Abstract
Objectives
18F-HX4 is a novel positron emission tomography (PET) tracer for imaging hypoxia. The purpose of this study was to determine the biodistribution and estimate the radiation dose from 18F-HX4 using whole body PET/CT scans in monkeys and humans.
Methods
Successive whole body PET/CT scans were performed following the injection of 18F-HX4 to four healthy humans (422 ± 142 MBq) and to three rhesus monkeys (189 ± 3 MBq). Biodistribution was determined from PET images and organ doses were estimated using OLINDA/EXM software.
Results
The bladder, liver and kidneys show the highest percentage of the injected radioactivity for humans and monkeys. For humans about 45% of the activity is eliminated by bladder voiding in 3.6 hours, and for monkeys 60% is in the bladder content after 3.0 hours. The critical organ is the urinary bladder wall with the highest absorbed radiation dose of 415 ± 18 μGy/MBq (monkeys) and 299±38 μGy/MBq (humans), in the 4.8 hour bladder voiding interval model. The average value of effective dose (ED) for the adult male was estimated at 42 ± 4.2 μSv/MBq from monkey data and 27 ± 2 μSv/MBq from human data.
Conclusions
Bladder, kidneys, and liver have the highest uptake of injected 18F-HX4 activity for both monkeys and humans. Urinary bladder wall receives the highest dose of 18F-HX4 and is the critical organ. Thus, patients should be encouraged to maintain adequate hydration and void frequently. The effective dose from 18F-HX4 is comparable to that of other 18F-based imaging agents.
Keywords: 18F-HX4, Biodistribution, Radiation Dosimetry, Hypoxia Marker, Positron Emission Tomography
Introduction
It is well known that hypoxia in tumors is associated with decreased radio-sensitivity [1, 2]. Radiation therapy is less effective in killing these tumor cells, leading to reduced local tumor control and patient survival. Increasing evidence points to the ability of hypoxia to induce the expression of gene products which confers aggressive tumor behaviors and promotes broad resistance to therapy [3]. Visualization of hypoxic regions in tumors prior to initiation of therapies would be helpful in identifying patients that may benefit from alternative treatment approaches for hypoxic tumors, e.g. use of radio-sensitizers[4], hyperthermia[5], or hypoxia-selective cytotoxins[6]. These approaches suggest that determining the presence or absence of tumor hypoxia is important in planning cancer therapy[1]. Recent advances in PET hypoxia imaging, conformal radiotherapy, and imaging-directed radiotherapy treatment planning now make it possible to perform hypoxia-directed radiotherapy [7]. A recent review has summarized the various PET imaging agents labeled with F-18 or Copper isotopes that have been developed for diagnostic assessment of hypoxia tissue[8]. The conclusion of this review is that the non-imidazole agent Cu-ATSM is the most promising among the hypoxia imaging agents. Among the 18F-based 2-nitroimidazole hypoxia agents, the best known and clinically investigated is fluoromisonidazole (18F-FMISO) [9]. Some of its limitations are slow accumulation in hypoxic tumors, low target to background ratio and considerable amount of metabolite products[8]. The putative hypoxia imaging agent 18F-HX4 that we are discussing here has been developed to overcome some of these limitations.
18F-HX4 represents a new, click chemistry derived generation of 2-nitroimidazole derivatives in which structure-activity relationships have been used to design an agent with preferred pharmacokinetic and clearance properties[10-12]. The present study (a Phase 0 clinical trial) is the first in human study with 18F-HX4. A phase I clinical trial conducted shortly thereafter has found 18F-HX4 to be safe for human use[13]. 18F-HX4 may serve as a clinically useful hypoxia marker to specifically define the size and location of tumor hypoxia in diagnostic imaging, allowing the rational application of hypoxia targeted therapies to those patients most likely to benefit from them.
The main goal of this study is to measure the biodistribution and dosimetry of 18F-HX4 in humans. A secondary goal is to determine if the biodistribution of 18F-HX4 in monkeys can adequately predict the biodistribution and dosimetry in humans.
There are a few reasons for doing the study in monkeys prior to humans. In monkeys, dosimetry estimates are readily made from dynamic PET imaging studies without the need for sacrificing the animals, whereas rodents would need to be sacrificed for such estimates. Secondly, monkeys are more closely related to humans compared to other species and are therefore more likely to provide accurate estimates of human biodistribution and dosimetry. If an unfavorable tissue distribution is observed in monkeys (e.g. high uptake in an area of interest for tumor imaging), the PET tracer development can be stopped at the preclinical stage saving unnecessary radiation burden to human subjects.
Methods
Radiopharmaceutical Preparation
3-[18F]fluoro-2-(4-((2-nitro-1H-imidazol-1-yl)methyl)-1H-1,2,3-triazol-l-yl)-propan-l-ol, also referred to as 18F-HX4, is a click chemistry derived, triazole-bearing 2-nitroimidazole useful for detecting hypoxic tissue in vivo. Production of 18F-HX4 suitable for human injection is carried out in an automated synthesis module. Briefly, azeotropically dried 18F-fluoride is reacted with the precursor in acetonitrile at 110°C for 10 minutes, displacing a primary nosylate group to afford the acetate-protected HX4 intermediate. The intermediate is then de-protected in the presence of 1N HCl at 105°C for 5 minutes to hydrolyze the primary acetate moiety (Fig. 1). The crude material is purified via RP-HPLC using 5% EtOH:95% 21 mM sodium phosphate. The isolate fraction is stabilized with ascorbic acid (50 mg/mL) and then sterilely filtered into a sterile, pyrogen-free vial. This protocol produces 18F-HX4 that is free of K222/pyrogens and passes residual solvent analyses for MeCN (≤ 0.4%) and EtOH (<10%). The product is also sterile and possesses an acceptable pH profile (pH = 5 to 7.5).
Fig. 1.
