Abstract
It is well-established that silent regions of the genome replicate late during S phase. In this issue of Molecular Cell, Black et al. uncover a conserved role for the JMJD2 family of histone demethylases in promoting replication within silent chromatin regions that contain histone H3 lysine 9 methylation and HP1.
The faithful replication of the genome during each cell division is a vital process that is highly coordinated and tightly controlled. In eukaryotic cells, origins of DNA replication, the sites where replication begins, are found throughout the genome and fire at different times throughout S phase. This timing is influenced by local chromatin structure and correlates with cell type-specific differences in gene expression and chromatin structure. Although there are exceptions, in general, transcriptionally active portions of the genome are replicated earlier in S phase than those found in silent, heterochromatic regions (MacAlpine and Bell, 2005). Histone H3 lysine 9 (H3K9) methylation is a conserved modification that is found in heterochromatin from fission yeast to human, and defines a binding site for Heterochromatin Protein 1 (HP1) family members, which help make heterochromatin inaccessible. Recent studies suggest that heterochromatin is more dynamic than previously appreciated and can be broached by machineries that transcribe and repair DNA (Kwon and Workman, 2008). However, how access to heterochromatin or other silent regions is regulated is poorly understood.
The discovery of histone lysine demethylases suggested new mechanisms for regulation of chromatin structure and, indeed, these enzymes have already been identified with numerous roles in regulation of transcription. Histone lysine demethylation is catalyzed by two distinct classes of evolutionarily conserved enzymes (Shi and Whetstine, 2007). JmjC domain proteins are metalloenzymes that catalyze oxidative reactions. Several years ago, a JmjC-domain protein of the JMJD2 family was found to catalyze the demethylation of tri-methylated H3K9, H3K36 and H1.4K26 (Fodor et al., 2006; Klose et al., 2006; Trojer et al., 2009; Whetstine et al., 2006). Depletion of JMJD-2 in C. elegans results in the activation of a DNA damage-induced apoptosis (Whetstine et al., 2006), but the molecular basis of this event had not been established.
In this issue of Molecular Cell, Black et al. describe a role for histone demethylation in cell cycle progression and DNA replication. They report that the expression of JMJD2A is cell cycle regulated with peak expression at the G1/S transition dropping off in S phase and lowest at G2/M. The overexpression of JMJD2A, but not catalytically inactive JMJD2A, which has a mutation at a key metal-coordinating residue required for demethylase activity (Whetstine et al., 2006), in human cell lines led to faster progression through S phase. In addition, the overexpression of JMJD2A resulted in both the early replication of a late-replicating satellite region, sat2 of chromosome 1 (Chr1 sat2), as well as faster recovery of these cells after treatment with hydroxyurea (HU), which causes replication stress. Similarly, mutating or knocking down the gene for the worm homolog, jmjd-2, resulted in increased sensitivity to HU. These results suggest that expression of JMJD2A/JMJD-2 during S phase facilitates DNA replication. Consistently, Black et al. also demonstrated that more single stranded DNA was observed during S phase in cells ectopically expressing JMJD2A, which suggests increased presence of replication forks. However, it remains unknown whether the faster S phase effects are at the level of initiation and/or fork progression.
Analysis of jmjd-2−/− worms revealed a decreased number of cells within the mitotic zone and an increased number of RAD-51 foci, an indication of stalled or collapsed replication forks. Furthermore, direct in vivo visualization of DNA replication using pulse-chase labeling experiments with cy3-dUTP/ALEXA-488-dUTP in the adult germlines of jmjd-2−/− worms showed that replication was slowed, suggesting that in vivo, JMJD-2 likely affects replication timing. This observation also explains the previously observed increased apoptosis in jmjd-2 mutants (Whetstine et al., 2006), a phenotype that Black et al. also show can be rescued by knock down of the p53 and ATR homologs, CEP-1 and ATL-1 respectively. However, the mutation of the ATM homolog did not provide a rescue of the observed phenotype, indicating that the increased apoptosis is mediated through the ATR pathway, which is activated after replication stress, but not the ATM pathway, which is typically activated in response to double strand breaks.
