TRAF2 regulates JNK and IKK activation in response to TNF-α stimulation. This study found that TNF-α and oxidative stress induce TRAF2 phosphorylation and that this phosphorylation inhibits apoptosis by promoting the prolonged phase of IKK activation while inhibiting the prolonged phase of JNK activation.
Abstract
Tumor necrosis factor α (TNF-α) receptor–associated factor 2 (TRAF2) regulates activation of the c-Jun N-terminal kinase (JNK)/c-Jun and the inhibitor of κB kinase (IKK)/nuclear factor κB (NF-κB) signaling cascades in response to TNF-α stimulation. Gene knockout studies have revealed that TRAF2 inhibits TNF-α–induced cell death but promotes oxidative stress–induced apoptosis. Here we report that TNF-α and oxidative stress both induce TRAF2 phosphorylation at serines 11 and 55 and that this dual phosphorylation promotes the prolonged phase of IKK activation while inhibiting the prolonged phase of JNK activation. Prolonged IKK activation trigged by TNF-α plays an essential role in efficient expression of a subset of NF-κB target genes but has no substantial role in TNF-α–induced cell death. On the other hand, TRAF2 phosphorylation in response to oxidative stress significantly promotes cell survival by inducing prolonged IKK activation and by inhibiting the prolonged phase of JNK activation. Notably, stable expression of phospho-null mutant TRAF2 in cancer cells leads to an increase in the basal and inducible JNK activation and B-cell lymphoma 2 (Bcl-2) phosphorylation. In addition, exposure of cells expressing phospho-null mutant TRAF2 to sublethal oxidative stress results in a rapid degradation of Bcl-2 and cellular inhibitor of apoptosis 1 as well as significantly increased cell death. These results suggest that TRAF2 phosphorylation is essential for cell survival under conditions of oxidative stress.
INTRODUCTION
The tumor necrosis factor receptor (TNFR)–associated factor (TRAF) family of proteins consists of six members that are characterized by a highly homologous TRAF domain at the protein C-terminus. With the exception of TRAF1, the TRAFs contain an N-terminal RING domain followed by several zinc-finger motifs (Bradley and Pober, 2001; Wajant et al., 2001). These TRAFs transduce signals that emanate from most members of the TNFR superfamily and the interleukin-1 receptor/Toll-like receptor superfamily, resulting in activation of the c-Jun N-terminal kinase (JNK) and the inhibitor of κB (IκB) kinase (IKK). JNK and IKK activate the AP-1 (e.g., c-Jun/ATF2) and nuclear factor κB (NF-κB) transcription factors, respectively, which in turn induce the expression of genes involved in inflammation, the immune response, and cell proliferation as well as genes that modulate death receptor– and stress-induced apoptosis (Davis, 2000; Bradley and Pober, 2001; Bonizzi and Karin, 2004).
TNFR family members activate NF-κB through canonical and noncanonical pathways. TNFR-associated factor 2 (TRAF2) is a prototypical member of the TRAF family that contributes to activation of both NF-κB pathways (Bonizzi and Karin, 2004; Hayden and Ghosh, 2008). Although the mechanism by which IKK activates NF-κB is well established, the signaling mechanisms that underlie TRAF2-mediated IKK activation are not yet fully understood. The current belief is that, in the canonical pathway, the RING domains of TRAF2 and/or TRAF5 catalyze K63-linked polyubiquitination of receptor-interacting protein 1 (RIP1) in response to TNF-α stimulation and that this noncanonical polyubiquitin chain recruits both the TGF-β–activated kinase 1 (TAK1) complex (consisting of TAK1, TAB2, and TAB3) and the IKK complex (consisting of IKKα, β, and γ), by binding directly to the ubiquitin-binding domains present on TAB2 and IKKγ, respectively. Once hooked by polyubiquitinated RIP1, TAK1 directly activates IKKβ through proximity-mediated phosphorylation (Chen, 2005; Ea et al., 2006; Wu et al., 2006). In the case of the noncanonical NF-κB pathway, recent studies revealed that its activation requires the accumulation of NF-κB–inducing kinase (NIK), which in unstimulated cells is constitutively targeted for ubiquitination-dependent degradation by TRAF2 and TRAF3 (Gardam et al., 2008; Vallabhapurapu et al., 2008). In either case, the RING domains of TRAF2, TRAF3, and TRAF5 seem to function as E3 ubiquitin ligases that regulate the basal and inducible activation of both the canonical and noncanonical NF-κB pathways.
Numerous studies have clearly demonstrated that the NF-κB pathway protects cells from TNF-α– and stress-induced apoptosis (Karin et al., 2002; Orlowski and Baldwin, 2002; Aggarwal, 2004; Greten et al., 2004). On the other hand, whereas transient JNK activation is preferentially involved in gene regulation, prolonged JNK activation leads to increased production of cytotoxic reactive oxygen species (ROS) and culminates in cell death, by both necrotic and apoptotic pathways (Davis, 2000; Ventura et al., 2006). Recent findings from several independent laboratories have demonstrated that one of the antiapoptotic functions of NF-κB is to suppress the prolonged phase of JNK activation, and also the production of ROS, by promoting the expression of X-chromosome–linked inhibitor of apoptosis (XIAP), Gadd45β, cellular FLICE inhibitory protein (cFLIP), and Mn-SOD (De Smaele et al., 2001; Tang et al., 2001; Sakon et al., 2003). Gene knockout studies have revealed that TRAF2-null cells exhibit normal NF-κB–dependent gene expression in response to TNF-α stimulation, but that they are nevertheless sensitive to TNF-α–induced cell death, due to impaired recruitment of cellular inhibitor of apoptosis 1 and 2 (cIAP1/2) to the TNFR1 complex (Yeh et al., 1997; Zhang et al., 2009). In contrast to their sensitivity to TNF-α, TRAF2-deficient cells display significant resistance to oxidative stress–induced cell death (Shen et al., 2004). However, the mechanism by which TRAF2 promotes oxidative stress–induced cell death is unknown.
In previous studies, we have shown that TNF-α induces TRAF2 phosphorylation at serines 11 and 55 and that mutation of either site to alanine leads to a decrease in the prolonged phase of IKK activity and NF-κB–dependent gene expression (Blackwell et al., 2009; Thomas et al., 2009). Here, we report that mutation of both Ser-11 and Ser-55 to alanine blunts the prolonged phase of IKK activation and promotes that of JNK, and it sensitizes cells to oxidative stress–induced cell death. This suggests that dual TRAF2 phosphorylation represents a new layer of the TRAF2 pathway that promotes cell survival under conditions of oxidative stress.
RESULTS
TRAF2 phosphorylation increases basal and inducible NF-κB activity
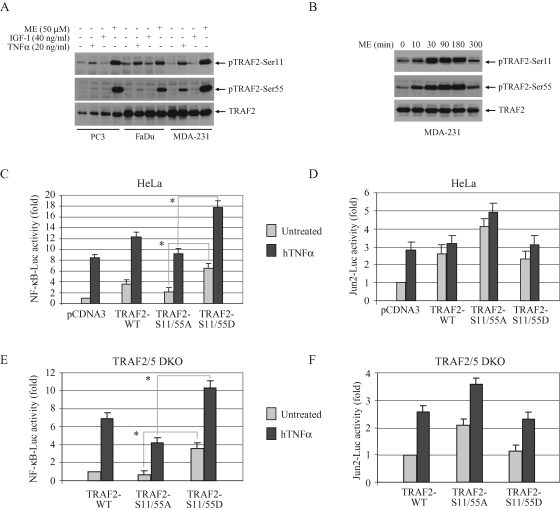
In previous studies, we reported the identification of two phosphorylation sites (Ser-11 and Ser-55) in the N-terminal region of TRAF2 by classical phosphopeptide mapping approaches and showed that mutation of either site to alanine leads to reduction, but not complete inhibition, of TNF-α–induced NF-κB activation (Blackwell et al., 2009; Thomas et al., 2009). These phosphorylation sites are conserved between mouse and human, and the Ser-55 residue is located in the middle of the TRAF2 RING domain (Supplemental Figure 1A). In addition to TNF-α, menadione (ME; an oxidative stress–inducing agent) and UV strongly induced TRAF2 phosphorylation at both sites in all cell types tested (Figure 1A and Supplemental Figure 1B). We also have reported previously that TRAF2 phosphorylation peaks within 30 min after TNF-α stimulation and declines thereafter (Blackwell et al., 2009; Thomas et al., 2009). Interestingly, ME-induced TRAF2 phosphorylation also peaked at the 30-min time point but remained high until 3 h after treatment, suggesting that ME induces strong and prolonged TRAF2 phosphorylation as opposed to the transient TRAF2 phosphorylation induced by TNF-α (Figure 1B). We speculated that mutation of both sites to alanine may completely eliminate TNF-α–induced NF-κB activation.
FIGURE 1:
TRAF2 phosphorylation increases basal and inducible NF-κB activation. (A) TRAF2 phosphorylation in cancer cell lines. PC3, FaDu, and MDA-MB-231 cells were either left untreated or treated with TNF-α, IGF-I, or ME for 30 min as indicated, and TRAF2 phosphorylation was then monitored by Western blotting, using phospho-specific antibodies (pTRAF2-Ser11 and pTRAF2-Ser55). (B) MDA-231 cells were either left untreated or treated with ME (50 μM) and harvested at various time points as indicated, and TRAF2 phosphorylation was then monitored by Western blotting. (C–F) NF-κB and c-Jun activity assay. HeLa cells and TRAF2/5 DKO MEFs were cotransfected with NF-κB-Luc (C and E) or Jun2-Luc (D and F), plus pRL-TK and pCDNA3, TRAF2-WT, ‑S11/55A, or ‑S11/55D as indicated. At 36 h after transfection, the cells were either mock treated or treated with hTNF-α (in the case of HeLa cells; 20 ng/ml for 6 h) or mTNF-α (in the case of DKO MEFs; 10 ng/ml for 4 h), after which the NF-κB-Luc or Jun2-Luc activity was measured and normalized to pRL-TK activity. Data shown are the mean ± SD of three experiments that were done in triplicate. *p < 0.05.
