The oncogenic signal transducer Gab2 mediates altered cytoskeletal organization and enhanced cell migration of mammary epithelial cells via down-regulation of RhoA activity. This sheds new light on the role of Gab2 in cancer cell metastasis.
Abstract
The docking protein Gab2 is overexpressed in several human malignancies, including breast cancer, and is associated with increased metastatic potential. Here we report that Gab2 overexpression in MCF-10A mammary epithelial cells led to delayed cell spreading, a decrease in stress fibers and mature focal adhesions, and enhanced cell migration. Expression of a Gab2 mutant uncoupled from 14-3-3-mediated negative feedback (Gab22×A) led to a more mesenchymal morphology and acquisition of invasive potential. Expression of either Gab2 or Gab22×A led to decreased activation of RhoA, but only the latter increased levels of Rac-GTP. Expression of constitutively active RhoA in MCF-10A/Gab2 cells restored stress fibers and focal adhesions, indicating that Gab2 signals upstream of RhoA to suppress these structures. Mutation of the two Shp2-binding sites to phenylalanine (Gab2ΔShp2) markedly reduced the effects of Gab2 on cellular phenotype and RhoA activation. Expression of Gab2 or Gab22×A, but not Gab2ΔShp2, promoted Vav2 phosphorylation and plasma membrane recruitment of p190A RhoGAP. Knockdown of p190A RhoGAP reversed Gab2-mediated effects on stress fibers and focal adhesions. The identification of a novel pathway downstream of Gab2 involving negative regulation of RhoA by p190A RhoGAP sheds new light on the role of Gab2 in cancer progression.
INTRODUCTION
Cell migration requires the coordinated interplay of several key processes: extension of a lamellipodium at the “leading edge,” establishment of focal contacts with the underlying matrix and their maturation into focal adhesions (FAs), contraction of the cell body, and finally detachment at the rear of the cell. Key regulators of these processes are members of the Rho family of GTPases (Heasman and Ridley, 2008). In particular, Cdc42 plays a critical role in establishment of cell polarity and extension of filopodia, Rac is required for both the assembly of the dendritic actin network that drives lamellipodial protrusion and focal contact assembly, and RhoA promotes the formation of contractile actomyosin stress fibers and FAs (Heasman and Ridley, 2008). Dysregulation of these processes contributes to the enhanced motility and invasiveness of cancer cells and thereby promotes their metastatic spread (Ellenbroek and Collard, 2007).
The action of specific cellular stimuli on Rho GTPase activation is mediated via a diverse array of guanine nucleotide exchange factors (GEFs), GTPase-activating proteins (GAPs), and guanine nucleotide dissociation inhibitors, which exhibit selectivity in terms of the regulatory stimulus to which they respond and the GTPase on which they act (Ellenbroek and Collard, 2007). For example, Rac is activated during cell spreading following integrin- and focal adhesion kinase (FAK)–dependent tyrosine phosphorylation of p130Cas, which leads to recruitment of a complex between the adaptor Crk and the Rac GEF DOCK180 (Defilippi et al., 2006). In contrast, stimulation of Rac GTP loading by particular receptor or receptor-associated tyrosine kinases is mediated, in part, via particular members of the Vav family of GEFs, which are directly regulated by tyrosine phosphorylation (Bos et al., 2007). In the case of RhoA, FAK can modulate its activation via regulation of both p190RhoGEF (Lim et al., 2008) and p190A RhoGAP (p190A) (Tomar et al., 2009), and v-Src signals via the latter protein to impair actin stress fiber formation (Fincham et al., 1999). The cellular activity of p190A toward RhoA is enhanced by tyrosine phosphorylation of the former protein, which promotes binding to the src homology 2 (SH2) domains of p120RasGAP, and hence plasma membrane recruitment of p190A (Bradley et al., 2006).
Receptor-type and cytoplasmic tyrosine kinases regulate pleiotropic cellular responses, including cell motility. One mechanism utilized by these kinases to diversify their signaling repertoire is phosphorylation of docking proteins of the Grb2-associated binder (Gab)/Daughter of sevenless family, which includes mammalian Gab1–3 (Wohrle et al., 2009). Structural features of these proteins include an N-terminal pleckstrin homology domain that mediates plasma membrane recruitment; multiple tyrosine phosphorylation sites that bind specific SH2 domain–containing effectors, including the protein tyrosine phosphatase Shp2 and the p85 subunit of phosphatidylinositol (PI) 3-kinase; and canonical and atypical binding sites for the C-terminal SH3 domain of Grb2. The latter adaptor binds tyrosine-phosphorylated targets via its SH2 domain and hence bridges Gab proteins to specific growth factor or cytokine receptors. A critical role of the Gab/Shp2 complex is to enhance and/or sustain Ras/Erk activation (Maroun et al., 2000; Wohrle et al., 2009), but Shp2 recruitment also regulates other effector pathways in a context-dependent manner, including PI3-kinase (Yu et al., 2002) and Rac/JNK (Yu et al., 2006; Samayawardhena and Pallen, 2008). Signaling via Gab proteins is implicated in morphological and/or migratory responses to a variety of stimuli. For example, Gab1 coupling to Shp2 is critical for Met-induced branching morphogenesis of Madin-Darby canine kidney cells (Maroun et al., 2000; Schaeper et al., 2000) and enhances platelet-derived growth factor–induced formation of lamellipodia (Kallin et al., 2004). In the case of Gab2, signaling via Shp2 promotes β1-integrin-induced adhesion and migration of Ba/F3 cells (Yu et al., 2002) as well as migration of mast cells (Samayawardhena and Pallen, 2008). Further work is required to delineate how Gab proteins regulate cytoskeletal organization and cell motility and the effector pathways involved.
Recent work has strongly implicated Gab2 in cellular transformation and cancer. Uncoupling of Gab2 from phosphorylation-mediated negative feedback regulation on S159, or S210 and T391, results in transforming properties in fibroblasts and MCF-10A cells, respectively (Lynch and Daly, 2002; Brummer et al., 2008). In addition, Gab2 is required for Bcr-Abl-mediated transformation of myeloid cells (Sattler et al., 2002) and erbB2-induced mammary tumorigenesis (Bentires-Alj et al., 2006) and metastatic spread (Ke et al., 2007). Finally, Gab2 is amplified at the gene level and/or overexpressed in breast and gastric cancers, metastatic melanoma, and acute myeloid leukemia (Daly et al., 2002; Zatkova et al., 2006; Lee et al., 2007; Chernoff et al., 2009; Horst et al., 2009; Bocanegra et al., 2010). Previously, we demonstrated that Gab2 overexpression in MCF-10A cells promotes proliferation and growth factor independence in three-dimensional (3D) culture (Brummer et al., 2006). In the current study, the demonstrated links between Gab2 and metastasis (Ke et al., 2007; Horst et al., 2009) have led us to determine whether Gab2 regulates cellular motility in MCF-10A cells and the underlying mechanisms. This has revealed that Gab2 markedly alters cytoskeletal organization, FA formation, and cell motility and identifies modulation of RhoA activity as the mechanism underpinning this phenotype.
RESULTS
Gab2 overexpression delays cell spreading and reduces the assembly of actin stress fibers and maturation of FAs
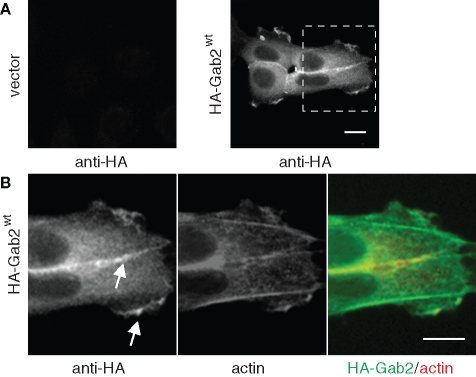
Previously, we generated stable pools of MCF-10A immortalized mammary epithelial cells expressing Gab2 at levels comparable to those detected in breast cancer cells (Brummer et al., 2006). Because parental MCF-10As express very low levels of this docking protein (Brummer et al., 2006), this provides a powerful system for characterizing the role of Gab2 in breast cancer progression. To determine the localization of Gab2 in MCF-10A/Gab2 cells, we utilized anti-HA staining because the expressed Gab2 exhibits a C-terminal HA tag. This revealed localization of Gab2 in the perinuclear region, at leading edge lamellipodia and at cell–cell contacts (Figure 1).
FIGURE 1:
(A) Subcellular localization of Gab2 in MCF-10A/Gab2 cells. Staining shown is anti-HA (Gab2). (B) Enlarged view of the inset in (A), showing both anti-HA and F-actin staining. Arrows indicate Gab2 localization at cell–cell contacts and lamellipodia. Scale bars are 10 μm.
