Abstract
Ongoing neurogenesis in discrete sectors of the adult central nervous system depends on the mitotic activity of an elusive population of adult stem cells. The existence of adult neural stem cells provides an alternative approach to transplantation of embryonic stem cells in cell-based therapies. Owing to the limited intrinsic fate of adult stem cells and inhibitory nature of the adult brain for neurogenesis, accommodation for circuit replacement in the brain will require genetic and epigenetic manipulation. Here, we show that a replication-incompetent Equine Infectious Anemia Virus (EIAV) is highly suitable for stable and persistent gene transfer to adult neural stem cells. The transduced regions were free of long-lasting neuroimmune responses to EIAV. Transduction in the subventricular zone was specific to the stem cell niche, but spared the progeny of adult neural stem cells that includes transit amplifying progenitors (TAPs) and migrating neuroblasts. With time, EIAV-transduced stem cells passed on the transgene to TAPs and migrating neuroblasts, which ultimately differentiated into neurons in the olfactory bulbs. We show that EIAV is highly suitable for discovery and assessment of mechanisms that regulate proliferation, migration and differentiation in the postnatal brain.
Keywords: adult neural stem cells, adult neurogenesis, lentivirus, EIAV, olfactory bulb, neuronal migration
Introduction
The ability of the central nervous system to replace its damaged or diseased tissue is limited and cell-based therapies will be required for replacement strategies in the central nervous system. Most neurons are born during prenatal gestation and early postnatal periods.1,2 However, cell-specific neurogenesis persists in the adult dentate gyrus of the hippocampus and the subventricular zone (SVZ) of the lateral ventricles in a number of mammalian species including humans.3–5 The existence of postnatal and adult neural stem cells offers a potential source for production of new neurons and glia in damaged or diseased neural tissue6–8 The endogenous nature of these stem cells is particularly attractive as they may eliminate the use of exogenous embryonic stem cells, and potentially bypass problems associated with transplanted cells and tissues.9,10 However, restricted differentiation potential of adult neural stem cells to generate multiple types of neurons in vivo11–13 must be overcome before their utilization in cell-based therapies. For example, only subsets of interneurons in the olfactory bulb (OB) and glial cell types in the white matter are generated in the adult SVZ.3 It is now clear that genetic/epigenetic manipulation for reprogramming the adult neural stem cells will be essential for generation and differentiation of distinct neuronal and glial cell types at the site of injury or disease. Thus, methods for cell-specific gene transfer are critical for utilization of adult stem cells in cell-based therapies.
Currently methods for cell-specific gene transfer primarily depend on promoter activity in target cells. However, utilization of promoters is technically difficult, and can be limiting for adult stem cells, which are a heterogeneous population with presumably distinct sets of active promoters. Thus, development of tools that specifically target adult neural stem cells while sparing their progeny during the initial transduction event will be highly valuable for both basic science and future therapeutic applications.
Viral vectors have proven to be the most effective tools for in vivo gene transfer,14,15 and recently electroporation techniques have also been established in embryonic and adult mice.16,17 The caveat with electroporation is the transient nature of gene expression inherent to this method, with mostly acute results in the targeted cells and subsequent ‘dilution’ of the transferred gene in the long run. Moreover, current methodologies involving electroporation in mice are unlikely to translate into larger mammalian species or humans owing to technical difficulties that arise with electroporation of large brains. A number of viral vectors have been used to study gene function and lineage tracing in postnatal and adult neurogenesis;18–22 however, a comprehensive analysis of their transduction targets is not clear. Moreover, target specificity of many of the vectors is thought to be based on their origin (for example, adeno- or lenti-based vectors) and pseudotyping. Of the tested viral vectors, lentiviruses have an efficient capacity to induce long-term changes, as their retroviral capacity dictates an efficient integration of carried genetic material into host chromosomal DNA.14
Here, we show that a replication-incompetent vector based on the Equine Infectious Anemia Virus (EIAV), expressing Enhanced Green Fluorescent Protein (EGFP) is highly specific for gene transfer to adult neural stem cells. We chose EIAV because of its high transduction capacity,23 the limited adverse effects of its viral particles after transduction in vivo,24 as well as its earlier use in neural tissue.25 The vector was pseudotyped with the vesicular stomatitis virus G-protein, and we show that it preferentially transduces adult neural stem cells in the SVZ. The specific targeting of adult stem cells allowed for assessment of proliferation, migration and differentiation of their progeny in the postnatal brain.
Results
EIAV targets the adult stem cell niche without adverse reactions
EIAV vectors encoding for the reporter gene EGFP under the human cytomegalovirus (EIAV-CMV-EGFP) or chicken β-actin (EIAV-pCA-EGFP) promoters were generated as described earlier23,24 (Figure 1a). To assess the ability of EIAV to target the adult neural stem cells, vectors were stereotactically injected into the anterior lateral ventricles of 6–8 weeks old adult mice. As neural stem cells in the postnatal brain give rise to a heterogeneous population of OB interneurons based on their position in the ventricular walls,26 bolus injections of EIAV were strategically placed to target the entire anterior/posterior and dorsal/ventral boundaries of the ventricular walls (5 μl, 5 × 107 total infectious units). The injected brains were assessed 24 h, 48 h, 1 week, 1 month and 6 months post-injection. No behavioral or health problems were detected in the injected mice at any survival time. EGFP expression in the SVZ was clearly detectable between 48 h and 1 week post-injection, but very few EGFP(+) cells were visible in the rostral migratory stream (RMS) or OB (Figure 1b). New born neurons migrate through the RMS to reach the OB in postnatal and adult brains. In contrast, in the 1 month and 6 months post-injected brains, the RMS and OB included large number of EGFP(+) cells indicating that EIAV had efficiently targeted stem cells in the SVZ, and that integrated transgene may have been passed on to their progeny (Figures 1c–d).
Figure 1.
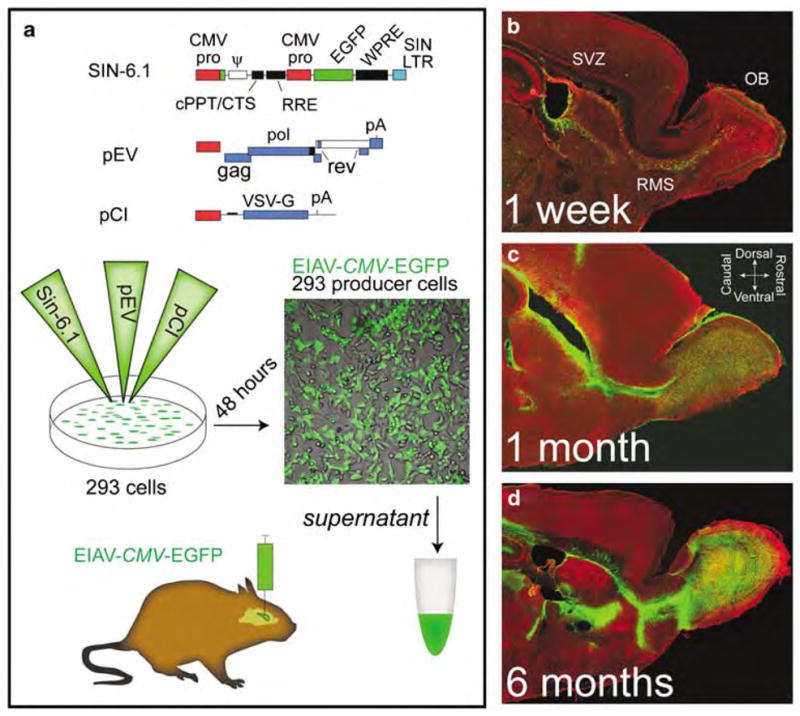
Equine infectious anemia virus (EIAV)-mediated gene transfer to the ventricular zones of the adult brain. (a) EIAV vectors were constructed as described before.23,24 Diagram illustrates the three plasmids for generating the EIAV-CMV-EGFP vector. A line of 293 cells was triple transfected with EIAV constructs and 48 h later EIAV-CMV-EGFP or EIAV-pCA-EGFP viruses were harvested from the supernatant, concentrated by ultracentrifugation and resuspended in a carrier medium for in vivo and in vitro transduction. Consistent yields of 1010 infectious units per mililiter of carrier medium were obtained. (b–d) EIAV-CMV-EGFP vectors were injected (5 μl per hemisphere; 5 × 107 total infectious units) into the anterior lateral ventricles of young adult mice (6 weeks old) using stereotaxic apparatus. Mice were then allowed to survive for 1 week (b), 1 month (c) or for 6 months (d). Sagittal views of the brains reveal a gradual increase in density of EGFP-positive cells (green) in the olfactory bulbs (OBs) with time. Note that the entire rostro-caudal and dorsal-ventral boundaries of the subventricular zone (SVZ) were transduced with the bolus injections. CMV, cytomegalovirus; EGFP, enhanced green fluorescent protein; pCA, chicken β-actin promoter; RMS, rostral migratory stream.