To test this hypothesis, we generated two phosphomutant TRAF2 plasmids: TRAF2-S11/55A, in which both Ser-11 and Ser-55 were mutated to alanine to abolish TRAF2 phosphorylation; and TRAF2-S11/55D, in which both Ser-11 and Ser-55 were mutated to aspartic acid to constitutively mimic phosphorylation. In luciferase reporter gene assays performed in HeLa cells, the expression of TRAF2-S11/55A reduced NF-κB activity by 30–35%, but increased c-Jun activity by 25–30%, compared with the levels measured in cells transfected with TRAF2-WT (Figure 1, C and D). Notably, expression of TRAF2-S11/55D significantly increased both the basal and inducible NF-κB activities compared with those measured in TRAF2-S11/55A–transfected HeLa cells (Figure 1C). To examine the role of TRAF2 phosphorylation in the absence of interference from endogenous TRAF2 and TRAF5, we performed luciferase reporter gene assays in TRAF2 and TRAF5 double-knockout (TRAF2/5 DKO) mouse embryonic fibroblasts (MEFs). Consistent with the results from HeLa cells, expression of TRAF2-S11/55A in TRAF2/5 DKO cells partially decreased NF-κB but increased c-Jun activities relative to the levels in cells transfected with TRAF2-WT, whereas expression of TRAF2-S11/55D significantly increased both the basal and inducible NF-κB activities compared with those measured in TRAF2-S11/55A–transfected cells (Figure 1, E and F). However, none of the constructs tested exhibited a dominant-negative effect to block TNF-α–induced c-Jun or NF-κB activation. Western blot analysis of the lysates used for these luciferase assays revealed that plasmids encoding WT and phosphomutant TRAF2 expressed TRAF2 proteins at similar levels in both HeLa and MEFs (Supplemental Figure 2, A and B). These findings suggest that TRAF2 phosphorylation to some extend regulates, but is not essential for, TNF-α–induced activation of NF-κB and c-Jun.
TRAF2 phosphorylation is essential for cell survival in the context of puromycin-triggered oxidative stress
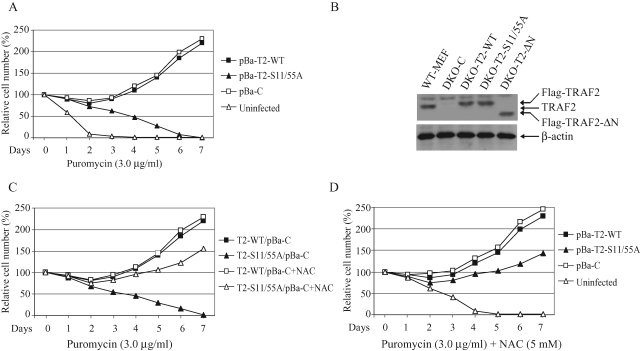
To examine NF-κB and c-Jun activation in cells that stably express TRAF2-S11/55A at a physiological level, we generated the retroviral vectors pBa-T2-WT and ‑S11/55A by subcloning TRAF2-WT and ‑S11/55A cDNAs into pBabe-puro. However, our attempts to establish TRAF2/5 DKO cells that stably express TRAF2-S11/55A failed repeatedly; all cells transduced with pBa-T2-S11/55A died within a week of puromycin selection (Figure 2A). The possibility that a deleterious mutation had arisen in the puromycin-resistance gene was ruled out by DNA sequencing and RT-PCR analysis (unpublished data). Interestingly, cells transduced with pBa-T2-S11/55A died slowly compared with cells that were not transduced with any vector. In addition, cells transduced with pBabe-puro empty vector (pBa-C) or the N-terminal RING domain–deleted TRAF2 (TRAF2-ΔN) survived puromycin selection (unpublished data). This suggests that TRAF2-S11/55A expression sensitizes cells to stress-induced apoptosis by promoting a certain proapoptotic signal. Notably, the aminonucleoside portion of puromycin was known to induce oxidative stress and cell death independent of its inhibition of protein synthesis (Gwinner et al., 1997; Liu et al., 2002).
FIGURE 2:
TRAF2 phosphorylation is essential for cell survival under the condition of puromycin selection. (A) Survival of transfected TRAF2/5 DKO cells under puromycin selection. TRAF2/5 DKO cells (cultured in 100-mm plates) were left uninfected or infected overnight with retroviral supernatants of empty pBabe-puro vector (pBa-C), pBa-TRAF2-WT, or ‑S11/55A. At 48 h after infection, each plate was divided among eight 60-mm dishes and was subjected to puromycin (3.0 μg/ml) selection for 7 d. Every other day, the medium was replaced with fresh medium containing puromycin, and on each day, live cells from individual plates were counted by trypan blue exclusion assay. (B) TRAF2/5 DKO MEFs were introduced with Flag-TRAF2-WT, ‑S11/55A, or ‑ΔN via hygromycin-selectable retroviral vectors (DKO-T2-WT, ‑S11/55A, or ‑ΔN), and expression of TRAF2 was then monitored by Western blotting using anti-TRAF2 antibody. (C) DKO-T2-WT and ‑S11/55A cells were infected overnight with a retroviral supernatant of pBa-C. At 48 h after infection, cells were selected with puromycin in the absence or presence of NAC, and live cells were counted as in (A). (D) TRAF2/5 DKO cells were left uninfected or infected overnight with retroviral supernatant of pBa-C, pBa-TRAF2-WT, or ‑S11/55A. At 48 h after infection, cells were selected with puromycin in the presence of NAC, and live cells were counted as in (A).
In light of this discovery, we subcloned TRAF2-WT, ‑S11/55A, and ‑ΔN into the hygromycin-selectable retroviral vector pQCXIH and transduced TRAF2/5 DKO cells with these new constructs. Over 80% of the cells transduced with vector pQCXIH-TRAF2-WT (DKO-T2-WT), ‑S11/55A (DKO-T2-S11/55A), or ‑ΔN (DKO-T2-ΔN) survived a week of hygromycin selection and expressed TRAF2-WT, ‑S11/55A, and ‑ΔN (Figure 2B). Next, we infected DKO-T2-WT and ‑S11/55A cells with the same retroviral supernatant of pBa-C (T2-WT/pBa-C and T2-S11/55A/pBa-C) and selected them with puromycin. As expected, all T2-S11/55A/pBa-C cells died within a week of puromycin selection, whereas over 80% of T2-WT/pBa-C cells survived (Figure 2C). These findings support the notion that TRAF2-S11/55A expression indeed sensitizes cells to apoptosis induced by puromycin, which is most likely mediated by low levels of oxidative stress triggered by puromycin. To confirm the involvement of ROS in puromycin-induced cell death in TRAF2-S11/55A–expressing cells, we treated T2-WT/pBa-C and T2-S11/55A/pBa-C cells with puromycin in the presence of the antioxidant N-acetyl cysteine (NAC). As shown in Figure 2C, many T2-S11/55A/pBa-C cells survived puromycin selection in the presence of NAC. In light of these findings, we transduced TRAF2/5 DKO cells with pBa-T2-WT and ‑S11/55A again and treated them with puromycin in the presence of NAC. Indeed, many TRAF2/5 DKO cells transduced with pBa-T2-S11/55A survived puromycin selection in the presence of NAC (Figure 2D). Notably, although TRAF2-S11/55A–expressing cells survived puromycin selection in the presence of NAC, they grew slowly compared with TRAF2-WT–expressing cells, indicating that TRAF2-S11/55A may also cause other types of cellular stress in addition to oxidative stress. Collectively, these data suggest that TRAF2 phosphorylation at Ser-11 and Ser-55 plays a critical role in cell survival in the context of oxidative stress.
TRAF2 phosphorylation is required for efficient expression of a subset of NF-κB target genes
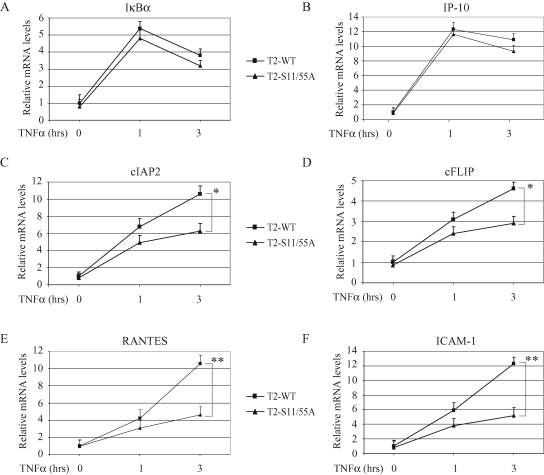
To assess the role of TRAF2 phosphorylation in TNF-α–induced NF-κB activation, we analyzed the expression of several NF-κB target genes in DKO-T2-WT and ‑S11/55A cell lines, by real-time RT-PCR. As shown in Figure 3, A–F, although the expression levels of IκBα and IP-10 did not differ substantially between these two cell lines, those of cIAP2, cFLIP, RANTES, and ICAM-I were significantly dampened in DKO-T2-S11/55A cells versus DKO-T2-WT cells. Notably, the differences in RANTES and ICAM-I expression levels between DKO-T2-WT and ‑S11/55A cells were more significant at the 3-h time point. These data indicate that TRAF2 phosphorylation is essential for efficient expression of certain NF-κB target genes.
FIGURE 3:
TRAF2 phosphorylation is essential for efficient TNF-α–induced expression of a subset of NF-κB target genes. (A–F) DKO-T2-WT and DKO-T2-S11/55A cells were left untreated or treated with mTNF-α (10 ng/ml) as indicated, and the expression levels of IκBα, IP-10, cIAP2, cFLIP, RANTES, and ICAM-I were determined by real-time RT-PCR. The relative expression level of each gene is presented as the ratio between it and the reference gene GAPDH, as an average from four independent experiments. *p < 0.05; **p < 0.01.