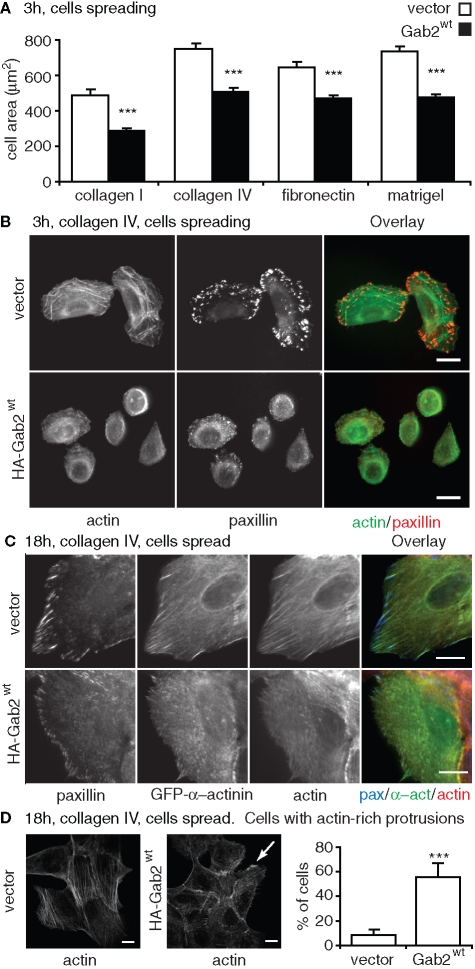
To determine how Gab2 modulates cell spreading, we allowed cells to attach and then spread for 3 h on either collagen I, collagen IV, fibronectin, or reconstituted basement membrane (Matrigel). On all these types of extracellular matrix, Gab2 overexpression resulted in delayed cell spreading, as indicated by a significant reduction in cell area at this time point (Figure 2, A and B). This delay was not due to a defect in integrin binding to the extracellular matrix, as cellular adhesion was unaltered (e.g., on collagen IV, cell adhesion in arbitrary units was 77.1 ± 4.0 for control cells and 80.5 ± 2.7 for Gab2-overexpressing cells). In addition, delayed spreading was observed when the cells were plated in either growth medium, which is serum- and growth factor–supplemented (Figure 2), or assay medium (unpublished data; see Materials and Methods for the detailed composition of both media). Subsequent experiments described in this article utilize growth medium. Using F-actin and paxillin staining, we characterized cytoskeletal organization together with size and density of FAs in cells plated for 3 h on collagen IV (Figure 2B). This revealed that stress fiber assembly was markedly reduced in MCF-10A/Gab2 cells. In addition, the formation of large FAs was significantly reduced in cells overexpressing Gab2 compared with vector controls.
FIGURE 2:
Delayed cell spreading and impaired actin stress fiber and FA assembly in cells overexpressing Gab2. (A) Effect of Gab2 on cell spreading. The histograms indicate cell area for cells spreading for 3 h on different extracellular matrices. Values are mean ± SE of 60–80 measurements in three independent experiments. (B) Effect on focal adhesions and stress fibers during spreading. Vector controls or cells overexpressing Gab2 were plated on collagen IV for 3 h, fixed, and then stained with FITC-phalloidin or an anti-paxillin antibody. (C) Effect in spread cells. Cells were transfected with a GFP-α-actinin construct, allowed to spread for 18 h on collagen IV, and then stained for paxillin and F-actin. (D) Effect on lamellipodia and ruffles. The histograms indicate the percentage of cells with actin-rich plasma membrane protrusions (lamellipodia and ruffles), as detected by F-actin staining (representative images flank graph, arrow highlights a ruffle). Values are mean ± SD of 50 measurements in four independent experiments. *** indicates p < 0.0001 for vector vs. MCF-10A/Gab2 (by unpaired Student’s t test). All scale bars are 10 μm.
To characterize this phenotype further, we used green fluorescent protein (GFP)–α-actinin as a marker of FA maturation (von Wichert et al., 2003; Herrera Abreu et al., 2006) and analyzed cells plated for 18 h, when both control and MCF-10A/Gab2 cells have fully spread. Control MCF-10A cells formed prominent actin stress fibers that terminated at mature FAs positive for both paxillin and α-actinin (Figure 2C). However, Gab2-overexpressing cells exhibited smaller FAs that failed to recruit α-actinin, and a disorganized cytoskeleton. In addition, they displayed a significant increase in the formation of lamellipodia and ruffles (Figure 2D). Therefore, in MCF-10A cells, Gab2 induces marked alterations in cell spreading, cell–matrix contacts, and cytoskeletal organization.
Modulation of FAs and stress fibers by Gab2 is dependent on the Shp2-binding sites
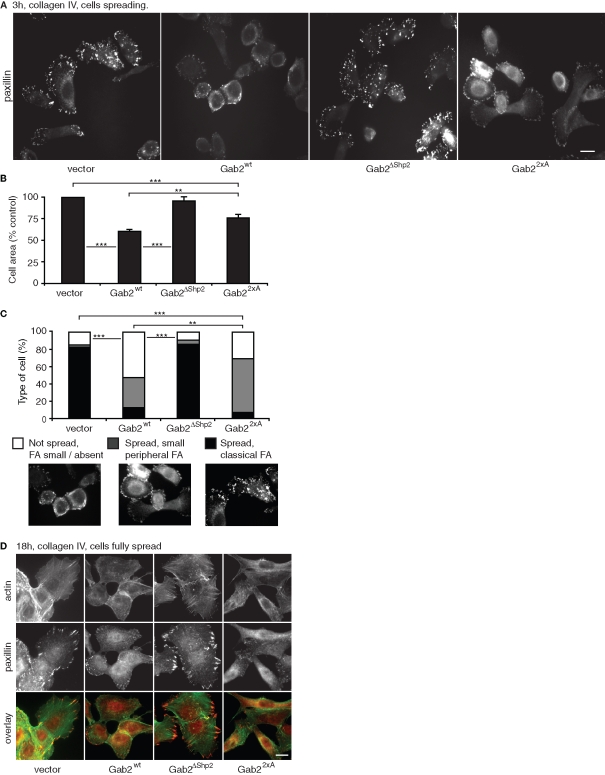
Because previous studies have identified roles for Shp2 in regulating the formation of stress fibers and FAs, we hypothesized that the Gab2-mediated phenotypic changes characterized in Figure 2 may reflect coupling of Gab2 to this protein tyrosine phosphatase. To test this hypothesis, we used MCF-10A cells stably expressing Gab2ΔShp2, a Gab2 mutant defective in binding Shp2 (Brummer et al., 2006). In addition, we determined the effects of Gab22×A, a mutant uncoupled from 14-3-3-mediated negative feedback regulation that when expressed in MCF-10A cells exhibits increased binding of specific SH2 domain–containing proteins, including Shp2, and promotes sustained downstream signaling (Brummer et al., 2008). Both of these mutants were expressed at levels similar to the wild-type protein (Brummer et al., 2006; Brummer et al., 2008). Mutation of the Shp2-binding sites restored normal cell spreading, FA maturation, and stress fiber formation (Figure 3, A–D). However, while Gab22×A mediated similar effects on FAs and stress fibers to wild-type Gab2 (Figure 3D), cell spreading was enhanced compared to MCF-10A/Gab2 cells (Figure 3, A–C), and expression of this mutant was associated with a more elongated, mesenchymal morphology (Figure 3D).
FIGURE 3:
Gab2-mediated modulation of cell spreading and cytoskeletal organization is dependent on the Shp2-binding sites. Effect on FAs (A) and cell spreading (B). The images in (A) represent paxillin staining for cells spreading for 3 h on collagen IV. In (B), the histograms indicate cell area for these cells. Values are mean ± SE of 60–80 measurements in three independent experiments. (C) Classification of cells based on FA formation and degree of spreading. More than 200 cells were classified in three independent experiments. Statistical tests compare data for spread cells exhibiting classical FAs. ** and *** indicate p < 0.001 and p < 0.0001, respectively. (D) Cytoskeletal organization and FAs in fully spread cells. Images are derived from cells allowed to spread for 18 h on collagen IV and then stained for actin or paxillin. All scale bars are 10 μm.
Gab2 overexpression promotes cell motility and reduces the formation and stability of epithelial colonies
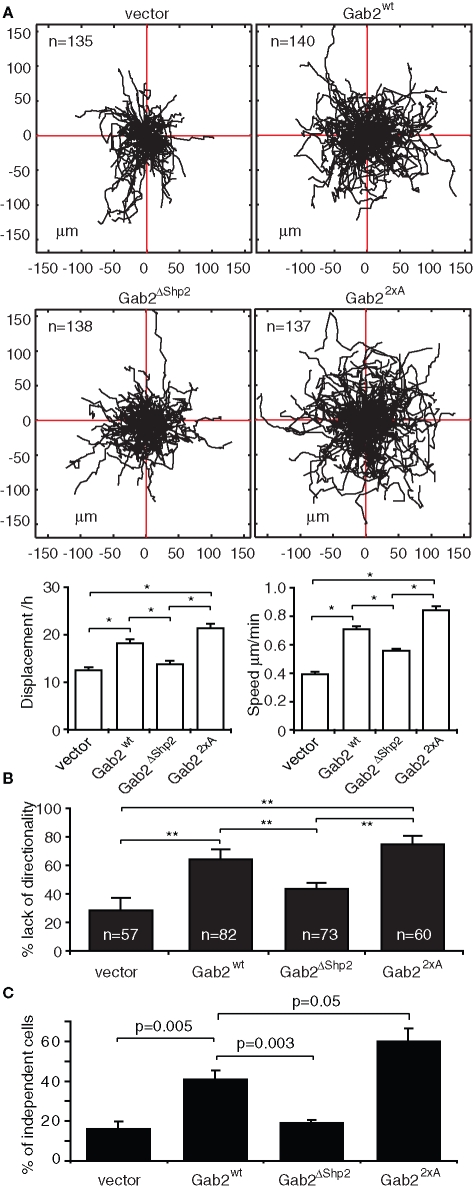
To characterize how Gab2-overexpressing cells move and establish cell–cell contacts, we undertook live cell imaging of randomly migrating cells. Tracking of cell paths revealed that MCF-10A/Gab2 cells exhibited a significant increase in displacement from the point of origin and speed of movement when compared with vector controls (Figure 4A). However, although Gab2-overexpressing cells exhibited a marked increase in lamellipodia formation and were highly motile, cellular protrusions often formed in opposing directions, leading to a lack of persistent directionality (see Supplemental Material: Video 1 for control, Video 2 for MCF-10A/Gab2; Figure 4B). Furthermore, small “islands” of these cells appeared less stable than their control counterparts and often partly dissociated (see Supplemental Material: Video 3 for control, Video 4 for MCF-10A/Gab2). Also, MCF-10A/Gab2 cells tended to disperse following cell division, rather than associating to form the epithelial colonies characteristic of control cells (see Supplemental Material: Video 5 for control, Video 6 for MCF-10A/Gab2), and had a reduced tendency to join preexisting colonies (see Supplemental Material: Video 3 for control, Video 7 for MCF-10A/Gab2). Analysis of the distribution of plated cells revealed an approximately twofold increase in independent cells, versus those in colonies, upon Gab2 overexpression (Figure 4C). Gab22×A exhibited similar or, in the case of cellular independence, enhanced effects on these parameters compared to the wild-type protein, while the activity of Gab2ΔShp2 was significantly impaired. Despite decreased formation of cell–cell contacts in MCF-10A/Gab2 cells, expression of the adherens junction proteins E-cadherin and α-, β-, and p120-catenin were similar to control cells (Supplemental Figure S1).
