. Letters in Applied Microbiology. 2001;32:36–41. doi: 10.1046/j.1472-765x.2001.00854.x. [DOI] [PubMed] [Google Scholar]
- Vasanthakumar A, Delalibera I, Handelsman J, Klepzig KD, Schloss PD, Raffa KF. Characterization of gut-associated bacteria in larvae and adults of the southern pine beetle, Dendroctonus frontalis Zimmermann. Environmental Entomology. 2006;35:1710–1717. [Google Scholar]
- Vasanthakumar A, Handelsman J, Schloss PD, Bauer L, Raffa KF. Gut microbiota of an invasive subcortical beetle, Agrilus planipennis Fairmaire, across various life stages. Environmental Entomology. 2008;37:1344–1353. doi: 10.1603/0046-225x(2008)37[1344:gmoais]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- Wang QG, Garrity M, Tiedje JM, Cole JR. Naïve Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Applied Environmental Microbiology. 2007;73:5261–5267. doi: 10.1128/AEM.00062-07. [DOI] [PMC free article] [PubMed] [Google Scholar]