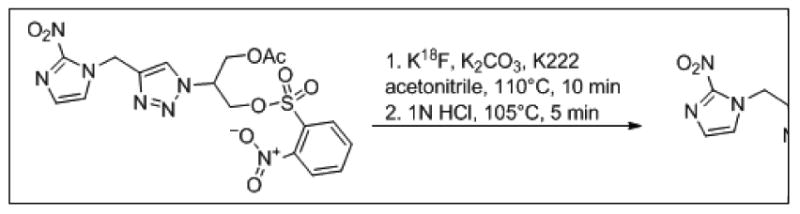
Synthesis of 18F-HX4 from Precursor
The production of the investigational product 18F-HX4 was conducted according to the exploratory New Drug Application 74,944, which was submitted and approved by the U.S. Food and Drug Administration. Each manufactured batch of 18F-HX4 was formulated to yield a minimum specific activity of 7.4 GBq / μmol.
Participants
The study was approved by the Research Review Committee (for scientific merit), Institutional Review Board and Radiation Safety Committee of Fox Chase Cancer Center. Four healthy volunteers (2 male, 2 female) with ages 52 ± 5 (age range 46-60) were included as part of the exploratory study. Written informed consent was obtained from each subject. The subjects' weights were 77 ± 11 kg (range 64-86 kg). All subjects were healthy based on history, physical examination, EKG, urinalysis, and blood tests (complete blood count with differential, and blood chemistry, with special attention to blood urea nitrogen and creatinine clearance). The subjects were free of significant renal diseases based on clinical history and the blood tests.
PET/CT Acquisition
The subjects were injected 422±142 MBq (range 240-636 MBq) of 18F-HX4. The large variation in the injected activity was due to operational reasons and was taken into account in the data processing and analysis. After the injection,, five successive whole body PET/CT scans were performed on a Discovery LS (GE Healthcare, Waukesha, WI) PET/CT scanner. To minimize the radiation exposure from the repeated CT scans, the lowest possible mA setting on the scanner was used to perform the CT scans for attenuation correction. The helical CT scan acquisition parameters were: 140 kVp, 10 mA, 0.8 sec rotation, 5 mm slice thickness, 0.75 pitch, and 4.25 mm interval.
The whole body PET scans were acquired in 2-D mode and ranged from the vertex of the head down to mid-thigh. The five whole-body scans were conducted at approximately 15, 80, 120, 150, 180 minutes after injection. The scan time was 6 minutes per bed position and each scan covered 7 bed positions with single slice overlap between the bed positions. In addition, after the first whole body scan, a PET/CT scan of mid-thigh to toes was done. Blood pressure, temperature, pulse and EKG were monitored before the administration of 18F-HX4 and following first and second PET/CT scans, and then at 24 hours after administration.
Blood and urine were collected prior to each PET scan. The urine samples were assayed with a well counter to estimate the total excreted activity. High-performance liquid chromatography and thin-layer chromatography assays were performed to determine the amounts of intact 18F-HX4 and its radioactive metabolites in the blood. In addition, the metabolites in urine of two of the subjects were assayed.
Data Analysis
The whole-body scans were reconstructed to 55 cm display field of view using ordered subsets expectation maximization (OSEM) algorithm with 28 subsets and 2 iterations using manufacturer supplied software. The reconstruction included corrections for randoms and scatter. Attenuation correction was applied based on the low-dose CT. The accuracy of the activity in the PET images was verified by summing the activity in the first whole-body PET scan and the PET scan from mid-thigh to toes, and comparing to the decayed injected activity. Samples of urine collected during the study were assayed in a well counter to estimate the excreted activity in urine.
The five whole body PET images for each volunteer were displayed on an image display workstation. The time point that had the largest uptake was identified for brain, kidneys, bladder, liver, gallbladder, and large intestine (lower and upper). Volume regions of interest (ROIs) were drawn on the displayed scan to cover the organs. For small intestine, which was mostly not visualized above background, a volume region of interest was determined by marking the boundaries of visualized adjacent organs. The PET images for other time points were displayed and registered with this PET scan using MIMVista (MIM Software Inc., Cleveland, OH) to transfer the volume ROI and determine the total activity in the organs. Adjustments to the individual ROIs were made to ensure the inclusion of visualized organs (e.g. bladder, gallbladder). For testes, the ROIs were drawn in the CT images and transferred to the registered PET images. The percentage injected activity (%IA) was determined for the organs for each of the time points.
Residence Times and Absorbed Dose Calculations
The %IA for each organ for each time point was fitted to exponential or sum of exponential functions in OLINDA/EXM (Organ Level Internal Dose Assessment) software [14] to determine the total number of disintegrations per unit administered activity, also known as residence time. The ‘remainder of body’ was calculated for each time point as the decay corrected injected activity minus the activity in all the source organs and in collected urine. The half-life of biological decay was computed by exponential fitting of injected activity minus accumulated urine (projected to injection time). The 1.0 hour and 4.8 hour bladder voiding models in OLINDA/EXM software were used to determine residence times in bladder. Absorbed doses in the various organs were calculated by entering the residence times of all source organs for each subject into OLINDA/EXM software and using the 70 kg adult male and female models. We selected this software based on its history of widespread use in the radiopharmaceutical industry and research community for internal dose calculations for radiopharmaceuticals and its validation from years of testing by medical, safety, and regulatory professionals.
Animal Subjects
The rhesus monkey studies were approved by the West Point Institutional Animal Care and Use Committee at Merck Research Laboratories. Three male rhesus monkeys (ages 6-8 years, 10-11 kg) were initially anesthetized with ketamine (10 mg/kg intramuscularly), then induced with propofol (5 mg/kg intravenously), intubated, and ventilated with medical grade air. Anesthesia was maintained with propofol (0.4 mg/kg/minute) for the duration of the study and the vital signs (EKG, expired tidal CO2, SPO2, and temperature) were monitored and maintained in the normal range for the duration of the study. Animals were administered an intravenous bolus of 189 ± 3 MBq of 18F-HX4 in 3 ml volume injected with a 10-15 sec bolus duration.
