Abstract
IL-2 complexes have substantial effects on the cellular immune system and this approach is being explored for therapeutic application in infection and cancer. However, the impact of such treatments on subsequent encounter with pathogens has not been investigated. Here, we report that naïve mice treated with a short course of IL-2 complexes show enhanced protection from newly encountered bacterial and viral infections. IL-2 complex treatment expands both the NK and CD8 memory cell pool, including a recently described population of preexisting memory-phenotype T cells responsive to previously unencountered foreign antigens. Surprisingly, prolonged IL-2 complex treatment decreased CD8 T cell function and protective immunity. These data reveal the impact of cytokine complex treatment on the primary response to infection.
Introduction
Cytokine therapy, especially involving IL-2 treatment, has long been proposed as a way of manipulating the cellular immune response to enhance immunity to tumors and pathogens (1, 2). Limitations due to high dose IL-2 toxicity may be circumvented by more recent approaches in which anti-IL-2 antibody/IL-2 complexes have been shown to be much more efficient at inducing changes in the cellular immune system (3). This has lead to promising results using IL-2 complex treatment in mouse models of cancer (4–6), reduction of persistent viral load (7) and as an adjuvant for enhancing immune responses (4, 8, 9). However, these studies all explore the effect of cytokine complex treatment on previously or simultaneously encountered antigens - the effect of such therapy on subsequent exposure to a novel pathogen has not yet been explored. Since substantial dysregulation of the immune system follows such treatments, it is not clear whether this is a benefit or a detriment to new immune responses.
IL-2 complex treatment has a dramatic effect on memory-phenotype (MP) (defined as CD44hiCD122hi) CD8+ T cells, leading to massive expansion of their numbers (3). Animals not exposed to an antigen previously would not be expected to contain antigen-specific memory T cells, yet our recent studies suggest that homeostatic processes produce memory-like T cells with specificities for unencountered antigens (10). While we have previously shown that “homeostatic” memory cells, produced during the response to lymphopenia can elicit effective protective immunity (11), it is not clear whether expansion of such cells influences the “primary” immune response to a infection with a novel pathogen. Experiments using OT-I TCR transgenic T cells showed some short-term protective benefit following IL-2 complex treatment, but deficiencies in cytokine production were noted, indicating these memory cells may not be fully functional (12). In addition to its effects on MP CD8+ T cells, IL-2 complex therapy also leads to transient expansion of the NK cell pool (3, 6), which may also contribute to innate immunity against newly encountered pathogens.
We sought to assess the protective potential of IL-2 complexes to elicit increased resistance against infection in naïve mice. Following short-term IL-2 complex immunization, naïve mice are significantly protected against high dose acute infections. We show that such IL-2 complex treatment effectively expands MP CD8+ T cells, increases their expression of granzyme B, and their capacity for IFNγ production upon stimulation. Enhanced protection against Listeria monocytogenes (LM) infection involved contributions by both CD8 and NK cells. Furthermore, we found that CD8 T cells primed following IL-2 complex treatment underwent more robust expansion upon a secondary infection. However, more extensive IL-2 complex therapy, despite producing even greater expansion of MP CD8 T cells, led to a loss of protective immunity. Overall, these results suggest appropriate IL-2 complex cytokine therapy may enhance immune resistance to previously unencountered pathogens, suggesting potential as a novel vaccine tool.
Materials and Methods
Mice
6–12 week old female C57BL/6 and B6.SJL mice were purchased from the National Cancer Institute or Jackson Labs. All mouse protocols were approved by the Institutional Animal Use and Care Committees at the University of Minnesota.
IL-2 Complex Treatment
Mice were injected i.v. with 1.0 μg of carrier-free recombinant mouse IL-2 (eBioscience) combined with 10 μg anti-IL-2 antibody (S4B6; BioXCell). Mice given 3 injections of complex were injected on days 0, 2, and 4. Mice given 5 injections of complex received treatment on days 0, 1, 2, 3, and 4. Control mice were injected on the same schedule with 10 μg of rat IgG2a (BioXCell) or left untreated.
In vitro stimulations
Splenocytes were incubated with PMA and ionomycin (Sigma) or OVA peptide (SIINFEKL), and brefeldin (BD Biosciences) for 6 hours. Surface staining followed by intracellular staining for IFNγ, TNF, and IL-2 was then performed. Cytokine stimulations with IL-2, IL-12, and IL-18 were performed as described previously (10).
Flow Cytometry and Magnetic Bead Isolation
B8R-Kb and OVA-Kb tetramers were produced as described previously (13). Endogenous, tetramer-specific CD8+ T cells were enriched by magnetic bead separation (10, 14). Cells were analyzed on an LSR II (BD) and data was evaluated using FlowJo software.
Bacterial and Viral Infections
Recombinant LM strains LM-OVA or attenuated LM-OVA (both expressing secreted ovalbumin protein) were provided by Hao Shen (University of Pennsylvania School of Medicine, Philadelphia, PA) and have been described (15). Attenuated strain LM-B8R (expressing the B8R CD8 epitope from vaccinia virus) was provided by Dr. Ross Kedl (National Jewish Medical Research Center, University of Colorado, Denver, CO). Bacteria were grown in tryptic soy broth with 50 μg/ml streptomycin to an OD600 of ~0.1. For LM-OVA challenges 2 × 105 CFU were injected i.v. For primary infection with attenuated LM-OVA or LM-B8R, 3 ×106 CFU were injected i.v. The actual number of bacteria injected was confirmed by dilution and growth on tryptic soy agar plates containing streptomycin. For vaccinia virus infections, 2×106 PFU of VV-WR was diluted in PBS and injected i.v.