FIGURE 4:
Effects of Gab2 on cell motility and epithelial colony stability and formation. (A) Effect of Gab2 and Gab2 mutants on random cell motility. Tree graphs show length (μm), direction, and displacement (distance from origin) of more than 150 paths. Graphs indicate effects on cell displacement and speed. (B) Effect of Gab2 on directionality of migration. (C) Effect of Gab2 on cellular independence. Data points are mean ± SE; * and ** indicate p < 0.01 and p < 0.001, respectively.
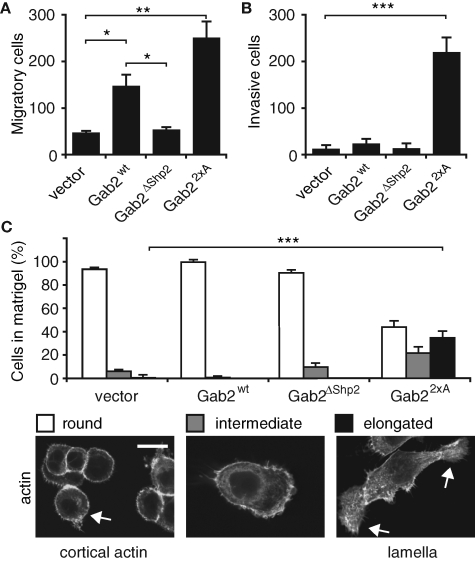
To further characterize how Gab2 overexpression affects cell motility, and to determine whether it increases cell invasion, transwell assays were performed. MCF-10A/Gab2 cells exhibited a significant increase in cell migration compared to vector controls. This effect was lost upon mutation of the Shp2-binding sites on Gab2 and enhanced further in MCF-10A/Gab22×A cells (Figure 5A). However, only the latter cells displayed significant invasive activity (Figure 5B). When the morphology of the different cell pools in Matrigel was determined, control cells, as well as those expressing Gab2 and Gab2ΔShp2, were rounded. In marked contrast, a significant proportion of MCF-10A/Gab22×A cells were elongated and mesenchymal (Figure 5C). Consequently, Gab2 overexpression in MCF-10A cells promotes enhanced cell migration that is dependent on the Shp2-binding sites, and further amplification of Gab2 signaling via abrogation of 14-3-3 binding leads to an invasive phenotype.
FIGURE 5:
Effects of Gab2 and Gab2 mutants on cell migration and invasion. (A) Results of transwell migration assays. (B) Results of transwell invasion assays. In (A) and (B), histograms represent mean number of cells/field ± SE. (C) Morphology of cells in Matrigel. Images show phalloidin staining of cells that have migrated for 6 h in a Matrigel matrix. Histograms indicate the proportion of cells exhibiting particular morphologies. For each cell pool, 80–100 cells were classified in three independent experiments. *, **, and *** indicate p < 0.01, p < 0.001, and p < 0.0001, respectively. Scale bar is 10 μm.
Regulation of Rho family GTPases by Gab2 in MCF-10A cells
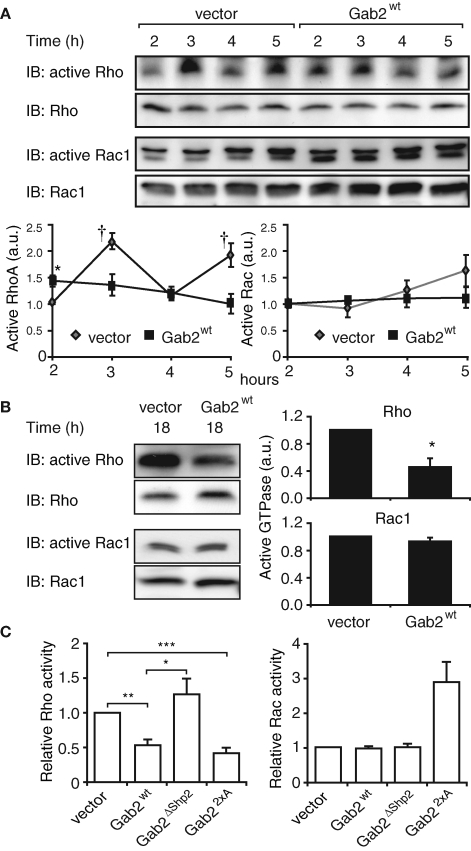
Although the effects of Gab2 on cellular phenotype were dependent on the presence of Shp2-binding sites, under the conditions of our experiments, activation of Erk was unaltered in MCF-10A/Gab2 cells (Supplemental Figure S2). However, the striking alterations in cytoskeletal organization, adhesion complexes, and migratory potential exhibited by these cells suggested that Gab2 signaling may modulate the activation status of particular Rho family GTPases. To test this hypothesis, levels of RhoA-GTP and Rac-GTP were determined at different times during cell spreading by pull-down assays. At 2 h postplating, when the cells are fully attached and start to spread, RhoA activation was significantly higher in Gab2-overexpressing cells (Figure 6A). However, while RhoA in the control cells exhibited two cycles of activation between 2 and 5 h, in MCF-10A/Gab2 cells the abundance of GTP-bound RhoA steadily decreased, so that at 3, 5, and 18 h RhoA activation was markedly lower in these cells than in vector controls (Figure 6, A and B). Global Rac1 activation was similar in control and Gab2-overexpressing cells at all time points examined (Figure 6, A and B).
FIGURE 6:
Activation of Rho family GTPases in Gab2-overexpressing cells. Effect of Gab2 on activation of RhoA and Rac1 (A) during cell spreading or (B) in spread cells (18 h). Cell lysates were used in pull-down assays for activation of specific GTPases. Levels of GTP-bound RhoA or Rac1 were normalized for their corresponding expression levels. Graphs indicate activation relative to that in control cells at 2 h. Values are mean ± SE of four independent experiments. † and * indicate p < 0.05 and p < 0.01, respectively, by unpaired Student’s t test. (C) Effect of Gab2 mutants on RhoA and Rac activity. Cells expressing Gab2 or the indicated mutants were allowed to spread on collagen IV for 18 h, and then activation of RhoA and Rac was assayed as in (A). Numbers indicate activation relative to vector controls, which are arbitrarily set at 1.0. Data are derived from n ≥ 3 experiments (RhoA) and n = 2 experiments (Rac). *, **, and *** indicate p < 0.01, p < 0.001, and p < 0.0001, respectively. a.u.: arbitrary units.
Next, we determined how expression of particular Gab2 mutants affected activation of RhoA and Rac. Gab2ΔShp2 did not alter the amount of GTP-bound RhoA or Rac1 in spread cells (Figure 6C), consistent with the lack of effect of this mutant on cytoskeletal organization and FAs (Figure 3). Expression of Gab22×A led to a similar reduction in RhoA activation to wild-type Gab2. However, Rac1-GTP levels were increased approximately threefold (Figure 6C).
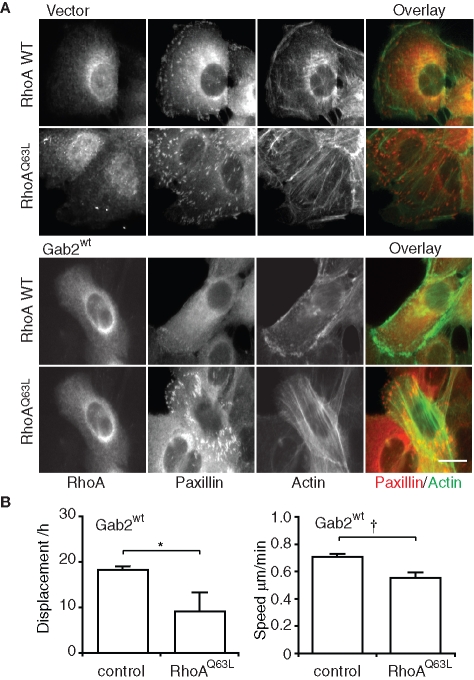
These data implicated RhoA in mediating the effects of Gab2 on stress fibers and FAs. To directly test this hypothesis, we transiently transfected MCF-10A/Gab2 cells with constructs encoding wild-type RhoA or an active form of this GTPase. While expression of wild-type RhoA did not alter the formation of stress fibers or FAs, these were restored upon expression of the active mutant RhoQ63L (Figure 7A). In addition, expression of active RhoA significantly reduced cell motility, as determined by live cell imaging (Figure 7B). Consequently, Gab2 must signal upstream of RhoA to regulate cytoskeletal organization and cell migration.