To assess the potential immune responses to EIAV, brain sections harvested from EIAV-injected mice were labeled with markers for immune cells (Supplementary Figures 1 and 2). Between 24 h and 1 week after EIAV injection, neuroimmune and neuroinflammatory responses were detectable in the periventricular tissue (Supplementary Figure 1). To determine whether insult during injection of vectors contributed to the initial immune responses, brains were sham injected with saline only. At 48 h after injection, a substantial degree of responses were noted using markers for immune cells (Supplementary Figure 2). Similar densities of immune cells were present in sham- and EIAV-injected brains at both 48 h and 7 days post-injection, suggesting that much of the immune responses in EIAV-injected brains were likely because of surgical manipulation rather than presentation of EIAV (Supplementary Figure 1). In further support of this interpretation, immune responses significantly dissipated by 1 month and were undetectable after 6 months post EIAV injection (Supplementary Figure 1). Therefore, EIAV-mediated gene transfer to cells in the SVZ does not induce long-term immune responses.
Slow proliferation in the adult SCN is sufficient to populate the RMS and OB with neuronal progeny
Newly generated neurons in the adult brain are thought to arise from populations of stem cells that reside in an adult stem cell niche (SCN) situated in the SVZ. The adult SCN extends from the SVZ to the OB along the RMS, in which adult stem cells continuously self-renew and sustain a distinct population of rapidly dividing transit amplifying progenitors (TAPs).27,28 A heterogeneous population of TAPs gives rise to migrating neurons, which in due course settle in the OB and differentiate into interneurons.8 The germinal zones in the SVZ and RMS have provided a suitable platform for investigating molecular and cellular mechanisms of proliferation, migration and differentiation in the post-natal brain.
On the basis of the effective transduction of the periventricular zones in the brain, we next characterized the cellular targets and transduction extent of EIAV in the adult SCN, in TAPs, and in their neuronal progeny during the multiple survival time points. Two important constituents of the SCN are ependymal cells and astrocytes. Ependymal cells within the SCN express the calcium-binding protein, S100β, but not the intermediate filament glial fibrillary acidic protein, GFAP (S100β(+)/mGFAP(−)). In contrast, S100β(−)/mGFAP(+) cells are the SCN astrocytes.29 Between 48 h and 1 week after injection, the majority of transduced cells in the SVZ were S100β(+)/mGFAP(−) ependymal cells (Figure 2a) and S100β(−)/mGFAP(+) SCN astrocytes (Figure 2b). Most TAPs in the adult SVZ express the transcription factor DLX2 (distaless-2) and EGFR (epidermal growth factor receptor).30 DLX2(+) and EGFR(+) cells were devoid of EGFP expression after 1 week suggesting that they were not targeted by EIAV (Figure 2c). Similarly, few migrating neuroblasts positive for PSA-NCAM (polysialylated neuronal cell adhesion molecule) or NeuN(+) mature neurons in the OB expressed EGFP after 1 week (Figures 2d and e). Curiously, we found a number of GFAP(+)/EGFP(+) in the RMS, which we reasoned may have been reactive astrocytes that had been transduced by EIAV and migrated into the RMS during the first week post-injection (Figure 2d).
Figure 2.
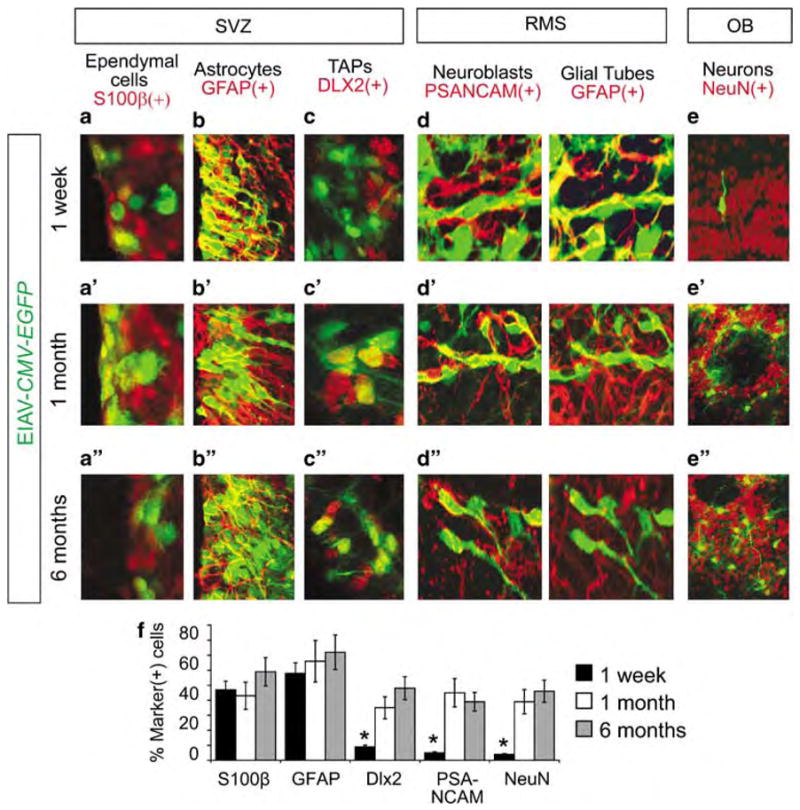
Cellular targets of Equine infectious anemia virus (EIAV) in the subventricular zone (SVZ). (a–e) At 1 week after EIAV-CMV-EGFP injections, the majority of transduced cells in the SVZ (positive for EGFP, green) were S100β(+) ependymal cells (red, a) and the glial fibrillary acidic protein (GFAP)(+) astrocytes (red, b). Few, if any, distaless 2 (DLX2)(+) transit amplifying progenitors (TAPs) (red, c), polysialylated neuronal cell adhesion molecule (PSA-NCAM)(+) migrating neuroblasts (red, d) or NeuN(+) olfactory neurons (red, e) expressed EGFP. EIAV-transduced cells in the rostral migratory stream (RMS) were largely GFAP(+) astrocytes (d). (a′/a″–e′/e″) 1 month (a′–b′) and 6 months (a″–b″) post EIAV-CMV-EGFP injection, ependymal and astrocytic cells maintained their expression of EGFP. In contrast to the 1-week survival time, a significant number of TAPs (c′/c″), migrating neuroblasts (d′/d″) and olfactory neurons (e′/e″) were EGFP(+), suggesting that they were derived from either the transduced astrocytes or ependymal cells. (f) Percentage of distinct cell types in the SVZ described above that express EGFP was calculated using stereological estimates of cell density for double-labeled EGFP(+)/marker(+) cells in the SVZ. Data are average±s.e.m. Asterisks indicate significance, Student’s t-test, P<0.001. CMV, cytomegalovirus; EGFP, enhanced green fluorescent protein; RMS, rostral migratory stream; OB, olfactory bulb.
At 1 month after EIAV injection, we noted a significant expansion of EGFP(+) cells beyond the ependymal and astrocytic cell types (Figures 2a′–d′). By this time, a significantly higher percentage of DLX2(+) TAPs (Figure 2c′), migrating neuroblasts (Figure 2d′) and OB neurons (Figure 2e′) expressed EGFP (Figure 2f). To ensure that the initial lack of EGFP expression in TAPs and migrating cells was not because of possible repression of the CMV promoter, we replaced CMV with the ubiquitously active chicken β-actin promoter (pCA) in the EIAV vector. Cellular targets and timing of EGFP expression with EIAV-pCA-EGFP was identical to EIAV-CMV-EGFP-transduced brains. This comparison indicated that labeled cells in the RMS and OB were indeed derived from either astrocytes or ependymal cells within the SCN. Finally, to determine the longevity of EIAV-mediated gene transfer to the adult SCN, the injected mice were allowed to survive for 6 months post-injection (Figure 2a″–d″). The cellular pattern and developmental progeny of adult neural stem cells in the SVZ, RMS and OB remained transduced after 6 months indicating that the cDNA introduced by EIAV had stably integrated into the genome of cells in these regions. Finally, GFAP(+)/EGFP(+) cells that had invaded the RMS during the first week of transduction could no longer be detected after 1 or 6 months post-injection (Figures 2d′/d″).
EIAV targets mitotically quiescent ependymal cells and astrocytic cell types in the SVZ
On the basis of the slow passage of transduced transgene into neuronal progeny in the OB, we next asked whether EIAV-CMV-EGFP-transduced cells in the SVZ included slowly dividing putative stem cells. After 1 and 6 months, mice from the injected groups were administered Bromodeoxyuridine (BrdU) for 7 days followed by a 2-week chase period (Figure 3). During this chase period, incorporated BrdU is diluted from cells undergoing consecutive rounds of mitosis; thus, levels of detectable BrdU is only retained in cells that exited the cell cycle during BrdU administration and in slowly dividing stem cells with prolonged cell cycle lengths.31
Figure 3.