Determination of Colony Forming Units (CFU)
On day 3 after infection, the spleen and liver were removed and placed in a 0.2% IGEPAL solution (Sigma-Aldrich). Organs were homogenized and serial dilutions were plated onto TSB plates containing 50μg/ml streptomycin. Bacterial colonies were counted following plate incubation for ~24 hours at 37°C. The limit of detection (approximately 100 organisms) is indicated on graphs by a dashed line.
Plaque Assay
On day 3 after infection with VV-WR, ovaries were harvested in PBS and frozen as a single cell suspension. After two freeze-thaw cycles, the ovary homogenate was incubated at 37°C for 45 minutes with 0.25mg/ml trypsin (Sigma). 143B cells (ATCC) were grown to confluence. Dilutions of the ovary homogenate were added in duplicate to the cellular monolayer and left for two days. Staining with 1% crystal violet was then performed. Plaques were counted and total viral load per ovaries was calculated.
Depletion of NK cells and CD8+ T cells
Mice were treated with 600 μg anti-NK1.1 (PK136; ATCC) or 1 mg anti-CD8β (2.43; BioXCell) on d-1, d1, and d3 relative to IL-2 complex treatment (d0, d2, and d4). Depletion of greater than 97% was confirmed by staining of the spleen and liver.
Statistics
A two-tailed, unpaired, Student's t-test was performed on the indicated data samples using Prism software. P values are displayed within figures.
Results
Short Term IL-2 complex treatment protects naïve mice from bacterial and viral infection
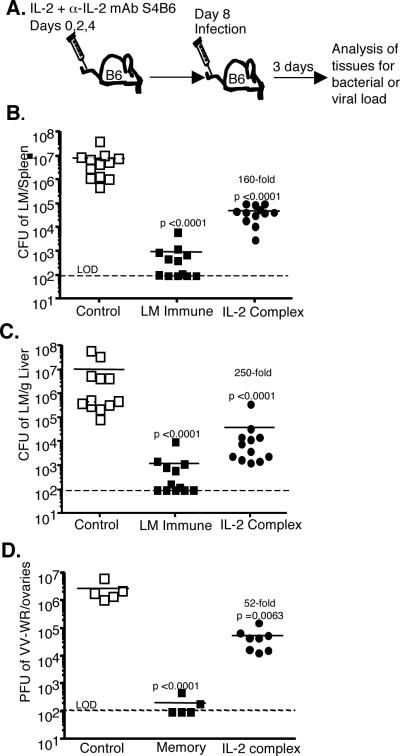
IL-2 complex treatment has been reported to selectively expand pre-existing MP CD8 T cells and NK cells (3). However, the significance of such treatment on subsequent infection with a pathogen has not been explored. We sought to determine if IL-2 complex treated naïve mice would exhibit increased protection when challenged with a high dose bacterial or viral infection. We treated mice with three injections of IL-2 combined with anti-IL-2 antibody S4B6 (as in Ref. 3) on days 0, 2, and 4. Four days after the last injection, mice were infected with a high dose of Listeria monocytogenes expressing ovalbumin (LM-OVA) (Fig. 1A). After three days, high bacterial loads were recovered in the spleen and liver of control treated mice, while LM-immune mice had reduced this bacterial burden by 3–4 logs (Fig. 1B and C). Remarkably, naïve mice that received IL-2 complex reduced the bacterial load by 2–3 logs in both organs, despite the fact that these animals had not been primed with specific antigen. In a second cohort of mice, 5/9 control treated mice died during the first week following infection, while all the IL-2 complex treated mice (9/9) survived the infective dose (data not shown). As a second model of infection, IL-2 complex treated naïve mice were infected with 2 ×106 PFU of vaccinia virus (strain WR). Three days after infection the ovaries (the major reservoir of infection) were harvested and assessed for viral load. Compared to controls, the IL-2 complex treated animals showed a significant decrease in vaccinia virus load (Fig. 1D). Therefore, in two separate infection models (bacterial and viral), with either lymphoid (LM infection) or peripheral (vaccinia virus) sites of infection, IL-2 complex afforded significantly better elimination of pathogens in naïve hosts.
Figure 1. Enhanced protection of naïve mice against LM and VV after IL-2 complex treatment.
(a) Schematic of the timing of IL-2 complex treatment and infection. (b and c) Colony forming units (CFU) of LM in the spleen (b) and liver (c) 3 days after infection with 8 × 104 CFU of LM-OVA. Each symbol represents an individual mouse. (d) Plaque forming units (PFU) of VV in the ovaries of mice 3 days after infection with 2 × 106 PFU of VV-WR. LOD = limit of detection.