FIGURE 7:
Expression of active RhoA reverses the Gab2-associated cellular phenotype. (A) Cytoskeletal organization. Vector control and MCF-10A/Gab2 cells were transfected with constructs encoding either myc-tagged RhoA wt or RhoA Q63L and then allowed to spread for 18 h on collagen IV. Transfected cells were identified by anti-myc staining. F-actin was stained with FITC-phalloidin and FAs with an anti-paxillin antibody. Scale bar is 10 μm. (B) Random cell motility. Control MCF-10A/Gab2 cells, and cells expressing RhoA Q63L, were subject to cell tracking analysis. Graphs indicate effects on cell displacement and speed. † and * indicate p < 0.05 and p < 0.01, respectively.
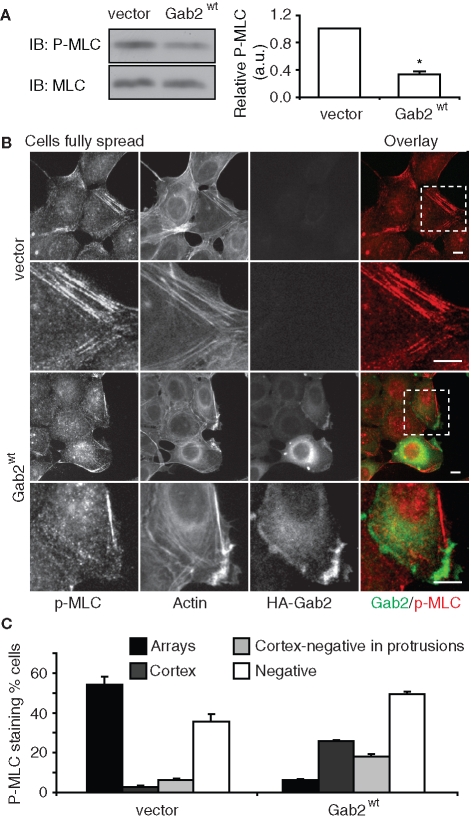
RhoA signals via Rho kinase to enhance the phosphorylation of myosin light chain (MLC) and thereby the formation of contractile actomyosin stress fibers (Ellenbroek and Collard, 2007). Consistent with the relative activation of RhoA in the two cell types (Figure 6), Western blotting revealed that total cellular levels of pMLC were significantly reduced in MCF-10A/Gab2 cells compared to controls (Figure 8A). Furthermore, following spreading, control cells exhibited strong pMLC staining at arrays of actin stress fibers, and this staining pattern was reduced in cells overexpressing Gab2 (Figure 8, B and C). Interestingly, in MCF-10A/Gab2 cells, pMLC staining was occasionally detected at the cell cortex (Figure 8C), but only in regions that were negative for Gab2 expression. This is highlighted in Figure 8B, where a region of cell protrusion that is strongly positive for Gab2 expression stains negative for pMLC. Overall, these data indicate that Gab2 overexpression leads to decreased RhoA activation and downstream signaling. In addition, they suggest that the increased formation of cell protrusions by MCF-10A/Gab2 cells (Figure 2D), which occurs without changes in Rac activation (Figure 6), reflects localized down-regulation of RhoA by Gab2 at the cell periphery. This effect generates zones of decreased contractility where cell protrusion can readily occur.
FIGURE 8:
Gab2 overexpression perturbs regulation of MLC. (A) MLC2 phosphorylation. Cells were allowed to spread for 18 h on collagen IV. Cell lysates were Western blotted as indicated. Phosphorylation of MLC2 was normalized for total protein expression and is expressed relative to the value for vector cells, which was arbitrarily set at 1.0. Data represent the mean ± SE of three independent experiments. * indicates p < 0.01 by unpaired Student’s t test. (B) Localization of pMLC. Cells treated as in (A) were stained as indicated. Enlarged views of the insets highlighted are shown in the lower panels for each cell type. Scale bar is 10 μm. (C) Quantification of pMLC staining patterns. The graph indicates the percentage of cells with pMLC in arrays (arrays), cortical pMLC (cortex), cortical pMLC but also pMLC-negative protrusions (cortex-negative in protrusions), and lacking pMLC staining (negative).
Regulation of Rho family GAP and GEF activities by Gab2
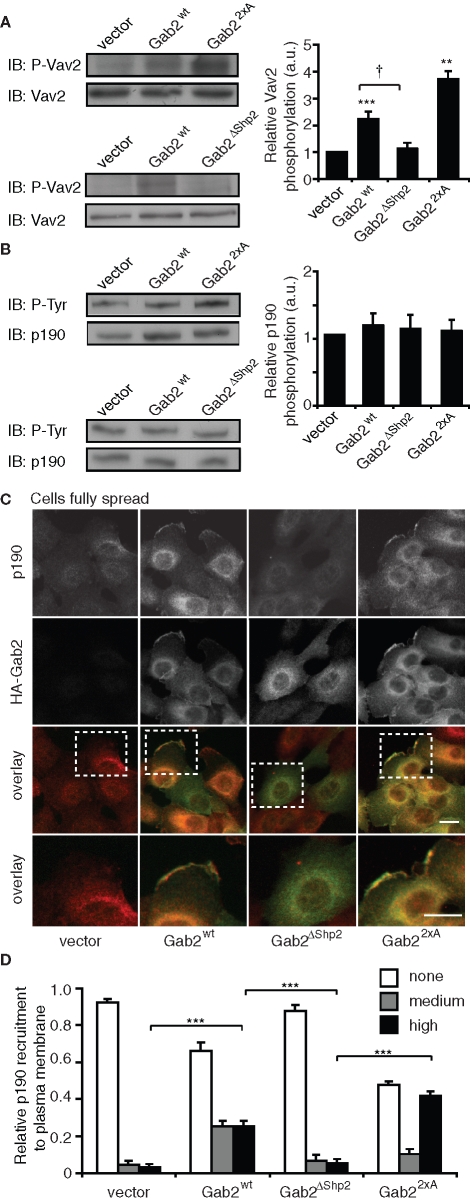
To delineate the mechanism for Gab2-mediated suppression of RhoA activity in spread cells (Figure 6), we initially focused on two known regulators of the RhoA family, Vav2 and p190A RhoGAP (p190A). The former is a GEF that stimulates both RhoA and Rac in vitro (Abe et al., 2000), and the related family member Vav1 signals downstream of Gab2 in mast cells (Samayawardhena and Pallen, 2008). The latter, which negatively regulates RhoA, plays a well-established role in mediating signaling between particular tyrosine kinases and the cytoskeleton (Fincham et al., 1999; Bradley et al., 2006; Tomar et al., 2009). The cellular activity of both proteins is positively regulated by tyrosine phosphorylation, although in the case of p190A, the effect is indirect and mediated via enhanced plasma membrane recruitment (Bradley et al., 2006; Bos et al., 2007).
Western blotting revealed that tyrosine phosphorylation of Vav2 was increased by approximately twofold and three- to fourfold in MCF-10A/Gab2 and MCF-10A/Gab22×A cells, respectively (Figure 9A). This effect was not observed in cells expressing Gab2ΔShp2. These data indicate that Vav2 is unlikely to mediate the effects of Gab2 on RhoA, although it may contribute to the increased Rac activation observed in MCF-10A/Gab22×A cells (Figure 6C). However, tyrosine phosphorylation of p190A was not significantly altered upon expression of Gab2 or Gab22×A (Figure 9B).
FIGURE 9:
Effects of Gab2 overexpression on regulation of Vav2 and p190A RhoGAP. (A) Tyrosine phosphorylation of Vav2. Cells were allowed to spread for 18 h on collagen IV. Cell lysates were then Western blotted for pVav2 or total Vav2. The graph indicates relative tyrosine phosphorylation of Vav2 in cells overexpressing Gab2 or the indicated mutants. Western blots were subjected to densitometry and tyrosine-phosphorylated Vav2 normalized for Vav2 levels. Data were then expressed relative to the value for vector controls, which was arbitrarily set at 1.0. †, **, and *** indicate p < 0.05, p ≤ 0.001, and p < 0.0001, respectively, for statistical significance relative to vector controls. (B) Tyrosine phosphorylation of p190A RhoGAP. Cells were treated as in (A). Cell lysates were subject to immunoprecipitation with an anti-p190A RhoGAP antibody, and immunoprecipitates were Western blotted with the indicated antibodies. The graph indicates relative tyrosine phosphorylation of p190A RhoGAP. Blots were subjected to densitometry and tyrosine-phosphorylated p190A RhoGAP normalized for total levels of protein. Data were then expressed relative to the value for vector controls, which was arbitrarily set at 1.0. (C) Gab2 promotes plasma membrane recruitment of p190A RhoGAP. Cells were plated on collagen IV for 18 h and then stained for HA (Gab2) and p190. The bottom panels provide an enlarged view of the highlighted insets. Scale bars are 10 μm. (D) Relative p190A RhoGAP membrane recruitment in the different cell pools. A total of 100–150 cells for each group were counted in three independent experiments. Data represent mean ± SE. *** indicates p < 0.0001.