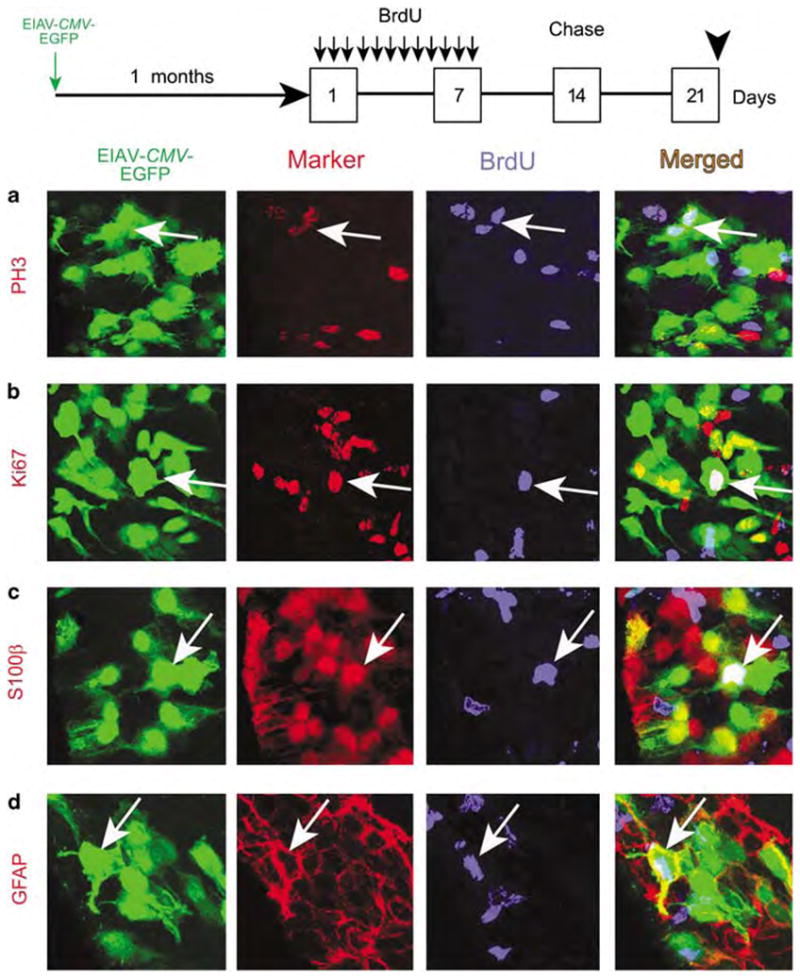
Equine infectious anemia virus (EIAV) targets slowly dividing cells in the adult stem cell niche (SCN). To assess the proliferative capacity of putative neural stem cells targeted by EIAV, Bromodeoxyuridine (BrdU) was administered thrice per day, for 7 days, in 1-month post EIAV-injected mice. Mice were allowed to survive a 2-week ‘chase’ period after the last BrdU injection so as to dilute out BrdU from TAPs and migrating neuroblasts. (a) In the subventricular zone (SVZ), some EIAV-CMV-EGFP-transduced cells (arrows) retained BrdU (blue) and co-labeled with the mitotic (M)-phase marker phosphohistone-3 (PH3) (red). (b) Likewise, some EGFP(+)/BrdU(+) cells expressed the cell cycle marker Ki67 (red, arrows), indicating that EIAV-transduced cells in the SVZ included slowly dividing stem cells. (c and d) EGFP(+)/BrdU(+) cells included S100β(+) ependymal cells and glial fibrillary acidic protein (GFAP)(+) astrocytes in the SVZ (both markers are red in their respective panels). CMV, cytomegalovirus; EGFP, enhanced green fluorescent protein.
Of all EGFP(+) cells in the SVZ and RMS, 18.7±3% retained BrdU, suggesting that they either were in the cell cycle or exited the cell cycle during the week of BrdU administration. To distinguish the fraction of EGFP(+)/BrdU(+) cells that had remained in the cell cycle, BrdU-stained brain sections were co-labeled with the cell cycle marker, Ki67, and the mitotic-phase marker, PH3 (phosphohistone-3). A total of 4.4±1% of BrdU(+)/EGFP(+) cells expressed PH3 (Figure 3a) and 6.3±2% were Ki67(+) (Figure 3b), indicating that a small fraction of the EIAV-transduced cells were indeed slowly dividing, putative stem cells. Moreover, 69.2±9% of BrdU(+)/Ki67(+) and 72.6±8% of BrdU(+)/PH3(+) cells were EGFP(+), indicating that EIAV targeted approximately 70% of the slowly dividing stem cell population in the SVZ (n = 3). Finally, to determine the cell identity of these BrdU retaining proliferating cells, we found that a small fraction of EGFP/BrdU cells in the SVZ and RMS co-expressed S100β and GFAP, suggesting that at least some of the EIAV-transduced cells are clearly the ependymal/astrocytic constituents of the SCN (Figure 3c–d).
To further assess the targeting of adult stem cells by EIAV, we used the neurosphere assay. Neurospheres are clones of neural stem cells with the ability to self renew and give rise to neuronal and glial cell types.32,33 EIAV-transduced SVZs were dissociated 2 weeks post-injection and subjected to fluorescence-activated cell sorting. Fluorescence-activated cell-sorted EGFP(+) cells were then clonally selected and cultured for neurosphere formation. Purified EIAV-CMV-EGFP-transduced cells gave rise to EGFP(+) neurospheres by 48 h. To ensure that the EIAV transgene had stably integrated into adult neural stem cells, neurospheres were passaged for 10 rounds and then assessed for EGFP expression (Figure 4a). A total of 95.8±4% (n = 3) of passaged neurospheres maintained EGFP expression, suggesting that indeed the initial transduction by EIAV had stably integrated into the stem cell genome (Figure 4a). Individual EGFP(+) neurospheres were then plated on laminin and poly-L-lysine-coated glass slides for 7 days to induce differentiation (Figure 4b). The differentiated cells included EGFP(+) neurons, EGFP(+) oligodendrocytes and EGFP(+) astrocytes (Figure 4c), indicating that EIAV-CMV-EGFP-sorted cells included adult neural stem cells with the potential to generate the three primary cell types of the brain.
Figure 4.
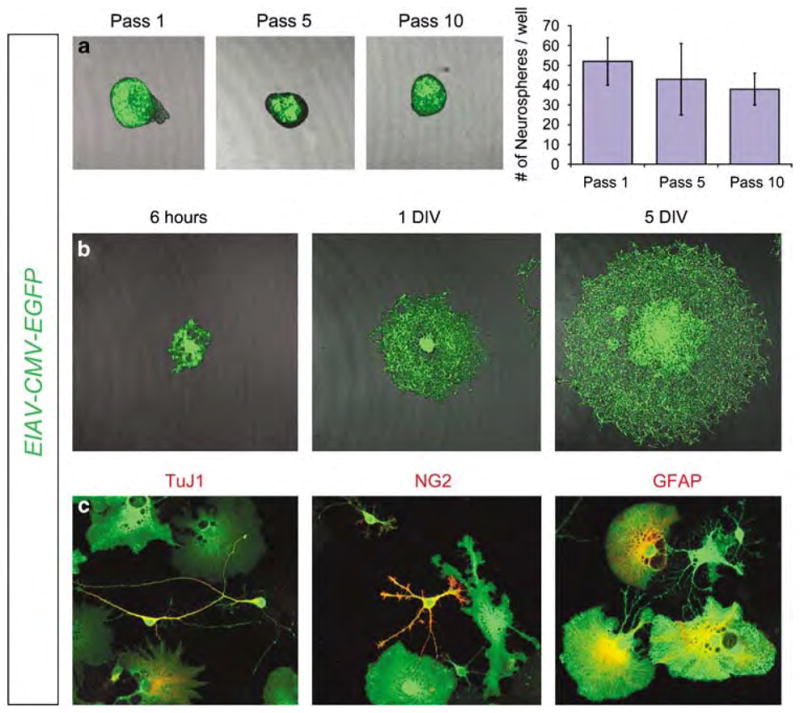
Equine infectious anemia virus (EIAV) transduced stem cells form neurospheres in vitro. EIAV-CMV-EGFP-transduced brains were harvested 2 weeks post EIAV injection, and EGFP(+) cells were fluorescence-activated cell sorted and cultured for neurosphere formation. (a) Neurospheres maintained EGFP expression after 10 passages (Pass 10) indicating that the EIAV transgene had stably integrated into the genome of the transduced neural stem cells. Data in chart are number of neurospheres per 6 mm culture dish after 1, 5 and 10 passages (error bars, standard error of the mean). (b) Passaged neurospheres were plated on laminin/poly-L-lysine-coated glass slides and after 7 days in vitro (DIV) differentiated cells were readily visible surrounding the core of the neurospheres. (c) Cells derived from the neurospheres included TuJ1(+) neurons (red); NG2(+) oligodendrocytes (red); and glial fibrillary acidic protein (GFAP)(+) astrocytes (red). CMV, cytomegalovirus; EGFP, enhanced green fluorescent protein.
Migratory behavior of EIAV-transduced cells in the RMS
After proliferation in the SVZ and RMS, newly generated cells must make their way to the OB to differentiate into appropriate neuronal types. To directly monitor the migration of progeny derived from the EIAV-transduced stem cells, organotypic slices were harvested from postnatal day 7 (P7) mice, which had received intraventricular injections of EIAV-CMV-EGFP immediately after birth at P0. Induction of the SVZ at this early postnatal time point allowed for detection of an adequate number of cells in the RMS and high-resolution analysis of their migratory behavior. Moreover, slice preparations of early postnatal brains are more viable, thus allowing us to bypass difficulties in the maintenance of adult brain slices in vitro. Slices were obtained in the sagittal plane to visualize the rostral-to-caudal extent of the RMS populated with transduced EGFP(+)-migrating cells. Migratory behavior of the isolated cells was observed in organotypic slices harvested from six EIAV-CMV-EGFP-injected mice using time-lapse confocal microscopy during a 2.5 h window (n = 50 cells per mouse; Figure 5a and b).
