Abstract
Purpose
To determine if optical coherence tomography (OCT) device-type influences clinical grading of OCT imaging in the context of exudative age-related macular degeneration (AMD).
Methods
Ninety-six paired OCT scans from 49 patients with active exudative AMD were obtained on both the time-domain Stratus™ OCT system and the spectral-domain Cirrus™ OCT system at the same visit. Three independent graders judged each scan for the presence of intraretinal fluid (IRF) or subretinal fluid (SRF). The degree of grader consensus was evaluated and the ability of the systems to detect the presence of disease activity was analyzed.
Results
Cirrus™ OCT generated a higher degree of inter-grader consensus than Stratus OCT with higher intraclass correlation coefficients (ICC) for all parameters analyzed. A pair-wise comparison of Cirrus™ OCT to Stratus™ OCT systems revealed that Cirrus™-based gradings more frequently reported the presence of SRF and IRF and detected overall neovascular activity at a higher rate (p<0.05) compared to Stratus™-based gradings
Conclusions
The choice of time-domain (Stratus™) versus spectra-domain (Cirrus™) OCT systems has a measurable impact on clinical decision making in exudative AMD. Spectral-domain OCT systems may be able to generate more consensus in clinical interpretation and, in particular cases, detect disease activity not detected by time-domain systems. Clinical trials employing OCT-based clinical evaluations of exudative AMD may need to account for these inter-system differences in planning and analysis.
Keywords: Age-related macular degeneration, Optical coherence tomography, Clinical trials, neovascularization, anti-angiogenic treatment
INTRODUCTION
Optical coherence tomography (OCT) evaluation of macular structure plays a central role in the clinical diagnosis and management of patients with age-related macular degeneration (AMD) 1. In clinical practice, the decision to recommend treatment for exudative AMD is dependent upon the ability of the clinician to detect and characterize the presence of intraretinal and/or subretinal fluid indicative of exudative activity. In particular, OCT imaging has been important in decisions regarding treatment with intravitreal anti-angiogenic agents 2, 3. While quantitative measure of retinal thickness has been used as secondary outcomes in clinical trials of AMD 4,5 a qualitative evaluation, relying on the visual inspection of the OCT image, has also been considered as a retreatment criterion in both clinical trials 4, and routine clinical care.
As such, the ability of OCT imaging to present the necessary data in a reliable and reproducible manner for clinical interpretation is essential. Currently, there are two OCT systems in popular clinical use in the form of time-domain and spectral-domain systems. A widely used time-domain OCT device is the Stratus™ OCT instrument (Carl Zeiss Meditec, Inc., Dublin, CA), that uses a common macular acquisition protocol called the FastMac protocol 6,7,8 9, 10, to survey macular anatomy rapidly and to generate macular thickness measurements. In a recent study analyzing the reading center-based grading of OCT images in exudative AMD (Stratus™ OCT system employing the FastMac scanning protocol), good inter-reader and intra-reader agreement was found 10. However, with the recent advent of spectral domain OCT systems, such as the Cirrus™ HD-OCT system (Carl Zeiss Meditec, Inc), the question of how these devices influence the nature and consistency of treatment decisions in AMD has been raised.
Both time-domain and spectral domain OCT devices employ the use of axial scans to create cross sectional images of the retina and to calculate retinal thicknesses averaged across standardized subfields in the macula. However, the number of scans performed, speed of acquisition, degree of axial resolution, and array of axial scan pattern vary between the two kinds of instruments. As spectral domain OCT instruments become more available, the retinal specialist often has the choice of using a time domain or a spectral domain OCT system. Also, multi-centered clinical trials often involve clinics that use different OCT systems. How different OCT systems influence, if at all, the consistency of clinical decision-making via their ability to elicit agreement among independent clinician-graders has not been previously analyzed. In this study, we analyzed the consistency of clinical decision-making by examining inter-grader agreement between independent graders separately for the Stratus™ OCT and Cirrus™ HD-OCT systems. We also directly compared pairs of Stratus™-based and Cirrus™-based OCT scans to measure agreement in clinical decisions regarding anti-VEGF treatment made between the systems. Further, we examined cases where the two OCT systems disagreed with each other and analyzed the factors contributing to the discordance in clinical decisions. The results of this study will provide information how the automated acquisition protocols on time-domain versus spectral-domain OCT system affects the qualitative physician-based decision to treat a patient with NV AMD and will clarify the significance of OCT system choice in clinical trials and routine patient care.