IL-2 complex treatment increases CD8 T cells and NK cells in both lymphoid and non-lymphoid organs
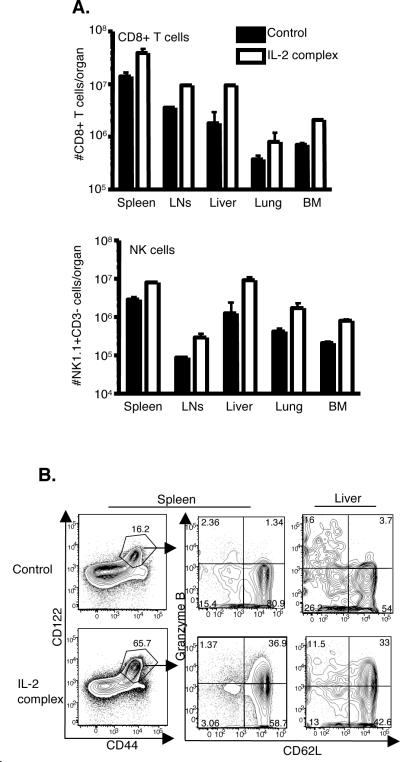
We next sought to more closely examine the phenotype of CD8 T cells expanded with IL-2 complex treatment. This resulted in increased numbers of CD8 T cells and NK cells (NK1.1+, CD3−) in all organs analyzed (spleen, lymph nodes, bone marrow, lung, and liver) (Fig. 2A). CD4 T cells and NKT cells (CD3lo, NK1.1+) were also slightly increased in number (data not shown). In addition, we found that the CD8 T cell population were overwhelmingly of central-memory phenotype (CD44hi, CD62Lhi) as well as expressing high levels of CD43 and CD27 (Fig. 2B and data not shown). Surprisingly, these cells also expressed high levels of granzyme B (Fig. 2B), which is typically associated with effector and effector-memory T cell populations, not central memory T cells. However, this phenotype has been described previously for IL-2 complex treated antigen-experienced memory cells in the context of a chronic infection (7). The enhanced number of cells and corresponding phenotypic changes appeared to last for approximately two (NK cells) to three (CD8 T cells) weeks after the start of treatment (Supplemental Fig. 1). Therefore, after three injections of IL-2 complex over the course of a week, we find transiently increased numbers of MP CD8 T cells and NK cells in multiple organs, some of which are positive for a critical effector molecule (granzyme B).
Figure 2. IL-2 complex treatment increases CD8 T cells and NK cells that exhibit increased granzyme B expression.
(a) Total numbers of CD8 T cells and NK cells in lymphoid and peripheral tissues post-IL-2 complex treatment. N = 6 per group (b) Phenotype of CD8 T cells in the spleen and liver. A representative plot is shown.
Antigen-Specific Memory Phenotype CD8 T cells are Increased in Number with IL-2 Complex Treatment
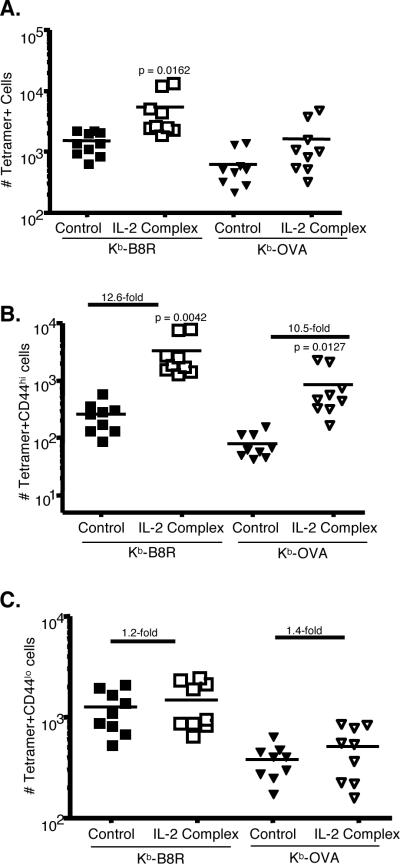
Our previous studies indicated that, in unimmunized animals CD8 T cells specific for foreign antigens can be detected in the MP pool (10). It was therefore of interest to know whether IL-2 complex treatment lead to an increase in numbers of MP CD8 T cells with specificities relevant to the pathogens studied. To examine endogenous, antigen-specific CD8 T cells, we employed the use of MHC class I tetramer staining followed by magnetic bead enrichment (10, 14). Using this technique, along with multi-parameter flow cytometry, we found increases in the number of both Kb-B8R and Kb-OVA specific CD8+ T cells (Figure 3A). These are dominant epitopes in the CD8 T cell response to vaccinia and LM-OVA respectively and are likely to contribute to protection of the host (Fig. 1). By costaining with the CD44 marker, we found that virtually all of the increase in cell number was due to an increase in the CD44hi compartment, which ranged from 10–12-fold (Fig. 3B and C). Importantly, the antigen-specific CD44lo compartment was conserved, but did not significantly change in number. This agrees with previous adoptive transfer data showing that naïve CD8 T cells do not divide in response to IL-2 complex treatment, which is likely due to their low expression of CD122 (3). However, our data contrasts with another report that indicated both naïve and memory OT-1 CD8 T cells were expanded by IL-2 complex (12). We conclude that in the endogenous, polyclonal repertoire IL-2 complex treatment increases the overall number of tetramer positive CD8 T cells by specifically amplifying MP (CD44hiCD122hi) cells.
Figure 3. Increased tetramer specific CD44hi cells after IL-2 complex treatment.
