Abstract
Chemical leukoderma is seen in a variety of clinical settings. We present a case of leukoderma associated with the phenolic derivative thymol found in a common over-the-counter medication for nasal congestion. The proposed mechanism for this type of leukoderma is presented along with other sources of phenolic and catecholic derivatives. Treatment is also briefly reviewed.
Contact or chemical leukoderma refers to depigmentation related to application of certain chemical compounds to the skin. Phenolic and catecholic derivatives are well-documented causes of leukoderma.1 They are commonly found in oral and topical medications, cosmetics, and a variety of nonmedicinal compounds.2 We describe what is believed to be the first reported case of chemical leukoderma associated with Vicks VapoRub® (Procter and Gamble).
Case
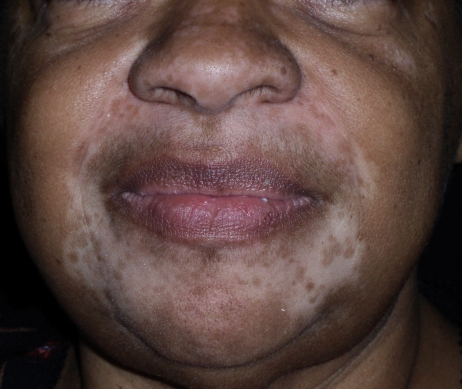
A 78-year-old, African-American female presented with newly acquired depigmented areas on the perinasal and perioral skin. The patient was blind, but caretakers noticed the change shortly after she applied Vicks VapoRub to her upper and lower cutaneous lip as well as on and around her nose for several consecutive days (Figure 1). She denied irritation or pruritus of the area, and there was no report of precipitating erythema or eczematous-type reaction in the depigmented areas.
Figure 1.
A 78-year-old, African-American woman presented with newly acquired depigmented areas on the perinasal and perioral skin.
She did not recall previous use of this particular product, and had no previous personal or family history of vitiligo. Beyond the areas of the face where the product was applied, she had not developed depigmentation on any other areas of her face and body. She was not applying any other topical medications or creams. She declined any treatment or further testing, such as patch tests or open application tests.
DISCUSSION
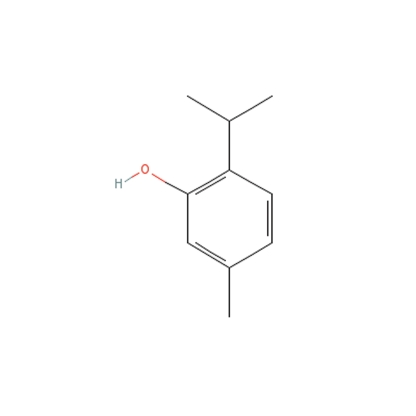
The main ingredients of Vicks VapoRub include camphor 4.8%, eucalyptus oil, menthol 2.6%, thymol, and several other ingredients.3 Thymol, or 5-methyl-2-(1-methylethyl) phenol, is a phenol derivative that can have antiseptic and preservative properties. It is found in sunscreens, hair dye, and a wide variety of other topical formulations.
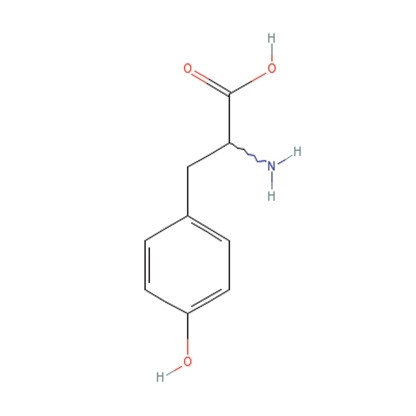
Phenol derivatives are structurally similar to the melanin precursor tyrosine and compete for tyrosinase (Figure 2).4 Tyrosinase hydroxylates phenols into damaging free radicals.5 A subset of people seems to be more affected by the free radicals created from phenolic derivatives. This might be due to their comparatively higher levels of tyrosinase-related protein-1 (Tyrp1), which also catalyzes the derivatives' conversion into reactive oxygen species. The cumulative oxidative stress is cytotoxic to melanocytes, resulting in hypopigmentation.1 Phenol has also been found to be toxic to other internal organs, as shown in factory workers exposed to large amounts of phenol-containing chemicals.1 Phenolic and catecholic derivatives are in a number of common substances, and many of these have been implicated in cases of chemical leukoderma (Table 1).