Figure 5.
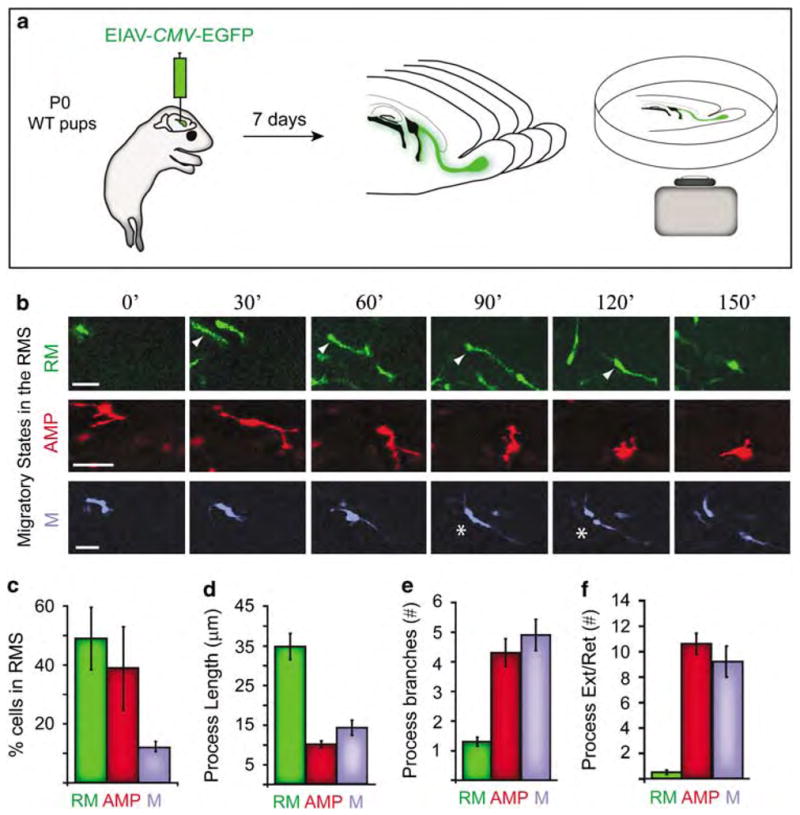
The life of migrating cells in the rostral migratory stream (RMS). (a) Organotypic slices harvested from EIAV-CMV-EGFP-injected newborn (P0) mice were time-lapse imaged (spanning 150 min) using a confocal microscope. Three dynamic migratory states in cells within the RMS were identified by assessment of EGFP cells in the RMS. Cells in the three states were colorized for diagrammatic purposes. (b) In the first state, rapidly migrating (RM) cells (green panel) rapidly translocate from one location to another, in a forward fashion toward the olfactory bulb (for example, cell indicated by arrowhead). Many RM cells entered a phase of non-directed, hyperactive motion, hallmarked by multi-process extensions and retractions (arborizing multiple processes, AMP cells, red panel). In this state, nuclear translocation is dramatically less rapid and covers less distance compared with the first state. AMP cells occasionally underwent mitosis (M cells, blue panel, asterisks indicate cell division). Bars = 50 μm for each panel. (c) Percentage of cells within each of the identified states in the RMS (n = 150 cells). (d) Length of processes(s) at each of the identified states (n = 25 cells per state). (e) Number of process branches in cells within each of the migratory states (n = 10 cell per state). (f) Average number of process extensions and process retractions during 150 min of observation (n = 30 cell per state). Data are average±s.e.m. CMV, cytomegalovirus; EGFP, Enhanced Green Fluorescent Protein.
We discovered remarkable harmony in clustered motility of cells within approximately 100 μm radii in the RMS. During the 2.5 h imaging period, migrating cells transitioned in unison in and out of three stereotyped dynamic states. (1) Of the observed EGFP(+) cells, 49±11% were rapidly migrating (RM cells), during which cells translocated in a forward fashion toward the OB (green cell indicated by arrowhead, Figure 5b, Supplementary movie 1). The average migration rate of RM cells was 76±11 μm h−1, as they sustained a steady morphology with a single polarized and long leading process that maintained its length during nuclear translocation (Figure 5b–d). There was limited branching (Figure 5e) or retraction/extension of the process (Figure 5f) compared with cells in other modes within the RMS. (2) Approximately 39±14% of EGFP(+) cells in the RMS were rapidly arborizing multiple processes (AMP cells) with limited nuclear translocation. We found that many RM cells often entered the AMP state after slowing to a near stop. These cells retracted their leading process and began to rapidly extend and retract multiple small processes (Figure 5b, red cell; Supplementary movie 2). (3) Of all the observed EGFP(+) cells in the RMS, a few (12±3%) underwent mitosis (M cells) (Figure 5b, blue cell; asterisks indicate when the cell divided). Nearly all M cells were in an AMP state before undergoing mitosis. AMP cells halted extending and retracting their processes, maintained one process, which was then followed by cytokinesis (supplementary movie 3). The unprecedented discovery of these transitory states was made possible by the highly efficient and stable transduction by EIAV in combination with high-power analysis of the temporal dynamics of migrating cells in the RMS.
A novel assay for the assessment of extracellular regulators of neuronal migration in the RMS
To ensure that EIAV-CMV-EGFP(+) migrating cells were responding to extracellular cues as do wild-type neuroblasts, we tested their responsiveness to chemotactic cues present in the RMS. We have earlier shown that neuregulins (NRGs) and their ErbB receptor tyrosine kinases play important roles in postnatal neuronal migration and differentiation.34,35 The majority of neuroblasts migrating in the RMS express the receptor tyrosine kinase, ErbB4.35 To assess the responsiveness of EIAV-transduced cells to NRGs, we devised an overlay assay in organotypic slices that included the entire extent of the RMS as described above (Figure 6a). The slices were overlaid with aggregates of Cos 7 cells, which were co-transfected with various NRG ligands and a reporter construct encoding for the monomeric red fluorescent protein (mRFP, Figure 6a). Explants were overlaid near the RMS to expose migrating cells to a confined source of secreted factors (as opposed to their application throughout the culture medium, which would not generate a gradient of factors). Responsiveness of EGFP(+)-migrating cells was then measured within a 100 μm perimeter in the RMS adjacent to the overlaid explants (Figure 6a). Responses to a secreted NRG1 isoform (NRG1 type I/II) confirmed our earlier finding that NRG1 functions as an attractive cue for migrating cells in the RMS35 (Figure 6b). However, the proportions of RM, AMP or M cells were not significantly altered when exposed to NRG1 explants compared with control exposures to mock-transfected Cos7 cells (Figure 6c–e). In contrast, ectopic exposure to another NRG subtype, NRG2, significantly increased the number of cells in the AMP state at the expense of the percentage of RM cells, and resulted in a significant increase in the number of observed M cells (Figure 6b–c). Thus, exposure to distinct factors can clearly modulate the behavior of cells in the RMS and alter their persistence in one of the states discovered in our initial analysis.
Figure 6.