TRAF2 phosphorylation has opposite effects on the prolonged phase of IKK and JNK activation
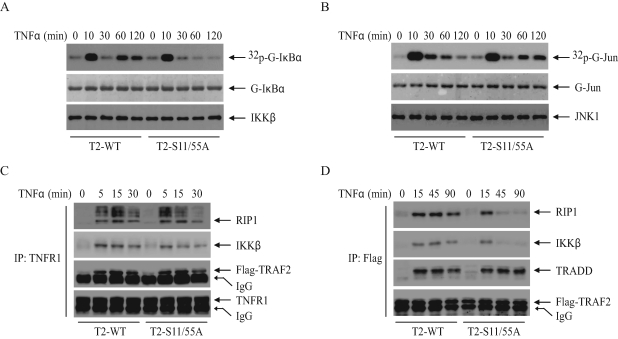
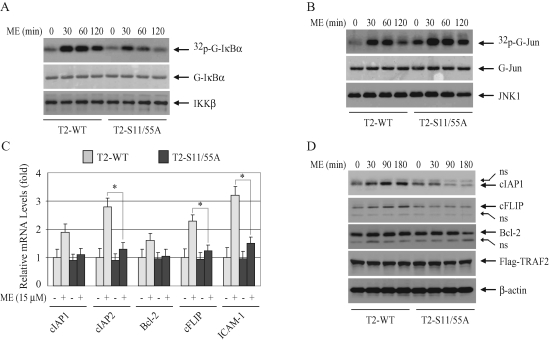
To determine the mechanism by which TRAF2 phosphorylation regulates NF-κB and c-Jun activities, we examined TNF-α–induced IKK and JNK activation by immunokinase assays. As shown in Figure 4A, in DKO-T2-WT cells TNF-α induced both immediate/transient and secondary/prolonged IKK activation, whereas in DKO-T2-S11/55A cells the TNF-α–induced transient IKK activation was normal, but the prolonged phase was completely inhibited. In the case of JNK activation, in contrast, prolonged JNK activation in response to TNF-α was enhanced in DKO-T2-S11/55A cells compared with that in DKO-T2-WT cells; TNF-α, as in the case of IKK activation, had no effect on transient JNK activation (Figure 4B). In vitro IKK and JNK kinase assays were repeated three times, and the average kinase activities are summarized in Supplemental Figure 3, A and B. Collectively, these data suggest that TRAF2 phosphorylation positively regulates the prolonged phase of IKK activation, while inhibiting that of JNK activation. These results explain why the expression of TRAF2-S11/55A leads to partial inhibition of NF-κB activity in response to TNF-α stimulation.
FIGURE 4:
TRAF2 phosphorylation regulates the prolonged phases of IKK and JNK activation in opposite directions. (A and B) DKO-T2-WT and ‑S11/55A cells were treated with mTNF-α (10 ng/ml) for the indicated times. The IKK complex or JNK1 was immunoprecipitated with anti-IKKγ or anti-JNK1 antibody, respectively, and subjected to in vitro kinase assays in which GST-IκBα1–55 served as the substrate for IKK and GST-jun1–87 served as the substrate for JNK1. Reaction mixtures were separated by SDS–PAGE, transferred onto a nitrocellulose membrane, and exposed to x-ray film for 6 h (32p-G-IκBα or 32p-G-jun). The same membrane was stained with Ponceau S (G-IκBα or G-jun) and then immunoblotted with anti-IKKβ or anti-JNK1 antibody (IKKβ or JNK1). (C) DKO-T2-WT and ‑S11/55A cells were treated with or without mTNF-α (10 ng/ml) as indicated, and the TNFR1 complexes were immunoprecipitated with anti-TNFR1 antibody. The recruitment of Flag-TRAF2, RIP1, and IKKβ to the receptor complex was monitored by Western blotting with the corresponding antibodies. (D) DKO-T2-WT and ‑S11/55A cells were treated with or without mTNF-α (10 ng/ml) as indicated. Flag-TRAF2 was then immunoprecipitated with anti-Flag antibody, and the recruitment of TRADD, RIP1, and IKK to TRAF2 was monitored by Western blotting with the corresponding antibodies.
TRAF2 phosphorylation is essential for retaining IKK in cytoplasmic complex II
TRAF2-mediated RIP1 ubiquitination is currently thought to play an essential role in TNF-α–induced IKK activation (Chen, 2005). To examine the role of TRAF2 phosphorylation in TNF-α–induced RIP1 ubiquitination, we analyzed the ubiquitination of RIP1 in DKO-T2-WT and ‑S11/55A cells by immunoprecipitating the TNFR1 complex followed by immunoblotting with an anti-RIP1 antibody. However, we did not observe any difference in RIP1 ubiquitination between DKO-T2-WT and ‑S11/55A cells (Figure 4C). In addition, both RIP1 and IKK were equally recruited to TNFR1 after TNF-α stimulation in these cells. On the other hand, when TRAF2 was immunoprecipitated, we observed that RIP1 and IKK remained associated with TRAF2-WT until 90 min after TNF-α stimulation, whereas RIP1 and IKK associated with TRAF2-S11/55A at early time points (5 min) but dissociated from TRAF2-S11/55A at later time points (Figure 4D). Notably, TNF-α–induced association of TRAF2 with TNFR type 1–associated death domain (TRADD) protein was comparable between DKO-T2-WT and ‑S11/55A cells. The complexes that were pulled down by TRAF2-WT at later time points correspond to cytoplasmic complex II (Micheau and Tschopp, 2003), and formation of this complex correlates kinetically well with the second phase of IKK activation. Collectively, these data suggest that TRAF2 phosphorylation is not essential for TNF-α–induced recruitment of TRAF2, RIP1, and IKK to the TNFR1 complex (complex I) at the plasma membrane but that it is required for the retention of RIP1 and IKK in complex II and subsequent induction of the second phase of IKK activation.
TRAF2 phosphorylation protects cells from cell death induced by oxidative stress
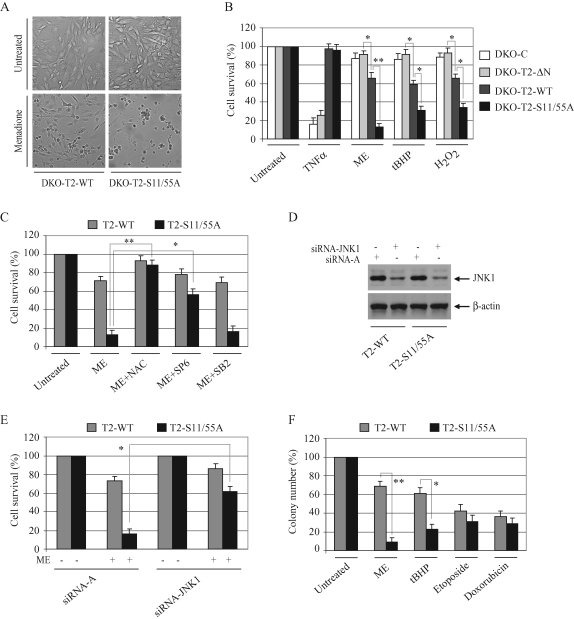
To confirm that puromycin-induced cell death in TRAF2-S11/55A–transfected cells is mediated by oxidative stress, we treated DKO-T2-WT and ‑S11/55A cells with low doses of ME (12.5 μM), tert-butyl hydroperoxide (tBHP; 75 μM), and H2O2 (0.075 mM). As expected, many DKO-T2-WT cells survived under the oxidative stress conditions induced by these agents, whereas most of the DKO-T2-S11/55A cells died under the same conditions (Figure 5, A and B). TNF-α stimulation has been shown to cause ROS accumulation and prolonged JNK activation in TRAF2/5 DKO cells; both of these events ultimately lead to necrotic and apoptotic cell death (Sakon et al., 2003). We also observed that about 80% of TRAF2/5 DKO MEFs transduced with pBa-C underwent cell death within 48 h of TNF-α treatment (Figure 5B). Stable expression of TRAF2-WT or ‑S11/55A in TRAF2/5 DKO MEFs completely inhibited this TNF-α–induced cell death, indicating that TRAF2 does not have to be phosphorylated to inhibit TNF-α–induced cell death. On the other hand, stable expression of TRAF2-ΔN in TRAF2/5 DKO cells failed to protect them from cell death induced by TNF-α, instead rendering them resistant to cell death induced by oxidative stress (compared with TRAF2-WT or ‑S11/55A in Figure 5B). As expected, the antioxidant NAC almost completely inhibited ME-induced cell death in DKO-T2-S11/55A cells (Figure 5C). Notably, application of the JNK-specific inhibitor (SP600125), but not of the p38-specific inhibitor (SB203580), also significantly inhibited ME-induced cell death in DKO-T2-S11/55A cells, albeit to a lesser degree than NAC (Figure 5C). To confirm that JNK signaling is involved in oxidative stress–induced cell death in DKO-T2-S11/55A cells, we knocked down endogenous JNK1 in DKO-T2-WT and ‑S11/55A cells using typical siRNA approaches (Figure 5D). As shown in Figure 5E, siRNA-mediated knockdown of JNK1 also significantly inhibited ME-induced cell death in DKO-T2-S11/55A cells. Colony formation assays were also undertaken and revealed that DKO-T2-S11/55A cells are significantly more sensitive to oxidative stress–induced cell death than are DKO-T2-WT cells (Figure 5F and Supplementary Figure 4). Although DKO-T2-S11/55A cells also displayed a certain susceptibility to apoptosis induced by DNA-damaging reagents (etoposide and doxorubicin), we did not see a statistically significant difference between DKO-T2-WT and ‑S11/55A cells in this case. Collectively, these data suggest that TRAF2 phosphorylation plays a critical role in cell survival in the context of oxidative stress.
FIGURE 5:
TRAF2 phosphorylation inhibits oxidative stress–induced cell death. (A) DKO-T2-WT and ‑S11/55A cells were left untreated or treated with ME (12.5 μM) for 30 h, at which point cell death was assessed by microscopy (200×). (B) TRAF2/5 DKO cells stably transduced with empty vector (DKO-C), TRAF2-WT (DKO-T2-WT), ‑S11/55A (DKO-T2-S11/55A), or ‑ΔN (DKO-T2-ΔN) were left untreated or were treated with mTNF-α (20 ng/ml), ME (12.5 μM), tBHP (75 μM), or H2O2 (0.075 mM) for 48 h. Total cell death was then assessed via the trypan blue exclusion assay. Data are presented as the average of three experiments performed in triplicate. (C) DKO-T2-WT and ‑S11/55A cells were left untreated or treated with ME (12.5 μM) in the absence or presence of antioxidant NAC (5 mM), the JNK inhibitor SP600125 (SP6; 15 μM), or the p38 inhibitor SB203580 (SB2; 15 μM) for 48 h. Total cell death was then assessed as in (B). (D) DKO-T2-WT and ‑S11/55A cells were transfected with a scrabbled control siRNA-A or an siRNA specific to mouse JNK1 (siRNA-JNK1), using Lipofectamine 2000. At 68 h after transfection, the expression level of JNK1 was assessed by Western blotting. (E) DKO-T2-WT and ‑S11/55A cells transfected with siRNA-A or siRNA-JNK1 were left untreated or treated with ME (12.5 μM), and total cell death was then assessed as in (B). (F) DKO-T2-WT and ‑S11/55A cells were left untreated or treated continuously with ME (10 μM) or tBHP (50 μM) for 14 d or were treated temporarily with etoposide (5 μM) or doxorubicin (5 μM) for 6 h. On day 14, colonies were stained and those containing more than 50 cells were counted. The averages are represented as mean ± SD. *p < 0.05; **p < 0.01.