PET/CT Acquisition
Following the injection of 189 ± 3 MBq of 18F-HX4 to the monkeys, a low-dose non-contrast CT (120 kVp, 60 mA, 0.5 sec rotation, 3.75 mm slice thickness, 0.938 pitch) was done followed by dynamic whole body PET scans on a Discovery ST (GE Healthcare, Waukesha, WI) PET/CT scanner. A total of 24 WB PET images were acquired in 2D mode for 5 bed positions covering 72.9 cm axially for a total duration of 200 minutes, time per bed ranging from 15 seconds to 4 minutes (3 × 15 s, 5 × 30 s, 7 × 60 s, 5 × 120 s, and 4 × 240 s).
After the completion of the WB PET, a contrast CT (120 kVp, 200 mA, 0.5 sec rotation, 3.75 mm slice thickness, 0.938 pitch) using 2.5 ml/kg Omnipaque 300 was performed to assist with organ identification.
Data Analysis
The whole-body scans were reconstructed using OSEM algorithm with 30 subsets and 2 iterations and 3 mm full-width half-maximum Gaussian post-processing filter, using manufacturer supplied software. The reconstruction included corrections for scatter and randoms from singles. Attenuation correction was applied based on the low-dose CT.
For each study, regions of interest (ROIs) were delineated using both the contrast CT and the summed PET. They included: brain (CT), liver (PET), kidneys (CT), testes (CT) and urinary bladder (PET). The gut ROI was drawn around the abdomen, excluding all other delineated organs. As such, the gut ROI included the gallbladder. ROIs were projected onto each whole body PET image at each time-point. Time-activity curves (TACs) were obtained by calculating the total activity in the ROI and expressing them as percentage of the total injected activity (%IA).
Residence Times and Absorbed Dose Calculations
The %IA in each source organ was iteratively fitted to bi-exponential function using a nonlinear least-squares regression algorithm (SAAM II v1.2 software)[15] to obtain the residence time.
Rhesus monkeys accumulated radioactivity in the urinary bladder over the duration of the study. The bladder %IA, obtained from the monkey images were used as input and the urinary bladder contents' residence time was calculated using Cloutier's dynamic bladder model[16]. Bladder voiding intervals of 1.0-hour and 4.8-hour were used.
In this analysis, the gut ROI was comprised of the gallbladder, small bowel, upper large intestine and lower large intestine. The value for fecal excretion fraction (fgut) was taken as the value at the last image time (≅ 200 minutes). The gut residence times were calculated using the International Commission on Radiation Protection's Publication 30 model for gastrointestinal tract kinetics, assuming the radioactivity entered via the small bowel.
To obtain an estimate of the corrected total-body TAC (which excludes the activity in the gut and the bladder), the total-body TAC was first constructed as decay corrected injected activity minus the urinary excreted activity and was fit to a biexponential function. Secondly, to correct the total body time-activity curve for fecal excretion, the total body curve was also multiplied by a factor of [1 – fgut] where fgut is the fecal excretion fraction. The corrected total body residence time was calculated by integrating the corrected total body time activity curve fit. The remainder of the body residence time was calculated as the corrected total body residence time minus all other residence times, except bladder and gut.
All absorbed radiation doses were calculated from these rhesus residence times using the OLINDA/EXM software package for the adult male phantom. Radiation doses were computed for each subject, using voiding intervals of 1.0 and 4.8 hours. No scaling of the rhesus biodistribution data was done to estimate human absorbed radiation doses. Thus, it is assumed that the biodistribution in these monkeys is the same as it would be in humans.
Results and Discussion
Human Studies
The injection of 422 ± 142 MBq of 18F-HX4 in four subjects produced no clinically significant effects on vital signs (blood pressure, temperature, pulse, and EKG) and blood tests during the first 3-4 hour observation period following administration and the follow up visit at 24 hours.
The analysis of plasma samples of 3 subjects showed that the level of un-metabolized 18F-HX4 decreased slowly from 94% at 5 minutes to 82% at 120 minutes after the 18F-HX4 injection. The analysis of the urine samples indicated that the level of un-metabolized 18F-HX4 decreased slowly from 99% at 0-35 minutes to approximately 84% at 105-140 minutes after 18F-HX4 injection. Thus, 18F-HX4 is seen to be essentially intact in the blood over the course of the HX4 PET/CT study.
The main characteristics of the radiotracer uptake are illustrated in PET maximum intensity projection (MIP) images for one of the subjects from the PET scans (Fig. 2). In the first scan, the predominant uptake is seen in the urinary bladder with moderate uptake in liver and kidneys. The gallbladder and large intestine show the 18F-HX4 uptake in later scans. All other organs have the background level of activity. In all subjects, the highest (decay-corrected) uptake of 18F-HX4 was found in the urinary bladder with peak values ranging from 10.9% to 16.4% IA. The second highest uptake was in liver, ranging from 4.3% to 6.4% IA. The %IA decreases significantly between 15 minute and 80 minute time points followed by a gradual decrease to the end of the study (Fig. 3). The radioactivity of 18F-HX4 was excreted primarily via the renal system. By the end of the study (∼3.6 hours) ∼45% of the injected activity of 18F-HX4 had been excreted in the urine, as determined by assaying urine samples in a well counter. The residence times for the organs are listed in Table 1.
Fig. 2.
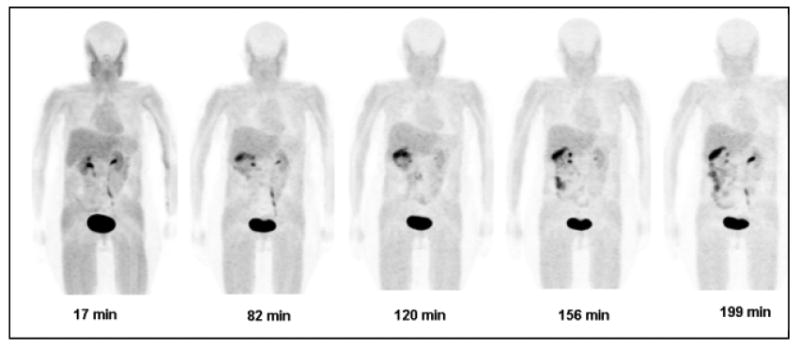
Decay-corrected anterior maximum-intensity projections of PET at 17, 82, 120, 156, and 199 min (from left to right) after injection of 18F-HX4 in a female volunteer. There is rapid clearance of activity in kidneys, liver and bladder. Gallbladder activity peaks at 82 min then decreases with time
Fig. 3.