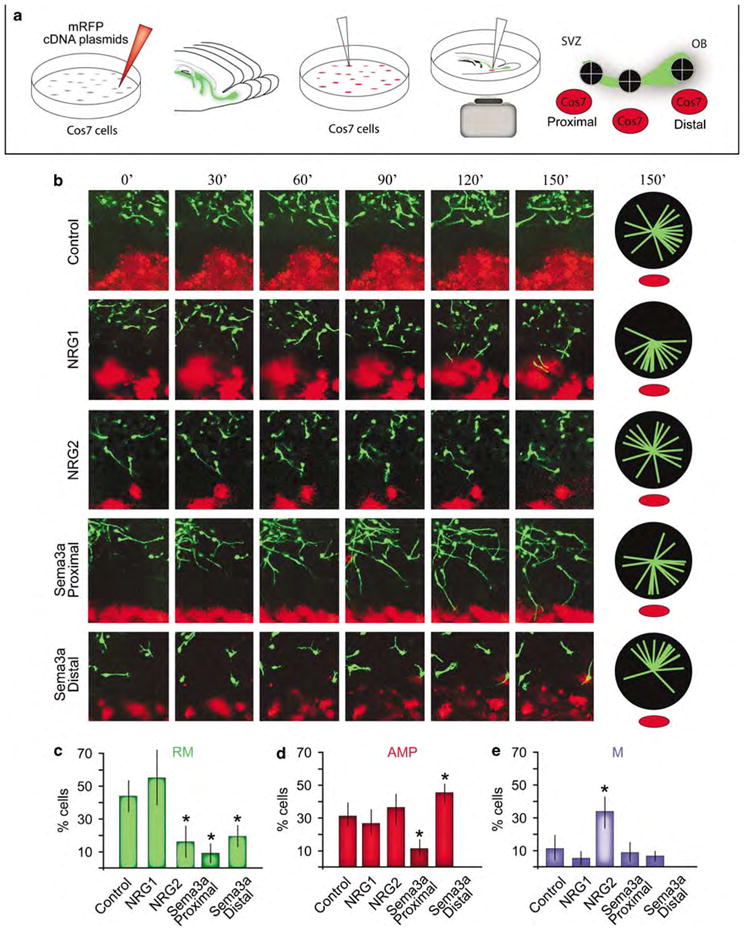
Equine infectious anemia virus (EIAV)-transduced cells in the rostral migratory stream (RMS) are highly suitable for assessment of chemotactic cues using a novel assay. (a) To assess the responsiveness of EIAV-CMV-EGFP-transduced cells in the RMS to chemotactic cues, Cos7 cells were transfected with various constructs encoded secreted factors and a monomeric red fluorescent protein (mRFP) reporter construct. Organotypic slices were harvested from P7 animals as described in Figure 5, and aggregates of Cos7 cells were explanted on the slices proximal to, in the middle or distal to the subventricular zone (SVZ). (b) Migrating cells do not respond to mock-transfected (mRFP alone) Cos7 cells. We earlier identified the expression and function of a class of growth factors called the neuregulins (NRGs) and their receptor tyrosine kinase, ErbB4, in migration and differentiation in the RMS and olfactory bulb (OB).34,35 The majority of neuroblasts migrating in the RMS express the receptor ErbB4. A secreted isoform of NRG1 has a potent attractive effect on the migrating cells in the RMS. Another ErbB4 ligand, NRG2, has a different effect on migrating neuroblasts, wherein it disrupts their rapid migration and increases the number of AMP and M cells in the RMS. Another secreted factor, the semaphorin type 3a protein (Sema3a) has distinct effects on migrating cells in the sector of the RMS proximal to the SVZ versus its effect on cells in the sectors of the RMS distal to the SVZ (near the OB). Pie orientation charts indicate direction of 20 randomly selected EGFP(+) cells in the RMS relative to the appropriate explants (red). (c–e) Percentage of cells within the identified migratory states in the RMS when exposed to different ligands (n =50 per experiment). Data are average±s.e.m. Asterisks indicate significance, Student’s t-test, P<0.01. AMP, arborizing multiple processes; CMV, cytomegalovirus; EGFP, Enhanced Green Fluorescent Protein; M, mitosis; RM, rapidly migrating.
To assess the effects of an established differentiation factor on EIAV-transduced migrating cells, we chose the secreted factor Semaphorin subtype 3a (Sema3a), which has a known function in migration and differentiation of neurons. Aggregates of Cos7 cells expressing Sema3a and monomeric red fluorescent protein were explanted either near the SVZ adjacent to the RMS (proximal, Figure 6a), mid-RMS or within the OB (distal, Figure 6a). Migrating cells in the proximal segment of the RMS showed dramatically different responses to Sema3a compared with EGFP(+) neuroblasts in the distal segment of the RMS. Proximal cells slowed their migration and many of them stopped, but their leading edges continued to extend (Figure 6b; Supplementary movie 4). Although some cells extended their leading process toward Sema3a-secreting explants, others did not appear attracted to the explant. In contrast, cells in the distal segment of the RMS slowed their migration to a near complete stop when presented with Cos7 cells expressing Sema3a. They then oriented their basal ends toward the source of Sema3a, whereas their apical process started to extend away from the source (Figure 6b; Supplementary movie 5).
The responses to Sema3a of EIAV-transduced cells in both the proximal and distal segments of the RMS resulted in significant changes in the proportion of cells in the three migratory states described earlier. The proportion of RM cells was massively decreased in both the proximal and the distal segments of the RMS when exposed to Sema3a (Figure 6c). However, proportion of cells in the AMP state was significantly different depending on location; hence, proportion of AMP cells significantly decreased in the proximal segment of the RMS, while an increase in AMP cells was noted in the distal segment (Figure 6d). No significant differences were found in proportion of M cells when exposed to Sema3a ectopically (Figure 6e). Hence, time of birth or the source of migrating cells in the RMS may dictate as to how they respond to Sema3a.
Differentiation of EIAV-transduced cells in the OB
On arrival into the OB, migrating neuroblasts disperse radially, settle into granule and periglomerular layers, and differentiate into GABAergic and dopaminergic interneurons (Figure 7a). Adult-derived neurons in the OB are thought to encompass interneuronal populations that express the calcium-binding proteins, CB (calbindin) and CR (calretinin), and the dopaminergic enzyme, TH (tyrosine hydroxylase). Analysis of cells in the OB 1 and 6 months post EIAV-CMV-EGFP injections revealed that 48±3% of EGFP(+) cells were positive for the neuronal marker, NeuN, which is expressed primarily by fully mature neurons. Nearly all EGFP(+) cells in the OB co-labeled with the cytoskeletal protein β type III tubulin (TuJ1), which is specific to immature and some mature neurons. This finding indicated that nearly 50% of newly arrived cells in the OB differentiate into fully mature neurons. Surprisingly, only a small fraction of the newly incorporated cells in the OB mature into CR(+), CB(+) or TH(+) neurons during adult periods (Figure 7b). Of all EGFP(+) cells in the OB, 13±4% were CR(+), 3±1% were TH(+) and <1% were CB(+). The percentage of cells that expressed CB, CR and TH remained low 6 months post EIAV-CMV-EGFP injections. We then asked whether or not some of the labeled cells were immature neurons on their way to differentiate in the OB. Approximately 22±8% of all EGFP(+) cells were PSA-NCAM(+) indicating that at least a subset of cells in the OB was undifferentiated (Figure 7b). However, a substantial number of mature cells remained unlabeled with any of the known markers, suggesting that many adult-derived neurons in the OB must belong to the yet-to-be identified neuronal cell types.
Figure 7.
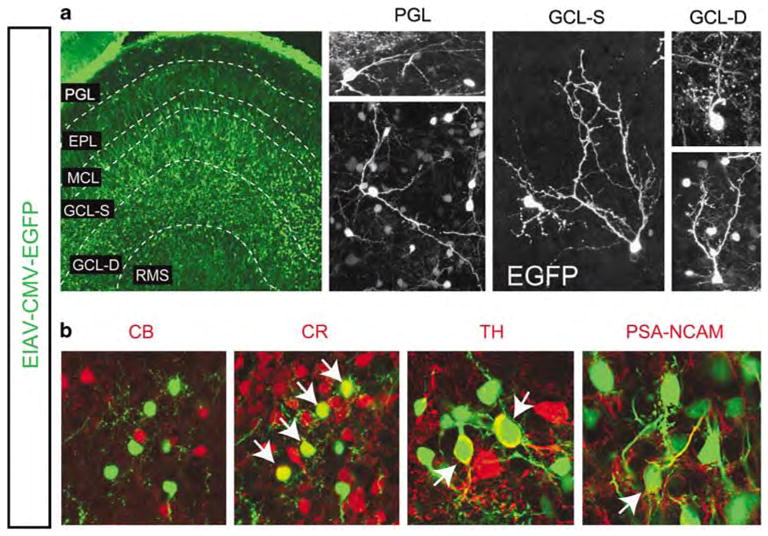
Equine infectious anemia virus (EIAV)-transduced cells differentiate into neurons in the olfactory bulbs (Obs). (a) EIAV-CMV-EGFP-transduced cells were distributed widely in the OB 1-month post-injection. Layers of the OB: PGL, periglomerular layer, EPL, external plexiform layer; MCL, mitral layer; GCL-S, superficial granule cell layer; GCL-D, deep granule cell layer; RMS, rostral migratory stream. Morphology of cell types in the distinct layers indicates that EIAV-transduced cells are highly suitable for morphometric assessment of differentiation in the OB. (b) The EGFP(+) population (green) in the OB includes some calretinin (CR, red)-expressing interneurons, a few tyrosine hydroxylase (TH, red)-expressing neurons in the PGL, but not calbindin (CB, red)-expressing neurons in the PGL. A portion of the EGFP cells expressed polysialylated neuronal cell adhesion molecule (PSA-NCAM) suggesting that they were undifferentiated cells. Arrows indicate double labeled cells for each marker. A significant number of EGFP(+) cells could not be labeled with any of the known markers. CMV, cytomegalovirus; EGFP, Enhanced Green Fluorescent Protein.
Discussion
EIAV targets adult neural stem cells without the use of cell-specific promoters
A number of viral species have been modified for gene transfer through deletion of their replication machinery. The most successful of these belong to the adeno-associated, lenti- and herpes simplex viruses.14,36 General consensus is that lenti-based vectors are effective for targeting both mitotic and post-mitotic cells, whereas adeno-associated and herpes simplex viruses largely target mitotic cells. Our study shows that EIAV is a powerful and cell-specific vector for gene delivery to the postnatal SCN, and for the subsequent labeling of neuronal progeny in the RMS and OB. These results match earlier findings by other investigators who have used lentiviruses to label progenitors in the SVZ.18–22 Our study provides a comprehensive assessment of timing and cell targets of the EIAV-mediated transduction. Moreover, we found that immune responses within the injected area, including the SCN, are at the level of sham injections of saline, suggesting that the EIAV viral particles may not elicit an immune response beyond what occurs because of surgical manipulation. The preferential targeting by EIAV proved highly valuable for gene transfer to quiescent and slowly dividing stem cells, and their cellular niche while sparing their progeny during the initial transduction.