Figure 2.
Phenol derivatives are structurally similar to the melanin precursor tyrosine and compete for tyrosinase.
Table 1.
Examples of Phenolic and Catecholic Derivatives1
| Rubber in clothing, aprons, hat bands |
| Adhesive tape |
| Neoprene |
| Varnish and lacquer resins |
| Formaldehyde resins |
| Printing inks |
| Plasticizers |
| Synthetic oils |
| Paints |
| Hydroquinone |
| Germicides |
Chemical leukoderma is histologically identical to vitiligo and may be hard to distinguish clinically except by specific exposure history of the area.6 The depigmentation can be precipitated by a contact dermatitis with eczematous changes or can arise without prior symptoms.7 Some authors distinguish chemical leukoderma from occupational vitiligo, the latter exhibiting depigmentation beyond the areas of contact, possibly via an immune-mediated mechanism.
Treatments for chemical leukoderma parallel those of vitiligo. These include psoralen and long-wave ultraviolet radiation (PUVA) or UVB phototherapy, epidermal surgical grafting, and topical or interlesional corticosteroids, all with varying, inconsistent results.8 A majority of the population is not susceptible to a chemical leukoderma induced by low concentrations of phenolic derivatives. However, these derivatives are found in a wide range of common products that touch the skin. The challenge for dermatologists is to appropriately recognize this association in the clinical setting.
References
- 1. Boissy RE, Manga P. On the etiology of contact/occupational vitiligo. Pigment Cell Res. 2004;17:208–214. doi: 10.1111/j.1600-0749.2004.00130.x. [DOI] [PubMed] [Google Scholar]
- 2. Svobodová A, Psotová J, Walterová D. Natural phenolics in the prevention of UV-induced skin damage. A review. Biomed Papers. 2003;147(2):137–145. [PubMed] [Google Scholar]
- 3. [October 15, 2008]. http://www.vicks.com/vaporub-ointment.php#ingredients.
- 4. Ros JR, Rodriguez-Lopez JN, Varon R, Garcia-Canovas F. Kinetic study of the oxidation of 4-tert-butylphenol by tyrosinase. Eur J Biochem. 1994;222:449–452. doi: 10.1111/j.1432-1033.1994.tb18884.x. [DOI] [PubMed] [Google Scholar]
- 5. Gellin GA, Maibach HI, Misiaszek MH. Detection of environmental depigmenting substances. Contact Dermatitis. 1979;5:201–213. doi: 10.1111/j.1600-0536.1979.tb04853.x. [DOI] [PubMed] [Google Scholar]
- 6. Banerjee K, Banerjee R, Mandal B. Amulet string contact leukoderma and its differentiation from vitiligo. Indian J Dermatol Venereol Leprol. 2004;70:180–181. [PubMed] [Google Scholar]
- 7. Kim YY, Kim MY, Park YM, Kim HO. Nasal cannula-induced chemical depigmentation. Contact Dermatitis. 2006;55:113–114. doi: 10.1111/j.0105-1873.2006.0866a.x. [DOI] [PubMed] [Google Scholar]
- 8. Singh P, Singh J, Agarwal US, Bhargava RK. Contact vitiligo: etiology and treatment. Indian J Dermatol Venereol Leprol. 2003;69(1):27–29. [PubMed] [Google Scholar]
- 9. [October 15, 2008]. http://chem.sis.nlm.nih.gov/chemidplus/jsp/common/ChemIn.