Next, we characterized the subcellular localization of p190A. Only a small fraction of control cells exhibited staining for p190A at the plasma membrane, but recruitment to this compartment was markedly enhanced in MCF-10A/Gab2 cells (Figure 9, C and D), and Gab2 and p190A colocalized at membrane protrusions (Figure 9C). Quantitative assessment revealed that p190A plasma membrane recruitment mirrored its colocalization with Gab2. This effect increased further upon expression of Gab22×A but was not observed with Gab2ΔShp2 (Figure 9, C and D). Although p190A and Gab2 colocalized, we were unable to coimmunoprecipitate these proteins, despite detection of a robust association between Gab2 and Shp2 (unpublished data). In addition, we could not detect association of Gab2 with the p190A-binding partner, p120RasGAP. Consequently, the altered subcellular localization of p190A upon Gab2 overexpression does not appear to reflect direct, or indirect, binding to this docking protein.
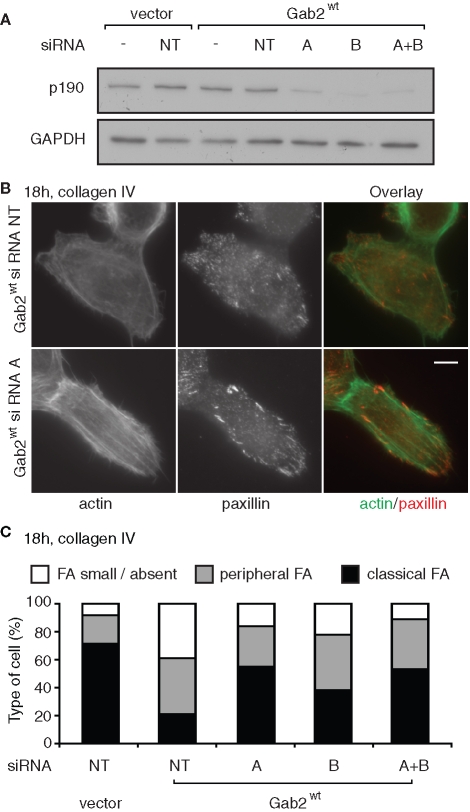
The inverse relationship between p190A plasma membrane recruitment in the different cell pools and RhoA-GTP levels (Figure 6) strongly implicates p190A in the suppression of RhoA activity upon Gab2 overexpression. However, dysregulation of other Rho regulators could render p190A ineffectual. To test whether p190A signaling contributes to the altered phenotype of MCF-10A/Gab2 cells, we reduced endogenous levels of this protein by small interfering RNA (siRNA)–mediated knockdown. Transfection of two selective siRNAs reduced protein expression by approximately 70% (Figure 10A). Following transfection, cells were allowed to spread overnight, and actin organization and FA formation were characterized by staining with phalloidin and anti-paxillin antibodies, respectively. Knockdown of p190A resulted in increased assembly of actin stress fibers and more pronounced paxillin staining at FAs (Figure 10B). Scoring of cells for FA size and distribution confirmed restoration of the normal MCF-10A phenotype in the Gab2-overexpressing cells (Figure 10C). These data are consistent with a model in which Gab2 signals via p190A to suppress RhoA activation and downstream signaling by this GTPase.
FIGURE 10:
Knockdown of p190A RhoGAP restores stress fibers and FAs in Gab2-overexpressing cells. (A) Reduction in p190A RhoGAP protein levels following transfection of targeting siRNAs. MCF-10A/Gab2 cells were transfected with the indicated siRNAs (see Materials and Methods), alone or in combination, and cell lysates were then Western blotted as indicated. NT indicates nontargeting control siRNA. (B) Knockdown of p190A RhoGAP reverses the Gab2-associated cellular phenotype. Cells were transfected as indicated, plated on collagen IV for 18 h, and then stained for actin with phalloidin or with anti-paxillin antibodies. (C) Scoring of FA size and distribution. Approximately 800 cells were classified from two independent experiments. Data represent the mean. For classical FAs, variation from the mean was <10% for vector cells and <4% for the other transfections.
DISCUSSION
Gab2 was recently implicated in the metastatic spread of both breast cancer and melanoma (Ke et al., 2007; Horst et al., 2009), but the contributing molecular mechanisms have yet to be fully characterized. In this study we demonstrate that Gab2-overexpressing mammary epithelial cells exhibit marked changes in cytoskeletal organization, maturation of cell–matrix and cell–cell contacts, and cell motility and that decreased RhoA activation underpins this altered phenotype. These novel findings regarding the biological effects of Gab2, and the extension of the Gab2 signaling network to include p190A-mediated down-regulation of RhoA activity, shed new light on the role of Gab2 in tumor progression.
Two previous studies reported a positive role for Gab2 in regulating migration of transformed mammary epithelial cells. Thus the motility of T47D breast cancer cells was reduced by Gab2 knockdown (Meng et al., 2005), and migration of neu-transformed Gab2−/− cells derived from mouse mammary tumors was diminished compared to controls expressing this docking protein (Ke et al., 2007). In both these cases, the effect of Gab2 correlated with its ability to enhance Erk activation. However, our detection of significantly reduced RhoA activation in MCF-10A/Gab2 cells identifies an additional mechanism whereby Gab2 enhances cell motility. It is likely that this effect is mediated via altered FA dynamics, with decreased assembly of larger, more stable cell–matrix adhesions, in combination with increased formation of lamellipodial protrusions, which is facilitated by localized reduction of contractility at the cell periphery. Our finding is consistent with work from other groups demonstrating a negative role for RhoA in regulating the motility of particular cell types. For example, knockdown of RhoA in MCF-10A cells (Simpson et al., 2008) or SUM159 and MCF-7 breast cancer cells (Simpson et al., 2004) led to increased cell migration or invasion. Furthermore, an additional study reported an important role for RhoA in regulating the formation of “leader” cells in epithelial wound healing assays, such that expression of a dominant-negative RhoA led to increased numbers of cells exhibiting this phenotype (Omelchenko et al., 2003). Interestingly, the phenotypic changes identified in these studies, which include increased lamellipodia and decreased formation of cell–cell contacts, are highly similar to those observed upon Gab2 overexpression. Finally, overexpression of p190A in fibroblasts decreased RhoA activity and enhanced cell motility without detectable effects on global Rac activation, again similar to the effects observed in this study (Arthur and Burridge, 2001). However, we note that the effects of RhoA on migration and invasion are context dependent and that in some cell types RhoA enhances these processes (Ellenbroek and Collard, 2007).
In addition to increased cell motility, other characteristics of MCF-10A/Gab2 cells were a lack of persistent directionality during cell migration, less stable intercellular junctions, and delayed cell spreading. With regard to the first effect, while Cdc42 represents an important regulator of cell polarity (Heasman and Ridley, 2008), activation of this GTPase was not altered in MCF-10A/Gab2 cells (unpublished data). However, evidence is accumulating that localized down-regulation of RhoA by p190A is required for cell polarization in the direction of movement (Arthur and Burridge, 2001; Tomar et al., 2009). Consequently, promiscuous down-regulation of RhoA in MCF-10A/Gab2 cells may facilitate extension of multiple cellular protrusions, leading to frequent changes in cell direction. The effect on adherens junctions may also be mediated via Gab2-mediated suppression of RhoA activity because Gab2 localizes to these sites, and RhoA is involved in expansion and completion of cell–cell contacts (Yamada and Nelson, 2007). However, the third phenotype noted for these cells, defective cell spreading, cannot be explained by decreased RhoA activation because a localized down-regulation of RhoA activity is actually required for this process (Arthur and Burridge, 2001). Instead, our finding that RhoA activation in MCF-10A/Gab2 cells was significantly enhanced early during the spreading process likely explains this effect. Of note, because we did not detect differences in Rac1-GTP levels between control and Gab2-overexpressing cells, the delay in cell spreading cannot be due to decreased Rac1 activation. However, the increased Rac activation in MCF-10A/Gab22×A cells may explain why the spreading defect is less pronounced in these cells.
Although both Gab2 and Gab22×A promoted cell migration, only the latter conferred invasive potential. In addition, cells expressing Gab22×A exhibited a more mesenchymal morphology, both in monolayer and in Matrigel, than MCF-10A/Gab2 cells. In combination with our previous work (Brummer et al., 2008), these findings indicate that deregulated Gab2 signaling can promote both anchorage-independent growth and invasion of immortalized mammary epithelial cells, further emphasizing the oncogenic potential of this docking protein. In cancers, this level of signal deregulation may occur when Gab2 is coexpressed with high levels of an activating kinase. This hypothesis is supported by findings from the Neel group, who reported that coexpression of Gab2 and erbB2 in MCF-10A cells leads to acquisition of an invasive phenotype upon 3D culture (Bentires-Alj et al., 2006). In terms of signaling, expression of Gab22×A enhanced Rac1 activation, an effect not observed with wild-type Gab2. While the effect of Gab22×A on Rac1 is likely to reflect increased signaling via Vav2 compared to the wild-type protein, the enhancement of PI3-kinase activation by Gab22×A (Brummer et al., 2008) may also contribute because PI3-kinase positively regulates the activity of additional Rac GEFs (Kolsch et al., 2008). The differential effect of Gab2 and Gab22×A on Rac1 is likely to contribute to the contrasting morphology and invasive potential of the corresponding cell types. Indeed, the mesenchymal mode of tumor cell movement in 3D matrices is known to be dependent on Rac activation (Sanz-Moreno et al., 2008). Given that this migratory mode also requires extracellular proteolysis, it will be interesting to characterize how Gab22×A expression affects the expression and secretion of proteases involved in degradation of extracellular matrix.