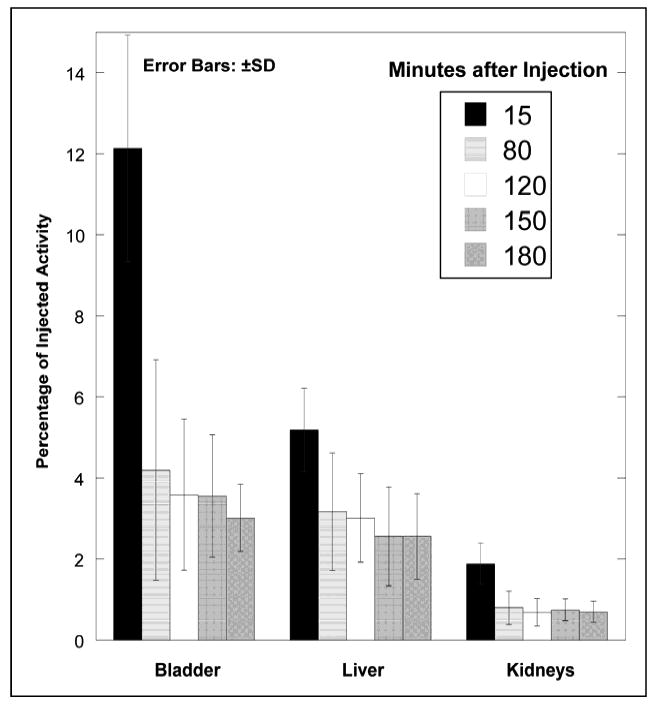
Mean percentage of injected activity and standard deviation (SD) for top 3 organs determined on the basis of 4 18F-HX4 PET emission scans in human volunteers, as a function of time after injection. Rapid clearance of activity is observed in the organs
TABLE 1.
Residence times of source organs for participants injected with 18F-HX4 (n=4, mean ± SD)
| Organ | Residence time (h) |
|---|---|
| Brain | 0.022±0.009 |
| Gallbladder | 0.0088±0.0045 |
| Kidneys | 0.024±0.004 |
| Liver | 0.083±0.023 |
| Distal Colon | 0.018±0.004 |
| Small Intestine | 0.022±0.004 |
| Testes | 0.0010±0.0003 |
| Proximal Colon | 0.016±0.007 |
| Bladder (1 hour void) | 0.163±0.021 |
| Bladder (4.8 hour void) | 0.615±0.082 |
| Remainder of Body | 1.52±0.15 |
The mean effective dose of 18F-HX4 was estimated to be 14 ± 1 μSv/MBq and 27 ± 2 μSv/MBq for 1 hour and 4.8 hour bladder voiding models respectively. The four organs with the highest radiation absorbed doses were urinary bladder wall, gallbladder wall, lower large intestine wall, and kidneys (Table 2).
TABLE 2.
Radiation Dosimetry Estimates for 18F-HX4 in 1 hour and 4.8 hour bladder voiding models, based on human participant data (n=4).
| Organ | 1 hour Void (μGy/MBq) |
4.8 hour Void (μGy/MBq) |
|---|---|---|
| Urinary Bladder Wall | 85±10 | 299±38 |
| Gallbladder Wall | 24±7 | 24±7 |
| LLI Wall | 22±2 | 28±3 |
| Kidneys | 19±2 | 20±2 |
| ULI Wall | 16±3 | 18±3 |
| Small Intestine | 15±0.4 | 17±0.3 |
| Uterus | 14±1 | 28±2 |
| Liver | 14±3 | 14±3 |
| Ovaries | 12±1 | 18±1 |
| Osteogenic Cells | 12±1 | 12±1 |
| Pancreas | 10±1 | 10±1 |
| Adrenals | 9±1 | 9.6±1 |
| Testes | 9±1 | 13±1 |
| Stomach Wall | 9±1 | 9±1 |
| Spleen | 9±1 | 9±1 |
| Heart Wall | 9±1 | 9±1 |
| Muscle | 8±1 | 10±1 |
| Thymus | 8±1 | 8±1 |
| Red Marrow | 8±1 | 9±1 |
| Lungs | 8±1 | 8±1 |
| Thyroid | 8±1 | 8±1 |
| Breasts | 6±1 | 6±1 |
| Skin | 6±1 | 7±0.5 |
| Brain | 5±1 | 5±1.4 |
| Total Body | 8±1 | 10±0.5 |
| Effective Dose (μSv/MBq) | 14±1 | 27±2 |
Values are expressed as mean ± SD.
LLI, lower large intestine; ULI, upper large intestine.
The hypoxia marker 18F-HX4 shows a biodistribution dominated by activity in the bladder and steady renal clearance, with 45% of IA excreted in the 3.6 hours of the study. In the intermediate scans gallbladder activity is noted, and in the last scans intestinal elimination as indicated by intestinal activity can be observed. The relatively wide range of activities seen in the various organs implies wide range of absorbed radiation doses. Apart from urinary bladder, liver, kidneys, gallbladder, and large intestine are seen to have above background level of activity. All other organs displayed near the background level of activity.
Urinary bladder received the highest dose of 18F-HX4 among all the organs. The mean urinary bladder wall dose was 85 ± 10 μGy/MBq and 299 ± 38 μGy/MBq for the 1 hour and 4.8 hour bladder voiding models, respectively. The absorbed doses of 18F-HX4 to the liver, large and small intestines, and gallbladder, uterus and ovaries were in the range of 14-28 μGy/MBq in the two models with the remaining organs having lower doses. The average values of effective dose (ED) for 18F-HX4 were 14±1 μSv/MBq and 27±2 μSv/MBq respectively for the two models. For a 740 MBq injected activity of 18F-HX4, the average effective dose for the 1 hour model is 10.4±0.7 mSv and the dose to the bladder is 63±7 mSv. The dose to the bladder may be reduced by encouraging adequate hydration and frequent voiding.