METHODS
Subjects
Patient records from the NEI eye clinic between July 1, 2007 and December 31, 2008 were retrospectively reviewed. During this period of time, patients in the retina clinic were scanned using both the Stratus™ OCT and Cirrus™ HD-OCT systems as part of the standard clinic operating procedure; patients were not pre-selected from a wider population to undergo scanning on these OCT systems. On retrospective analysis, records meeting all the following inclusion criteria were collected: (a) a diagnosis of exudative AMD, as verified by clinical examination and angiographic studies, and undergoing treatment with intraocular anti-VEGF agents (bevacizumab and/or ranibizumab); (b) OCT scans that were of sufficient quality (signal strength ≥ 5 and the absence of imaging artifacts or distortions); and (c) OCT scans performed on both Stratus™ OCT and Cirrus™ HD-OCT systems on the same visit. OCT scans included were representative of the overall clinic patient population with exudative AMD, including eyes with wide range of visual acuities. The criterion to include eyes that are undergoing anti-VEGF treatment enables the selection of eyes with active exudative disease, and is relevant to the currently widespread use of OCT imaging in clinical trials and clinical practice in which anti-VEGF agents are used as first-line agents. Patients identified in this way contributed only one eye to the analysis; if both eyes of a patient were eligible, the study eye was randomly selected. Informed patient consent and local institutional review board (IRB) approval were obtained for this retrospective study which was conducted in accordance with the ethical standards of the 1964 Declaration of Helsinki. We certify that all applicable institutional and governmental regulations concerning the ethical use of human volunteers were followed during this research.
Optical Coherence Tomography Imaging
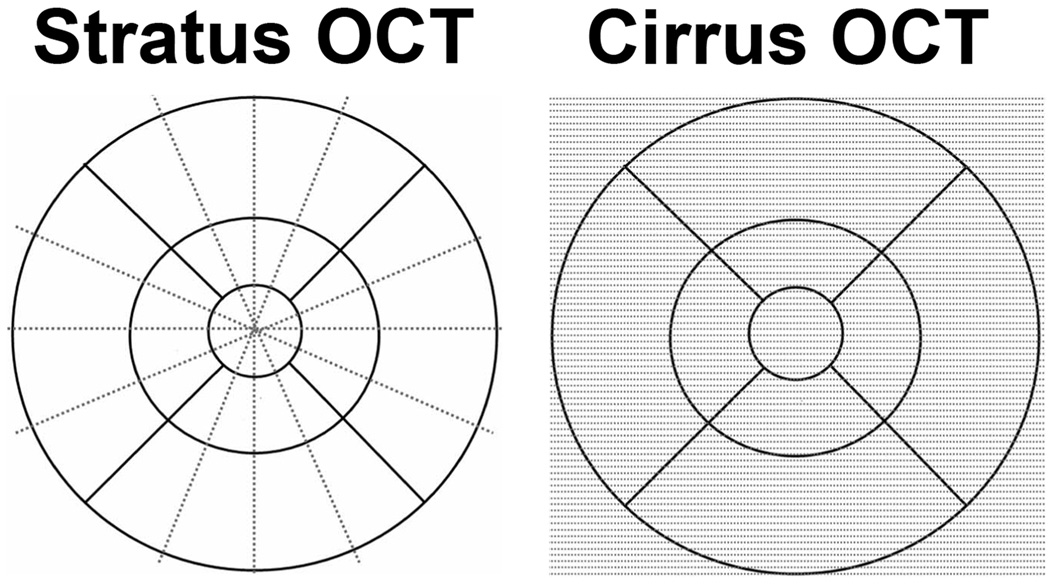
The two OCT scanners examined in this study were the Stratus™ OCT (software version 5.0), a time domain OCT instrument, and the Cirrus™ HD-OCT (software version 2.0), a spectral domain OCT instrument (both commercially available from Carl Zeiss Meditec, Dublin, CA). Information on scanning modes and image analyses were obtained from the manufacturer. Scanning with the Stratus™ OCT was performed using the fast macular thickness map (FastMac) protocol, which acquires six evenly distributed 6mm radial lines, consisting of 128 A-scans per line, intersecting at the fovea (total of 768 sampled points) within a scan time of 1.9 seconds. Scanning with the Cirrus™ HD-OCT was performed using the 512X128 scan pattern (Macular Cube protocol) where a 6x6mm area on the retina was scanned with 128 horizontal lines, each consisting of 512 A-scans per line (total of 65,536 sampled points) within a scan time of 2.4 seconds. A schematic providing a comparison of the Stratus™ OCT and Cirrus™ HD-OCT scanning patterns is shown in Figure 1.
Figure 1.
Retinal sampling patterns employed by the Stratus™ OCT and Cirrus™ HD-OCT systems. The Stratus™ OCT (FastMac protocol) samples points along 6 intersecting radial lines while the Cirrus™ HD- OCT (512x128 protocol) samples the points in horizontal rows (128 rows) in a consecutive manner evenly throughout the macular grid.
A total of 96 pairs of OCT scans from 49 patients (mean = 1.94 scans per patient, range = 1 to 5 scans per patient) were identified and admitted for clinical grading that was performed in a masked fashion.