(a) Numbers of Kb-B8R-specific or Kb-OVA-specific CD8 T cells in the spleen and lymph nodes of control or IL-2 complex treated mice. (b) Numbers of CD44hi tetramer positive cells. (c) Numbers of CD44lo tetramer positive cells. Numbers indicate the fold increase compared to control animals. Each symbol represents an individual mouse.
IL-2 complex protection is mediated by both CD8 T cells and NK cells and lasts for several weeks
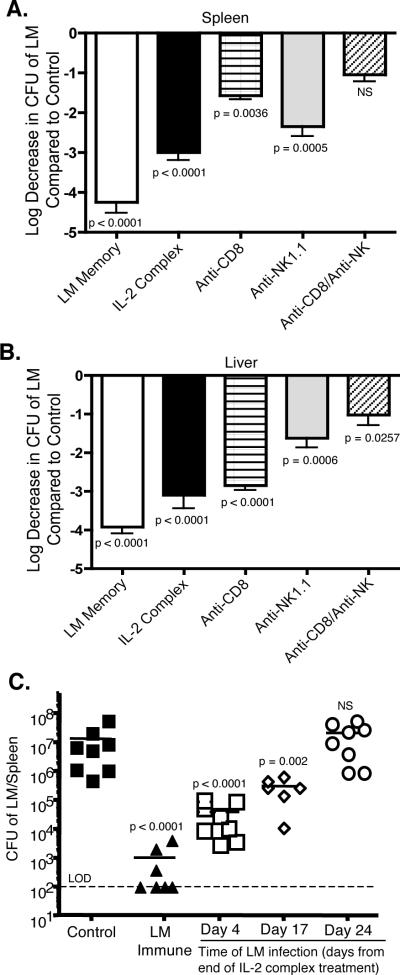
We next investigated the mechanism behind IL-2 complex mediated protection during high dose infection with LM-OVA. CD8 T cells are known to be critical mediators of LM clearance (16). IL-2 complex treatment increased both the overall number of MP CD8 T cells as well as the number of Kb-OVA-specific CD8 T cells. However, given the large increase in numbers of NK cells post-IL-2 complex treatment, it was possible that these cells were also important mediators of protection. To address this, mice were depleted of NK1.1+ cells or CD8+ cells or both during the week of IL-2 complex treatment. Following the week of injections and confirmation of greater than 90% depletion of target cells (data not shown), mice were challenged with LM-OVA. Three days after infection, IL-2 complex treated mice displayed strong clearance (~3 logs) over control treated mice (Fig. 4A and B). Depletion of either CD8+ cells or NK1.1+ cells alone decreased clearance of LM in both the spleen and liver. CD8+ T cells can display NK1.1 with activation (17). However, the frequency of MP CD8+ T cells expressing NK1.1 in IL-2 complex treated mice was not elevated compared to control animals (data not shown), indicating that NK1.1 depletion in these experiments is not depleting a significant numbers of CD8+ T cells. CD8+ cells were more critical in the spleen, whereas NK1.1+ cells appeared to contribute more to clearance in the liver. However, when both populations were removed, the number of bacteria found in the spleen was no longer significantly different from control treated mice. Bacterial loads remained significantly lower in the liver even after removal of CD8+ and NK1.1+ cells, indicating other cell populations are actively contributing to clearance in this organ.
Figure 4. Both CD8 T cells and NK cells mediate protection against LM which lasts for several weeks.
(a and b) Mice were depleted of CD8+ cells or NK1.1+ cells or both during the IL-2 complex treatment phase. Depletion was confirmed in the spleen and liver to be >90%. Mice were then challenged with LM-OVA. Clearance of LM in the spleen (a) and liver (b) 3 days after infection compared to the control treated group. Data are combined from three experiments. N = 10/ group (c) Time after completion of IL-2 complex treatment was varied as indicated followed by infection with LM. CFU of LM in the spleen three days after infection is displayed. Each symbol represents an individual mouse.
We also examined how long the protective effect was maintained following one week (3 injections) of IL-2 complex. Challenging mice with a high dose of LM-OVA on day 8 after the start of treatment continued to convey impressive clearance of bacterial within three days (Fig. 4C). When a greater interval of time was allowed between IL-2 complex treatment and challenge with LM, we found that protection lasted for at least 3 weeks after the start of IL-2 complex treatment but waned after that time period. These data are supported by the observation that elevated numbers of CD8 T cells and NK cells are maintained for 2–3 weeks following treatment (Supplemental Fig. 1). These data demonstrate that short-term treatment with IL-2 complex provides measurable protection against infection for several weeks.