How does JMJD2A-mediated histone demethylation contribute to DNA replication timing? The overexpression of JMJD2A causes the redistribution of HP1 proteins (Klose et al., 2006) likely by removing the chromatin mark to which HP1 proteins bind, trimethylated H3K9. So, potentially JMJD2A may regulate chromatin structure and replication timing via effects on HP1 localization. Indeed, using micrococcal nuclease digestions of isolated nuclei, Black et al. observed increased DNA accessibility in cells overexpressing catalytically active JMJD2A, specifically during S phase. In addition, in these cells, the chromatin structure of the late-replicating locus Chr1 sat2 was found to be more open corresponding to its observed earlier replication. These data support the notion that altering chromatin accessibility can regulate cell cycle progression and replication timing, providing another way to regulate cell cycle that is independent or complimented by altered transcriptional programs.
Interestingly, it appears that JMJD2A/JMJD-2 act by antagonizing a specific HP1 isoform. Most cells contain two or more HP1 homologs, HP1α, β, and HP1γ in human, and HPL-1 and -2 in worms. The overexpression of HP1γ, but not HP1α or HP1β, suppressed the JMJD2A-dependent increased number of cells in late S phase, an effect that depended on both the chromodomain and chromoshadow domain of HP1γ. Also, at the Chr1 sat2 locus, the overexpression of JMJD2A decreased HP1γ localization. In worms, the jmjd-2−/− phenotypes of increased RAD-51 foci, decreased number of nuclei in the mitotic zone, slowed replication, and increased apoptosis are all rescued by the depletion of HP1 homolog HPL-2, establishing a conserved antagonistic relationship between these two proteins.
Together the results suggest that JMJD2A/JMJD-2 modulate replication timing by opposing reduced DNA accessibility to the replication machinery in DNA domains that are decorated by H3K9 trimethylation and HP1γ (Figure 1). It remains to be determined whether the activation of DNA damage checkpoint and apoptosis in jmjd-2−/− cells result from a widespread failure to complete DNA replication or from a failure to do so at specific heterochromatic regions, analogous to human satellite repeats. The findings also add to an emerging body of evidence that suggests distinct roles for different HP1 proteins in regulation of DNA replication and other heterochromatin functions, which may relate to protein interactions and/or genomic location. For example, the two fission yeast homologs, Swi6 and Chp2, play distinct roles in replication of heterochromatin and gene silencing (Hayashi et al., 2009; Motamedi et al., 2008). Swi6HP1 specifically recruits the Cdc7 kinase to promote origin firing and early replication of heterochromatin at pericentromeric DNA regions and the mating type locus (Hayashi et al., 2009). Surprisingly, Black et al. found that deletion of the worm HP1γ homolog (HPL-2) had the same delayed replication phenotypes as jmjd-2−/− cells, suggesting that intricacies in the balance between the two worm HP1 proteins are also important for proper replication timing. We can therefore look forward to future studies that will explore possible direct links between different HP1 proteins and activation or silencing of DNA replication.