TRAF2 phosphorylation promotes IKK activation but suppresses JNK activation in response to oxidative stress
To understand the mechanism by which TRAF2 phosphorylation promotes cell survival in the context of oxidative stress, we examined IKK and JNK activation in DKO-T2-WT and ‑S11/55A cells following treatment with ME. As shown in Figure 6A, ME clearly induced prolonged IKK activation in DKO-T2-WT cells, whereas in DKO-T2-S11/55A cells ME induced IKK activation only weakly at an early time point (30 min), and this activity subsided quickly thereafter. In contrast to IKK activity, JNK activity (both basal and inducible) was significantly higher in DKO-T2-S11/55A cells than in DKO-T2-WT cells (Figure 6B). In vitro IKK and JNK kinase assays were repeated three times, and the average kinase activities are summarized in Supplemental Figure 5. Collectively, these data suggest that TRAF2 phosphorylation also positively regulates the prolonged phase of IKK activation, while inhibiting that of JNK activation, in the context of oxidative stress.
FIGURE 6:
TRAF2 phosphorylation promotes IKK but inhibits JNK activation in response to oxidative stress. (A and B) DKO-T2-WT and ‑S11/55A cells were left untreated or treated with ME (30 μM) as indicated, and IKK (A) and JNK (B) activities were assessed by kinase assays as in Figure 4, A and B. (C) DKO-T2-WT and ‑S11/55A cells were treated with ME (15 μM) for 3 h as indicated, and the expression levels of cIAP1, cIAP2, Bcl-2, cFLIP, and ICAM-I were determined by real-time RT-PCR as in Figure 3. *p < 0.05. (D) DKO-T2-WT and ‑S11/55A cells were left untreated or treated with ME (30 μM) as indicated, and the expression levels of cIAP1, Bcl-2, and cFLIP protein were monitored by Western blotting. ns: nonspecific bands.
TRAF2 phosphorylation promotes the expression of NF-κB target genes in response to stimulation by oxidative stress
To assess the role of TRAF2 phosphorylation in oxidative stress–induced NF-κB activation, we stimulated DKO-T2-WT and ‑S11/55A cells with ME and then analyzed the expression of several NF-κB target genes by real-time RT-PCR. As shown in Figure 6C, the expression levels of cIAP2, cFLIP, and ICAM-I were significantly higher in DKO-T2-WT cells than in DKO-T2-S11/55A cells. Although the expression levels of cIAP1 and B-cell lymphoma 2 (Bcl-2) were also higher in DKO-T2-WT cells than in DKO-T2-S11/55A cells, there was no statistically significant difference. Notably, oxidative stress–induced up-regulation of NF-κB target genes (1- to 3-fold) was found to be much weaker than that induced by TNF-α (5- to 15-fold). Therefore, we also examined the expression of antiapoptotic proteins by Western blotting. As shown in Figure 6D, in DKO-T2-WT cells ME stimulation slightly increased the expression of cIAP1 and cFLIP proteins, whereas in DKO-T2-S11/55A cells ME stimulation failed to do so. In fact, in DKO-T2-S11/55A cells, ME stimulation led to a decrease in the protein expression of cIAP1 and Bcl-2 at the later time points to a level that is lower than in unstimulated cells (Figure 6D). This suggests that ME may cause degradation of cIAP1 and Bcl-2 in DKO-T2-S11/55A cells. Nevertheless, these data indicate that TRAF2 phosphorylation promotes the expression of certain NF-κB–dependent antiapoptotic proteins in response to stimulation by oxidative stress.
TRAF2 phosphorylation is essential for its interaction with IKK under conditions of oxidative stress
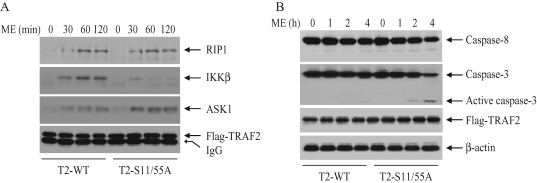
To examine the possible mechanism for TRAF2 phosphorylation-dependent activation of IKK in response to oxidative stress, we analyzed the recruitment of RIP1, apoptosis signal-regulated kinase 1 (ASK1), and IKK to TRAF2 in DKO-T2-WT and ‑S11/55A cells following ME stimulation. As shown in Figure 7A, RIP1 and ASK1 were equally recruited to TRAF2 in both DKO-T2-WT and ‑S11/55A cells in response to ME stimulation. On the other hand, ME stimulation clearly induced the recruitment of IKK to TRAF2 in DKO-T2-WT cells but not in DKO-T2-S11/55A cells. These data suggest that TRAF2 phosphorylation is required for its interaction with IKK under conditions of oxidative stress and that this interaction may lead to IKK activation and suppression of the prolonged phase of JNK activation.
FIGURE 7:
(A) TRAF2 phosphorylation regulates its interaction with IKK in response to oxidative stress. DKO-T2-WT and ‑S11/55A cells were treated with or without ME (30 μM) as indicated. Flag-TRAF2 was then immunoprecipitated with anti-Flag antibody, and the recruitment of RIP1, IKKβ, and ASK1 to TRAF2 was monitored by Western blotting. (B) DKO-T2-WT and ‑S11/55A cells were treated with or without ME (15 μM) as indicated, and cleavage of caspase-3 and ‑8 was monitored by Western blotting.
TRAF2 phosphorylation inhibits the intrinsic pathway of apoptosis
TRAF2 and RIP1 are components of the death receptor signaling complexes and are known to regulate the extrinsic pathway of apoptosis (Karin et al., 2002; Hayden and Ghosh, 2008). Therefore, to examine the possible mechanism for TRAF2 phosphorylation-dependent suppression of cell death under the oxidative stress condition, we examined the activation of caspase-8 and caspase-3 in DKO-T2-WT and ‑S11/55A cells following ME stimulation. As shown in Figure 7B, ME stimulation induced caspase-3 activation within 4 h of treatment in DKO-T2-S11/55A cells but not in DKO-T2-WT cells. Caspase-8 activation was not observed clearly in either cell line in response to ME stimulation. In addition, treatment of the cells with ME in the presence of TNF-α–neutralizing antibody did not inhibit ME-induced cell death in DKO-T2-S11/55A cells (unpublished data). These data suggest that TRAF2 phosphorylation inhibits the intrinsic apoptotic pathway under conditions of oxidative stress.
TRAF2 phosphorylation protects cancer cells from stress-induced cell death
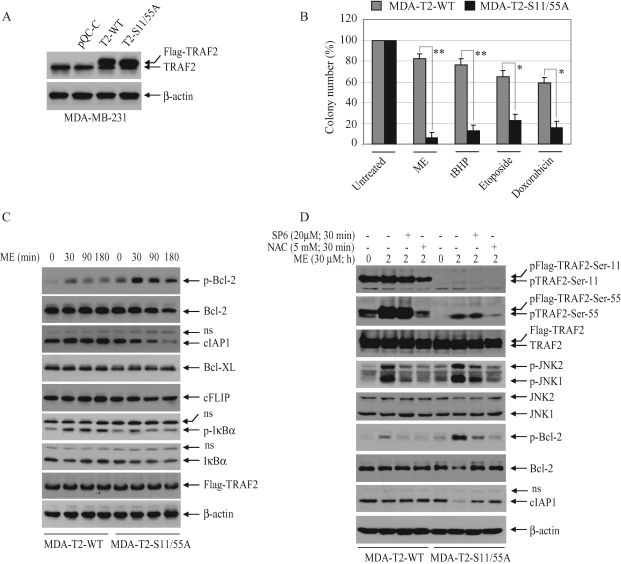
To examine whether TRAF2-S11/55A also sensitizes cancer cells to apoptosis under conditions of oxidative stress, we stably transfected MDA-MB-231 and FaDu cells with TRAF2-WT and ‑S11/55A using hygromycin-selectable retroviral vectors. Unexpectedly, FaDu cells stably transfected with TRAF2-S11/55A did not grow well under standard cell culture conditions, although FaDu cells stably transfected with TRAF2-WT grew well under the same conditions. On the other hand, MDA-MB-231 cells stably transfected with either TRAF2-WT (MDA-T2-WT) or ‑S11/55A (MDA-T2-S11/55A) grew well under standard cell culture conditions and expressed TRAF2 proteins at similar levels (Figure 8A). Although the MDA-T2-S11/55A cells express endogenous TRAF2, both cytotoxicity (unpublished data) and clonogenic assays revealed that MDA-T2-S11/55A cells are more sensitive to cell death induced by oxidative stress than are MDA-T2-WT cells (Figure 8B). Notably, MDA-T2-S11/55A cells also exhibited increased susceptibility to cell death induced by DNA-damaging reagents compared with MDA-T2-WT cells, albeit to a lesser degree than in the context of oxidative stress. These data suggest that TRAF2 phosphorylation plays a critical role in protecting cancer cells from stress-induced cell death.
FIGURE 8:
TRAF2 phosphorylation inhibits oxidative stress–induced death of cancer cells by suppressing the degradation of Bcl-2 and cIAP1. (A) MDA-MB-231 cells were introduced with TRAF2-WT (MDA-T2-WT) or ‑S11/55A (MDA-T2-S11/55A) in a hygromycin-selectable retroviral vector. TRAF2 protein levels were monitored by Western blotting using an anti-TRAF2 antibody. (B) MDA-T2-WT or ‑S11/55A cells were left untreated or treated with ME, tBHP, etoposide, or doxorubicin as in Figure 5F, and the number of colonies of 50 cells or greater was counted on day 14. *p < 0.05; **p < 0.01. (C) MDA-T2-WT or ‑S11/55A cells were left untreated or treated with ME (30 μM) as indicated, and phosphorylation of Bcl-2 and IκBα, and the levels of Bcl-2, cIAP1, Bcl-XL, cFLIP, and IκBα proteins, were monitored by Western blotting. (D) MDA-T2-WT or ‑S11/55A cells were pretreated with or without NAC or a JNK inhibitor SP600125 (SP6) for 30 min and then treated with ME (30 μM) for 2 h as indicated. Phosphorylation of TRAF2, JNK1/2, and Bcl-2 and the levels of TRAF2, JNK1/2, Bcl-2, and cIAP1 proteins were monitored by Western blotting.