Clinical grading of OCT images
OCT scans admitted for analysis (96 scans each from Stratus™ OCT and Cirrus™ HD-OCT) were shuffled and arranged in random order. De-identified scans (with patient name and scan date obscured from view) were called up and displayed on a computer monitor for grading. Three masked graders, experienced in the clinical use of OCT scans for patient care and for clinical research, performed the grading. The graders viewed the OCT images using display modes intrinsic to the Stratus™ and Cirrus™ OCT software packages. For the Cirrus™ HD-OCT, individual scans from the set in 512x128 macular cube series were displayed. Graders were allowed to scroll through the individual images ad libitum but any additional use of imaging rendering algorithms (e.g. three-dimensional analysis, image segmentation, retinal thickness measurements) was not permitted. For the Stratus™ OCT, the 6 individual scans captured using the FastMac mode were displayed. Graders were able to examine each of the 6 scans ad libitum, and again, no additional depictions of the individual scans were used.
Based on the above inspection mode, graders scored the individual scans in the following aspects: (a) presence or absence of intraretinal fluid (IRF) and (b) presence or absence of subretinal fluid (SRF). No explicit predetermined criteria for IRF and SRF were imposed; graders were asked to judge their presence as they would in a clinical context. Graders were also asked to determine whether treatment with anti-VEGF therapy would be administered using the OCT scan alone and were given the criteria that if either IRF or SRF were detected, exudative activity was deemed to be present, and treatment recommended. Time taken for grading was not constrained, and graders were at liberty to use the same amount of time as they would in arriving at a treatment decision in a clinical scenario. Each grader performed the grading in the same setting using identical display monitors. Gradings of individual scans were conducted by each grader independently, without communication between individual graders. The above grading and treatment guidelines were modeled after the reinjection criteria from OCT-based decision making in clinical trials presently ongoing or planned for the treatment of exudative AMD (i.e. the intervention substudy in the Age-Related Eye Study 2 (AREDS2) and the Comparison of Age-Related Macular Degeneration Treatments Trial (CATT) study). According to the CATT study’s manual of procedures, patient assigned to the variable schedule dosing regimen are evaluated for retreatment at study visits and retreatment decisions are driven by the presence or absence of fluid on OCT scans. Graders in this study are asked to qualitatively score for the presence of fluid on OCT scans in a manner similar to that performed by study investigators in the above trials.
Statistical Analysis
In order to assess the correlation between the individual graders for OCT images, the intraclass correlation coefficient (ICC) values were calculated for both Stratus™ and Cirrus™ systems using SPSS (version 16.0). To compare agreement between the Stratus™ and Cirrus™ systems, for each parameter, paired two-tailed t-tests were calculated using a statistical program (Graph PadPrism, version 5).
RESULTS
Comparison of inter-grader consensus obtained with Stratus™ versus Cirrus™ systems
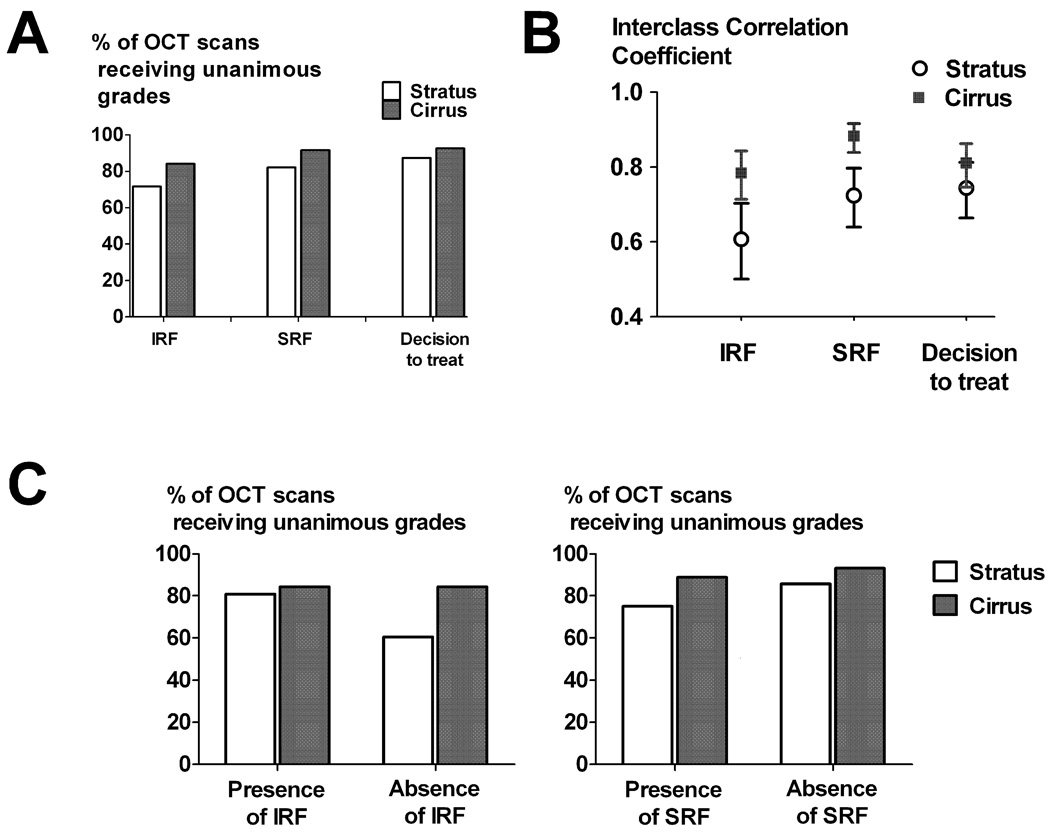
The results of the grading of OCT images were analyzed to discover differences between the Stratus™ and the Cirrus™ systems in generating consensus in clinical decisions made by the 3 graders. Individual grades (for the presence/absence of IRF and SRF, and the decision to recommend/defer treatment) from the independent 3 graders were tabulated and a tally was made of 1) the number of scans receiving unanimous grades (all graders awarding the same grade) and 2) the number of scans receiving non-unanimous grades (two of the graders disagreeing with the third). The percentage of OCT scans with unanimous grades was computed for each OCT system and for each activity parameter. As shown in Figure 2A, in all three parameters graded (IRF, SRF, and decision to treat), OCT scans obtained using the Cirrus™ system had a higher rate of unanimous grades. Inter-grader agreement was also computed by the computation of the intraclass correlation coefficient (ICC). As shown in Figure 2B, for gradings of IRF and SRF, a statistically higher ICC value, indicating greater inter-grader agreement, was found for the Cirrus™ compared to the Stratus™ system (Fig 2B).
