Abstract
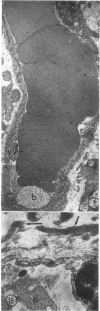
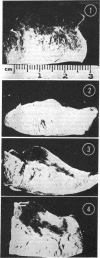
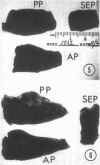
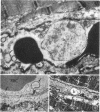
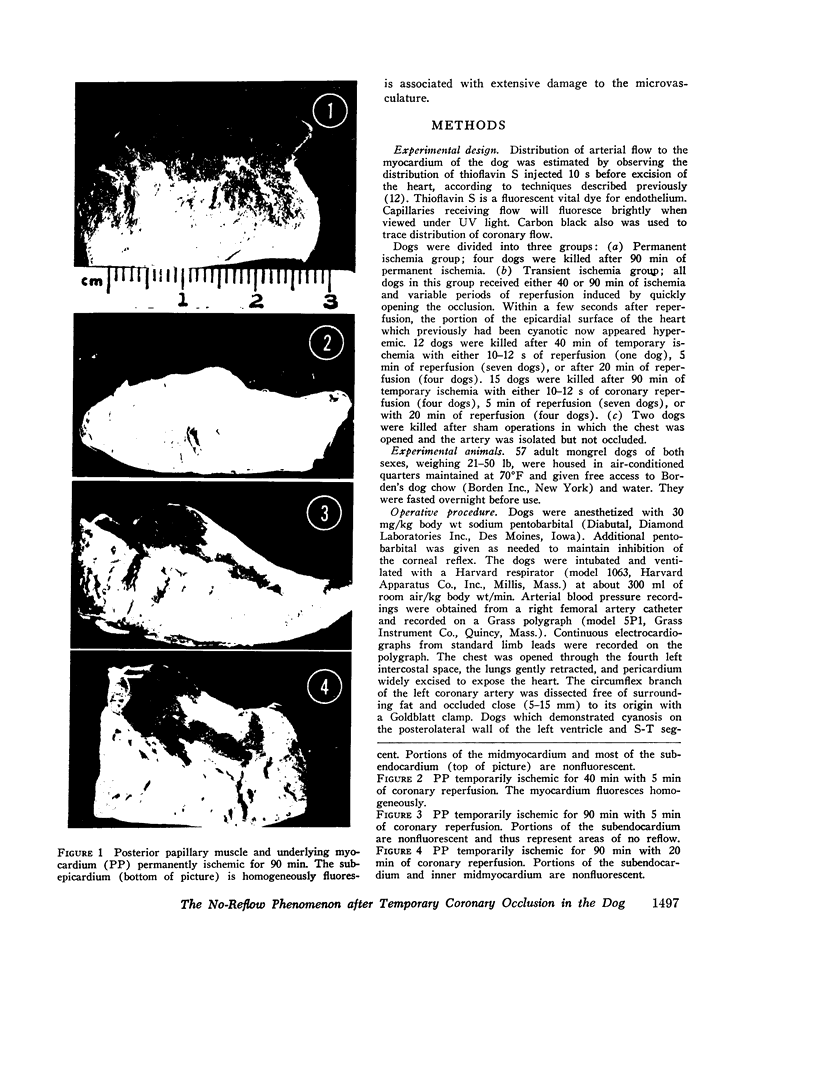
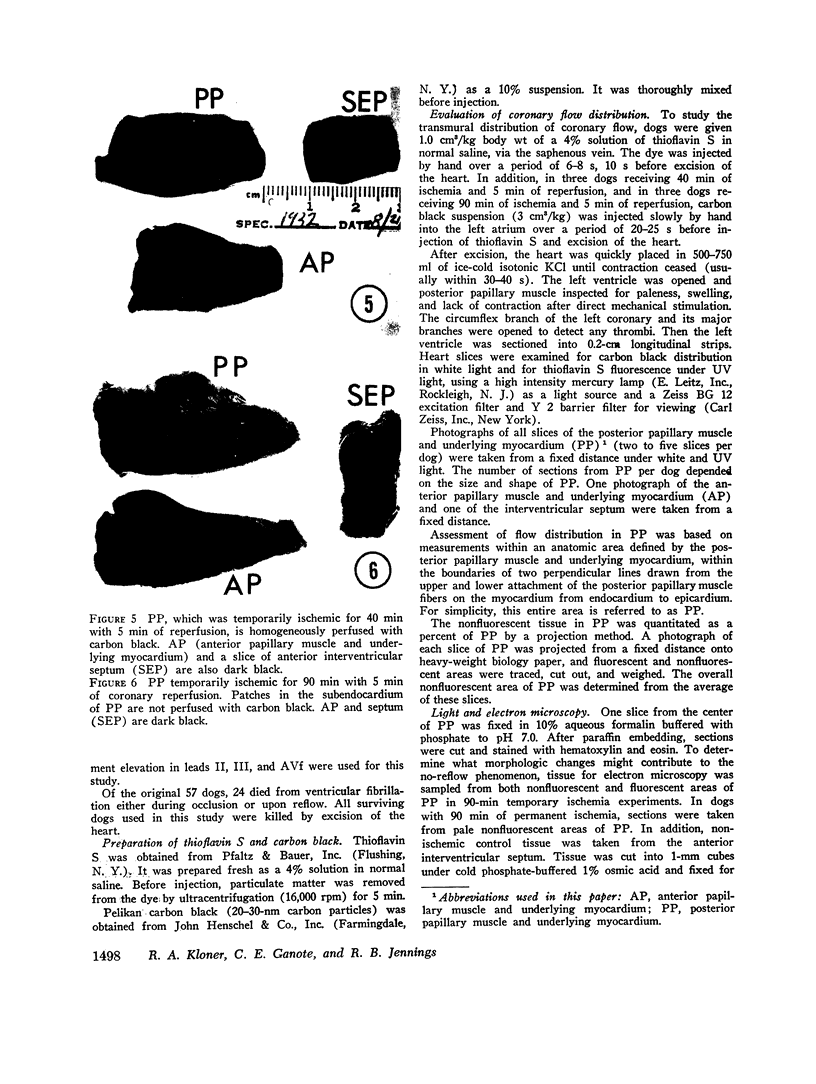
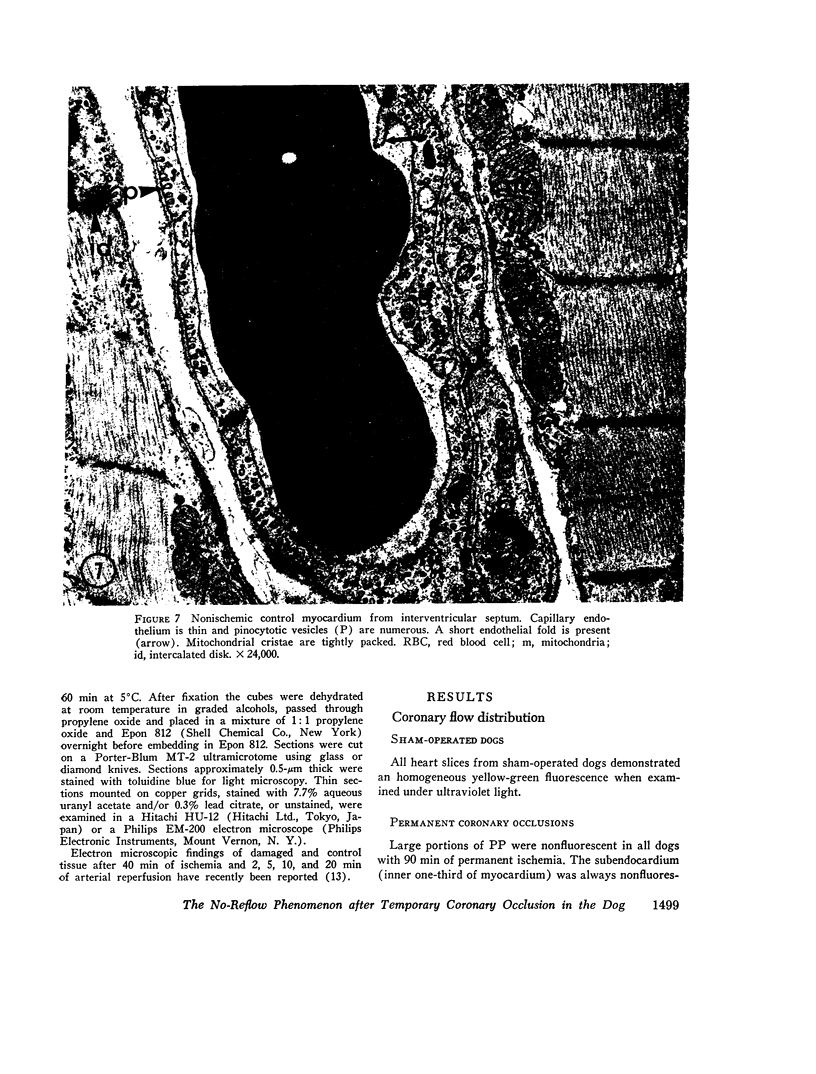
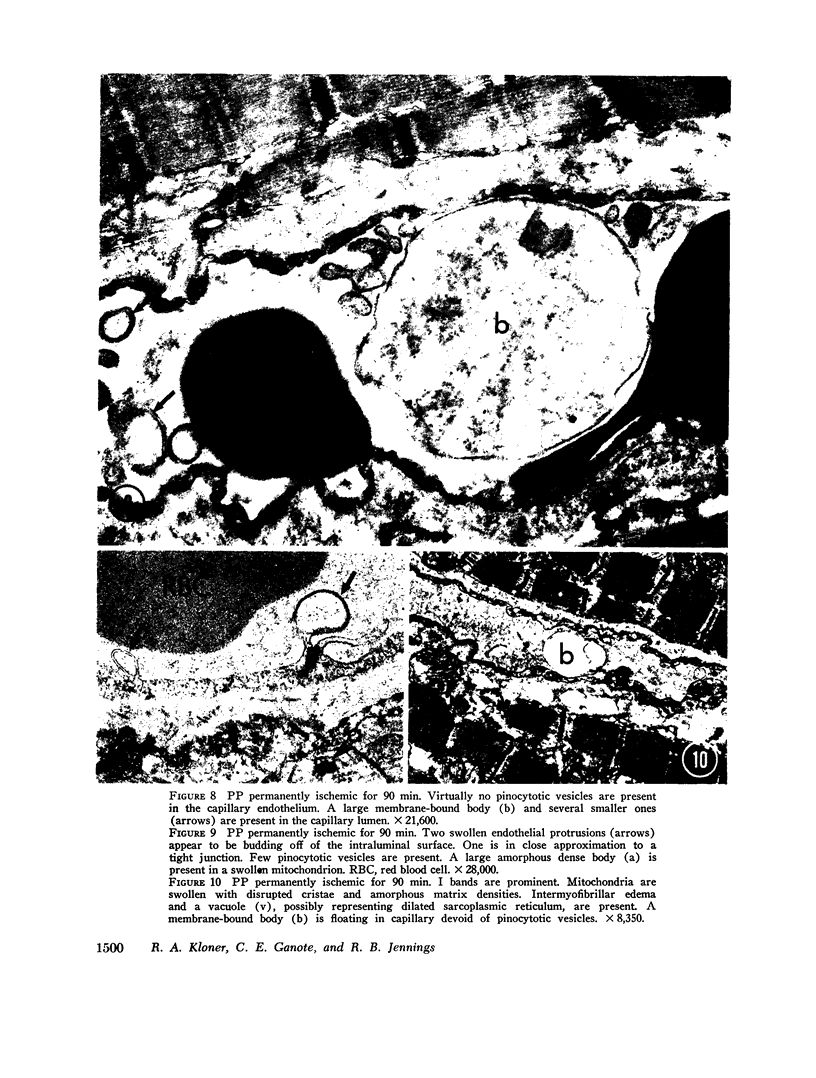
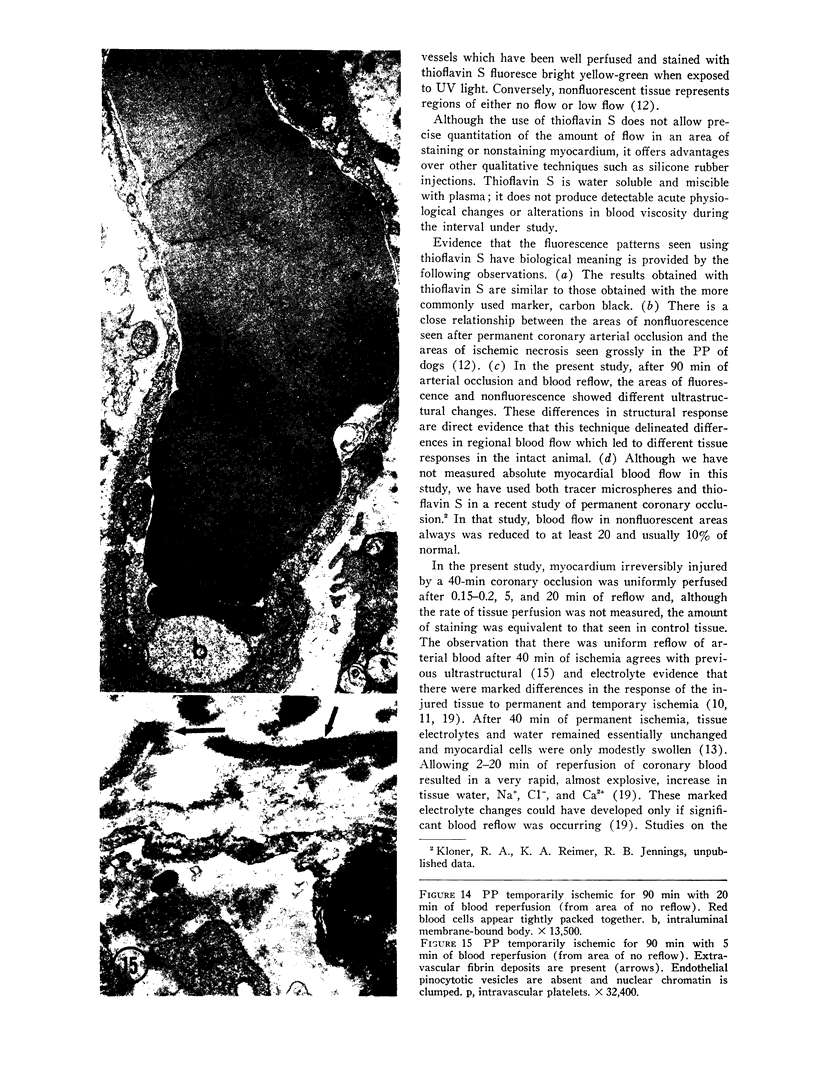
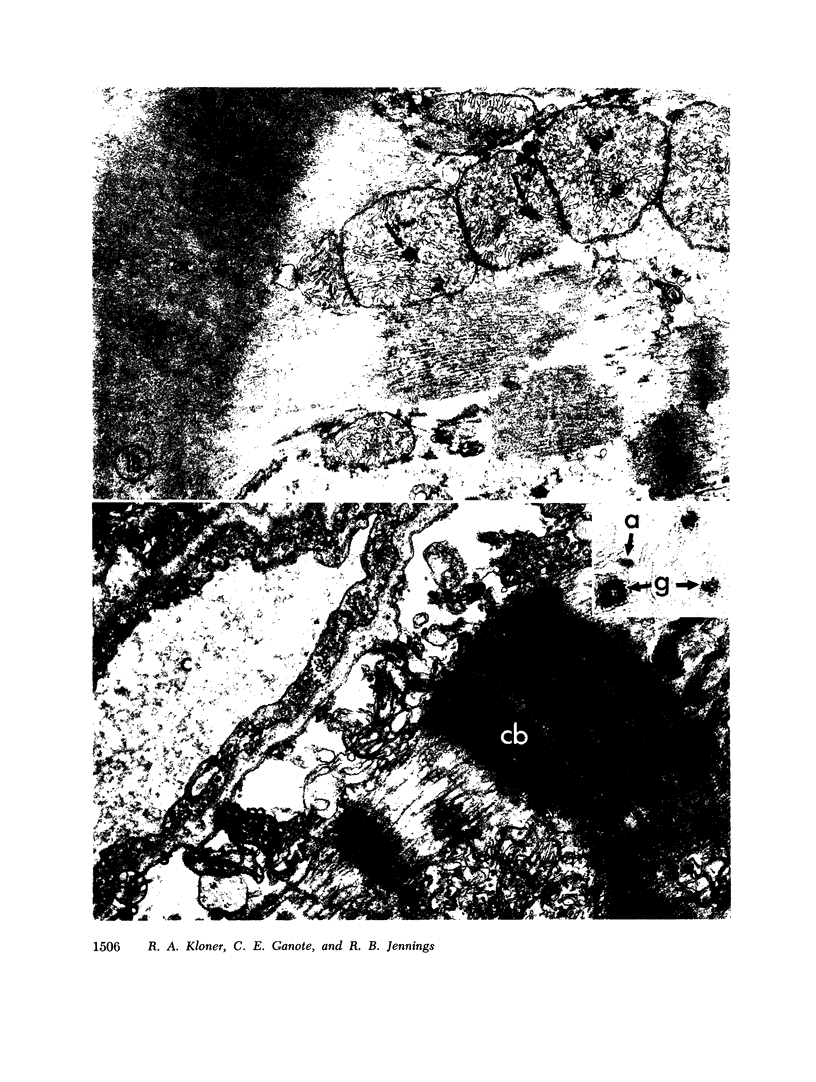
The role of microvascular damage in the genesis of the “no-reflow” phenomenon was investigated in the left ventricular myocardium of dogs subjected to temporary occlusions of a major coronary artery for 40 and 90 min. Intravenous carbon black or thioflavin S (a fluorescent vital stain for endothelium) were used to