TRAF2 phosphorylation suppresses Bcl-2 phosphorylation and degradation in response to stimulation by oxidative stress
Oxidative stress has been shown to induce Bcl-2 phosphorylation and degradation in cardiac myocytes (Markou et al., 2009). Therefore, we examined the phosphorylation of Bcl-2, as well as the expression of other antiapoptotic proteins, in MDA-T2-WT and ‑S11/55A cells following ME treatment. As shown in Figure 8C, MDA-T2-S11/55A cells exhibited basal Bcl-2 phosphorylation even in the absence of ME stimulation, and ME stimulation increased Bcl-2 phosphorylation to a greater extent than in MDA-T2-WT cells. In addition, the level of Bcl-2 and cIAP1 proteins decreased in MDA-T2-S11/55A cells at the later time points following ME treatment. On the other hand, ME treatment slightly increased the level of cFLIP protein in MDA-T2-WT cells compared with that in MDA-T2-S11/55A cells. Notably, in contrast to the increase in Bcl-2 phosphorylation, IκBα phosphorylation was clearly reduced in MDA-T2-S11/55A cells compared with that in MDA-T2-WT cells. In addition, IκBα was degraded at 30 min and recovered to the basal level at 120 min after ME treatment in MDA-T2-WT cells, whereas in MDA-T2-S11/55A cells, IκBα was slowly degraded but did not fully recover back to the basal level. These data suggest that TRAF2 phosphorylation promotes NF-κB activation and suppresses the degradation of Bcl-2 and cIAP1 in response to ME treatment.
Oxidative stress–induced Bcl-2 phosphorylation and degradation is mediated by JNK
Strong and prolonged JNK activation has been shown to induce apoptosis via the mitochondria pathway, by directly phosphorylating and inactivating the antiapoptotic function of Bcl-2 (Yamamoto et al., 1999; Fan et al., 2000). Therefore, to confirm that Bcl-2 phosphorylation and degradation in MDA-T2-S11/55A cells under conditions of oxidative stress are due to the reduced TRAF2 phosphorylation and increased JNK activation, we examined TRAF2 and Bcl-2 phosphorylation in MDA-T2-WT and ‑S11/55A cells in the presence or absence of NAC or JNK inhibitor. Interestingly, TRAF2 is constitutively phosphorylated at Ser-11 to a maximum level in MDA-T2-WT cells, and ME treatment did not further increase Ser-11 phosphorylation (Figure 8D). On the other hand, although TRAF2 is also constitutively phosphorylated at Ser-55 in MDA-T2-WT cells, this phosphorylation was further increased by ME treatment. In addition, ME-induced TRAF2 Ser-11 phosphorylation was almost completely inhibited and Ser-55 phosphorylation was reduced significantly in MDA-T2-S11/55A cells. Notably, the overall levels of TRAF2 phosphorylation were much higher in MDA-T2-WT cells than in MDA-T2-S11/55A cells, and pretreatment of MDA-T2-WT cells with NAC, but not with JNK inhibitor, reduced both the basal and inducible levels of TRAF2 phosphorylation (Figure 8D). Moreover, ME-induced Bcl-2 phosphorylation in MDA-T2-S11/55A cells was almost completely blocked by NAC and JNK inhibitor. Collectively, these data suggest that JNK is the kinase responsible for Bcl-2 phosphorylation and that TRAF2 phosphorylation promotes cell survival by suppressing prolonged JNK activation and Bcl-2 degradation in the context of oxidative stress.
DISCUSSION
TRAF2, RIP1, and ASK1 regulate TNF-α– and oxidative stress–induced activation of the JNK and NF-κB pathways. Both TNF-α and oxidative stress induced TRAF2 phosphorylation in all cell types tested; thus, to better understand the mechanisms by which TRAF2 phosphorylation regulates JNK and IKK activation, we concomitantly analyzed JNK and IKK activation in TRAF2-null cells reconstituted with WT or phosphomutant TRAF2 following treatment of the cells with TNF-α or oxidative stress.
Ligation of TNFR1 by TNF-α recruits TRADD, RIP1, TRAF2, and IKK to form membrane-bound complex I, which immediately activates the JNK and NF-κB pathways. Thereafter, the TRADD/RIP1/TRAF2/IKK complex dissociates from TNFR1 and recruits Fas-associated death domain protein and caspase-8 to form cytoplasmic complex II, which causes apoptosis if the NF-κB pathway is inhibited (Micheau and Tschopp, 2003). It is currently believed that K63-linked RIP1 ubiquitination serves as a platform to recruit both the TAK1/TAB1/TAB2 and IKKα/IKKβ /NEMO complexes to TNFR1 and plays an essential role in TNF-α–induced and TAK1-mediated IKK activation (Ea et al., 2006; Wu et al., 2006; Chen and Sun, 2009). However, Wong et al. (2010) have recently reported that TNF-α can normally activate NF-κB in both primary and immortalized RIP1-deficient cells. In fact, it has been shown that TRAF2 can recruit IKK to TNFR1 in RIP1-deficient cells in response to TNF-α stimulation by interacting with IKKα and IKKβ (Devin et al., 2000). Therefore, it is likely that TRAF2 and RIP1 can independently recruit IKK to TNFR1 and that they can independently induce IKK activation in response to TNF-α stimulation.
Notably, whereas RIP1-mediated IKK recruitment to TNFR1 is dependent on K63-linked ubiquitination of RIP1, TRAF2-mediated IKK recruitment is dependent on the N-terminal RING domain of TRAF2 (Devin et al., 2000; Ea et al., 2006; Wu et al., 2006). As both Ser-11 and Ser-55 are located at the TRAF2 N-terminal region, we speculated that TRAF2 phosphorylation might regulate IKK recruitment to TNFR1 upon TNF-α stimulation. However, we did not observe any difference between TRAF2-WT– and TRAF2-S11/55A–expressing cells with respect to IKK recruitment to TNFR1 (Figure 4C). On the other hand, when TRAF2 was immunoprecipitated from TRAF2-WT– and ‑S11/55A–expressing cells following TNF-α stimulation, both RIP1 and IKK were found to be associated with TRAF2-WT (complex I and II) until 90 min after TNF-α stimulation, whereas RIP1 and IKK associated with TRAF2-S11/55A at early time points (complex I) but quickly dissociated from them (complex II) at later time points (Figure 4D). These data suggest that TRAF2 phosphorylation is essential for retaining IKK in complex II. The absence of RIP1 in complex II in TRAF2-S11/55A–expressing cells is unexpected, and the underlying mechanism is not clear. One possibility is that in TRAF2-S11/55A–expressing cells K63-ubiquitinated RIP1 in complex II is deubiquitinated and then ubiquitinated through K48-linkage by A20, resulting in its rapid degradation. In TRAF2-WT–expressing cells, TRAF2 phosphorylation may interfere with A20-mediated RIP1 degradation.
Although complex II has been regarded as an apoptosis-inducing complex, it does not cause cell death in wild-type cells unless protein synthesis or the NF-κB pathway is blocked. Thus, the physiological function of complex II has been unclear. TRAF2-S11/55A–expressing cells are still sensitive to TNF-α–induced cell death in the presence of cycloheximide (unpublished data), indicating that TRAF2 phosphorylation is not required for TNF-α–induced complex-II formation. Likewise, TRAF2 expression is not essential for complex-II formation, as TNF-α–induced cell death is not impaired but augmented in TRAF2-deficient cells. TNF-α induces NF-κB activation in a biphasic (an immediate/transient phase followed by a secondary/prolonged phase) manner, and both phases are dependent on IKK activity (Hoffmann et al., 2002; Ladner et al., 2003; Schmidt et al., 2003; Werner et al., 2005). With respect to IκBα phosphorylation, the membrane-bound IKK was reported to be less effective than the IKK recovered from the whole cell lysates, indicating that the IKK complex retains kinase activity when it is dissociated from TNFR1 (Zhang et al., 2000). Kinetically, TRAF2 phosphorylation-dependent retention of IKK in complex II correlates well with the prolonged phase of IKK activation. This suggests that the physiological function of complex II is most likely to trigger the second phase of IKK activation in a TRAF2 phosphorylation-dependent manner.
In addition to inflammatory cytokines such as TNF-α, growth factors, oxidative stress, and DNA-damaging agents also activate both the JNK and NF-κB pathways (Davis, 2000; Hayden and Ghosh, 2008). Although these stimuli activate the same c-Jun and NF-κB pathways, they nevertheless induce different patterns of gene expression and thus elicit different cellular responses. Thus, c-Jun– and NF-κB–dependent gene expression may be controlled by extremely sensitive and fine-tuned mechanisms that involve sensing the strength and duration of their transcriptional activities (Davis, 2000; Hoffmann et al., 2002; Werner et al., 2005; Hayden and Ghosh, 2008). Hoffmann et al. (2002) have shown that transient NF-κB activation is sufficient to activate IP-10 expression, whereas prolonged NF-κB activation is required for the efficient expression of RANTES. Protein kinase Cζ (PKCζ) and TRAF2-associated kinase (T2K/TBK1/NAK) have also been implicated in TNF-α–induced NF-κB activation (Bonnard et al., 2000; Moscat et al., 2003). However, targeted disruption of PKCζ results in reduced NF-κB activation without abolishing TNF-α–induced transient IKK activation (Leitges et al., 2001). Although the T2K knockout likewise has no effect on TNF-α–induced transient IKK activation, it significantly reduces TNF-α–induced expression of NF-κB target genes such as TLR2 and ICAM-1 (Bonnard et al., 2000).