Use of mutants defective in coupling to specific effector pathways demonstrated that the Shp2-binding sites were essential for Gab2 to delay spreading, reduce stress fibers and FAs, and enhance motility as well as to down-regulate RhoA. In an attempt to confirm that these effects were mediated via Shp2, we undertook Shp2 knockdowns with two independent siRNAs. In both vector control cells, which express very low levels of Gab2 (Brummer et al., 2006), and Gab2-overexpressing cells, this caused marked cell rounding and detachment from the matrix as well as a failure of the cells to spread once replated (Supplemental Figure S3). While consistent with the spreading defect in Shp2 gene-targeted fibroblasts (Yu et al., 1998; Oh et al., 1999; von Wichert et al., 2003), this contrasts with the behavior of cells expressing Gab2ΔShp2, which exhibit normal cell spreading (the cells expressing Gab2 are impaired). One explanation is that the spreading defect in the Gab2-overexpressing cells may be due to Gab2-mediated sequestration of Shp2 away from a critical target. However, the presence of such a marked effect on the vector controls raises the possibility that Shp2 knockdown affects targets not normally regulated by the Gab2/Shp2 complex. Due to this confounding issue, we cannot definitively identify Shp2 as the effector involved in mediating the observed effects of Gab2 on cellular phenotype, although it remains a very strong candidate. Supporting evidence is that Shp2 negatively regulates RhoA activity, stress fibers, and FAs and promotes motility in other models (Inagaki et al., 2000; Kodama et al., 2000; Schoenwaelder et al., 2000; Chang et al., 2002).
In a previous study, down-regulation of RhoA signaling in aortic smooth muscle cells was associated with dephosphorylation of Vav (Wakino et al., 2004). However, in the MCF-10A model, Gab2 overexpression, while decreasing RhoA activation, increased Vav2 phosphorylation. These data are consistent with a previous study that reported activation of Vav1 downstream of Gab2 (Samayawardhena and Pallen, 2008) and indicate that Vav2 is unlikely to function as a GEF for RhoA in our model. Instead, it is more likely that Vav2 positively regulates Rac, and while amplification of this pathway by wild-type Gab2 is not detectable by the pull-down assay, the further enhancement of signaling that occurs when Gab22×A is expressed leads to a demonstrable elevation of Rac-GTP. This hypothesis is supported by a recent study that demonstrated increased activation of Rac and Cdc42, but not RhoA, in mammary epithelial cells expressing an activated Vav2 mutant (Duan et al., 2010).
Recent evidence indicates that cell spreading is promoted by recruitment of the p120RasGAP/p190A complex to tyrosine-phosphorylated FAK at leading edge FAs, which suppresses RhoA activation and hence cell contractility at these sites (Tomar et al., 2009). Whether perturbed regulation of p190A in Gab2-overexpressing cells contributes to their spreading defect is currently under investigation. However, in fully spread cells, plasma membrane recruitment of p190A was enhanced in MCF-10A/Gab2 cells, and this effect, like the observed down-regulation of RhoA, was reduced upon expression of Gab2ΔShp2. So how does Gab2 regulate the localization and hence activation of p190A? One possibility is that Gab2 overexpression leads to increased plasma membrane targeting of this complex. This might reflect increased tyrosine phosphorylation of one or more targets of the p120RasGAP SH2 domains or association of p120RasGAP with other types of binding partners, such as annexin A6 (Vila de Muga et al., 2009). Alternatively, serine/threonine phosphorylation of p190A, which is known to regulate its localization (Pullikuth and Catling, 2010), may be altered upon Gab2 overexpression. Interestingly, Gab2ΔShp2, which fails to regulate p190A localization and down-regulate RhoA, is itself impaired in plasma membrane recruitment, a characteristic that may reflect decreased Grb2 binding (Brummer et al., 2006). This suggests that plasma membrane relocalization of p190A requires generation of localized signals in this subcellular compartment by Gab2.
In summary, we have identified a novel mechanism whereby Gab2 regulates cytoskeletal organization and cell motility. The finding that Gab2 regulates RhoA expands our knowledge of the Gab2 signaling network and emphasizes the ability of this docking protein to regulate diverse signaling pathways depending on cellular context. In addition, the identification of Shp2 as a likely target of Gab2 in promotion of cell migration expands the potential benefits of targeting this phosphatase therapeutically.
MATERIALS AND METHODS
Reagents and antibodies
Reconstituted basement membrane (Matrigel) was from Becton Dickinson, North Ryde, NSW, Australia. TRITC-phalloidin, FITC-phalloidin, collagen I, collagen IV, and fibronectin, together with β-actin antibody, were from Sigma, Castle Hill, NSW, Australia. RhoA (67B9), phosphotyrosine (P-Tyr-100), phospho(Thr18/Ser19)-myosin light chain 2 (pMLC2), myosin light chain 2 (MLC2), phospho(Thr202/Tyr204)-Erk, and total Erk antibodies were from Cell Signaling Technology (Danvers, MA). Anti-HA rat antibody was from Roche Diagnostics, Castle Hill, NSW, Australia. Paxillin, Rac1, p190A RhoGAP, E-cadherin (clone 36), and α-, β-, and p120-catenin antibodies were from BD Transduction Laboratories, North Ryde, NSW, Australia. Paxillin, p120RasGAP (171), p120RasGAP (B4F8), Vav2, and p-Vav2 (Tyr172)-R antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA).
Cell culture
Generation of MCF-10A/EcoR cells expressing HA-tagged Gab2, or HA-tagged Gab2 mutants ΔSHP2 and 2xA, has been described previously (Brummer et al., 2006; Brummer et al., 2008). Cultures of MCF-10A cells were maintained in DMEM/nutrient mixture F-12 (Invitrogen, Mulgrave, VIC, Australia) supplemented with 5% (vol/vol) horse serum (Invitrogen), 20 ng/ml human recombinant epidermal growth factor (R&D Systems, Minneapolis, MN), 0.5 μg/ml hydrocortisone (Sigma), 100 ng/ml cholera toxin (Sigma), 10 μg/ml bovine insulin (Sigma), 50 U/ml penicillin G (Invitrogen), and 50 μg/ml streptomycin sulfate (Invitrogen), hereafter referred to as growth medium. Certain experiments required the use of assay medium: DMEM F-12 supplemented with 0.4% (vol/vol) horse serum, 0.5 μg/ml hydrocortisone, 100 ng/ml cholera toxin, 50 U/ml penicillin G, and 50 μg/ml streptomycin sulfate. Tissue culture plates were coated with different extracellular matrices at the following concentrations: collagen IV, 10 μg/ml; fibronectin, 10 μg/ml; collagen I, 10 μg/ml; and Matrigel, 100 μg/ml.
Transfections and retroviral infections
Plasmids encoding GFP-α-actinin (provided by Gregory Downey, National Jewish Medical and Research Center, Denver, CO), myc-tagged RhoA, and myc-tagged RhoA Q63L were transfected for 18 h using the cell line T Nucleofector kit (Amaxa, Mt. Waverly, VIC, Australia) according to the manufacturer’s instructions. To generate RhoA Q63L-expressing MCF-10A cells for tracking, the corresponding cDNA was subcloned into the bicistronic retroviral vector pRetroX-IRES-DsRed Express (Clontech, Mountain View, CA), and the resulting construct transfected into PlatE packaging cells. After 48 h, the viral supernatant was added to MCF-10A/Gab2 cells (Brummer et al., 2006). Positive cells were selected 48 h later by cell sorting on a FACS Vantage (Becton Dickinson), based on expression of DsRed. Sorted cells were plated on collagen IV–coated 12-well plates.
Transfections with siRNAs
p190A RhoGAP siRNAs, designed by Barberis et al. (2005), and a nontargeting control siRNA (siGENOME Non-Targeting siRNA #3) were from Dharmacon, Lafayette, CO. Shp2 siRNAs (Wu et al., 2009) were from Sigma. Cell lines were transfected with 10 nM siRNA using Lipofectamine (Invitrogen) according to the manufacturer’s instructions. Forty-eight hours later, cells were replated onto collagen IV–coated plates.
Immunoblotting, immunoprecipitation, and Rho GTPase activation assays
Cell lysates for Western blotting and immunoprecipitation were prepared in RIPA buffer (Bennett et al., 2008). These procedures were undertaken as described previously (Janes et al., 1994; Brummer et al., 2006). Pull-down assays for activated Rac1 and RhoA utilized GST-PAK CRIB and GST-rhotekin fusion proteins, respectively, and were performed as previously described (Edlund et al., 2002). Western blotting of whole cell lysates for Rac1 and RhoA served as a loading control.
Immunofluorescence microscopy
Cells were grown on collagen IV–coated coverslips and mounted for observation in a sealed chamber (Becton Dickinson) containing normal growth medium. In initial spreading experiments we also used fibronectin-, collagen I–, and Matrigel-coated coverslips. Cells were routinely fixed with 4% paraformaldehyde and blocked for 30 min with 0.1% Triton X-100/1% bovine serum albumin (BSA) in phosphate-buffered saline (PBS). Primary antibodies were diluted at the following concentrations in 1% BSA/PBS: paxillin monoclonal, 1:60 dilution; p190A RhoGAP, 1:100; myc, 1:200; HA, 1:200; and p(Thr18/Ser19)-MLC2, 1:60. Staining was for 1 h. Fluorescence staining of actin filaments was performed with TRITC-phalloidin and FITC-phalloidin (1 μM) for 40 min. Slides were viewed with a Zeiss Axiovert 200M inverted fluorescence microscope. Digital images were processed with AxioVision Rel 4.7 software. Cell area was estimated by measurements on paxillin-stained cells using the same software. Images of cells stained with anti-paxillin antibodies were magnified electronically, and >200 cells were classified visually based on their type of FAs and degree of spreading.
