Abstract
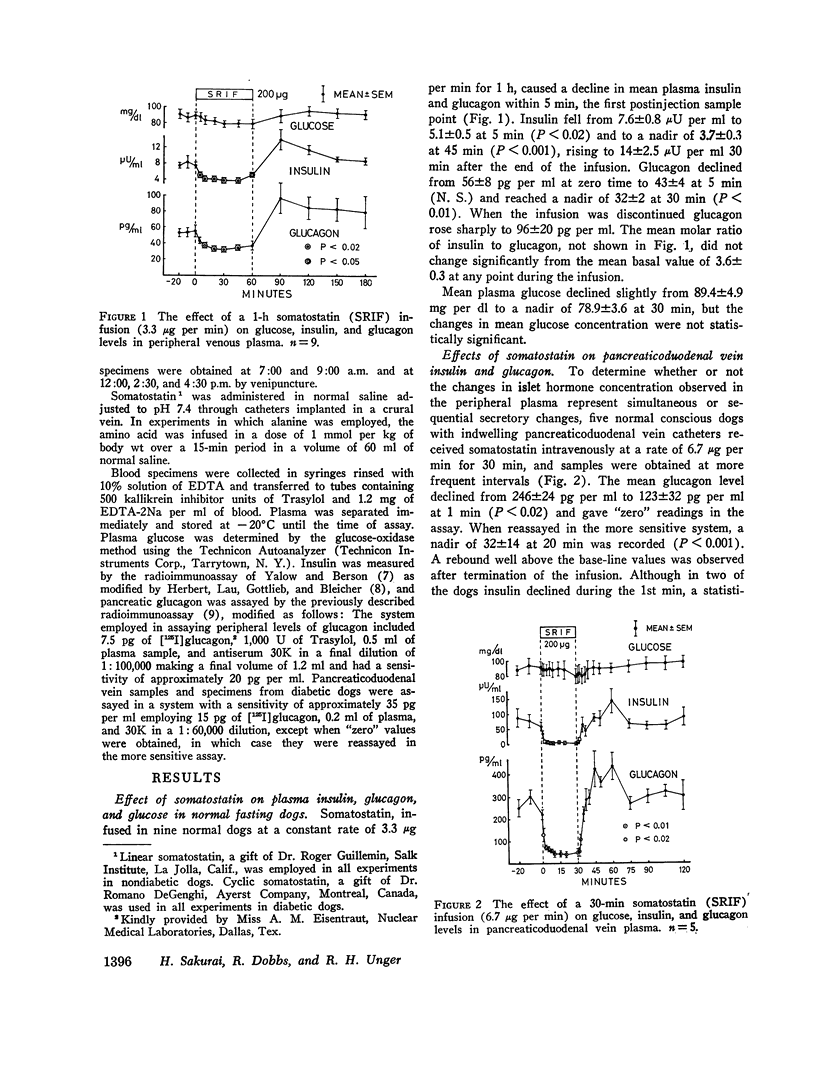
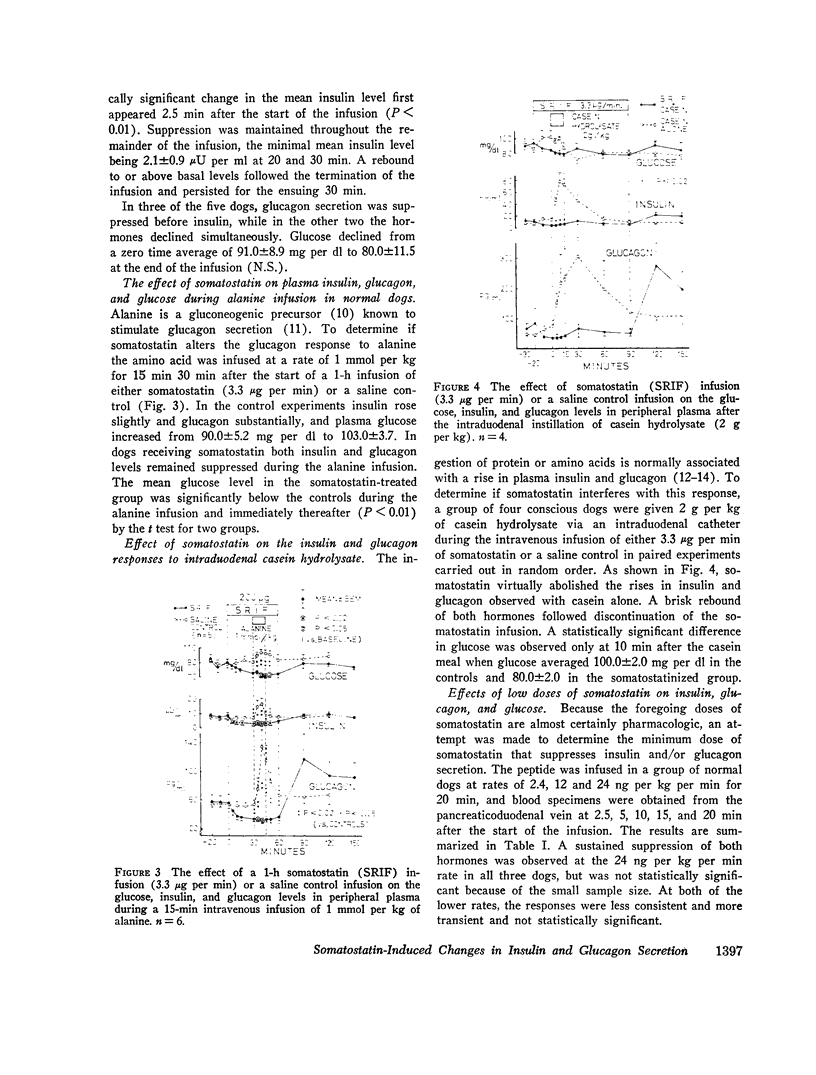
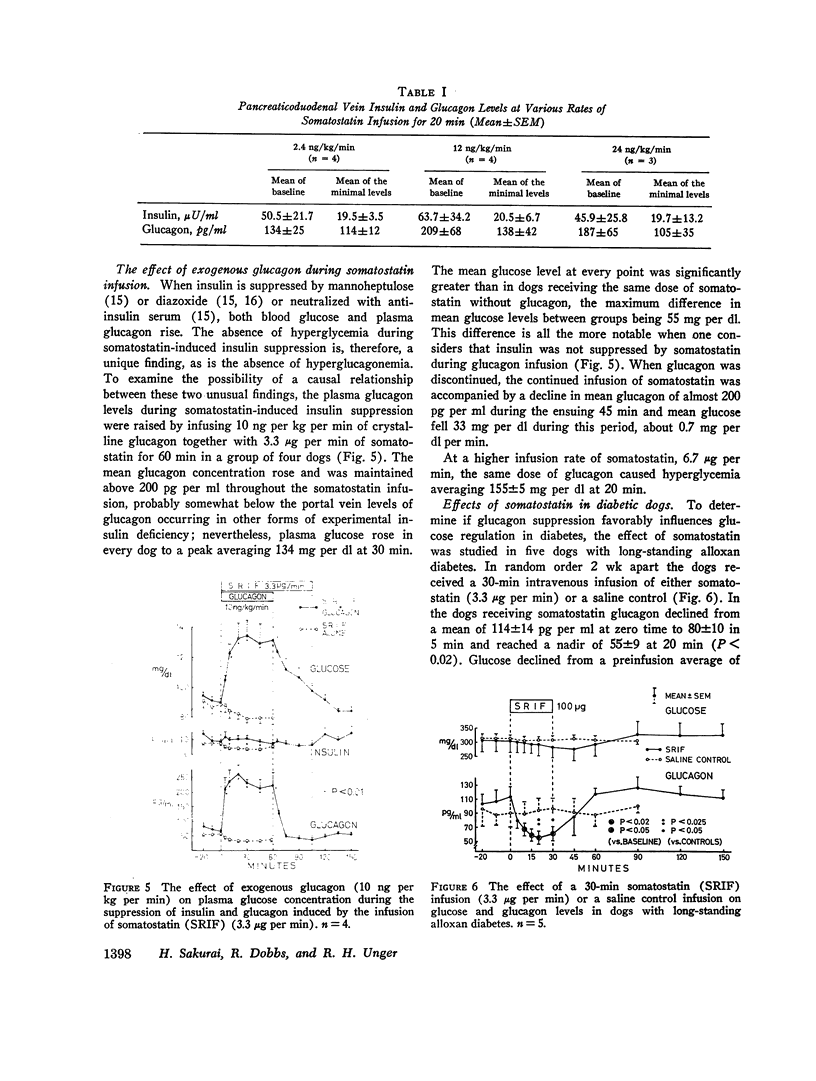
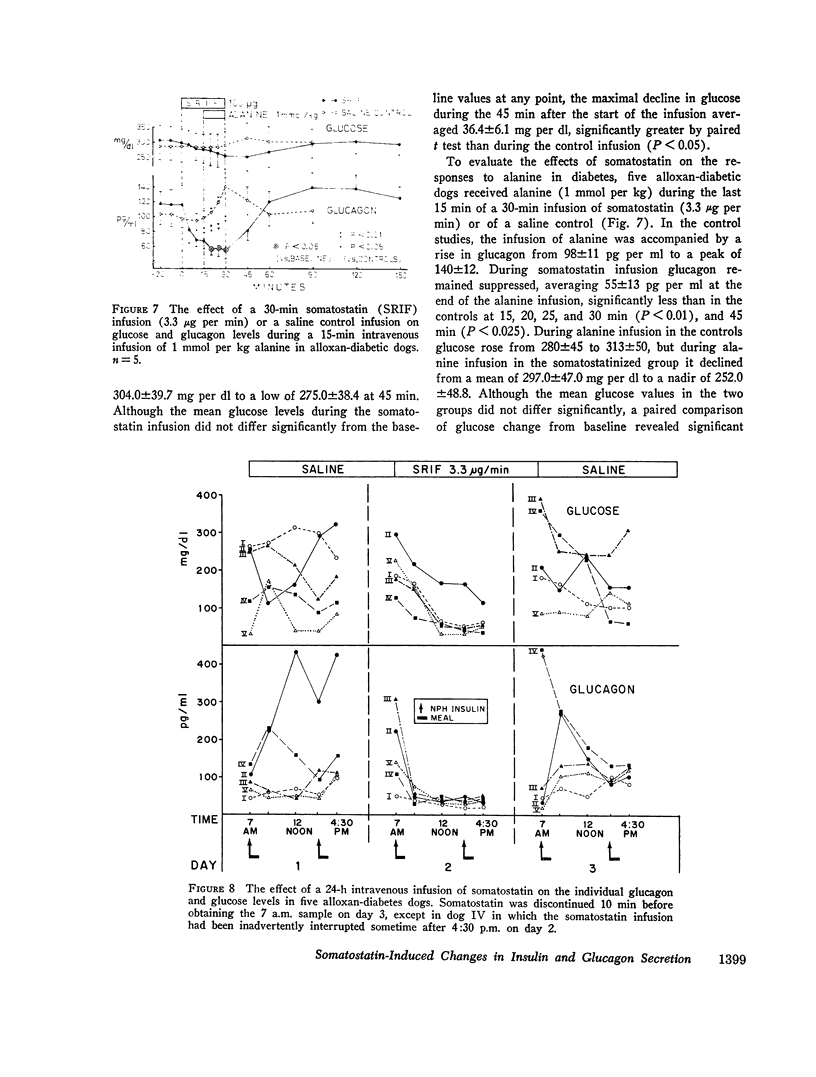
In conscious dogs intravenously infused somatostatin (3.3 μg per min for 1 h) caused prompt and sustained declines in mean plasma insulin and glucagon, even during alanine infusion and intraduodenal casein hydrolysate feeding; plasma glucose declined, but not significantly. 6.7 μg per min of somatostatin significantly lowered pancreatoduodenal vein glucagon and insulin within 2.5 min and profoundly suppressed their secretion throughout the infusion. Consistent bihormonal suppression occurred at rates as low as 24 ng per kg per min, but was variable at 12 and 2.4 ng per kg per min. When somatostatin-induced (3.3 μg per min) hypoglucagonemia was corrected by exogenous glucagon, hyperglycemia occurred. In dogs with long-standing insulin-requiring alloxan diabetes 3.3 μg per min of somatostatin suppressed glucagon to 55 pg per ml throughout the 30-min infusion and lowered glucose by 36.4±6.1 mg per dl, about 1 mg per dl per min. Glucagon suppression was maintained despite alanine infusion, and glucose, which rose 29 mg per dl during alanine infusion without somatostatin, declined 58 mg per dl in the somatostatin-treated diabetic dogs despite alanine. Continuous infusion of somatostatin for 24 h in five insulin-requiring alloxan-diabetic dogs suppressed glucagon and lowered glucose significantly, usually to below normal.