This suggests that many kinases are involved in TNF-α–induced NF-κB activation and gene expression, although these kinases have no direct role in TNF-α–induced transient IKK activation. Analysis of the expression of well-known NF-κB target genes by real-time RT-PCR revealed that TRAF2 phosphorylation is not essential for TNF-α–induced expression of IκBα and IP-10 but that it is required for efficient expression of RANTES, ICAM-1, cIAP2, and cFLIP at later time points (Figure 3, A–F). We found that TNF-α–induced IκBα and IP-10 expression occurred very quickly, peaking within 1 h after stimulation, whereas ICAM-1, cIAP2, and cFLIP expression continuously increased through 3 h, the final time point that was tested. Thus, it seems that the transient IKK activation that occurs in the absence of TRAF2 phosphorylation is sufficient to trigger maximal expression of some NF-κB target genes such as IκBα and IP-10 but that efficient expression of some other NF-κB target genes such as ICAM-1 and cIAP2 requires TRAF2 phosphorylation-dependent prolonged IKK activation. Notably, TRAF2 phosphorylation was found to play a critical role in oxidative stress–induced expression of NF-κB target genes, although the expression levels of NF-κB target genes induced by oxidative stress were much lower than that induced by TNF-α. Nevertheless, these data suggest that TRAF2 phosphorylation does contribute to efficient expression of some NF-κB–dependent genes.
TRAF2 KO and TRAF2/5 DKO cells are not defective in NF-κB activation but are still sensitive to TNF-α–induced cell death due to the impaired recruitment of cIAP1/2 to the TNFR1 complex in the absence of TRAF2 (Yeh et al., 1997; Zhang et al., 2009). In contrast to the susceptibility to TNF-α–induced cell death, TRAF2 KO cells exhibit increased resistance to cell death triggered by oxidative stress (Shen et al., 2004; Noguchi et al., 2005). A study in ASK1-deficient MEFs revealed that ASK1 plays a critical role in sustained but not immediate JNK activation in response to TNF-α and H2O2 treatment and that ASK1-mediated sustained JNK activation is essential for H2O2-induced cell death (Tobiume et al., 2001). It seems that when cells are subjected to oxidative stress, TRAF2 and ASK1 form a high-molecular-mass complex that in turn induces prolonged JNK activation (Noguchi et al., 2005). On the other hand, Shen et al. (2004) have shown that oxidative stress triggers TRAF2 and RIP1 to form a complex that causes cell death by inducing sustained JNK activation. Data from our study demonstrate that TRAF2 phosphorylation is required for its interaction with IKK but not with RIP1 and ASK1 in response to ME treatment (Figure 7A). We did not see any difference in TNF-α–induced TRAF2 interaction with ASK1 between TRAF2-WT and TRAF2-S11/55A cells (unpublished data). TRAF2 binds RIP1 and ASK1 through its C-terminal TRAF domain and interacts with IKK through its N-terminal RING domain (Wajant et al., 2001; Noguchi et al., 2005). TRAF2 phosphorylation occurs on its N-terminal region, which may explain why TRAF2 phosphorylation has no effect on its interaction with RIP1 and ASK1. Notably, in contrast to the absence of RIP1 in TNF-α–induced complex II in TRAF2-S11/55A–expressing cells, RIP1 was found to be stably associated with TRAF2 in TRAF2-S11/55A–expressing cells in response to ME stimulation. This is most likely due to the fact that ME, unlike TNF-α, does not induce RIP1 ubiquitination and that the interaction between TRAF2 and RIP1 does not require phosphorylation of TRAF2. Importantly, TRAF2 phosphorylation-dependent recruitment of IKK to the TRAF2/ASK1/RIP1 complex seems to play a critical role in efficient activation of IKK and expression of NF-κB–dependent genes in response to treatment by ME. This NF-κB activation most likely in turn inhibits cell death by suppressing prolonged activation of JNK and accumulation of ROS, as application of antioxidant NAC or a JNK inhibitor significantly inhibited ME-induced prolonged JNK activation and cell death (Figures 5C and 8D).
TRAF2/5 DKO cells reconstituted with TRAF2-ΔN or pBa-C exhibited significantly increased resistance to oxidative stress–induced cell death compared with cells reconstituted with TRAF2-WT (Figure 5B). In TRAF2 KO and TRAF2/5 DKO cells, noncanonical NF-κB is constitutively activated due to accumulation of NIK in the absence of TRAF2 (Grech et al., 2004; Zhang et al., 2009). Recently we reported that stable expression of TRAF2-WT, but not of TRAF2-ΔN, in TRAF2/5 DKO cells completely inhibits constitutive p100 processing (Zhang et al., 2010). This suggests that the TRAF2 RING domain is essential for degradation of NIK and suppression of the noncanonical NF-κB pathway in unstimulated cells. Noncanonical NF-κB has been reported to play an important role in elevated expression of NF-κB–dependent antiapoptotic genes in multiple myeloma cell lines (Annunziata et al., 2007; Keats et al., 2007). Therefore, it is most likely that constitutive activation of noncanonical NF-κB in TRAF2/5 DKO cells reconstituted with TRAF2-ΔN or pBa-C account for the increased resistance of these cells to ROS-induced cell death.
It is well established that JNK activation is critical for TNF-α–stimulated and AP-1–dependent gene expression; however, the role of JNK in TNF-α–induced cell death in physiological settings remains controversial (Ventura et al., 2006; Das et al., 2009). Recent studies have revealed the existence of cross talk between the JNK and NF-κB pathways (Nakano et al., 2006). In normal cells, NF-κB inhibits the prolonged phase of JNK activation by inducing the expression of XIAP, Gadd45β, and cFLIP as well as by suppressing the accumulation of ROS (De Smaele et al., 2001; Tang et al., 2001; Nakano et al., 2006). The prolonged JNK activation observed following inhibition of the NF-κB pathway appears to promote both the necrotic and apoptotic cell death induced by TNF-α and oxidative stress (Nakano et al., 2006). This prolonged JNK activation is mediated by ASK1 activation and also by phosphatase inhibition (Tobiume et al., 2001; Kamata et al., 2005). Although numerous studies have shown that prolonged JNK activation is critical in ROS-induced cell death, the underlying mechanisms are still elusive (Davis, 2000; Nakano et al., 2006). Several lines of evidence demonstrate that strong and sustained JNK activation induces cell death by directly phosphorylating Bcl-2 and thus inducing its proteasome-dependent degradation (Yamamoto et al., 1999; Fan et al., 2000; Markou et al., 2009).
We have found that unstimulated MDA-TRAF2-S11/55A cells exhibit elevated basal JNK activity and constitutive Bcl-2 phosphorylation and that ROS stimulation increases JNK activation and Bcl-2 phosphorylation to higher levels in these cells than in their TRAF2-WT–expressing counterparts (Figures 6B and 8C). In addition, application of NAC or a JNK inhibitor almost completely inhibited ME-induced JNK activation and Bcl-2 degradation in MDA-TRAF2-S11/55A cells (Figure 8D). This suggests that TRAF2 phosphorylation inhibits ROS-induced Bcl-2 phosphorylation and degradation by suppressing prolonged JNK activation. Unexpectedly, the level of cIAP1 protein decreased much more rapidly and significantly than that of Bcl-2 protein in MDA-TRAF2-S11/55A cells following ME treatment. cIAP1 has been reported to be a highly unstable protein that undergoes constitutive autoubiquitination and degradation in the absence of TRAF2 expression (Csomos et al., 2009). Therefore, it is likely that TRAF2 phosphorylation suppresses cIAP1 autoubiquitination and degradation under conditions of oxidative stress. Notably, compared with DKO-TRAF2-S11/55A cells, MDA-TRAF2-S11/55A cells displayed not only increased susceptibility to cell death induced by ROS but also susceptibility to death induced by DNA-damaging agents. TRAF2 is constitutively phosphorylated at both Ser-11 and Ser-55 in MDA-TRAF2-WT cells even in unstimulated conditions, whereas in MDA-TRAF2-S11/55A cells TRAF2 phosphorylation is completely inhibited in the same conditions (Figure 8D). Therefore, although TRAF2 phosphorylation may not be directly involved in DNA damage–induced cell death, the elevated basal JNK and Bcl-2 phosphorylation in MDA-TRAF2-S11/55A cells in contrast to the constitutive phosphorylation of TRAF2 in MDA-TRAF2-WT cells may account for the increased susceptibility of MDA-TRAF2-S11/55A cells to DNA damage–induced cell death.
In previous studies, we showed that TRAF2 is constitutively phosphorylated at both Ser-11 and Ser-55 in Hodgkin’s lymphomas (Blackwell et al., 2009; Thomas et al., 2009). The tumor microenvironment is characterized by hypoxia, low glucose, and free radicals—factors known to perturb cellular homeostasis and to trigger chronic cellular stress (Hanahan and Weinberg, 2000). In the study presented here, we show that ROS effectively induces TRAF2 phosphorylation and that this phosphorylation plays a critical role in cell survival in the context of oxidative stress. This suggests that TRAF2 phosphorylation may contribute to cancer cell adaptation to the tumor microenvironment and that it may do so by promoting NF-κB activation and simultaneously suppressing JNK activation. In this light, inhibiting TRAF2 phosphorylation might be an attractive method for selectively sensitizing cancer cells to anticancer drugs. Thus, it would be interesting to generate TRAF2 phosphomutant knockin mice in which to investigate whether the resulting inhibition of TRAF2 phosphorylation suppresses cancer development and/or tumor progression.
MATERIAL AND METHODS
Cell lines, plasmids, and reagents
TRAF2/5 DKO MEFs, HeLa, 293T, and NIH 3T3 cells were maintained in DMEM supplemented with 10% bovine calf serum (BCS; Hyclone, Logan, UT) and antibiotics. MDA-MB-231 (breast cancer), PC3 (prostate cancer), and FaDu (head and neck squamous cell carcinoma) cancer cell lines were maintained in DMEM/F-12 medium supplemented with 5% fetal calf serum (Hyclone) and antibiotics. Antibodies (Abs) and reagents were purchased as follows: anti-TRAF2, anti-JNK1, anti-IKKγ, anti-IKKβ, anti-cIAP1, anti-Mn-SOD Abs, siRNA for mouse JNK1, and scrambled control siRNA-A from Santa Cruz Biotechnology (Santa Cruz, CA); mouse and human TNF-α (hTNF-α and mTNF-α) from Roche (Indianapolis, IN); anti-Flag Ab, puromycin, ME, tBHP, etoposide, doxorubicin, and NAC from Sigma (St. Louis, MO); and JNK inhibitor (SP600125) and p38 inhibitor (SB203580) from EMB Chemicals (Gibbstown, NJ). Anti-TRAF2 phosphoantibodies and constructs encoding Flag-TRAF2, NF-κB, and c-Jun firefly luciferase reporter gene (NF-κB-Luc and Jun2-Luc) have been described (Blackwell et al., 2009). Mutations were introduced into the Flag-TRAF2 expression vector using the QuikChange Site-Directed Mutagenesis Kit (Stratagene, La Jolla, CA) and were confirmed by DNA sequencing. Retroviral vectors for the transduction of Flag-TRAF2 were generated by subcloning the TRAF2 cDNA into a pBabe-puro and pQCXIH-hygro plasmids, respectively.