Figure 2.
Comparison of measures of agreement between three independent graders obtained on the Stratus™ OCT and Cirrus™ HD-OCT systems. (A) Percentages of OCT scans receiving unanimously identical grades (with all 3 graders in agreement) scoring for intraretinal fluid (IRF), subretinal fluid (SRF), and on the decision to treat. (B) Interclass correlation coefficients (ICC) reflecting agreement between graders calculated the same parameters as in (A). Error bars indicate 95% confidence intervals. (C) Sub-analysis of the data in (A), broken down into subcategories of the presence or absence of IRF and SRF for both OCT systems.
The OCT scan grades were also analyzed to ascertain whether the degree of grader unanimity varied based on the presence or absence of the anatomical finding scored (IRF or SRF) (Table 1). The percentages of scans receiving unanimous grades were higher for those obtained on the Cirrus™ system compared to the Stratus™ system for both the subgroup in which either IRF or SRF was judged to be present, as well as for the subgroup in which either IRF or SRF was judged to be absent (Fig 2C). This analysis demonstrated that greater grader agreement was found for the Cirrus™ system in both clinical situations in which exudative activity was present or absent.
Table 1.
Grading consensus for grades of Intraretinal fluid (IRF) and Subretinal fluid (SRF): Stratus™ OCT versus the Cirrus™ HD-OCT systems.
| Presence of finding | Absence of finding | ||||||
|---|---|---|---|---|---|---|---|
| Number of Unanimous Decisions (% of total) |
Number of Non- unanimous Decisions (% of total) |
Total | Number of Unanimous Decisions (% of total) |
Number of Non- unanimous Decisions (% of total) |
Total | ||
| Intraretinal fluid (IRF) |
Stratus OCT |
42(80.8%) | 10(19.2%) | 52 | 26 (60.5%) | 17 (39.5%) | 43 |
| Cirrus OCT |
48(84.2%) | 9(15.8%) | 57 | 32 (84.2%) | 6 (15.8%) | 38 | |
| Subretinal fluid (SRF) |
Stratus OCT |
24(75.0%) | 8(25.0%) | 32 | 54 (85.7%) | 9 (14.3%) | 63 |
| Cirrus OCT |
32(88.9%) | 4(11.1%) | 36 | 55 (93.2%) | 4 (6.8%) | 59 | |
Measurement of agreement between Stratus™ and Cirrus™ OCT systems
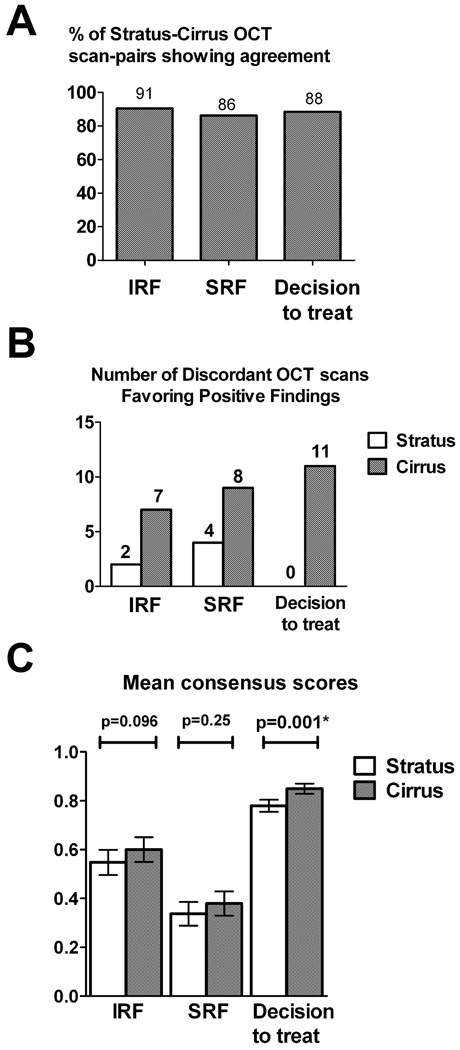
As all OCT scans evaluated in this study are paired (i.e. each OCT scan obtained on the Cirrus™ system is paired with an OCT scan obtained on the Stratus system for the same eye on the same patient visit), we were able to evaluate the correlation in clinical grading between the Cirrus™ and the Stratus™ systems. For each OCT scan and clinical parameter (IRF, SRF, and decision to treat), a consensus grade was derived from the majority of the 3 separate grades awarded by the individual graders (e.g. SRF was given the consensus grade of present when at least 2 of the graders considered it present). A summary of the distribution of consensus grades is shown in Table 2. As can be seen in Figure 3A, in the majority (86–90%) of Cirrus™-Stratus™ OCT scan pairs, the consensus grades were in agreement. Analysis of the remaining OCT scan pairs where the grades were not in agreement (i.e. discordant OCT pairs) (Fig. 3B) demonstrated that these were mostly comprised of Cirrus™ OCT scans that detected evidence of exudative activity where the corresponding Stratus™ OCT scans did not. Statistical analysis of all scan pairs also demonstrated an overall statistical difference (paired t-test, p<0.05) between the 2 OCT systems in the detection of exudative activity, with the Cirrus™ OCT scan detecting activity at a greater rate than the corresponding Stratus™ OCT scan (Fig. 3C).