Our study shows that EIAV may serve as an alternative gene transfer approach to the use of promoters for targeted gene transfer to the adult neural SCN. The quiescent and slow-dividing nature of adult stem cells is likely the basis of their affinity for lentiviruses. The absence of initial transduction in the progeny of adult stem cells will be of great value for cell-specific study of stem cell mechanisms while sparing other progenitors from the targeted manipulation in gene expression. To ensure that the absence of apparent transduction was not because of unbeknownst repression of promoter activity in TAPs or the migrating neuroblasts, we used two distinct ubiquitous promoters (CMV and chicken β-actin) and showed that the initial transduction of the adult SCN was indeed confined to its ependymal and astrocytic constituents. However, with time, a large number of TAPs, migrating neuroblasts and interneurons in the OB became EGFP(+) suggesting that these cells were derived from the transduced SCN.
The precise identity of adult neural stem cells in the central nervous system has remained largely elusive. Ependymal cells are thought to include subsets of stem cells;37,38 however, this view has been challenged by a number of studies which suggest that subpopulations of GFAP(+) astrocytes are ‘the’ adult stem cells.29,39,40 EIAV vectors targeted both the ependymal and astrocytic constituents of the SCN. The 50–70% transduction efficiency consistently achieved with our injections was sufficient to sequentially label the progeny of SVZ stem cells in the RMS and OB genetically. Given that ependymal cells are post-mitotic after birth,29 our results are highly suggestive that the progeny labeled by our initial transduction must be largely derived from transduced astrocytes in the adult SCN.
EIAV-transduced progeny are highly suitable for analysis and discovery of mechanisms of neuronal migration
Our study shows that EIAV-mediated transduction is highly suitable for high-resolution molecular and cellular analysis of neuronal migration in the early postnatal brain. Time-lapse examination of EIAV-transduced cells migrating in the RMS led to the discovery of three behaviorally distinct cell types. Altogether, these cells encompass the dynamic state of nearly every cell within the RMS. Furthermore, we postulate that cells in the RMS transit through these states until they reach their differentiation zone within the OB. Our results suggest that after neuroblasts are born in the SVZ or RMS, they enter an RM mode and migrate rapidly for 1–2 h moving for 100–200 μm. RM cells may then enter an AMP state during which some may divide (M cells), whereas others enter the RM state again. This finding raises the possibility that migrating neuroblasts are intrinsically programmed to transit in and out of the three dynamic modes for sufficient number of neurons to reach their target. Remarkably, cells within approximately 100 μm radii of the RMS move in and out of the identified modes in unison, suggesting that nearby neuroblasts respond to migratory cues in a similar fashion. Intrinsic and extrinsic molecular and physiological mechanisms that may regulate these dynamic states will be of great interest in future studies.
In addition, we developed a novel overlay assay to assess extracellular mechanisms that regulate postnatal migration in the RMS. Using this tool we determined that exposure to distinct extracellular cues preferentially pushes neuroblasts in or out of the identified dynamic modes. We have earlier shown that NRG-ErbB4 signaling influence neuronal migration in the RMS.34,35 Here, we found that NRG1-ErbB4 signaling may, in part, regulate persistence of cells in the RM state. In contrast, NRG2-ErbB4 signaling may push neuroblasts into the AMP and mitotic phases at the expense of their passage through the RM phase. The precise signaling mechanisms used by NRG1 and NRG2 to transmit their distinct effects through ErbB4 activation remain to be determined.
Another important signaling cascade that involves Sema ligands, and their Plexin and Neuropilin receptors may govern differentiation of neurons by regulating process extension and orientation of their polarity. Semas have been shown to exert both attractive and repulsive effects on process extension in developing neurons.41 Moreover, Sema–Neuropilin signaling has been shown to be essential for axon path finding from the olfactory epithelium to the OBs during development.42,43 Sema3a mRNA is discretely present in the OB with its highest expression confined to the mitral cell layer (Allen Brain Atlas; http://mouse.brain-map.org). A number of known Sema3a receptors that belong to the Neuropilin and Plexin family of proteins are expressed within the SVZ, RMS and OB regions (Allen Brain Atlas and Gene Paint, http://www.genepaint.org). The differential responses of EIAV-labeled cells in the proximal and distal portions of the RMS to Sema3A raises two intriguing possibilities; either the age of migrating cells is a determinant of their responsiveness to Sema3A (through potentially transient expression of distinct Sema3A receptors) or neuronal populations derived from distinct stem cell populations along the SVZ and RMS may differentially respond to Sema3A. These two possibilities can be tested by more careful characterization of Sema3A expression and function in different regions of the RMS. However, this future endeavor will greatly benefit from development of reliable markers of distinct stem cell populations along the SVZ and RMS. Together, our findings show that combination of EIAV-mediated transduction with our novel overlay assay and categorization of dynamic states during neuronal migration in the RMS is a highly suitable platform for discovery and delineation of mechanisms of neuronal migration in the postnatal brain.
Differentiation of EIAV-transduced cell in the OBs
With the aid of stably integrated EIAV transgene, we found that a large number of adult-derived neurons in the OB survived and appeared to be added to the existing circuitry in the OB. We found that a large number of EGFP(+) cells continued to be present in the OB even after 6 months, despite the massive reduction in adult neurogenesis after 2 months of age in mice.44,45 This suggests that adult-born neurons may remain functional for protracted periods during adult life, challenging the current view that interneurons are recycled in the OB continuously.44,46 Moreover, only a few adult-derived neurons differentiated into CR(+) interneurons, and even fewer were dopaminergic or express CB, which are currently thought to include the majority of interneurons in the OB. Thus, the stable integration of reporter transgene through EIAV transduction allowed for identification of an earlier unappreciated diversity in adult-derived neuronal populations in the OB. In support of our findings, a recent study has also shown that approximately 30% of newly generated cells labeled in the SVZ are positive for the same series of mature interneuronal markers tested in our study after 1 month.47 The characteristics of these neurons and their functional significance for olfactory function has begun to be assessed,48–50 but for the most part remains elusive.
Summary
Our study establishes that EIAV-mediated transduction of the postnatal SCN is highly suitable for stable and long-lasting gene transfer to adult-derived neurons in the OB. Preferential transduction of cells in the SCN, while sparing the TAP and neuroblasts populations, was sufficient for gene transfer to a substantial number of neuronal cell types in the OB with time. In addition, EIAV-mediated labeling of migrating cells in the RMS allowed for discovery of three dynamic states. This categorization will be of great value in future discovery and characterization of mechanisms required for neuronal migration in the RMS. Identification of such mechanisms will be essential for future utilization of adult neural stem cells and their progeny in cell-based therapies in non-regenerative regions of the adult brain. Moreover, on the basis of the recent focus on endogenous adult neural stem cells as potential sources of brain tumors,51 the methodologies presented here will be of great value toward molecular dissection and therapeutic targeting of cancerous stem cells in the brain.
Materials and methods
EIAV vector construction and administration
The EIAV-CMV-EGFP and EIAV-pCA-EGFP vectors were generated by triple transfection of EIAV constructs as described earlier23,24 (Figure 1). A total of 5 μl of EIAV-CMV-EGFP and EIAV-pCA-EGFP vectors (total of 5 × 107 infectious units) were injected into the anterior lateral ventricles of young adult mice (6–8 weeks old) using stereotaxic surgery. Newborn mice (P0) received 1 μl injections of the same EIAV-CMV-EGFP intraventricularly. See Supplementary Methods for more details.
Tissue processing and immunohistochemistry
Sagittal brain sections from EIAV-injected mice were obtained using a vibratome (Leica Instruments, Bannockburn, IL, USA) and collected in series. Each series was then immunostained for identification of distinct cell types in the SVZ, RMS and OB. Injection sites were mapped to decipher the position of origin in each case as distinct domains within the SVZ have been recently shown to give rise to distinct neuronal types in the OB.26 Only brains with transduction of the entire rostro-caudal and ventro-dorsal domains of the SVZ were analyzed for progeny analysis in the OBs (n = 3). Sections were blocked with 10% Goat serum in 0.1 M phosphate buffer saline and 1% Triton X for 1 h. Sections were then placed in primary antibodies overnight at the appropriate dilutions (Supplementary methods). After several washes, secondary antibodies (Alexa 488 anti-chicken, Alexa 647 anti-rabbit and anti-mouse Cy3; Invitrogen, Carlsbad, CA, USA) were applied for 1–2 h at room temperature. Sections were then washed and mounted for imaging.