Luciferase reporter gene assays
Cells cultured in six-well plates were transfected with an NF-κB or c-Jun firefly luciferase reporter plasmid (NF-κB-Luc or Jun2-Luc; 0.2 μg), together with a Flag-TRAF2-WT, ‑S11/55A, or ‑S11/55D (0.2 μg) plasmid and a Renilla luciferase reporter plasmid (pRL-TK; 0.01 μg), using Lipofectamine 2000 reagents. At 36 h after transfection, test cells were treated with hTNF-α (10 ng/ml) or mTNF-α (5 ng/ml), and protein samples were prepared at 6 (HeLa) or 4 (MEFs) h after treatment. The firefly and Renilla luciferase activities were then measured using the Dual-Luciferase assay system according to the manufacturer’s instructions (Promega, Madison, WI).
JNK and IKK immunokinase assays
Cells were treated with mTNF-α (10 ng/ml) or ME (25 μM), and protein samples were extracted using TNE lysis buffer (20 mM HEPES, pH 7.4, 350 mM NaCl, 1% Triton X-100, 1 mM dithiothreitol, 1 mM EDTA, 20% glycerol, and a cocktail of protease and phosphatase inhibitors). Endogenous JNK1 or IKK complex was immunoprecipitated using anti-JNK1 or anti-IKKγ antibody, respectively, and then these samples were subjected to in vitro kinase assays in which GST-Jun1–87 (for JNK) or GST-IκBα1–55 (for IKK) served as substrates, as described previously (Habelhah et al., 2004).
Retrovirus-mediated TRAF2 transfection into TRAF2/5 DKO or MDA-MB-231 cells
TRAF2/5 DKO and MDA-MB-231 cell lines that stably express TRAF2-WT or TRAF2-S11/55A at a physiological level were generated as described previously (Blackwell et al., 2009). Briefly, 293T cells at 60–70% confluence were cotransfected with 2 μg of pMD.OGP (encoding gag-pol), 2 μg of pMD.G (encoding vesicular stomatitis virus G protein), and 2 μg of pQCXIH-Flag-TRAF2-WT or ‑S11/55A, using the standard calcium phosphate precipitation method. At 48 h after transfection, the viral supernatant was collected and filtered through a 0.45-μm filter. The retroviral supernatants were diluted threefold with 10% fetal bovine serum/DMEM and then immediately used to infect TRAF2/5 DKO and MDA-MB-231 cells in the presence of 4 μg/ml polybrene overnight. At 48 h after infection, cells were selected with hygromycin (200–300 μg/ml) for 6 d. Resistant cells were pooled and used for functional experiments within 1 mo of establishment.
Real-time PCR
MEFs were treated with mTNF-α (10 ng/ml), and total RNA was prepared using the RNeasy Mini Kit (Qiagen, Valencia, CA). Then 5 μg of total RNA was treated with RQ1 RNase-free DNase for 30 min at 37°C and reverse transcribed using an oligo(dT) primer. The resulting cDNA was subjected to quantitative real-time PCR using the Power SYBR Green AB Master Mix and an ABI Prism 7700 Sequence Detector (Applied Biosystems, Foster City, CA). Mouse GAPDH-specific primers were used to generate an internal control, and the average threshold cycle for samples in triplicate was used in the subsequent calculations. Relative expression levels of NF-κB target genes were calculated as the ratio with respect to the GAPDH expression level. The mean ± SD of four independent experiments was considered to be statistically significant at p < 0.05. All primers used in this study are exactly the same as reported previously (Blackwell et al., 2009; Zhang et al., 2009).
Immunoblot analysis
For the detection of TRAF2 phosphorylation, cells were treated as indicated and protein samples were extracted with TNE lysis buffer containing cocktail inhibitors of protease and phosphatase, for 30 min on ice. Then 30 μg of cleared lysates were separated by SDS–PAGE and transferred onto nitrocellulose membranes. The blots were blocked with 0.2% Tween 20/Tris-buffered saline containing 3% bovine serum albumin for 4 h and then incubated with TRAF2 phosphoantibody overnight at 4°C. The phosphorylation status of TRAF2 was then assessed using horseradish peroxidase–labeled secondary antibody and ECL solution. The same membranes were then stripped and reprobed with anti-TRAF2 antibody.
Cytotoxicity assay
Cells were plated on six-well plates at a density of 2 × 104 cells/well. The next day, cells were left untreated or were treated as indicated. At 24 and 48 h after treatment, all cells including those floating in the medium were harvested, and total cell death was assessed via the trypan blue exclusion assay, as described previously (Blackwell et al., 2009).
Colony formation assay
Cells were plated (at 500 cells/well) in six-well plates and treated the next day as indicated. The medium was replaced with fresh medium containing vehicle or the test reagent every 3 d. Then, 12–14 d later, cells were fixed with 3% paraformaldehyde and stained for 30 min with 0.25% crystal violet in 50% methanol. Colonies containing more than 50 cells were counted, and relative survival was calculated as the ratio of the number of colonies on the treated plates to the number of colonies on the corresponding untreated plates.
Supplementary Material
Acknowledgments
We thank Oksana Zagorodna (University of Iowa) for technical assistance. Support by NCI grant CA78419 (to H.H.) is gratefully acknowledged.
Abbreviations used:
- ASK1
apoptosis signal-regulated kinase
- Bcl-2
B-cell lymphoma 2
- cIAP
cellular inhibitor of apoptosis
- IKK
inhibitor of κB kinase
- JNK
c-Jun N-terminal kinase
- ME
menadione
- NF-κB
nuclear factor κB
- RIP1
receptor-interacting protein 1
- ROS
reactive oxygen species
- TNF-α
tumor necrosis factor α
- TNFR1
TNF receptor 1
- TRAF2
TNF receptor–associated factor 2
Footnotes
This article was published online ahead of print in MBoC in Press (http://www.molbiolcell.org/cgi/doi/10.1091/mbc.E10-06-0556) on November 30, 2010.
REFERENCES
- Aggarwal BB. Nuclear factor-kappaB: the enemy within. Cancer Cell. 2004;6:203–208. doi: 10.1016/j.ccr.2004.09.003. [DOI] [PubMed] [Google Scholar]
- Annunziata CM, et al. Frequent engagement of the classical and alternative NF-kappaB pathways by diverse genetic abnormalities in multiple myeloma. Cancer Cell. 2007;12:115–130. doi: 10.1016/j.ccr.2007.07.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell K, Zhang L, Thomas GS, Sun S, Nakano H, Habelhah H. TRAF2 phosphorylation modulates tumor necrosis factor alpha-induced gene expression and cell resistance to apoptosis. Mol Cell Biol. 2009;29:303–314. doi: 10.1128/MCB.00699-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonizzi G, Karin M. The two NF-kappaB activation pathways and their role in innate and adaptive immunity. Trends Immunol. 2004;25:280–288. doi: 10.1016/j.it.2004.03.008. [DOI] [PubMed] [Google Scholar]
- Bonnard M, et al. Deficiency of T2K leads to apoptotic liver degeneration and impaired NF-kappaB-dependent gene transcription. EMBO J. 2000;19:4976–4985. doi: 10.1093/emboj/19.18.4976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bradley JR, Pober JS. Tumor necrosis factor receptor-associated factors (TRAFs) Oncogene. 2001;20:6482–6491. doi: 10.1038/sj.onc.1204788. [DOI] [PubMed] [Google Scholar]
- Chen ZJ. Ubiquitin signalling in the NF-kappaB pathway. Nat Cell Biol. 2005;7:758–765. doi: 10.1038/ncb0805-758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen ZJ, Sun LJ. Nonproteolytic functions of ubiquitin in cell signaling. Mol Cell. 2009;33:275–286. doi: 10.1016/j.molcel.2009.01.014. [DOI] [PubMed] [Google Scholar]
- Csomos RA, Brady GF, Duckett CS. Enhanced cytoprotective effects of the inhibitor of apoptosis protein cellular IAP1 through stabilization with TRAF2. J Biol Chem. 2009;284:20531–20539. doi: 10.1074/jbc.M109.029983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Das M, Sabio G, Jiang F, Rincon M, Flavell RA, Davis RJ. Induction of hepatitis by JNK-mediated expression of TNF-alpha. Cell. 2009;136:249–260. doi: 10.1016/j.cell.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis RJ. Signal transduction by the JNK group of MAP kinases. Cell. 2000;103:239–252. doi: 10.1016/s0092-8674(00)00116-1. [DOI] [PubMed] [Google Scholar]
- De Smaele E, Zazzeroni F, Papa S, Nguyen DU, Jin R, Jones J, Cong R, Franzoso G. Induction of gadd45beta by NF-kappaB downregulates pro-apoptotic JNK signalling. Nature. 2001;414:308–313. doi: 10.1038/35104560. [DOI] [PubMed] [Google Scholar]
- Devin A, Cook A, Lin Y, Rodriguez Y, Kelliher M, Liu Z. The distinct roles of TRAF2 and RIP in IKK activation by TNF-R1: TRAF2 recruits IKK to TNF-R1 while RIP mediates IKK activation. Immunity. 2000;12:419–429. doi: 10.1016/s1074-7613(00)80194-6. [DOI] [PubMed] [Google Scholar]
- Ea CK, Deng L, Xia ZP, Pineda G, Chen ZJ. Activation of IKK by TNFalpha requires site-specific ubiquitination of RIP1 and polyubiquitin binding by NEMO. Mol Cell. 2006;22:245–257. doi: 10.1016/j.molcel.2006.03.026. [DOI] [PubMed] [Google Scholar]
- Fan M, Goodwin M, Vu T, Brantley-Finley C, Gaarde WA, Chambers TC. Vinblastine-induced phosphorylation of Bcl-2 and Bcl-XL is mediated by JNK and occurs in parallel with inactivation of the Raf-1/MEK/ERK cascade. J Biol Chem. 2000;275:29980–29985. doi: 10.1074/jbc.M003776200. [DOI] [PubMed] [Google Scholar]