Table 2.
Comparison of Optical Coherence Tomography (OCT) Findings on Time-Domain (Stratus™) and Spectral-Domain (Cirrus™) Optical Coherence Tomography Using Consensus Grades
| Presence on Time-Domain (Stratus™) OCT |
Absence on Time-Domain (Stratus™) OCT |
|||
|---|---|---|---|---|
| Presence on Spectral- Domain (Cirrus™) OCT |
Absence on Spectral- Domain (Cirrus™) OCT |
Presence on Spectral- Domain (Cirrus™) OCT |
Absence on Spectral- Domain (Cirrus™) OCT |
|
| Intraretinal Fluid (IRF) |
50/52 (96%) | 2/52 (4%) | 7/43 (16%) | 36/43 (84%) |
| Subretinal Fluid (SRF) |
28/32 (87.5%) | 4/32 (12.5%) | 8/63 (13%) | 55/63 (87%) |
| Presence of Exudative Activity (Presence of either IRF or SRF) |
72/72 (100%) | 0/72 (0%) | 11/23 (48%) | 12/23 (52%) |
Figure 3.
Agreement between pairs of OCT scans obtained on the Stratus™ OCT and Cirrus™ HD-OCT based on consensus grades (A) Percentage of OCT scan-pairs whose consensus grades are in agreement, with respect to intraretinal fluid (IRF), subretinal fluid (SRF), and on the decision to treat (based on the presence of exudative activity, as evidenced by the presence of either IRF or SRF). (B) Distribution of OCT scan-pairs whose consensus grades were discordant. The number of discordant pairs favoring the presence of intraretinal fluid (IRF), subretinal fluid (SRF), and on the decision to treat is shown in each column. (C) Comparison of OCT scan-pairs in the consensus detection of intraretinal fluid (IRF), subretinal fluid (SRF), or either (resulting in a decision to treat).
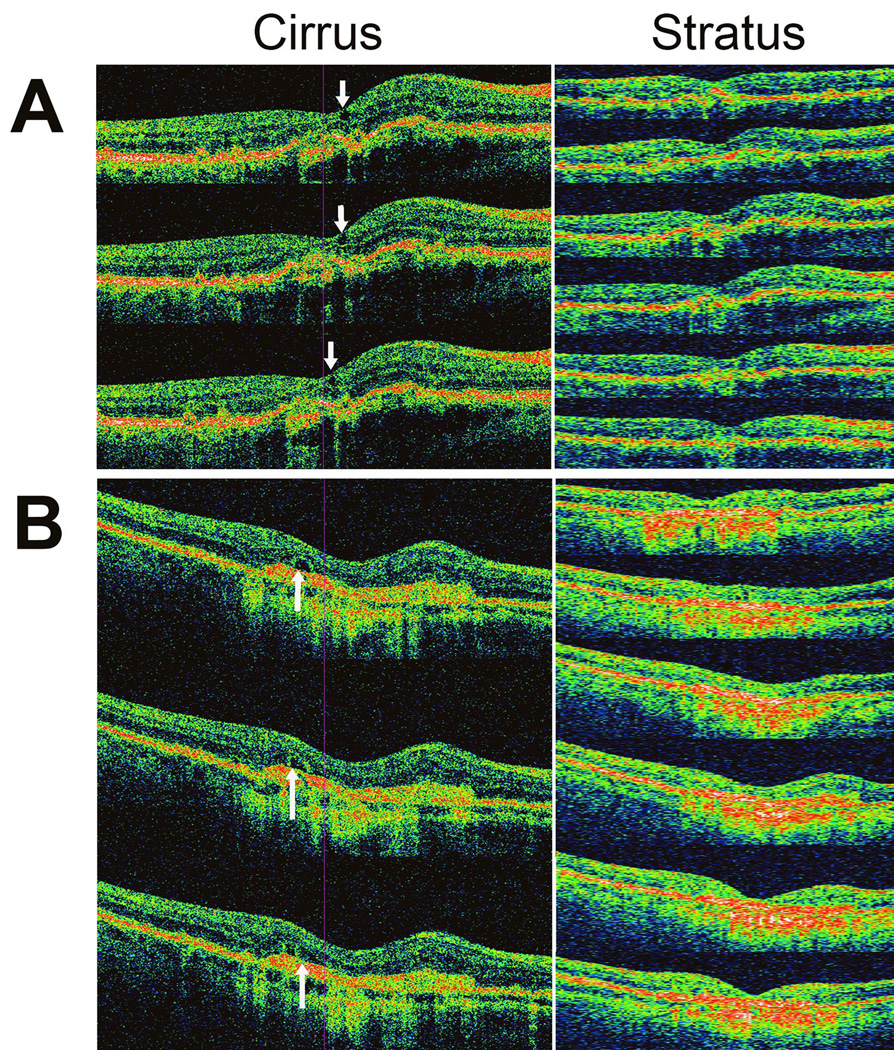
Individual review of the discordant OCT scan pairs demonstrated a number of anatomical features which may have led to differing grades obtained between OCT systems. These consisted of the following 3 categories: (1) small areas of IRF, (2) areas of SRF that are small in area and thickness, and (3) cystic fluid spaces in the outer retina that were graded as IRF on the Cirrus™ HD-OCT system but were graded as SRF on the Stratus™ OCT. Examples of each of these are shown in Figure 4. Overall, 8 cases had subtle IRF that was not scored on the Stratus scans, 7 cases of subtle SRF that were not scored on the Stratus scans, and 5 cases where the fluid appeared as outer retinal cysts (IRF) on the Cirrus™ HD-OCT systems but were graded as subretinal fluid (SRF) on the Stratus™ OCT system.
Figure 4.
Sample OCT scans from two study eyes in with discordant consensus grades were awarded between the Cirrus™ HD-OCT (left) and Stratus™ OCT (right) systems. Three representative and consecutive horizontal scans are shown for the Cirrus™ HD-OCT scans while all 6 radial scans are shown fo the Stratus™ OCT scans. (A) Study eye in which intraretinal fluid (IRF) was graded as present on Cirrus™ HD-OCT but absent on Stratus™ OCT. Arrows indicate position of IRF on the Cirrus™ HD-OCT. (B) Study eye in which subretinal fluid (SRF) was graded as present on Cirrus™ HD-OCT but absent on Stratus™ OCT. Arrows indicate position of SRF on the Cirrus™ HD-OCT.
DISCUSSION