Monkey Studies
The injection of 189 ± 3 MBq of 18F-HX4 in three male monkeys produced no clinically significant effects on vital signs (blood pressure, pulse, EKG) and blood tests during the 3 hour observation period following administration. In the rhesus, the liver was best visualized at approximately 4 minutes post injection, while the bladder content became visible approximately 7 minutes post injection.
PET MIP images for selected time points illustrate the nature of organ uptake as a function of time (Fig. 4). At 3 minutes, rapid uptake of 18F-HX4 is observed in liver, heart, kidneys, and bladder, with patchy uptake in head. Bladder activity continues to accumulate with time, as the rhesuses are under anesthesia and do not void. Rapid clearance of activities in other organs is observed in the images at subsequent time points.
Fig. 4.
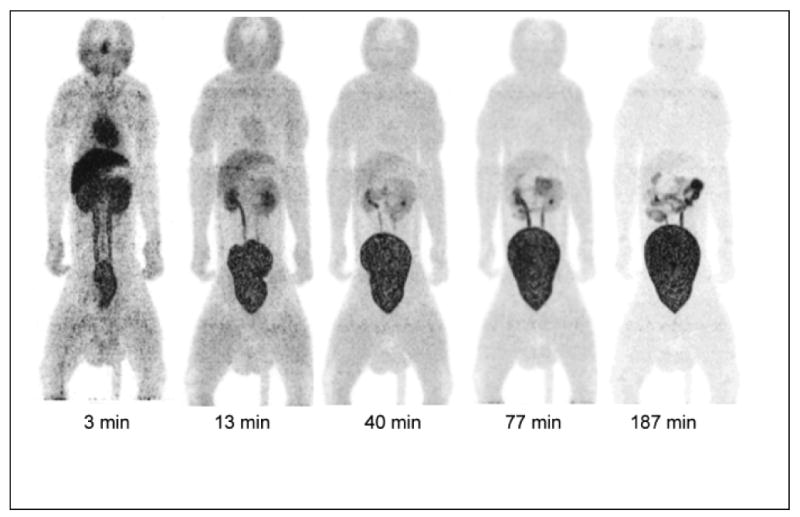
Decay-corrected anterior maximum-intensity projections of PET at 3, 13, 40, 77 and 187 min (from left to right) after injection of 18F-HX4 in a rhesus monkey. The liver and kidney activities decrease rapidly with time, and bladder accumulates activity with time (there is no voiding of bladder as the monkey is anesthetized)
Urinary bladder had the highest uptake, with 60 ± 2% IA at the end of the 3 hour 18F-HX4 PET/CT acquisition. Peak values of %IA in the liver and kidneys were 9.7% and 7.3% (Fig. 5). At the end of the study, 6.2 ± 0.1% IA was found in the gut which included the gallbladder, small intestine, proximal and distal colons' contents. The residence times for the organs are listed in Table 3. The estimated mean organ doses from 18F-HX4 are given in Table 4.
Fig. 5.
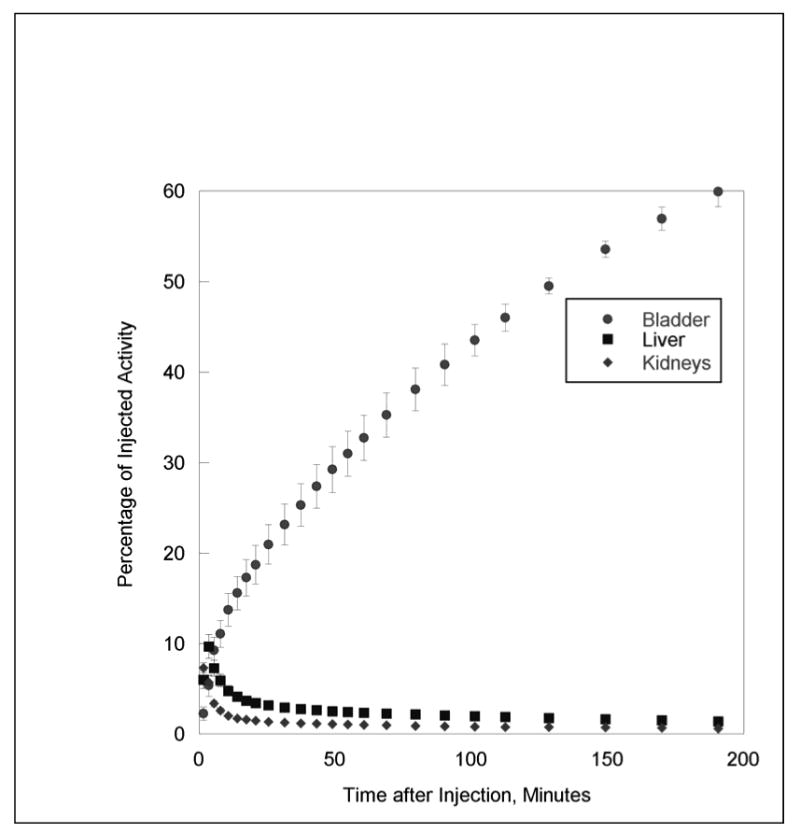
Mean percentage of injected activity and standard deviation (SD) for top three organs determined on the basis of three rhesus monkey 18F-HX4 PET emission scans, as a function of time after injection. Liver and kidney activities decrease rapidly with time, and bladder activity increases with time (there is no voiding as monkeys are anesthetized)
TABLE 3.
Residence times of source organs for rhesus monkeys injected with 18F-HX4 (n=3, mean ± SD).
| Organ | Residence time (hour) |
|---|---|
| Brain | 0.007 ± 0.000 |
| Liver | 0.058 ± 0.009 |
| Kidneys | 0.026 ± 0.002 |
| Testes | 0.013 ± 0.004 |
| Urinary Bladder Contents | |
| (1.0-hour void) | 0.263 ± 0.014 |
| (4.8-hour void) | 0.863 ± 0.043 |
| Total Body | 1.39 ± 0.028 |
| Total Body-corrected | 1.31 ± 0.026 |
| Remainder of the body | 1.20 ± 0.036 |
Values are mean ± SD of 3 subjects
Note: % IA to feces 6.2 ± 0.7%
%IA, percentage of injected activity; SD, standard deviation.