Cell adhesion assay
Cells were harvested and resuspended at a density of 2 × 105/ml. A total of 100 μl of the cell suspension was seeded into individual wells of a 96-well plate that had been previously coated with collagen IV. The plates were then incubated for either 30 min or 1 h at 37°C. After incubation, the supernatant was discarded and the wells were gently washed twice with PBS. The cells that adhered to the wells were incubated with 100 μl of calcein AM dye (Merck, Kilsyth, VIC, Australia) for 1 h at 37°C. The fluorescence of the samples was measured using a fluorescence plate reader at an excitation wavelength of 485 nm and emission wavelength of 520 nm.
Cell migration assay
Cells were harvested and resuspended at a density of 2 × 105/ml in assay medium. A total of 200 μl of the cell suspension was plated in the top chamber of polyethylene terephthalate transwells (24-well insert, pore size of 8 μm; Becton Dickinson). The underside of the membrane was previously coated with collagen IV (10 μg/ml). The bottom chamber contained 0.6 ml of growth medium. The cells were incubated for 18 h at 37°C, and the cells that did not migrate through the pores in the membrane were removed by wiping the membrane with a cotton swab. Cells that passed through the membrane pores were stained with a Diff-Quick cell staining kit (Lab Aids, Castle Hill, NSW, Australia). Cells in six random fields of view at magnification 100× were counted. Five independent experiments were undertaken.
Matrigel invasion assay
A total of 6 × 104 cells in 300 μl of assay medium were added to individual wells of a 24-well, 8-μm-pore-size transwell chamber that had previously been coated with 100 μl of Matrigel (1:5 dilution in serum-free medium). Growth medium (600 μl) was added to the bottom chamber. After 18 h, cells that had migrated through the Matrigel and filter membrane were fixed and stained. Cells in six random fields of view at magnification 100× were counted. Six independent experiments were undertaken.
Determination of cell morphology in Matrigel
This was analyzed by plating cells on top of a deformable gel. We used 3.2 mg/ml Matrigel (dilution 1:2 in 2× growth medium) on chamber slides (Becton Dickinson). After a 6-h incubation, cells were fixed in 4% paraformaldehyde, permeabilized using 0.2% Triton X-100 in PBS for 30 min, stained with TRITC-phalloidin (0.2 mg/ml), and imaged with a Leica SP2 (DM IRE2) confocal microscope.
Live cell microscopy and tracking analysis
Cells on collagen IV–coated chambers were imaged using an Axiovert 200M (Zeiss, Jena, Germany) equipped with an incubator (XL, Pecon, Waltham, MA) maintained at 37°C, using a 10× NA 0.3 EC Plan Neofluar objective. Tracking was performed either on bright-field image series transformed so that each cell was associated with one spot using HCA-Vision (Vallotton et al., 2007; Vallotton and Small, 2009) or on phase contrast image series using ImageJ (http://rsb.info.nih.gov/ij/) and Imaris software (http://bitplane.com).
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council of Australia and Cancer Institute New South Wales.
Abbreviations used:
- FA
focal adhesion
- FAK
focal adhesion kinase
- Gab
Grb2-associated binder
- GAP
GTPase-activating protein
- GEF
guanine nucleotide exchange factor
- MLC
myosin light chain
- PI3-kinase
phosphatidylinositol 3-kinase
- SH
src homology.
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-03-0185) on November 30, 2010.
REFERENCES
- Abe K, Rossman KL, Liu B, Ritola KD, Chiang D, Campbell SL, Burridge K, Der CJ. Vav2 is an activator of Cdc42, Rac1, and RhoA. J Biol Chem. 2000;275:10141–10149. doi: 10.1074/jbc.275.14.10141. [DOI] [PubMed] [Google Scholar]
- Arthur WT, Burridge K. RhoA inactivation by p190RhoGAP regulates cell spreading and migration by promoting membrane protrusion and polarity. Mol Biol Cell. 2001;12:2711–2720. doi: 10.1091/mbc.12.9.2711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barberis D, Casazza A, Sordella R, Corso S, Artigiani S, Settleman J, Comoglio PM, Tamagnone L. p190 Rho-GTPase activating protein associates with plexins and it is required for semaphorin signalling. J Cell Sci. 2005;118:4689–4700. doi: 10.1242/jcs.02590. [DOI] [PubMed] [Google Scholar]
- Bennett HL, Brummer T, Jeanes A, Yap AS, Daly RJ. Gab2 and Src co-operate in human mammary epithelial cells to promote growth factor independence and disruption of acinar morphogenesis. Oncogene. 2008;27:2693–2704. doi: 10.1038/sj.onc.1210928. [DOI] [PubMed] [Google Scholar]
- Bentires-Alj M, Gil SG, Chan R, Wang ZC, Wang Y, Imanaka N, Harris LN, Richardson A, Neel BG, Gu H. A role for the scaffolding adapter GAB2 in breast cancer. Nature Med. 2006;12:114–121. doi: 10.1038/nm1341. [DOI] [PubMed] [Google Scholar]
- Bocanegra M, et al. Focal amplification and oncogene dependency of GAB2 in breast cancer. Oncogene. 2010;29:774–779. doi: 10.1038/onc.2009.364. [DOI] [PubMed] [Google Scholar]
- Bos JL, Rehmann H, Wittinghofer A. GEFs and GAPs: critical elements in the control of small G proteins. Cell. 2007;129:865–877. doi: 10.1016/j.cell.2007.05.018. [DOI] [PubMed] [Google Scholar]
- Bradley WD, Hernandez SE, Settleman J, Koleske AJ. Integrin signaling through Arg activates p190RhoGAP by promoting its binding to p120RasGAP and recruitment to the membrane. Mol Biol Cell. 2006;17:4827–4836. doi: 10.1091/mbc.E06-02-0132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer T, et al. Phosphorylation-dependent binding of 14-3-3 terminates signalling by the Gab2 docking protein. EMBO J. 2008;27:2305–2316. doi: 10.1038/emboj.2008.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brummer T, Schramek D, Hayes VM, Bennett HL, Caldon CE, Musgrove EA, Daly RJ. Increased proliferation and altered growth factor dependence of human mammary epithelial cells overexpressing the Gab2 docking protein. J Biol Chem. 2006;281:626–637. doi: 10.1074/jbc.M509567200. [DOI] [PubMed] [Google Scholar]
- Chang Y, Ceacareanu B, Dixit M, Sreejayan N, Hassid A. Nitric oxide-induced motility in aortic smooth muscle cells: role of protein tyrosine phosphatase SHP-2 and GTP-binding protein Rho. Circulation Res. 2002;91:390–397. doi: 10.1161/01.res.0000033524.92083.64. [DOI] [PubMed] [Google Scholar]
- Chernoff KA, et al. GAB2 amplifications refine molecular classification of melanoma. Clin Cancer Res. 2009;15:4288–4291. doi: 10.1158/1078-0432.CCR-09-0280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Daly RJ, Gu H, Parmar J, Malaney S, Lyons RJ, Kairouz R, Head DR, Henshall SM, Neel BG, Sutherland RL. The docking protein Gab2 is overexpressed and estrogen regulated in human breast cancer. Oncogene. 2002;21:5175–5181. doi: 10.1038/sj.onc.1205522. [DOI] [PubMed] [Google Scholar]
- Defilippi P, Di Stefano P, Cabodi S. p130Cas: a versatile scaffold in signaling networks. Trends Cell Biol. 2006;16:257–263. doi: 10.1016/j.tcb.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Duan L, et al. Distinct roles for Rho versus Rac/Cdc42 GTPases downstream of Vav2 in regulating mammary epithelial acinar architecture. J Biol Chem. 2010;285:1555–1568. doi: 10.1074/jbc.M109.057976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edlund S, Landstrom M, Heldin CH, Aspenstrom P. Transforming growth factor-β-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13:902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellenbroek SI, Collard JG. Rho GTPases: functions and association with cancer. Clin Exp Metastasis. 2007;24:657–672. doi: 10.1007/s10585-007-9119-1. [DOI] [PubMed] [Google Scholar]
- Fincham VJ, Chudleigh A, Frame MC. Regulation of p190 Rho-GAP by v-Src is linked to cytoskeletal disruption during transformation. J Cell Sci. 1999;112((Pt 6),):947–956. doi: 10.1242/jcs.112.6.947. [DOI] [PubMed] [Google Scholar]
- Heasman SJ, Ridley AJ. Mammalian Rho GTPases: new insights into their functions from in vivo studies. Nature Rev Mol Cell Biol. 2008;9:690–701. doi: 10.1038/nrm2476. [DOI] [PubMed] [Google Scholar]
- Herrera Abreu MT, Wang Q, Vachon E, Suzuki T, Chow CW, Wang Y, Hong O, Villar J, McCulloch CA, Downey GP. Tyrosine phosphatase SHP-2 regulates IL-1 signaling in fibroblasts through focal adhesions. J Cell Physiol. 2006;207:132–143. doi: 10.1002/jcp.20544. [DOI] [PubMed] [Google Scholar]