The role of OCT imaging in diagnosis and management of exudative AMD is currently a large one, and has a significant impact on how patients on variable anti-angiogenic treatment regimens are treated. Treatment decisions in AMD typically rely upon qualitative visual inspection of OCT images for fluid accumulation. The quantitative measurement of absolute retinal thickness by OCT, although suitable as an outcome measure for clinical studies, is less easily employed in everyday practice and is not easily automated11. Fluorescein angiography is often used as a confirmatory test of exudative activity, however, it is not always required by study protocols 3, 4, 12, 13 and has variable utilization and reproducibility in daily clinical practice 14. Despite their utility, there is some diversity in how OCT images are presently captured, rendered, and displayed visually to the clinician by different OCT systems, and these factors may have an important impact on how OCT data is interpreted for management decisions.
Multi-centered clinical trials in which separate centers employ different OCT systems may potentially be affected by these non-uniformities between systems. In a number of clinical trials on exudative AMD, the detection of neovascular activity and the decision to treat an eye with anti-VEGF treatment have often to be made at that particular visit with the information at hand, and may not amenable to consultation with other investigators or review by a reading center before the decision is made to treat. If the number of treatments delivered is computed as one outcome measure in the trial, then the superior ability of one system to generate consensus between individual practitioners is likely to make this measure more reliable. Also, if different OCT systems consistently generate different overall rates of decisions favoring treatment, then their non-uniform use is likely to introduce systematic biases into the study.
The two OCT systems used in this study differ in resolution and in the pattern of sampling. The Stratus™ OCT produces images with 10-µm axial resolution, compared to 5-µm resolution in the Cirrus™ system. In addition, the pattern and density of A-scans used differ significantly between the two systems. The Stratus™ OCT with the FastMac acquisition protocol uses 6 radial lines, each consisting of 128 A-scans whereas Cirrus™ HD-OCT scanning (with the Macular Cube protocol in the 512x128 mode) uses 128 evenly distributed horizontal scan-lines, each comprising of 512 A-scan. As a result, Stratus™ OCT samples the retina more densely near the center of the macula but much less so at the edges of the scan field, in contrast to the Macular Cube protocol on the Cirrus™ HD-OCT which samples the entire scan field uniformly but also at a high density per B-scan. We have chosen the use the FastMac protocol for the Stratus™ OCT system in preference to higher resolution scans available, such as the radial line scan (RLS), as the latter need to be manually placed, have a longer total scan time (6 × 1.28 sec, for 7.7 sec of total scan time), and are more subject to decentration.