Antigen-specific memory cells are long-lasting and exhibit increased secondary expansion
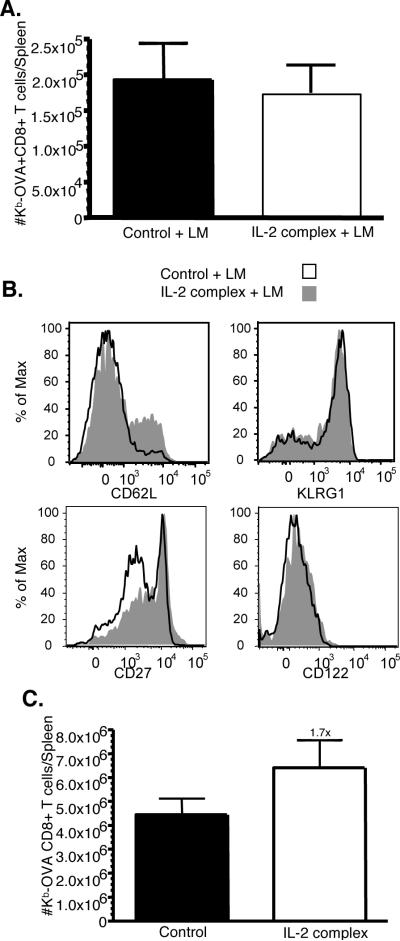
Our data shows that IL-2 complex treatment can induce acute protection against bacterial or viral pathogens. We next sought to determine if after that encounter with infection, a long-lasting and effective memory compartment was created. Mice were injected with IL-2 complexes over the course of 1 week and then infected with LM-OVA as shown in Figure 1. Unexpectedly, despite the increase in antigen specific CD8 T cells following IL-2 complex treatment (Fig. 3A), the number of Kb-OVA-specific memory cells in the spleen at six weeks after infection was similar in mice that did or did not receive IL-2 complex treatment (Fig. 5A). This might suggest the IL-2 complex treatment results in responding cells with decreased potential to become memory cells following antigen encounter. Interestingly, studies in which IL-2 or IL-15 complexes were given during the contraction phase of an acute response indicated that cytokine therapy lead to increased frequencies of KLRG-1hi CD127lo short-lived effector cells (SLEC) (8). To explore this in our system, we analyzed the phenotype of antigen-specific memory cells following LM-OVA infection. As shown (Fig. 5B) the OVA-Kb specific memory cells had a similar phenotype regardless of prior IL-2 complex treatment, except for a slight increase in the frequency of CD62Lhi and CD27hi cells in IL-2 complex treated animals (Fig. 5B). Importantly, the frequency of KLRG-1hi cells was similar regardless of IL-2 complex treatment (Fig. 5B), suggesting no change in SLEC frequency. We also found that IL-2 complex treatment did not alter the patterns of KLRG-1 (and CD127) expression on MP CD8 T cells prior to infection, nor on effector cells at day 5 during following LM-OVA infection (data not shown).
Figure 5. Increased central memory pool and secondary expansion after IL-2 complex treatment.
(a) Mice were control treated or treated with IL-2 complex followed by infection with LM-OVA. Six weeks after infection, the number of Kb-OVA specific CD8 T cells in the spleen was determined by tetramer staining. N = 5/group (b) Surface staining for the indicated markers was performed on Kb-OVA specific memory CD8 T cells. (c) Mice were challenged with 2 × 105 LM-OVA. Five days after infection the number of Kb-OVA specific CD8 T cells was enumerated by tetramer staining. N = 5/group.
We next asked whether these cells would respond if a second encounter with pathogen occurred. After LM-OVA rechallenge, vigorous expansion of Kb-OVA specific CD8 T cells occurred with a slightly higher fold-expansion (1.7-fold) occurring in mice which previously received IL-2 complex treatment (Fig. 5C). These data indicate that strong IL-2 signals delivered by complex treatment do not impair (or strongly enhance) the development of long-term memory cells that effectively respond to a reencounter with antigen.
Increasing the number of cytokine complex injections has a detrimental impact on protective immunity
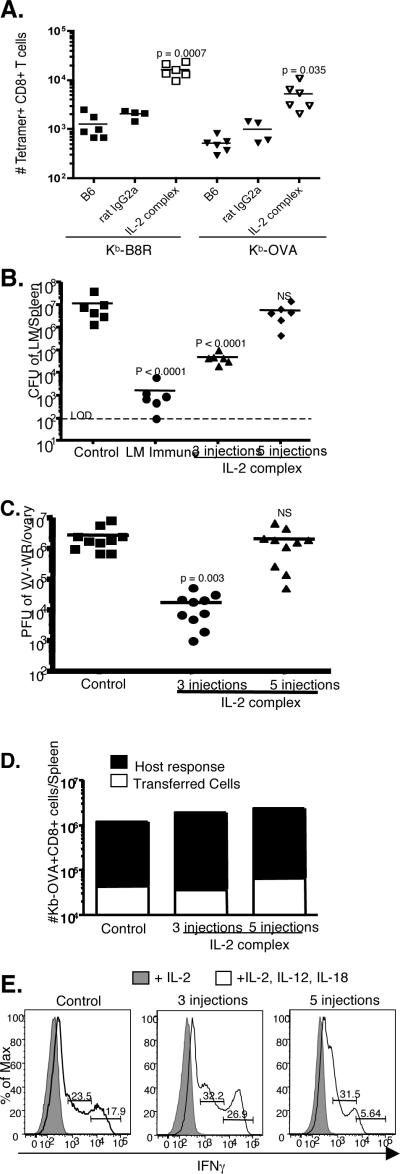
Lastly, we asked whether increasing the number of IL-2 complex injections would even further enhance protective immunity. Increasing the number of IL-2 complex injections to five total (given on days 0,1,2,3, and 4) did enhance the number of CD8 T cells and the frequency of cells that were MP (Supplemental Fig. 2A and B). This resulted in a CD8 T cell compartment that was 90% or greater CD44hiCD122hi in nature. As expected, antigen specific CD8+ T cells also went up in number (Fig. 6A), and reached impressive precursor frequencies (~15,000 Kb-B8R and ~5,000 Kb-OVA). This was again predominantly due to an increase in the CD44hi compartment, which increased 32-fold (for Kb-B8R) and 17-fold (for Kb-OVA) compared to control treated mice (Supplemental Fig. 2C). We also saw a slight increase in antigen-specific CD44lo cells which was not observed after 3 injections of IL-2 complex (Supplemental Fig. 2D and Fig. 3C). The basis for this change is currently unclear.