- Horst B, et al. Gab2-mediated signaling promotes melanoma metastasis. Am J Pathol. 2009;174:1524–1533. doi: 10.2353/ajpath.2009.080543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki K, Noguchi T, Matozaki T, Horikawa T, Fukunaga K, Tsuda M, Ichihashi M, Kasuga M. Roles for the protein tyrosine phosphatase SHP-2 in cytoskeletal organization, cell adhesion and cell migration revealed by overexpression of a dominant negative mutant. Oncogene. 2000;19:75–84. doi: 10.1038/sj.onc.1203204. [DOI] [PubMed] [Google Scholar]
- Janes PW, Daly RJ, deFazio A, Sutherland RL. Activation of the Ras signalling pathway in human breast cancer cells overexpressing erbB-2. Oncogene. 1994;9:3601–3608. [PubMed] [Google Scholar]
- Kallin A, Demoulin JB, Nishida K, Hirano T, Ronnstrand L, Heldin CH. Gab1 contributes to cytoskeletal reorganization and chemotaxis in response to platelet-derived growth factor. J Biol Chem. 2004;279:17897–17904. doi: 10.1074/jbc.M312996200. [DOI] [PubMed] [Google Scholar]
- Ke Y, Wu D, Princen F, Nguyen T, Pang Y, Lesperance J, Muller WJ, Oshima RG, Feng GS. Role of Gab2 in mammary tumorigenesis and metastasis. Oncogene. 2007;26:4951–4960. doi: 10.1038/sj.onc.1210315. [DOI] [PubMed] [Google Scholar]
- Kodama A, Matozaki T, Fukuhara A, Kikyo M, Ichihashi M, Takai Y. Involvement of an SHP-2-Rho small G protein pathway in hepatocyte growth factor/scatter factor-induced cell scattering. Mol Biol Cell. 2000;11:2565–2575. doi: 10.1091/mbc.11.8.2565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kolsch V, Charest PG, Firtel RA. The regulation of cell motility and chemotaxis by phospholipid signaling. J Cell Sci. 2008;121:551–559. doi: 10.1242/jcs.023333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee SH, Jeong EG, Nam SW, Lee JY, Yoo NJ, Lee SH. Increased expression of Gab2, a scaffolding adaptor of the tyrosine kinase signalling, in gastric carcinomas. Pathology. 2007;39:326–329. doi: 10.1080/00313020701329773. [DOI] [PubMed] [Google Scholar]
- Lim Y, et al. PyK2 and FAK connections to p190Rho guanine nucleotide exchange factor regulate RhoA activity, focal adhesion formation, and cell motility. J Cell Biol. 2008;180:187–203. doi: 10.1083/jcb.200708194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch DK, Daly RJ. PKB-mediated negative feedback tightly regulates mitogenic signalling via Gab2. EMBO J. 2002;21:72–82. doi: 10.1093/emboj/21.1.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroun CR, Naujokas MA, Holgado-Madruga M, Wong AJ, Park M. The tyrosine phosphatase SHP-2 is required for sustained activation of extracellular signal-regulated kinase and epithelial morphogenesis downstream from the met receptor tyrosine kinase. Mol Cell Biol. 2000;20:8513–8525. doi: 10.1128/mcb.20.22.8513-8525.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meng S, Chen Z, Munoz-Antonia T, Wu J. Participation of both Gab1 and Gab2 in the activation of the ERK/MAPK pathway by epidermal growth factor. Biochem J. 2005;391:143–151. doi: 10.1042/BJ20050229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oh ES, Gu H, Saxton TM, Timms JF, Hausdorff S, Frevert EU, Kahn BB, Pawson T, Neel BG, Thomas SM. Regulation of early events in integrin signaling by protein tyrosine phosphatase SHP-2. Mol Cell Biol. 1999;19:3205–3215. doi: 10.1128/mcb.19.4.3205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omelchenko T, Vasiliev JM, Gelfand IM, Feder HH, Bonder EM. Rho-dependent formation of epithelial “leader” cells during wound healing. Proc Natl Acad Sci USA. 2003;100:10788–10793. doi: 10.1073/pnas.1834401100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullikuth AK, Catling AD. Extracellular signal-regulated kinase promotes Rho-dependent focal adhesion formation by suppressing p190A RhoGAP. Mol Cell Biol. 2010;30:3233–3248. doi: 10.1128/MCB.01178-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Samayawardhena LA, Pallen CJ. Protein-tyrosine phosphatase α regulates stem cell factor-dependent c-Kit activation and migration of mast cells. J Biol Chem. 2008;283:29175–29185. doi: 10.1074/jbc.M804077200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanz-Moreno V, Gadea G, Ahn J, Paterson H, Marra P, Pinner S, Sahai E, Marshall CJ. Rac activation and inactivation control plasticity of tumor cell movement. Cell. 2008;135:510–523. doi: 10.1016/j.cell.2008.09.043. [DOI] [PubMed] [Google Scholar]
- Sattler M, et al. Critical role for Gab2 in transformation by BCR/ABL. Cancer Cell. 2002;1:479–492. doi: 10.1016/s1535-6108(02)00074-0. [DOI] [PubMed] [Google Scholar]
- Schaeper U, Gehring NH, Fuchs KP, Sachs M, Kempkes B, Birchmeier W. Coupling of Gab1 to c-Met, Grb2, and Shp2 mediates biological responses. J Cell Biol. 2000;149:1419–1432. doi: 10.1083/jcb.149.7.1419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schoenwaelder SM, Petch LA, Williamson D, Shen R, Feng GS, Burridge K. The protein tyrosine phosphatase Shp-2 regulates RhoA activity. Curr Biol. 2000;10:1523–1526. doi: 10.1016/s0960-9822(00)00831-9. [DOI] [PubMed] [Google Scholar]
- Simpson KJ, Dugan AS, Mercurio AM. Functional analysis of the contribution of RhoA and RhoC GTPases to invasive breast carcinoma. Cancer Res. 2004;64:8694–8701. doi: 10.1158/0008-5472.CAN-04-2247. [DOI] [PubMed] [Google Scholar]
- Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nature Cell Biol. 2008;10:1027–1038. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- Tomar A, Lim ST, Lim Y, Schlaepfer DD. A FAK-p120RasGAP-p190RhoGAP complex regulates polarity in migrating cells. J Cell Sci. 2009;122:1852–1862. doi: 10.1242/jcs.046870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallotton P, Lagerstrom R, Sun C, Buckley M, Wang D, De Silva M, Tan SS, Gunnersen JM. Automated analysis of neurite branching in cultured cortical neurons using HCA-Vision. Cytometry A. 2007;71:889–895. doi: 10.1002/cyto.a.20462. [DOI] [PubMed] [Google Scholar]
- Vallotton P, Small JV. Shifting views on the leading role of the lamellipodium in cell migration: speckle tracking revisited. J Cell Sci. 2009;122:1955–1958. doi: 10.1242/jcs.042036. [DOI] [PubMed] [Google Scholar]
- Vila de Muga S, et al. Annexin A6 inhibits Ras signalling in breast cancer cells. Oncogene. 2009;28:363–377. doi: 10.1038/onc.2008.386. [DOI] [PubMed] [Google Scholar]
- von Wichert G, Haimovich B, Feng GS, Sheetz MP. Force-dependent integrin-cytoskeleton linkage formation requires downregulation of focal complex dynamics by Shp2. EMBO J. 2003;22:5023–5035. doi: 10.1093/emboj/cdg492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakino S, Hayashi K, Kanda T, Tatematsu S, Homma K, Yoshioka K, Takamatsu I, Saruta T. Peroxisome proliferator-activated receptor γ ligands inhibit Rho/Rho kinase pathway by inducing protein tyrosine phosphatase SHP-2. Circ Res. 2004;95:e45–55. doi: 10.1161/01.RES.0000142313.68389.92. [DOI] [PubMed] [Google Scholar]
- Wohrle FU, Daly RJ, Brummer T. Function, regulation and pathological roles of the Gab/DOS docking proteins. Cell Commun Signal. 2009;7:22. doi: 10.1186/1478-811X-7-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu D, Pang Y, Ke Y, Yu J, He Z, Tautz L, Mustelin T, Ding S, Huang Z, Feng GS. A conserved mechanism for control of human and mouse embryonic stem cell pluripotency and differentiation by Shp2 tyrosine phosphatase. PLoS One. 2009;4:e4914. doi: 10.1371/journal.pone.0004914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamada S, Nelson WJ. Localized zones of Rho and Rac activities drive initiation and expansion of epithelial cell-cell adhesion. J Cell Biol. 2007;178:517–527. doi: 10.1083/jcb.200701058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu DH, Qu CK, Henegariu O, Lu X, Feng GS. Protein-tyrosine phosphatase Shp-2 regulates cell spreading, migration, and focal adhesion. J Biol Chem. 1998;273:21125–21131. doi: 10.1074/jbc.273.33.21125. [DOI] [PubMed] [Google Scholar]
- Yu M, Luo J, Yang W, Wang Y, Mizuki M, Kanakura Y, Besmer P, Neel BG, Gu H. The scaffolding adapter Gab2, via Shp-2, regulates kit-evoked mast cell proliferation by activating the Rac/JNK pathway. J Biol Chem. 2006;281:28615–28626. doi: 10.1074/jbc.M603742200. [DOI] [PubMed] [Google Scholar]
- Yu WM, Hawley TS, Hawley RG, Qu CK. Role of the docking protein Gab2 in beta(1)-integrin signaling pathway-mediated hematopoietic cell adhesion and migration. Blood. 2002;99:2351–2359. doi: 10.1182/blood.v99.7.2351. [DOI] [PubMed] [Google Scholar]
- Zatkova A, Schoch C, Speleman F, Poppe B, Mannhalter C, Fonatsch C, Wimmer K. GAB2 is a novel target of 11q amplification in AML/MDS. Genes Chromosomes Cancer. 2006;45:798–807. doi: 10.1002/gcc.20344. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.