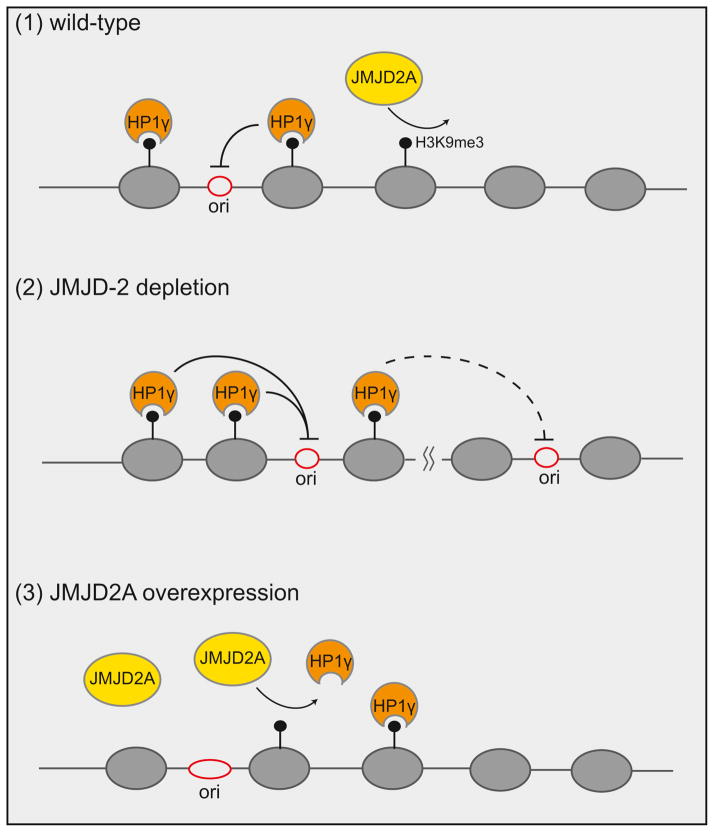
Figure 1. Human JMJD2A antagonizes HP1γ and promotes DNA replication.
(1) HP1γ binds trimethylated histone H3 lysine 9 (H3K9me3), maintaining a closed chromatin structure, which inhibits the firing of origins of replication or slows the progress of replication forks. Demethylation of H3K9me3 results in dissociation of HP1γ, thus promoting DNA replication and cell cycle progression. (2) In JMJD-2 depleted germline nuclei, H3K9me3 levels and HPL-2/HP1γ association increase, leading to delayed replication origin firing. Potential spreading of HPL-2/HP1γ may also prevent the firing of distally located origins of replication. (3) During S phase or when JMJD2A is overexpressed, demethylation of H3K9me3 and HP1γ displacement allows origin firing resulting in faster progression through S phase.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Black JC, Allen A, Capucine VR, Forbes E, Longworth M, Tschop K, Rinehart C, Quiton J, Walsh R, Smallwood A, Dyson NJ, Whetstine JR. 2010 doi: 10.1016/j.molcel.2010.11.008. This issue. [DOI] [PubMed] [Google Scholar]
- Fodor BD, Kubicek S, Yonezawa M, O’Sullivan RJ, Sengupta R, Perez-Burgos L, Opravil S, Mechtler K, Schotta G, Jenuwein T. Genes Dev. 2006;20:1557–1562. doi: 10.1101/gad.388206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi MT, Takahashi TS, Nakagawa T, Nakayama J, Masukata H. Nat Cell Biol. 2009;11:357–362. doi: 10.1038/ncb1845. [DOI] [PubMed] [Google Scholar]
- Klose RJ, Yamane K, Bae Y, Zhang D, Erdjument-Bromage H, Tempst P, Wong J, Zhang Y. Nature. 2006;442:312–316. doi: 10.1038/nature04853. [DOI] [PubMed] [Google Scholar]
- Kwon SH, Workman JL. Mol Cells. 2008;26:217–227. [PubMed] [Google Scholar]
- MacAlpine DM, Bell SP. Chromosome Res. 2005;13:309–326. doi: 10.1007/s10577-005-1508-1. [DOI] [PubMed] [Google Scholar]
- Motamedi MR, Hong EJ, Li X, Gerber S, Denison C, Gygi S, Moazed D. Mol Cell. 2008;32:778–790. doi: 10.1016/j.molcel.2008.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y, Whetstine JR. Mol Cell. 2007;25:1–14. doi: 10.1016/j.molcel.2006.12.010. [DOI] [PubMed] [Google Scholar]
- Trojer P, Zhang J, Yonezawa M, Schmidt A, Zheng H, Jenuwein T, Reinberg D. J Biol Chem. 2009;284:8395–8405. doi: 10.1074/jbc.M807818200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whetstine JR, Nottke A, Lan F, Huarte M, Smolikov S, Chen Z, Spooner E, Li E, Zhang G, Colaiacovo M, et al. Cell. 2006;125:467–481. doi: 10.1016/j.cell.2006.03.028. [DOI] [PubMed] [Google Scholar]