TABLE 4.
Radiation Doses for 18F-HX4 for the Human Adult Male (1.0 and 4.8 Hour bladder voiding Interval) [based on male primate (n=3) biodistribution data]
| Target Organs | 1.0 hour model μGy/MBq |
4.8 hour model μGy/MBq |
|---|---|---|
| Urinary Bladder Wall | 131 ± 6.1 | 415 ± 18 |
| Testes | 60 ± 17 | 65 ± 17 |
| ULI Wall | 32 ± 2.9 | 34± 2.7 |
| Small Intestine | 30 ± 2.5 | 33 ± 2.4 |
| Kidneys | 20 ± 1.5 | 21 ± 1.5 |
| LLI Wall | 18 ± 0.80 | 26 ± 0.40 |
| Uterus | 17 ± 0.17 | 35 ± 0.71 |
| Ovaries | 14 ± 0.36 | 21 ± 0.10 |
| Liver | 10 ± 1.1 | 11 ± 1.1 |
| Gallbladder Wall | 10 ± 0.37 | 11 ± 0.35 |
| Osteogenic Cells | 10 ± 0.21 | 11 ± 0.21 |
| Pancreas | 8.3 ± 0.20 | 8.6 ± 0.18 |
| Stomach Wall | 8.0 ± 0.23 | 8.4 ± 0.22 |
| Adrenals | 7.8 ± 0.17 | 8.1 ± 0.16 |
| Spleen | 7.4 ± 0.18 | 7.6 ± 0.18 |
| Muscle | 7.2 ± 0.14 | 9.3 ± 0.08 |
| Red Marrow | 7.0 ± 0.17 | 8.3 ± 0.12 |
| Heart Wall | 6.9 ± 0.14 | 7.0 ± 0.15 |
| Thymus | 6.2 ± 0.15 | 6.2 ± 0.15 |
| Lungs | 6.1 ± 0.13 | 6.2 ± 0.13 |
| Thyroid | 6.0 ± 0.16 | 6.0 ± 0.16 |
| Skin | 5.1 ± 0.10 | 5.9 ± 0.08 |
| Breasts | 5.1 ± 0.12 | 5.1 ± 0.12 |
| Brain | 2.5 ± 0.02 | 2.5 ± 0.0 |
| Total Body | 7.6 ± 0.14 | 9.5 ± 0.06 |
| Effective Dose (μSv/MBq) | 25 ± 3.5 | 42.0 ± 4.2 |
Values are expressed as mean ± standard deviation.
The four organs with the highest radiation absorbed doses of 18F-HX4 were urinary bladder wall, testes, ULI wall, and small intestine. The mean effective dose of 18F-HX4 was 25 ± 3.5 μSv/MBq and 42 ± 4.2 μSv/MBq for 1 hour and 4.8 hour bladder voiding models respectively.
18F-HX4 shows a biodistribution dominated by activity in the bladder and steady renal clearance, with 60% of IA excreted in the 3 hours of the study. In the intermediate scans rapid clearance of activity is noted in all the organs, and in the last scans intestinal elimination as indicated by gut activity can be observed. Apart from urinary bladder, liver, kidneys, heart are seen to have above background activities in the early stages, with gut activity showing in later time points. All other organs displayed near the background level of activity.
For the adult male model, only the urinary bladder wall was estimated to receive radiation doses larger than 100 μGy/MBq. The mean absorbed dose of bladder wall was 131 ± 6.1 μGy/MBq and 415 ± 18 μGy/MBq for 1.0-hour and 4.8-hour bladder voiding intervals respectively. Testes received the second highest dose at ∼60 μGy/MBq. The organs in the digestive system has doses in the range of 8-32 μGy/MBq. The rest of the organs had lower doses. The mean values of the ED for the adult male were 25 ± 3.5 μSv/MBq (1.0 hour void interval) and 42 ± 4.2 μSv/MBq (4.8 hour void interval), respectively.
Comparison of Human and Monkey data
The monkey data includes fast sampling/dynamic PET scans (15 seconds per bed position) during the first few minutes followed by longer PET scans (4 minutes per bed position). The human data does not include the fast sampling but consists of sequential whole body PET scans at 6 minutes per bed position. A comparison of dosimetry for another 18F based imaging agent using similar data sets has shown that consistent dosimetry results are obtained without the fast sampling [17]. Hence the comparison of results between monkey and human data may be justified in spite of the difference in the acquisitions.
There are many similarities between the monkey and human data. The organs with the highest number of disintegrations per unit injected activity of 18F-HX4 are the bladder, liver and kidneys for both human and monkey data. The organ with the highest dose of 18F-HX4 is the bladder wall in both human and monkey cases. For both data the various organs of the digestive tract have similar doses. For humans, the doses of 18F-HX4 are in the range 9-24 μGy/MBq, whereas for monkeys, the doses of 18F-HX4 are in the range of 8-34 μGy/MBq. The effective dose is somewhat higher for monkeys (42 μSv/MBq) in comparison to humans (27 μSv/MBq). The estimated dose to testes is much higher in monkey data (60-65 μGy/MBq) than in human data (9-13 μGy/MBq). The monkey data is expected to overestimate the absorbed radiation dose to the testes, as monkey testes are at least twice as large as human testes.
Comparison to other 18F PET radiopharmaceuticals
Table 5 shows the doses per injected activity to individual organs for 18F-HX4, 18F-FDG (4.8 hour bladder voiding model)[18], 18F-FMISO and 18F-labeled fluoroerythronitroimidazole (FETNIM) (4 hour bladder voiding model) [9, 19]. The absorbed doses in heart and brain are much lower for 18F-HX4 compared to 18F-FDG, and the absorbed dose in urinary bladder is higher for 18F-HX4 than 18F-FDG. A large fraction (∼45% in 3.6 hours) of 18F-HX4 is excreted through urinary system whereas 18F-FMISO, has little urinary excretion (∼4% in 5 hours). 18F-FETNIM has an intermediate level of urinary excretion. Hence the urinary bladder dose for 18F-HX4 (299 μGy/MBq) is much higher than that for 18F-FETNIM and 18F-FMISO (127 μGy/MBq and 29 μGy/MBq respectively) (for 4.8/4 hour bladder voiding models). However, several steps can be taken to reduce the bladder dose. Patients can be encouraged to void frequently as the bladder dose reduces to ∼85±10 μGy/MBq in the 1 hour bladder voiding model. Having a near full bladder prior to injection can also reduce the bladder dose by diluting the bladder activity [20].