In this study, we found that the two different OCT systems examined influenced clinical decision making in two aspects: (1) the spectral-domain Cirrus™ HD-OCT system, compared to the time-domain Stratus™ OCT system, consistently produces a higher degree of consensus between independent clinical graders in both decisions involving the presence, as well as the absence, of findings; and (2) in OCT pairs where the 2 systems disagree, the spectral-domain Cirrus™ HD-OCT system reported the presence of activity (in terms of presence of IRF or SRF, or both) at a statistically higher rate than the time-domain Stratus™ OCT system. On the whole, the use of the Cirrus™ HD-OCT system, instead of the Stratus™ OCT system, for the same group of patients, resulted in a higher degree of consensus between clinical graders and a more sensitive detection of small positive anatomical findings leading to a higher overall incidence of decisions favoring treatment.
The differences between the Cirrus™ HD-OCT system compared to the Stratus™ OCT system may have arisen from a number of sources. One relates to the higher linear resolution and the spatial resolution of adjacent A-scans in individual B scans for the Cirrus™ HD-OCT. This may have conferred on graders with better ability to detect retinal fluid when it is present and also to diagnose normal retinal anatomy when fluid spaces are absent. The Stratus™ OCT system, when employing scanning protocols (e.g. the RLS protocol) other than the FastMac protocol, can acquire higher resolution line scans (512 A-scans per line). As Taban et al.,15 has shown, this RLS protocol produces scans fewer segmentation errors compared to the FastMac protocol. However, these protocols require the manual placement of each line scan, and are not able to survey the macular area in a rapid and automated fashion as performed by the FastMac protocol. Here we have compared available automated macular scanning protocols in the respective systems to avoid variability inherent in the manual placement of individual line scans. This choice may have limited the resolution of the individual scans in the case of the Stratus™ OCT system, and contributed to the differences seen here. On the other hand, the features of more rapid and automated macular scanning of FastMac protocol may have possibly reduced scanning errors resulting from eye movements and operator-dependent errors of line scan placement.
Another source of differences seen in the comparison relates to the differences in uniformity and density of individual A-scans across the scan field. In the examination of the entire series of individual B-scans that make up the imaging session (6 in Stratus™ OCT and 128 in the Cirrus™ HD-OCT), graders can more readily examine contiguous retinal areas in the Cirrus™ HD-OCT in similar orientation than is possible in the Stratus™ OCT. In cases where areas of retina fluid are shallow and/or small in size, having the area of interest persist on consecutive/contiguous B-scans in the Cirrus™ HD-OCT enabled graders to determine their presence with greater confidence and consistency. The greater “sensitivity” of the Cirrus™ HD-OCT compared to the Stratus™ OCT in detecting these small areas of retinal fluid derives from the greater likelihood of the Stratus™ OCT to “miss” positive findings when they are located in retinal areas lying between and not traversed by the more widely-spaced radial B-scans.
The timeliness of the subject is reflected in a recent article that recently appeared in-press during the time of manuscript preparation of the present study. Sayangi et al16 compared time domain and spectral domain devices in their ability to detect choroidal neovascularization activity in AMD and found that spectral domain OCT devices may be superior in the detection of exudative activity. The findings of our study support this conclusion and extend it by examining how the inter-reader agreement varies between the two devices. These conclusions are dependent on the specific scan protocols chosen using each OCT system, and should be validated in a larger, prospective clinical trial, perhaps as an ancillary study in a subset of participants enrolled in an interventional trial. The overall conclusion is likely to have an effect on the design of future protocols involving clinical decisions made with the help of OCT imaging.
Acknowledgments
This work has been supported by the National Eye Institute Intramural Research Program. None of the authors has proprietary interests related to the material in this manuscript.
Footnotes
Conflict of interest
The authors declare no conflicts of interest.