It is concluded that in normal dogs pharmacologic doses of somatostatin virtually abolish insulin and glucagon secretion in the basal state and during hyperaminoacidemia. Hyperglycemia occurs during somatostatin-induced insulin lack only if hypoglucagonemia is corrected. Somatostatin suppresses glucagon in diabetic dogs and lowers their plasma glucose approximately 1 mg per dl per min, even when the gluconeogenic substrate alanine is abundant. Glucagon suppression can be maintained for several hours in such dogs and hyperglycemia is thereby reduced.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alberti K. G., Christensen N. J., Christensen S. E., Hansen A. P., Iversen J., Lundbaek K., Seyer-Hansen K., Orskov H. Inhibition of insulin secretion by somatostatin. Lancet. 1973 Dec 8;2(7841):1299–1301. doi: 10.1016/s0140-6736(73)92873-0. [DOI] [PubMed] [Google Scholar]
- Brazeau P., Vale W., Burgus R., Ling N., Butcher M., Rivier J., Guillemin R. Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone. Science. 1973 Jan 5;179(4068):77–79. doi: 10.1126/science.179.4068.77. [DOI] [PubMed] [Google Scholar]
- Cherrington A. D., Vranic M. Effect of arginine on glucose turnover and plasma free fatty acids in normal dogs. Diabetes. 1973 Jul;22(7):537–543. doi: 10.2337/diab.22.7.537. [DOI] [PubMed] [Google Scholar]
- Fajans S. S., Floyd J. C., Jr, Knopf R. F., Conn F. W. Effect of amino acids and proteins on insulin secretion in man. Recent Prog Horm Res. 1967;23:617–662. doi: 10.1016/b978-1-4831-9826-2.50017-9. [DOI] [PubMed] [Google Scholar]
- Felig P., Pozefsky T., Marliss E., Cahill G. F., Jr Alanine: key role in gluconeogenesis. Science. 1970 Feb 13;167(3920):1003–1004. doi: 10.1126/science.167.3920.1003. [DOI] [PubMed] [Google Scholar]
- Gerich J. E., Lorenzi M., Schneider V., Karam J. H., Rivier J., Guillemin R., Forsham P. H. Effects of somatostatin on plasma glucose and glucagon levels in human diabetes mellitus. Pathophysiologic and therapeutic implications. N Engl J Med. 1974 Sep 12;291(11):544–547. doi: 10.1056/NEJM197409122911102. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Iversen J. Inhibition of pancreatic glucagon release by somatostatin: in vitro. Scand J Clin Lab Invest. 1974 Apr;33(2):125–129. [PubMed] [Google Scholar]
- Mortimer C. H., Tunbridge W. M., Carr D., Yeomans L., Lind T., Coy D. H., Bloom S. R., Kastin A., Mallinson C. N., Besser G. M. Effects of growth-hormone release-inhibiting hormone on circulating glucagon, insulin, and growth hormone in normal, diabetic, acromegalic, and hypopituitary patients. Lancet. 1974 Apr 20;1(7860):697–701. doi: 10.1016/s0140-6736(74)92903-1. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Aguilar-Parada E., Unger R. H. Abnormal alpha-cell function in diabetes. Response to carbohydrate and protein ingestion. N Engl J Med. 1970 Jul 16;283(3):109–115. doi: 10.1056/NEJM197007162830301. [DOI] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of alanine on glucagon secretion. J Clin Invest. 1971 Oct;50(10):2215–2218. doi: 10.1172/JCI106716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller W. A., Faloona G. R., Unger R. H. The effect of experimental insulin deficiency on glucagon secretion. J Clin Invest. 1971 Sep;50(9):1992–1999. doi: 10.1172/JCI106691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohneda A., Parada E., Eisentraut A. M., Unger R. H. Characterization of response of circulating glucagon to intraduodenal and intravenous administration of amino acids. J Clin Invest. 1968 Oct;47(10):2305–2322. doi: 10.1172/JCI105916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santeusanio F., Faloona G. R., Unger R. H. Inhibition of alanine-stimulated glucagon secretion by secretin in the dog. Horm Metab Res. 1973 Nov;5(6):425–427. doi: 10.1055/s-0028-1093916. [DOI] [PubMed] [Google Scholar]
- Santeusanio F., Faloona G. R., Unger R. H. Suppressive effect of secretin upon pancreatic alpha cell function. J Clin Invest. 1972 Jul;51(7):1743–1749. doi: 10.1172/JCI106975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unger R. H., Aguilar-Parada E., Müller W. A., Eisentraut A. M. Studies of pancreatic alpha cell function in normal and diabetic subjects. J Clin Invest. 1970 Apr;49(4):837–848. doi: 10.1172/JCI106297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- YALOW R. S., BERSON S. A. Immunoassay of endogenous plasma insulin in man. J Clin Invest. 1960 Jul;39:1157–1175. doi: 10.1172/JCI104130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yen S. S., Siler T. M., DeVane G. W. Effect of somatostatin in patients with acromegaly: suppression of growth hormone, prolactin, insulin and glucose levels. N Engl J Med. 1974 Apr 25;290(17):935–938. doi: 10.1056/NEJM197404252901704. [DOI] [PubMed] [Google Scholar]