TABLE 5.
Organ doses in μGy/MBq for 18F-HX4, 18F-FDG, 18F-FMISO, 18F-FETNIM.
| HX4 | FDG | FMISO | FETNIM | |
|---|---|---|---|---|
| Adrenals | 9.6 | 13 | 16.6 | 12 |
| Brain | 5.3 | 19 | 8.6 | 6 |
| Breasts | 6.4 | 9.2 | 12.3 | 7 |
| Gallbladder Wall | 24 | 14 | 14.8 | 14 |
| Heart Wall | 8.7 | 60 | 18.5 | 11 |
| Kidneys | 20 | 20 | 15.7 | 27 |
| Liver | 14 | 16 | 18.3 | 24 |
| LLI Wall | 28 | 17 | 14.3 | 14 |
| Lungs | 7.7 | 17 | 9.9 | 8 |
| Muscle | 9.6 | 11 | 14.2 | 12 |
| Osteogenic Cells | 12 | 12 | 7.7 | 11 |
| Ovaries | 18 | 17 | 17.6 | 14 |
| Pancreas | 10 | 26 | 17.9 | 19 |
| Red Marrow | 8.8 | 13 | 10.9 | 12 |
| Skin | 6.7 | 8.4 | 4.8 | 7 |
| Small Intestine | 17 | 14 | 13.2 | 12 |
| Spleen | 8.9 | 37 | 16.3 | 20 |
| Stomach Wall | 9.4 | 13 | 12.6 | 12 |
| Testes | 13 | 13 | 14.6 | 11 |
| Thymus | 7.8 | 12 | 15.5 | 9 |
| Thyroid | 7.7 | 10 | 15.1 | 9 |
| ULI Wall | 18 | 13 | 14 | 15 |
| Urinary Bladder Wall | 299 | 190 | 29 | 127 |
| Uterus | 28 | 23 | 18.3 | 18 |
HX4 and FDG data are using 4.8-h bladder voiding model, FMISO and FETNIM data using 4-h bladder voiding model.
Table 6 compares whole body radiation dose and effective dose from 18F-HX4 to dose from a few 18F based radiopharmaceuticals [9, 18, 19]. As shown in these tables, the radiation dose from 18F-HX4 is comparable to that from other 18F-based imaging agents, assuming similar injected activities for all the imaging agents. The effective dose of 18F-HX4 can be reduced considerably with frequent voiding.
Table 6.
| HX4 1 h void |
HX4 4.8 h void |
FDG 4.8 h void |
FMISO 4 h void |
FETNIM 2 h void |
FETNIM 4 h void |
|
|---|---|---|---|---|---|---|
| Total Body Dose (μGy/MBq) | 8 | 10 | 11 | 13 | 11 | 11 |
| Effective Dose (μSv/MBq) | 14 | 27 | 19 | - | 15 | 19 |
In summary, biodistribution of the hypoxia PET imaging agent 18F-HX4 has been measured in four humans and three monkeys using sequential PET/CT scans. Over 80% of 18F-HX4 maintains its integrity during the two hours following injection and it clears quickly through the renal system. The urinary bladder wall has the highest absorbed dose among all the organs. The dose to bladder can be reduced by encouraging adequate hydration and frequent voiding. The absorbed radiation dose from 18F-HX4 is similar to that of 18F-FDG and 18F-based hypoxia-imaging agents. Our results indicate that high quality clinical PET/CT images can be obtained shortly after the injection of 18F-HX4. 18F-HX4 biodistribution in monkeys has many similarities to its biodistribution in humans, with the estimated effective dose determined from monkey data being somewhat higher than that from human data. Considered together, its radiation absorbed dose, integrity in vivo, and rapid renal clearance make the putative hypoxia tracer 18F-HX4 favorable for further use in humans.
Acknowledgments
We thank Donna Mosley, Fox Chase Cancer Center, for help with patient data acquisition. We thank Stephen M. Krause, Merck and Co. for rhesus data acquisition. We thank Mary Benetz and staff from Protocol Management Office and Clinical Research Unit at Fox Chase Cancer Center for their help with the research protocol. We thank Dr. Joseph Walsh from Siemens for the review of description and synthesis of 18F-HX4. We also thank Dinko González Trotter, Ph.D. for careful review of the manuscript. The human part of the study was conducted at Fox Chase Cancer Center under the ClinicalTrials.gov Identifier NCT00606424. This study was supported by a grant from Siemens Molecular Imaging Biomarker Research.
Contributor Information
Mohan Doss, Diagnostic Imaging, Fox Chase Cancer Center, Philadelphia. PA.
James J. Zhang, Biomarker Research, Siemens Molecular Imaging Inc., Culver City, CA
Marie-José Bélanger, Imaging, Merck and Co., West Point, PA.