References
- 1.Hee MR, Baumal CR, Puliafito CA, Duker JS, Reichel E, Wilkins JR, et al. Optical coherence tomography of age-related macular degeneration and choroidal neovascularization. Ophthalmology. 1996;103:1260–1270. doi: 10.1016/s0161-6420(96)30512-5. [DOI] [PubMed] [Google Scholar]
- 2.Jaffe GJ, Caprioli J. Optical coherence tomography to detect and manage retinal disease and glaucoma. Am J Ophthalmol. 2004;137:156–169. doi: 10.1016/s0002-9394(03)00792-x. [DOI] [PubMed] [Google Scholar]
- 3.Moshfeghi AA, Rosenfeld PJ, Puliafito CA, Michels S, Marcus EN, Lenchus JD, et al. Systemic bevacizumab (Avastin) therapy for neovascular age-related macular degeneration: twenty-four-week results of an uncontrolled open-label clinical study. Ophthalmology. 2006;113(2002):e2001–e2012. doi: 10.1016/j.ophtha.2006.05.070. [DOI] [PubMed] [Google Scholar]
- 4.Fung AE, Lalwani GA, Rosenfeld PJ, Dubovy SR, Michels S, Feuer WJ, et al. An optical coherence tomography-guided, variable dosing regimen with intravitreal ranibizumab (Lucentis) for neovascular age-related macular degeneration. Am J Ophthalmol. 2007;143:566–583. doi: 10.1016/j.ajo.2007.01.028. [DOI] [PubMed] [Google Scholar]
- 5.Kaiser PK, Blodi BA, Shapiro H, Acharya NR. Angiographic and optical coherence tomographic results of the MARINA study of ranibizumab in neovascular age-related macular degeneration. Ophthalmology. 2007;114:1868–1875. doi: 10.1016/j.ophtha.2007.04.030. [DOI] [PubMed] [Google Scholar]
- 6.Polito A, Del Borrello M, Isola M, Zemella N, Bandello F. Repeatability and reproducibility of fast macular thickness mapping with stratus optical coherence tomography. Arch Ophthalmol. 2005;123:1330–1337. doi: 10.1001/archopht.123.10.1330. [DOI] [PubMed] [Google Scholar]
- 7.Danis RP, Fisher MR, Lambert E, Goulding A, Wu D, Lee LY. Results and repeatability of retinal thickness measurements from certification submissions. Arch Ophthalmol. 2008;126:45–50. doi: 10.1001/archopht.126.1.45. [DOI] [PubMed] [Google Scholar]
- 8.Massin P, Vicaut E, Haouchine B, Erginay A, Paques M, Gaudric A. Reproducibility of retinal mapping using optical coherence tomography. Arch Ophthalmol. 2001;119:1135–1142. doi: 10.1001/archopht.119.8.1135. [DOI] [PubMed] [Google Scholar]
- 9.Bernardes R, Santos T, Cunha-Vaz J. Increased-resolution OCT thickness mapping of the human macula: a statistically based registration. Invest Ophthalmol Vis Sci. 2008;49:2046–2052. doi: 10.1167/iovs.07-0467. [DOI] [PubMed] [Google Scholar]
- 10.Zhang N, Hoffmeyer GC, Young ES, Burns RE, Winter KP, Stinnett SS, et al. Optical coherence tomography reader agreement in neovascular age-related macular degeneration. Am J Ophthalmol. 2007;144:37–44. doi: 10.1016/j.ajo.2007.03.056. [DOI] [PubMed] [Google Scholar]
- 11.Keane PA, Liakopoulos S, Jivrajka RV, Chang KT, Alasil T, Walsh AC, et al. Evaluation of Optical Coherence Tomography Central Retinal Thickness Parameters for use as Anatomic Outcomes in Clinical Trials for Neovascular Age-Related Macular Degeneration. Invest Ophthalmol Vis Sci. 2009 doi: 10.1167/iovs.08-2728. (in press) [DOI] [PubMed] [Google Scholar]
- 12.Brown DM, Regillo CD. Anti-VEGF agents in the treatment of neovascular age-related macular degeneration: applying clinical trial results to the treatment of everyday patients. Am J Ophthalmol. 2007;144:627–637. doi: 10.1016/j.ajo.2007.06.039. [DOI] [PubMed] [Google Scholar]
- 13.Weigert G, Michels S, Sacu S, Varga A, Prager F, Geitzenauer W, et al. Intravitreal bevacizumab (Avastin) therapy versus photodynamic therapy plus intravitreal triamcinolone for neovascular age-related macular degeneration: 6-month results of a prospective, randomised, controlled clinical study. Br J Ophthalmol. 2008;92:356–360. doi: 10.1136/bjo.2007.125823. [DOI] [PubMed] [Google Scholar]
- 14.Holz FG, Jorzik J, Schutt F, Flach U, Unnebrink K. Agreement among ophthalmologists in evaluating fluorescein angiograms in patients with neovascular age-related macular degeneration for photodynamic therapy eligibility (FLAP-study) Ophthalmology. 2003;110:400–405. doi: 10.1016/S0161-6420(02)01770-0. [DOI] [PubMed] [Google Scholar]
- 15.Taban M, Sharma S, Williams DR, Waheed N, Kaiser PK. Comparing retinal thickness measurements using automated fast macular thickness map versus six-radial line scans with manual measurements. Ophthalmology. 2009;116:964–970. doi: 10.1016/j.ophtha.2008.12.033. [DOI] [PubMed] [Google Scholar]
- 16.Sayanagi K, Sharma S, Yamamoto T, Kaiser PK. Comparison of Spectral-Domain versus Time-Domain Optical Coherence Tomography in Management of Age-Related Macular Degeneration with Ranibizumab. Ophthalmology. 2009 doi: 10.1016/j.ophtha.2008.11.002. (in press) [DOI] [PubMed] [Google Scholar]