Figure 6. Multiple injections of IL-2 complex negatively impacts protection and functional responses.
Mice were injected with 3 or 5 doses of IL-2 complex over the course of one week. (a) The number of endogenous Kb-B8R or Kb-0VA specific CD8 T cells in the spleen and lymph node was determined on day 7. (b) On day 8 after IL-2 complex treatment mice were infected with LM-OVA. Three days after infection, the CFU in the spleen was determined. (c) On day 8 after IL-2 complex treatment, mice were infected with VV-WR. Three days after infection, the PFU in the ovary was determined. (d) On day 7, spleen and lymph nodes were harvested from the indicated groups. ~2 × 106 CD8+ CD44hi cells were adoptive transferred into new B6.SJL hosts. The next day, mice were infected with attenuated LM-OVA ActA-. Eight days after infection, the number of donor and host Kb-OVA specific cells was enumerated. N = 3/group; Data is representative of two experiments. (e) Day 7 splenocytes from the indicated groups were cultured in either: IL-2 or IL-2, IL-12, and IL-18 for ~18 hours. Surface staining followed by intracellular staining for IFNγ was performed. Numbers indicate the frequency of CD8+ CD44hi cells producing IFNγ. Data is representative of two experiments.
We next asked how increasing the number of IL-2 complex injections would impact protection against LM infection. We treated mice with either 3 or 5 injections of IL-2 complex and followed this with a high dose LM-OVA challenge on day 8. Mice in both groups received their last injection of IL-2 complex on day 4 and then had 4 days with no treatment prior to infection. Unexpectedly, we found a complete loss of protective effect when mice were treated 5 times with IL-2 complex (Fig. 6B). A similar loss of protection was also seen after vaccinia virus challenge (Fig. 6C). In the LM-Ova model we noted a slightly decreased expansion of antigen specific CD8 T cells in animals treated with 5 doses of IL-2 complex (Supplemental Fig. 3). Since this change in expansion might arise because of disparities in the frequency of responsive cells and/or changes in the bacterial loads sustained by the animals, we performed adoptive transfer of CD44hi cells from 3 injection and 5 injection mice into new hosts and infection with an attenuated strain of LM (LM-OVA ActA-) which is equivalently cleared by all the mice. In this model, antigen specific CD44hi cells from animals treated with 3 or 5 doses of IL-2 complex underwent similar expansion (Fig. 6D).
The loss of protection by animals given multiple doses of IL-2 complex might correlate with expression of inhibitory molecules, but neither PD-1 and LAG-3 (both of which are implicated in decreased functionality of “exhausted” CD8 T cells (18)) were induced in CD8 T cells treated with 5 injections of IL-2 complex (Supplemental Fig. 4). Reduced protection might also correlate with loss of effector responses. While granzyme B expression was actually greater in CD8 T cells from mice treated with 5 injections of IL-2 complex (Supplemental Fig. 4), we observed a defect in cytokine-induced production of IFN-γ in this population. During early phases of an immune response, memory phenotype CD8 T cells produce IFN-γ in response to IL-12 and IL-18, a function which can be mimicked in vitro (19). When comparing 3 injection and control treated mice in this system, we found a large increase in CD8 T cells from IL-2 complex treated mice that were high producers of IFNγ (Fig. 6E). In contrast, mice treated with 5 injections of complex exhibited decreased overall production of IFNγ, and were particularly deficient in the highest IFNγ producing cells. Interestingly, a similar deficit was also noted in the NK cell compartment (Supplemental Fig. 5). We conclude that expansion of protective MP CD8 T cells and NK cells can be achieved through IL-2 complex injections, but that overstimulation can occur, leading to functional deficiencies in both lymphocyte populations.
Discussion
Here we demonstrate that treatment of naïve mice with IL-2 complexes can increase resistance to newly acquired acute infections. Short-term treatment over the course of a week enhanced clearance of LM and protected mice from a lethal infection. A similar improvement in clearance was also seen after VV infection, a situation in which CD8 T cells and NK cells can mediate protective immunity (20). This indicates the potential usefulness of cytokine complexes against a wide range of infections. Additionally, the increased presence of MP CD8 T cells and NK cells in multiple organs (both lymphoid and non-lymphoid) also raises the possibility that IL-2 complex treatment could be beneficial during infections with peripheral reservoirs of infection. Others have shown cytokine complexes can improve responses to established tumor or chronic infection (4–7). Here we show for the first time that cytokine complex treatment can be used to positively manipulate the immune system prior to encounter with infection.