James B. Stubbs, Radiation Dosimetry Systems, Inc, Palo Alto, CA
Eric D. Hostetler, Imaging, Merck and Co., West Point, PA
R. Katherine Alpaugh, Protocol Lab, Fox Chase Cancer Center, Philadelphia, PA.
Hartmuth C. Kolb, Biomarker Research, Siemens Molecular Imaging Inc., Culver City, CA
Jian Q. Yu, Diagnostic Imaging, Fox Chase Cancer Center, Philadelphia. PA
References
- 1.Lucignani G. PET imaging with hypoxia tracers: a must in radiation therapy. Eur J Nucl Med Mol Imaging. 2008;35:838–42. doi: 10.1007/s00259-008-0740-2. [DOI] [PubMed] [Google Scholar]
- 2.Tatum JL, Kelloff GJ, Gillies RJ, Arbeit JM, Brown JM, Chao KS, et al. Hypoxia: importance in tumor biology, noninvasive measurement by imaging, and value of its measurement in the management of cancer therapy. Int J Radiat Biol. 2006;82:699–757. doi: 10.1080/09553000601002324. [DOI] [PubMed] [Google Scholar]
- 3.Vaupel P. Hypoxia and aggressive tumor phenotype: implications for therapy and prognosis. Oncologist. 2008;13 3:21–6. doi: 10.1634/theoncologist.13-S3-21. [DOI] [PubMed] [Google Scholar]
- 4.Zeng L, Ou G, Itasaka S, Harada H, Xie X, Shibuya K, et al. TS-1 enhances the effect of radiotherapy by suppressing radiation-induced hypoxia-inducible factor-1 activation and inducing endothelial cell apoptosis. Cancer Sci. 2008;99:2327–35. doi: 10.1111/j.1349-7006.2008.00943.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zaffaroni N, Fiorentini G, De Giorgi U. Hyperthermia and hypoxia: new developments in anticancer chemotherapy. Eur J Surg Oncol. 2001;27:340–2. doi: 10.1053/ejso.2000.1040. [DOI] [PubMed] [Google Scholar]
- 6.Yamazaki Y, Kunimoto S, Ikeda D. Rakicidin A: a hypoxia-selective cytotoxin. Biol Pharm Bull. 2007;30:261–5. doi: 10.1248/bpb.30.261. [DOI] [PubMed] [Google Scholar]
- 7.Rajendran JG, Hendrickson KR, Spence AM, Muzi M, Krohn KA, Mankoff DA. Hypoxia imaging-directed radiation treatment planning. Eur J Nucl Med Mol Imaging. 2006;33 1:44–53. doi: 10.1007/s00259-006-0135-1. [DOI] [PubMed] [Google Scholar]
- 8.Mees G, Dierckx R, Vangestel C, Van de Wiele C. Molecular imaging of hypoxia with radiolabelled agents. Eur J Nucl Med Mol Imaging. 2009;36:1674–86. doi: 10.1007/s00259-009-1195-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Graham MM, Peterson LM, Link JM, Evans ML, Rasey JS, Koh WJ, et al. Fluorine-18-fluoromisonidazole radiation dosimetry in imaging studies. J Nucl Med. 1997;38:1631–6. [PubMed] [Google Scholar]
- 10.Kolb HC, Finn MG, Sharpless KB. Click Chemistry: Diverse Chemical Function from a Few Good Reactions. Angew Chem Int Ed Engl. 2001;40:2004–21. doi: 10.1002/1521-3773(20010601)40:11<2004::AID-ANIE2004>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 11.Rostovtsev VV, Green LG, Fokin VV, Sharpless KB. A stepwise huisgen cycloaddition process: copper(I)-catalyzed regioselective “ligation” of azides and terminal alkynes. Angew Chem Int Ed Engl. 2002;41:2596–9. doi: 10.1002/1521-3773(20020715)41:14<2596::AID-ANIE2596>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 12.Tornoe CW, Christensen C, Meldal M. Peptidotriazoles on solid phase: [1,2,3]-triazoles by regiospecific copper(i)-catalyzed 1,3-dipolar cycloadditions of terminal alkynes to azides. J Org Chem. 2002;67:3057–64. doi: 10.1021/jo011148j. [DOI] [PubMed] [Google Scholar]
- 13.van Loon J, Janssen MH, Ollers M, Aerts HJ, Dubois L, Hochstenbag M, et al. PET imaging of hypoxia using [18F]HX4: a phase I trial. Eur J Nucl Med Mol Imaging. 37:1663–8. doi: 10.1007/s00259-010-1437-x. [DOI] [PubMed] [Google Scholar]
- 14.Stabin MG, Sparks RB, Crowe E. OLINDA/EXM: the second-generation personal computer software for internal dose assessment in nuclear medicine. J Nucl Med. 2005;46:1023–7. [PubMed] [Google Scholar]
- 15.Barrett PH, Bell BM, Cobelli C, Golde H, Schumitzky A, Vicini P, et al. SAAM II: Simulation, Analysis, and Modeling Software for tracer and pharmacokinetic studies. Metabolism. 1998;47:484–92. doi: 10.1016/s0026-0495(98)90064-6. [DOI] [PubMed] [Google Scholar]
- 16.Cloutier RJ, Smith SA, Watson EE, Snyder WS, Warner GG. Dose to the fetus from radionuclides in the bladder. Health Phys. 1973;25:147–61. doi: 10.1097/00004032-197308000-00009. [DOI] [PubMed] [Google Scholar]
- 17.Herzog H, Elmenhorst D, Winz O, Bauer A. Biodistribution and radiation dosimetry of the A1 adenosine receptor ligand 18F-CPFPX determined from human whole-body PET. Eur J Nucl Med Mol Imaging. 2008;35:1499–506. doi: 10.1007/s00259-008-0753-x. [DOI] [PubMed] [Google Scholar]
- 18.Stabin M, Stubbs J, Toohey R. Radiation Dose Estimates from Radiopharmaceuticals, Report NUREG/CR-6345 prepared for US Nuclear Regulatory Commission by Oak Ridge Institute for Science and Education. Oak Ridge, TN: 1996. URL: http://www.nrc.gov/reading-rm/doc-collections/nuregs/contract/cr6345/cr6345.pdf. [Google Scholar]
- 19.Tolvanen T, Lehtio K, Kulmala J, Oikonen V, Eskola O, Bergman J, et al. 18F-Fluoroerythronitroimidazole radiation dosimetry in cancer studies. J Nucl Med. 2002;43:1674–80. [PubMed] [Google Scholar]
- 20.Dowd MT, Chen CT, Wendel MJ, Faulhaber PJ, Cooper MD. Radiation dose to the bladder wall from 2-[18F]fluoro-2-deoxy-D-glucose in adult humans. J Nucl Med. 1991;32:707–12. [PubMed] [Google Scholar]