Data analysis
Tissue analyses were carried out using a confocal microscope (Nikon Eclipse C1, Nikon Instruments, Melville, NY, USA), and data were quantified using standard stereological estimation and fluorescence intensity quantification methods as described earlier.35 Significance was determined using Student’s t-tests and all values were expressed as mean±standard error of the mean (s.e.m). Fluorescence intensity index (Supplementary Figure 1) was calculated by subtracting background pixel density from measurements of pixel density in fluorescently labeled tissues using the Metamorph image analysis software (Molecular Devices, Sunnyvale, CA, USA) as described before.35
Neurosphere cultures
EIAV-transduced brains were harvested 2 weeks post EIAV injection. The brains were rapidly collected in Hanks’ balanced salt solution, the medial surface of the brain was exposed after hemi-dissection, and the septum and choroid plexus were removed under a dissecting microscope with fluorescence capability (Discovery V12, Carl Zeiss MicroImaging, Inc., Thornwood, NY, USA). The surface of the SVZ and the region corresponding to the RMS were then microdissected using microforceps and isolated cells were obtained by means of mechanical dissociation followed by enzymatic isolation of cells into individual cells (see Supplementary information for details). Cells were then sorted using a Dako Cytomation Modular Flow cytometer (NCSU Flow Cytometry Core) and immediately cultured in Neurobasal medium (Gibco Invitrogen Cell Culture, Carlsbad, CA, USA) supplemented with growth factors (see Supplementary information for details). After 2–3 days, EGFP(+) neurospheres appeared. Neurospheres were passaged after 5 days by dissociation into individual cells followed by culturing in neurosphere medium as above. Percentage of EGFP(+) neurospheres was calculated after every passage by counting the number of spheres in each well (n = 6 for each passage). Neurospheres were differentiated on laminin and poly-L-lysine-coated chambered glass slides in Neurobasal medium without growth factors for 10 days. Differentiated cultures were fixed with 4% paraformaldehyde and immunostained as described above.
Slice overlay assays
Newborn mice (P0) were anesthetized by means of hypothermia, and 1 μl of EIAV-CMV-EGFP or EIAV-pCA-EGFP was injected into the anterior horn of the lateral ventricles (see Supplementary information for details). Six days later Cos7 cells plated at high density were transfected (Effective, Qiagen Inc., Valencia, CA, USA) with constructs encoding NRGs isoforms and Sema3A. All constructs were co-transfected with an expression vector encoding for monomeric red fluorescent protein under the chicken β-actin promoter for visualization. The next day (7 days post EIAV injection) the brains from the injected animals were harvested and sagittal organotypic slices that included the entire extent of the RMS were obtained on a vibratome. Slices were placed on Whatman filters (8 μm pores, Cat# 09-300-63, Fisher Scientific, Pittsburgh, PA, USA) adhered on top of the glass-bottom portion of 25 mm Mat Tek culture dishes (Cat# NC9711297). After 5 min of incubation, aggregates of the transfected Cos7 cells with high density of monomeric red fluorescent protein expression were overlaid next to EIAV-transduced RMS-containing EGFP(+) cells. The dishes were immediately placed in an incubation chamber attached to a heated stage of a C1 Nikon confocal microscope. Dual-laser confocal scans were then obtained every 5 min for 3–4 h.
Supplementary Material
Acknowledgments
We thank Dr David Eisenstat (University of Manitoba) for providing the DLX2 antibody. We are grateful to Dr Roman Giger (University of Michigan) for providing the Sema3A construct. Drs Rick Meeker (UNC-Chapel Hill) and Lola Hudson (North Carolina State University) provided invaluable antibodies and discussion on neuroimmune and neuroinflammatory responses to EIAV. Natalie Surzenko helped with the NRG2 analysis. We also thank Dr Chris McGahan for critical reading of the manuscript. JCO acknowledges research support from the National Institutes of Health HL 51818 and sponsored research funds from the Oxford BioMedica Ltd. The initial stages of this work was supported by National Institute of Health Grant MH060929 to ESA. Most of this work was supported by NCSU start up funds to TG.
Footnotes
Conflict of interest
The authors declare no conflict of interest.
Supplementary Information accompanies the paper on Gene Therapy website (http://www.nature.com/gt)
References
- 1.Bhardwaj RD, Curtis MA, Spalding KL, Buchholz BA, Fink D, Bjork-Eriksson T, et al. Neocortical neurogenesis in humans is restricted to development. Proc Natl Acad Sci USA. 2006;103:12564–12568. doi: 10.1073/pnas.0605177103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Rakic P. Neurogenesis in adult primate neocortex: an evaluation of the evidence. Nat Rev Neurosci. 2002;3:65–71. doi: 10.1038/nrn700. [DOI] [PubMed] [Google Scholar]
- 3.Lledo PM, Merkle FT, Alvarez-Buylla A. Origin and function of olfactory bulb interneuron diversity. Trends Neurosci. 2008;31:392–400. doi: 10.1016/j.tins.2008.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Duan X, Kang E, Liu CY, Ming GL, Song H. Development of neural stem cell in the adult brain. Curr Opin Neurobiol. 2008;18:108–115. doi: 10.1016/j.conb.2008.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Curtis MA, Kam M, Nannmark U, Anderson MF, Axell MZ, Wikkelso C, et al. Human neuroblasts migrate to the olfactory bulb via a lateral ventricular extension. Science. 2007;315:1243–1249. doi: 10.1126/science.1136281. [DOI] [PubMed] [Google Scholar]
- 6.Emsley JG, Mitchell BD, Kempermann G, Macklis JD. Adult neurogenesis and repair of the adult CNS with neural progenitors, precursors, and stem cells. Prog Neurobiol. 2005;75:321–341. doi: 10.1016/j.pneurobio.2005.04.002. [DOI] [PubMed] [Google Scholar]
- 7.Abrahams JM, Gokhan S, Flamm ES, Mehler MF. De novo neurogenesis and acute stroke: are exogenous stem cells really necessary? Neurosurgery. 2004;54:150–155. doi: 10.1227/01.neu.0000097515.27930.5e. [DOI] [PubMed] [Google Scholar]
- 8.Ghashghaei HT, Lai C, Anton ES. Neuronal migration in the adult brain: are we there yet? Nat Rev Neurosci. 2007;8:141–151. doi: 10.1038/nrn2074. [DOI] [PubMed] [Google Scholar]
- 9.Li JY, Englund E, Holton JL, Soulet D, Hagell P, Lees AJ, et al. Lewy bodies in grafted neurons in subjects with Parkinson’s disease suggest host-to-graft disease propagation. Nat Med. 2008;14:501–503. doi: 10.1038/nm1746. [DOI] [PubMed] [Google Scholar]
- 10.Barker RA, Widner H. Immune problems in central nervous system cell therapy. NeuroRx. 2004;1:472–481. doi: 10.1602/neurorx.1.4.472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McConnell SK. Fates of visual cortical neurons in the ferret after isochronic and heterochronic transplantation. J Neurosci. 1988;8:945–974. doi: 10.1523/JNEUROSCI.08-03-00945.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ninkovic J, Gotz M. Signaling in adult neurogenesis: from stem cell niche to neuronal networks. Curr Opin Neurobiol. 2007;17:338–344. doi: 10.1016/j.conb.2007.04.006. [DOI] [PubMed] [Google Scholar]
- 13.Guillemot F. Cell fate specification in the mammalian telencephalon. Prog Neurobiol. 2007;83:37–52. doi: 10.1016/j.pneurobio.2007.02.009. [DOI] [PubMed] [Google Scholar]
- 14.Davidson BL, Breakefield XO. Viral vectors for gene delivery to the nervous system. Nat Rev Neurosci. 2003;4:353–364. doi: 10.1038/nrn1104. [DOI] [PubMed] [Google Scholar]
- 15.Waehler R, Russell SJ, Curiel DT. Engineering targeted viral vectors for gene therapy. Nat Rev Genet. 2007;8:573–587. doi: 10.1038/nrg2141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tabata H, Nakajima K. Efficient in utero gene transfer system to the developing mouse brain using electroporation: visualization of neuronal migration in the developing cortex. Neuroscience. 2001;103:865–872. doi: 10.1016/s0306-4522(01)00016-1. [DOI] [PubMed] [Google Scholar]
- 17.Barnabe-Heider F, Meletis K, Eriksson M, Bergmann O, Sabelstrom H, Harvey MA, et al. Genetic manipulation of adult mouse neurogenic niches by in vivo electroporation. Nat Methods. 2008;5:189–196. doi: 10.1038/nmeth.1174. [DOI] [PubMed] [Google Scholar]
- 18.Geraerts M, Eggermont K, Hernandez-Acosta P, Garcia-Verdugo JM, Baekelandt V, Debyser Z. Lentiviral vectors mediate efficient and stable gene transfer in adult neural stem cells in vivo. Hum Gene Therapy. 2006;17:635–650. doi: 10.1089/hum.2006.17.635. [DOI] [PubMed] [Google Scholar]