How does IL-2 complex treatment enhance protective immunity? We found that both CD8 T cells and NK cells were important for maximal clearance in the LM model. IL-2 complex treatment increased total numbers of CD8 T cells, including the numbers of precursors specific for the antigens studied (Kb-OVA and Kb-B8R). Whether IL-2 complex treatment amplifies CD8 T cells of all specificities equally is of interest and is currently being explored. The amplified pool of CD8 T cells overwhelmingly displayed a central memory phenotype, and earlier studies indicated that IL-2 complex treatment selectively induces proliferation of MP (rather than naïve) CD8 T cells (3): However, other cytokine therapies (for example IL-15/IL-15Rα complexes) can promote proliferation of naïve (CD44lo) CD8 T cells (21, 22), and IL-2 complexes have been shown to induce proliferation of some naïve CD8 T cell clones (12). Hence it is currently unclear whether the expanded antigen-specific MP CD8 T cell pool we detect following IL-2 complex therapy is derived from pre-exisiting MP cells, naïve cells or both populations. In apparent contrast to their central memory phenotype, the CD8 T cells induced by IL-2 complex treatment showed elevated expression of granzyme B, an important effector molecule. These findings mirror those from another report examining the effects of IL-2 complex therapy on antigen-primed memory CD8 T cells (7). We observe improved protection lasted for 2–3 weeks following termination of cytokine therapy, which correlated with the reestablishment of normal numbers and phenotype of CD8 T cells and NK cells. Therefore, protection via this therapy is relatively short-lived, but has the benefit of not irrevocably changing the immune system. In future studies it will be interesting to determine how quickly enhanced protection is acquired after the start of IL-2 complex treatment, as well as the response by CD8 T cells and NK cells to infection while concurrently responding to IL-2 complexes.
Given the elevation in antigen specific CD8 T cell numbers following IL-2 complex treatment, it was surprising that this did not translate into increased numbers of antigen specific memory cells following primary infection, or a substantial increase in the recall response (Fig 5). This does not appear to reflect alterations in the production of SLEC versus memory precursor cells at the memory stage (Fig 5B), but might instead reflect the more efficient and rapid elimination of the pathogen used for priming by animals treated with IL-2 complexes. Further studies will be required to investigate this issue.
Unexpectedly, we found that the improved outcome observed after treatment with three injections of IL-2 complex was negated by the delivery of two additional doses (5 injections over one week). We noted that increased IL-2 complex treatment generated MP CD8 T cells and NK cells with a reduced capacity to produce IFN-γ, potentially suggesting some form of exhaustion. On the other hand, antigen reactive CD8 T cells retained their expression of Granzyme B and their capacity to expand after adoptive transfer and priming. Earlier studies using TCR transgenic models suggested that CD8 T cells expanded by IL-2 complex treatment had inferior functional capacity (12), but our data suggest that the functional state of the cells expanded by IL-2 complex depends on the dosing regimen. Whether additional cell types are compromised by sustained IL-2 complex therapy is not clear.
Given that suitable IL-2 complex treatment enhanced protective immunity in unvaccinated animals, it is interesting to speculate on potential use of cytokine therapy as a novel vaccine. Traditional approaches to vaccination have been unable to effectively combat many serious infections, particularly in situations where a strong cellular immune response needs to be generated (23, 24). Additionally, newly emerging or rapidly mutating pathogens make the time needed for determining correct antigenic targets for cellular immune responses unavailable. Here, we show an alternative approach, using cytokine complexes to globally expand both innate and adaptive immune cells. This treatment proved to be an effective vaccine in two models of infections, and removed the need to pre-determine targets of the response.
Supplementary Material
Acknowledgments
We thank J. Vevea for technical support, V. Vezys and M. Jenkins for critical reading of the manuscript and the Jamequist lab for input during this project.
This work was supported by a Leukemia and Lymphoma Society Special Fellow Award (S.E.H.), Predoctoral Training Grant T32 AI07313 (to A.D.A.) and NIH grant AI075168 (to S.C.J.).