- Gardam S, Sierro F, Basten A, Mackay F, Brink R. TRAF2 and TRAF3 signal adapters act cooperatively to control the maturation and survival signals delivered to B cells by the BAFF receptor. Immunity. 2008;28:391–401. doi: 10.1016/j.immuni.2008.01.009. [DOI] [PubMed] [Google Scholar]
- Grech AP, Amesbury M, Chan T, Gardam S, Basten A, Brink R. TRAF2 differentially regulates the canonical and noncanonical pathways of NF-kappaB activation in mature B cells. Immunity. 2004;21:629–642. doi: 10.1016/j.immuni.2004.09.011. [DOI] [PubMed] [Google Scholar]
- Greten FR, Eckmann L, Greten TF, Park JM, Li ZW, Egan LJ, Kagnoff MF, Karin M. IKKbeta links inflammation and tumorigenesis in a mouse model of colitis-associated cancer. Cell. 2004;118:285–296. doi: 10.1016/j.cell.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Gwinner W, Landmesser U, Brandes RP, Kubat B, Plasger J, Eberhard O, Koch KM, Olbricht CJ. Reactive oxygen species and antioxidant defense in puromycin aminonucleoside glomerulopathy. J Am Soc Nephrol. 1997;8:1722–1731. doi: 10.1681/ASN.V8111722. [DOI] [PubMed] [Google Scholar]
- Habelhah H, Takahashi S, Cho SG, Kadoya T, Watanabe T, Ronai Z. Ubiquitination and translocation of TRAF2 is required for activation of JNK but not of p38 or NF-kappaB. EMBO J. 2004;23:322–332. doi: 10.1038/sj.emboj.7600044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–362. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- Hoffmann A, Levchenko A, Scott ML, Baltimore D. The IkappaB-NF-kappaB signaling module: temporal control and selective gene activation. Science. 2002;298:1241–1245. doi: 10.1126/science.1071914. [DOI] [PubMed] [Google Scholar]
- Kamata H, Honda S, Maeda S, Chang L, Hirata H, Karin M. Reactive oxygen species promote TNFalpha-induced death and sustained JNK activation by inhibiting MAP kinase phosphatases. Cell. 2005;120:649–661. doi: 10.1016/j.cell.2004.12.041. [DOI] [PubMed] [Google Scholar]
- Karin M, Cao Y, Greten FR, Li ZW. NF-kappaB in cancer: from innocent bystander to major culprit. Nat Rev Cancer. 2002;2:301–310. doi: 10.1038/nrc780. [DOI] [PubMed] [Google Scholar]
- Keats JJ, et al. Promiscuous mutations activate the noncanonical NF-kappaB pathway in multiple myeloma. Cancer Cell. 2007;12:131–144. doi: 10.1016/j.ccr.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ladner KJ, Caligiuri MA, Guttridge DC. Tumor necrosis factor-regulated biphasic activation of NF-kappa B is required for cytokine-induced loss of skeletal muscle gene products. J Biol Chem. 2003;278:2294–2303. doi: 10.1074/jbc.M207129200. [DOI] [PubMed] [Google Scholar]
- Leitges M, Sanz L, Martin P, Duran A, Braun U, Garcia JF, Camacho F, Diaz-Meco MT, Rennert PD, Moscat J. Targeted disruption of the zetaPKC gene results in the impairment of the NF-kappaB pathway. Mol Cell. 2001;8:771–780. doi: 10.1016/s1097-2765(01)00361-6. [DOI] [PubMed] [Google Scholar]
- Liu H, Bigler SA, Henegar JR, Baliga R. Cytochrome P450 2B1 mediates oxidant injury in puromycin-induced nephrotic syndrome. Kidney Int. 2002;62:868–876. doi: 10.1046/j.1523-1755.2002.00515.x. [DOI] [PubMed] [Google Scholar]
- Markou T, Dowling AA, Kelly T, Lazou A. Regulation of Bcl-2 phosphorylation in response to oxidative stress in cardiac myocytes. Free Radic Res. 2009;43:809–816. doi: 10.1080/10715760903071649. [DOI] [PubMed] [Google Scholar]
- Micheau O, Tschopp J. Induction of TNF receptor I-mediated apoptosis via two sequential signaling complexes. Cell. 2003;114:181–190. doi: 10.1016/s0092-8674(03)00521-x. [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT, Rennert P. NF-kappaB activation by protein kinase C isoforms and B-cell function. EMBO Rep. 2003;4:31–36. doi: 10.1038/sj.embor.embor704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakano H, Nakajima A, Sakon-Komazawa S, Piao JH, Xue X, Okumura K. Reactive oxygen species mediate crosstalk between NF-kappaB and JNK. Cell Death Differ. 2006;13:730–737. doi: 10.1038/sj.cdd.4401830. [DOI] [PubMed] [Google Scholar]
- Noguchi T, Takeda K, Matsuzawa A, Saegusa K, Nakano H, Gohda J, Inoue J, Ichijo H. Recruitment of tumor necrosis factor receptor-associated factor family proteins to apoptosis signal-regulating kinase 1 signalosome is essential for oxidative stress-induced cell death. J Biol Chem. 2005;280:37033–37040. doi: 10.1074/jbc.M506771200. [DOI] [PubMed] [Google Scholar]
- Orlowski RZ, Baldwin AS., Jr NF-kappaB as a therapeutic target in cancer. Trends Mol Med. 2002;8:385–389. doi: 10.1016/s1471-4914(02)02375-4. [DOI] [PubMed] [Google Scholar]
- Sakon S, et al. NF-kappaB inhibits TNF-induced accumulation of ROS that mediate prolonged MAPK activation and necrotic cell death. EMBO J. 2003;22:3898–3909. doi: 10.1093/emboj/cdg379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt C, Peng B, Li Z, Sclabas GM, Fujioka S, Niu J, Schmidt-Supprian M, Evans DB, Abbruzzese JL, Chiao PJ. Mechanisms of proinflammatory cytokine-induced biphasic NF-kappaB activation. Mol Cell. 2003;12:1287–1300. doi: 10.1016/s1097-2765(03)00390-3. [DOI] [PubMed] [Google Scholar]
- Shen HM, Lin Y, Choksi S, Tran J, Jin T, Chang L, Karin M, Zhang J, Liu ZG. Essential roles of receptor-interacting protein and TRAF2 in oxidative stress-induced cell death. Mol Cell Biol. 2004;24:5914–5922. doi: 10.1128/MCB.24.13.5914-5922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang G, Minemoto Y, Dibling B, Purcell NH, Li Z, Karin M, Lin A. Inhibition of JNK activation through NF-kappaB target genes. Nature. 2001;414:313–317. doi: 10.1038/35104568. [DOI] [PubMed] [Google Scholar]
- Thomas GS, Zhang L, Blackwell K, Habelhah H. Phosphorylation of TRAF2 within its RING domain inhibits stress-induced cell death by promoting IKK and suppressing JNK activation. Cancer Res. 2009;69:3665–3672. doi: 10.1158/0008-5472.CAN-08-4867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tobiume K, Matsuzawa A, Takahashi T, Nishitoh H, Morita K, Takeda K, Minowa O, Miyazono K, Noda T, Ichijo H. ASK1 is required for sustained activations of JNK/p38 MAP kinases and apoptosis. EMBO Rep. 2001;2:222–228. doi: 10.1093/embo-reports/kve046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallabhapurapu S, Matsuzawa A, Zhang W, Tseng PH, Keats JJ, Wang H, Vignali DA, Bergsagel PL, Karin M. Nonredundant and complementary functions of TRAF2 and TRAF3 in a ubiquitination cascade that activates NIK-dependent alternative NF-kappaB signaling. Nat Immunol. 2008;9:1364–1370. doi: 10.1038/ni.1678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ventura JJ, Hubner A, Zhang C, Flavell RA, Shokat KM, Davis RJ. Chemical genetic analysis of the time course of signal transduction by JNK. Mol Cell. 2006;21:701–710. doi: 10.1016/j.molcel.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Wajant H, Henkler F, Scheurich P. The TNF-receptor-associated factor family: scaffold molecules for cytokine receptors, kinases and their regulators. Cell Signal. 2001;13:389–400. doi: 10.1016/s0898-6568(01)00160-7. [DOI] [PubMed] [Google Scholar]
- Werner SL, Barken D, Hoffmann A. Stimulus specificity of gene expression programs determined by temporal control of IKK activity. Science. 2005;309:1857–1861. doi: 10.1126/science.1113319. [DOI] [PubMed] [Google Scholar]
- Wong WW, Gentle IE, Nachbur U, Anderton H, Vaux DL, Silke J. RIPK1 is not essential for TNFR1-induced activation of NF-kappaB. Cell Death Differ. 2010;17:482–487. doi: 10.1038/cdd.2009.178. [DOI] [PubMed] [Google Scholar]
- Wu CJ, Conze DB, Li T, Srinivasula SM, Ashwell JD. Sensing of Lys 63-linked polyubiquitination by NEMO is a key event in NF-kappaB activation [corrected] Nat Cell Biol. 2006;8:398–406. doi: 10.1038/ncb1384. [DOI] [PubMed] [Google Scholar]
- Yamamoto K, Ichijo H, Korsmeyer SJ. BCL-2 is phosphorylated and inactivated by an ASK1/Jun N-terminal protein kinase pathway normally activated at G(2)/M. Mol Cell Biol. 1999;19:8469–8478. doi: 10.1128/mcb.19.12.8469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh WC, et al. Early lethality, functional NF-kappaB activation, and increased sensitivity to TNF-induced cell death in TRAF2-deficient mice. Immunity. 1997;7:715–725. doi: 10.1016/s1074-7613(00)80391-x. [DOI] [PubMed] [Google Scholar]
- Zhang L, Blackwell K, Shi Z, Habelhah H. The RING domain of TRAF2 plays an essential role in the inhibition of TNFalpha-induced cell death but not in the activation of NF-kappaB. J Mol Biol. 2010;396:528–539. doi: 10.1016/j.jmb.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Blackwell K, Thomas GS, Sun S, Yeh WC, Habelhah H. TRAF2 suppresses basal IKK activity in resting cells and TNFalpha can activate IKK in TRAF2 and TRAF5 double knockout cells. J Mol Biol. 2009;389:495–510. doi: 10.1016/j.jmb.2009.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang SQ, Kovalenko A, Cantarella G, Wallach D. Recruitment of the IKK signalosome to the p55 TNF receptor: RIP and A20 bind to NEMO (IKKgamma) upon receptor stimulation. Immunity. 2000;12:301–311. doi: 10.1016/s1074-7613(00)80183-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.