- 19.Colak D, Mori T, Brill MS, Pfeifer A, Falk S, Deng C, et al. Adult neurogenesis requires Smad4-mediated bone morphogenic protein signaling in stem cells. J Neurosci. 2008;28:434–446. doi: 10.1523/JNEUROSCI.4374-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Suh H, Consiglio A, Ray J, Sawai T, D’Amour KA, Gage FH. In vivo fate analysis reveals the multipotent and self-renewal capacities of Sox2+ neural stem cells in the adult hippocampus. Cell Stem Cell. 2007;1:515–528. doi: 10.1016/j.stem.2007.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alonso M, Ortega-Perez I, Grubb MS, Bourgeois JP, Charneau P, Lledo PM. Turning astrocytes from the rostral migratory stream into neurons: a role for the olfactory sensory organ. J Neurosci. 2008;28:11089–11102. doi: 10.1523/JNEUROSCI.3713-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Consiglio A, Gritti A, Dolcetta D, Follenzi A, Bordignon C, Gage FH, et al. Robust in vivo gene transfer into adult mammalian neural stem cells by lentiviral vectors. Proc Natl Acad Sci USA. 2004;101:14835–14840. doi: 10.1073/pnas.0404180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Olsen JC. Gene transfer vectors derived from equine infectious anemia virus. Gene Therapy. 1998;5:1481–1487. doi: 10.1038/sj.gt.3300768. [DOI] [PubMed] [Google Scholar]
- 24.O’Rourke JP, Olsen JC, Bunnell BA. Optimization of equine infectious anemia derived vectors for hematopoietic cell lineage gene transfer. Gene Therapy. 2005;12:22–29. doi: 10.1038/sj.gt.3302350. [DOI] [PubMed] [Google Scholar]
- 25.Mazarakis ND, Azzouz M, Rohll JB, Ellard FM, Wilkes FJ, Olsen AL, et al. Rabies virus glycoprotein pseudotyping of lentiviral vectors enables retrograde axonal transport and access to the nervous system after peripheral delivery. Hum Mol Genet. 2001;10:2109–2121. doi: 10.1093/hmg/10.19.2109. [DOI] [PubMed] [Google Scholar]
- 26.Merkle FT, Mirzadeh Z, Alvarez-Buylla A. Mosaic organization of neural stem cells in the adult brain. Science. 2007;317:381–384. doi: 10.1126/science.1144914. [DOI] [PubMed] [Google Scholar]
- 27.Tramontin AD, Garcia-Verdugo JM, Lim DA, Alvarez-Buylla A. Postnatal development of radial glia and the ventricular zone (VZ): a continuum of the neural stem cell compartment. Cereb Cortex. 2003;13:580–587. doi: 10.1093/cercor/13.6.580. [DOI] [PubMed] [Google Scholar]
- 28.Hartfuss E, Galli R, Heins N, Gotz M. Characterization of CNS precursor subtypes and radial glia. Dev Biol. 2001;229:15–30. doi: 10.1006/dbio.2000.9962. [DOI] [PubMed] [Google Scholar]
- 29.Spassky N, Merkle FT, Flames N, Tramontin AD, Garcia-Verdugo JM, Alvarez-Buylla A. Adult ependymal cells are postmitotic and are derived from radial glial cells during embryogenesis. J Neurosci. 2005;25:10–18. doi: 10.1523/JNEUROSCI.1108-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Doetsch F, Petreanu L, Caille I, Garcia-Verdugo JM, Alvarez-Buylla A. EGF converts transit-amplifying neurogenic precursors in the adult brain into multipotent stem cells. Neuron. 2002;36:1021–1034. doi: 10.1016/s0896-6273(02)01133-9. [DOI] [PubMed] [Google Scholar]
- 31.Holmberg J, Genander M, Halford MM, Anneren C, Sondell M, Chumley MJ, et al. EphB receptors coordinate migration and proliferation in the intestinal stem cell niche. Cell. 2006;125:1151–1163. doi: 10.1016/j.cell.2006.04.030. [DOI] [PubMed] [Google Scholar]
- 32.Reynolds BA, Weiss S. Generation of neurons and astrocytes from isolated cells of the adult mammalian central nervous system. Science. 1992;255:1707–1710. doi: 10.1126/science.1553558. [DOI] [PubMed] [Google Scholar]
- 33.Richards LJ, Kilpatrick TJ, Bartlett PF. De novo generation of neuronal cells from the adult mouse brain. Proc Natl Acad Sci USA. 1992;89:8591–8595. doi: 10.1073/pnas.89.18.8591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Anton ES, Ghashghaei HT, Weber JL, McCann C, Fischer TM, Cheung ID, et al. Receptor tyrosine kinase ErbB4 modulates neuroblast migration and placement in the adult forebrain. Nat Neurosci. 2004;7:1319–1328. doi: 10.1038/nn1345. [DOI] [PubMed] [Google Scholar]
- 35.Ghashghaei HT, Weber J, Pevny L, Schmid R, Schwab MH, Lloyd KC, et al. The role of neuregulin-ErbB4 interactions on the proliferation and organization of cells in the subventricular zone. Proc Natl Acad Sci USA. 2006;103:1930–1935. doi: 10.1073/pnas.0510410103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wong LF, Goodhead L, Prat C, Mitrophanous KA, Kingsman SM, Mazarakis ND. Lentivirus-mediated gene transfer to the central nervous system: therapeutic and research applications. Hum Gene Ther. 2006;17:1–9. doi: 10.1089/hum.2006.17.1. [DOI] [PubMed] [Google Scholar]
- 37.Johansson CB, Momma S, Clarke DL, Risling M, Lendahl U, Frisen J. Identification of a neural stem cell in the adult mammalian central nervous system. Cell. 1999;96:25–34. doi: 10.1016/s0092-8674(00)80956-3. [DOI] [PubMed] [Google Scholar]
- 38.Coskun V, Wu H, Blanchi B, Tsao S, Kim K, Zhao J, et al. CD133+ neural stem cells in the ependyma of mammalian postnatal forebrain. Proc Natl Acad Sci USA. 2008;105:1026–1031. doi: 10.1073/pnas.0710000105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Doetsch F, Caille I, Lim DA, Garcia-Verdugo JM, Alvarez-Buylla A. Subventricular zone astrocytes are neural stem cells in the adult mammalian brain. Cell. 1999;97:703–716. doi: 10.1016/s0092-8674(00)80783-7. [DOI] [PubMed] [Google Scholar]
- 40.Ganat YM, Silbereis J, Cave C, Ngu H, Anderson GM, Ohkubo Y, et al. Early postnatal astroglial cells produce multilineage precursors and neural stem cells in vivo. J Neurosci. 2006;26:8609–8621. doi: 10.1523/JNEUROSCI.2532-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kruger RP, Aurandt J, Guan KL. Semaphorins command cells to move. Nat Rev Mol Cell Biol. 2005;6:789–800. doi: 10.1038/nrm1740. [DOI] [PubMed] [Google Scholar]
- 42.de Castro F, Hu L, Drabkin H, Sotelo C, Chedotal A. Chemoattraction and chemorepulsion of olfactory bulb axons by different secreted semaphorins. J Neurosci. 1999;19:4428–4436. doi: 10.1523/JNEUROSCI.19-11-04428.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Schwarting GA, Kostek C, Ahmad N, Dibble C, Pays L, Puschel AW. Semaphorin 3A is required for guidance of olfactory axons in mice. J Neurosci. 2000;20:7691–7697. doi: 10.1523/JNEUROSCI.20-20-07691.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Lemasson M, Saghatelyan A, Olivo-Marin JC, Lledo PM. Neonatal and adult neurogenesis provide two distinct populations of newborn neurons to the mouse olfactory bulb. J Neurosci. 2005;25:6816–6825. doi: 10.1523/JNEUROSCI.1114-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kuhn HG, Dickinson-Anson H, Gage FH. Neurogenesis in the dentate gyrus of the adult rat: age-related decrease of neuronal progenitor proliferation. J Neurosci. 1996;16:2027–2033. doi: 10.1523/JNEUROSCI.16-06-02027.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Petreanu L, Alvarez-Buylla A. Maturation and death of adult-born olfactory bulb granule neurons: role of olfaction. J Neurosci. 2002;22:6106–6113. doi: 10.1523/JNEUROSCI.22-14-06106.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.De Marchis S, Bovetti S, Carletti B, Hsieh YC, Garzotto D, Peretto P, et al. Generation of distinct types of periglomerular olfactory bulb interneurons during development and in adult mice: implication for intrinsic properties of the subventricular zone progenitor population. J Neurosci. 2007;27:657–664. doi: 10.1523/JNEUROSCI.2870-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hayes NL, Nowakowski RS. Dynamics of cell proliferation in the adult dentate gyrus of two inbred strains of mice. Brain Res Dev Brain Res. 2002;134:77–85. doi: 10.1016/s0165-3806(01)00324-8. [DOI] [PubMed] [Google Scholar]
- 49.Mouret A, Gheusi G, Gabellec MM, de Chaumont F, Olivo-Marin JC, Lledo PM. Learning and survival of newly generated neurons: when time matters. J Neurosci. 2008;28:11511–11516. doi: 10.1523/JNEUROSCI.2954-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Lledo PM, Lazarini F. Neuronal replacement in microcircuits of the adult olfactory system. C R Biol. 2007;330:510–520. doi: 10.1016/j.crvi.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 51.Stiles CD, Rowitch DH. Glioma stem cells: a midterm exam. Neuron. 2008;58:832–846. doi: 10.1016/j.neuron.2008.05.031. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