Abbreviations
- MP
memory phenotype
- LM-OVA
Listeria monocytogenes expressing ovalbumin
References
- 1.Overwijk WW, Theoret MR, Restifo NP. The future of interleukin-2: enhancing therapeutic anticancer vaccines. Cancer J Sci Am. 2000;6(Suppl 1):S76–80. [PMC free article] [PubMed] [Google Scholar]
- 2.Blattman JN, Grayson JM, Wherry EJ, Kaech SM, Smith KA, Ahmed R. Therapeutic use of IL-2 to enhance antiviral T-cell responses in vivo. Nat Med. 2003;9:540–547. doi: 10.1038/nm866. [DOI] [PubMed] [Google Scholar]
- 3.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 4.Verdeil G, Marquardt K, Surh CD, Sherman LA. Adjuvants targeting innate and adaptive immunity synergize to enhance tumor immunotherapy. Proc Natl Acad Sci U S A. 2008;105:16683–16688. doi: 10.1073/pnas.0805054105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Jin GH, Hirano T, Murakami M. Combination treatment with IL-2 and anti-IL-2 mAbs reduces tumor metastasis via NK cell activation. Int Immunol. 2008;20:783–789. doi: 10.1093/intimm/dxn036. [DOI] [PubMed] [Google Scholar]
- 6.Tomala J, Chmelova H, Mrkvan T, Rihova B, Kovar M. In vivo expansion of activated naive CD8+ T cells and NK cells driven by complexes of IL-2 and anti-IL-2 monoclonal antibody as novel approach of cancer immunotherapy. J Immunol. 2009;183:4904–4912. doi: 10.4049/jimmunol.0900284. [DOI] [PubMed] [Google Scholar]
- 7.Molloy MJ, Zhang W, Usherwood EJ. Cutting Edge: IL-2 Immune Complexes As a Therapy for Persistent Virus Infection. J Immunol. 2009;182:4512–4515. doi: 10.4049/jimmunol.0804175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rubinstein MP, Lind NA, Purton JF, Filippou P, Best JA, McGhee PA, Surh CD, Goldrath AW. IL-7 and IL-15 differentially regulate CD8+ T-cell subsets during contraction of the immune response. Blood. 2008;112:3704–3712. doi: 10.1182/blood-2008-06-160945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Mostbock S, Lutsiak ME, Milenic DE, Baidoo K, Schlom J, Sabzevari H. IL-2/anti-IL-2 antibody complex enhances vaccine-mediated antigen-specific CD8(+) T cell responses and increases the ratio of effector/memory CD8(+) T cells to regulatory T cells. J Immunol. 2008;180:5118–5129. doi: 10.4049/jimmunol.180.7.5118. [DOI] [PubMed] [Google Scholar]
- 10.Haluszczak C, Akue AD, Hamilton SE, Johnson LDS, Puanauski L, Teodorovic L, Jameson SC, Kedl RM. The antigen-specific CD8+ T cell repertoire in unimmunized mice includes memory-phenotype cells bearing markers of homeostatic expansion. J Exp Med. 2009;206:435–448. doi: 10.1084/jem.20081829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hamilton SE, Wolkers MC, Schoenberger SP, Jameson SC. The generation of protective memory-like CD8+ T cells during homeostatic proliferation requires CD4+ T cells. Nat. Immunol. 2006;7:475–481. doi: 10.1038/ni1326. [DOI] [PubMed] [Google Scholar]
- 12.Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Daniels MA, Jameson SC. Critical role for CD8 in T cell receptor binding and activation by peptide/major histocompatibility complex multimers. J Exp Med. 2000;191:335–346. doi: 10.1084/jem.191.2.335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Moon JJ, Chu HH, Pepper M, McSorley SJ, Jameson SC, Kedl RM, Jenkins MK. Naive CD4(+) T cell frequency varies for different epitopes and predicts repertoire diversity and response magnitude. Immunity. 2007;27:203–213. doi: 10.1016/j.immuni.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pope C, Kim S, Marzo A, Williams K, Jiang J, Shen H, Lefrancois L. Organ-specific regulation of the CD8 T cell response to Listeria monocytogenes infection. J Immunol. 2001;166:3402–3409. doi: 10.4049/jimmunol.166.5.3402. [DOI] [PubMed] [Google Scholar]
- 16.Harty JT, Tvinnereim AR, White DW. CD8+ T cell effector mechanisms in resistance to infection. Ann. Rev. Immunol. 2000;18:275–308. doi: 10.1146/annurev.immunol.18.1.275. [DOI] [PubMed] [Google Scholar]
- 17.Assarsson E, Kambayashi T, Sandberg JK, Hong S, Taniguchi M, Van Kaer L, Ljunggren HG, Chambers BJ. CD8+ T cells rapidly acquire NK1.1 and NK cell-associated molecules upon stimulation in vitro and in vivo. J Immunol. 2000;165:3673–3679. doi: 10.4049/jimmunol.165.7.3673. [DOI] [PubMed] [Google Scholar]
- 18.Crawford A, Wherry EJ. The diversity of costimulatory and inhibitory receptor pathways and the regulation of antiviral T cell responses. Curr Opin Immunol. 2009;21:179–186. doi: 10.1016/j.coi.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berg RE, Crossley E, Murray S, Forman J. Memory CD8+ T cells provide innate immune protection against Listeria monocytogenes in the absence of cognate antigen. J Exp Med. 2003;198:1583–1593. doi: 10.1084/jem.20031051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Xu R, Johnson AJ, Liggitt D, Bevan MJ. Cellular and humoral immunity against vaccinia virus infection of mice. J Immunol. 2004;172:6265–6271. doi: 10.4049/jimmunol.172.10.6265. [DOI] [PubMed] [Google Scholar]
- 21.Rubinstein MP, Kovar M, Purton JF, Cho JH, Boyman O, Surh CD, Sprent J. Converting IL-15 to a superagonist by binding to soluble IL-15R{alpha} Proc Natl Acad Sci U S A. 2006;103:9166–9171. doi: 10.1073/pnas.0600240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Stoklasek TA, Schluns KS, Lefrancois L. Combined IL-15/IL-15Ralpha immunotherapy maximizes IL-15 activity in vivo. J Immunol. 2006;177:6072–6080. doi: 10.4049/jimmunol.177.9.6072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Appay V, Douek DC, Price DA. CD8+ T cell efficacy in vaccination and disease. Nat Med. 2008;14:623–628. doi: 10.1038/nm.f.1774. [DOI] [PubMed] [Google Scholar]
- 24.Ahlers JD, Belyakov IM. Lessons learned from natural infection: focusing on the design of protective T cell vaccines for HIV/AIDS. Trends Immunol. 2010 doi: 10.1016/j.it.2009.12.003. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.