demonstrate the distribution of coronary arterial flow in control and damaged myocardium. These tracers were injected simultaneously with release of the coronary occlusion or after 5 or 20 min of reflow of coronary arterial blood. After 40 min of ischemia plus arterial reperfusion, usually the tracers were evenly distributed throughout the damaged tissue at each time of reperfusion. On the other hand, when reflow was allowed after 90 min of ischemia, portions of the inner half of damaged myocardium were not penetrated by the tracers. Electron microscopic study of this poorly perfused tissue revealed severe capillary damage; endothelial cells with large intraluminal protrusions and decreased pinocytic vesicles were common. Also, occasional intraluminal fibrin thrombi were noted, as well as extravascular fibrin deposits and erythrocytes. Myocardial cells were swollen in both poorly perfused and well-perfused irreversibly injured tissue. Contraction bands and mitochondrial Ca2+ accumulation were prominent features of irreversible injury with reflow at 40 min but were not noted after 90 min of ischemia in areas with poor perfusion. These results suggest that 40 min of ischemia were tolerated by the capillary bed of the dog heart without serious capillary damage or perfusion defects, but that 90 min of ischemic injury was associated with the “no-reflow” phenomenon, i.e., failure to achieve uniform reperfusion. This failure of reflow was associated with extensive capillary damage and myocardial cell swelling. Death of severely ischemic myocardial cells in this model occurs before the onset of capillary damage and the no-reflow phenomenon.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Buckberg G. D., Luck J. C., Payne D. B., Hoffman J. I., Archie J. P., Fixler D. E. Some sources of error in measuring regional blood flow with radioactive microspheres. J Appl Physiol. 1971 Oct;31(4):598–604. doi: 10.1152/jappl.1971.31.4.598. [DOI] [PubMed] [Google Scholar]
- CAULFIELD J., KLIONSKY B. Myocardial ischemia and early infarction: an electron microscopic study. Am J Pathol. 1959 May-Jun;35(3):489–523. [PMC free article] [PubMed] [Google Scholar]
- Chiang J., Kowada M., Ames A., 3rd, Wright R. L., Majno G. Cerebral ischemia. III. Vascular changes. Am J Pathol. 1968 Feb;52(2):455–476. [PMC free article] [PubMed] [Google Scholar]
- Dohrmann G. J., Wick K. M., Bucy P. C. Spinal cord blood flow patterns in experimental traumatic paraplegia. J Neurosurg. 1973 Jan;38(1):52–58. doi: 10.3171/jns.1973.38.1.0052. [DOI] [PubMed] [Google Scholar]
- Fischer E. G., Ames 3d A. Studies on mechanisms of impairment of cerebral circulation following ischemia: effect of hemodilution and perfusion pressure. Stroke. 1972 Sep-Oct;3(5):538–542. doi: 10.1161/01.str.3.5.538. [DOI] [PubMed] [Google Scholar]
- Flores J., DiBona D. R., Beck C. H., Leaf A. The role of cell swelling in ischemic renal damage and the protective effect of hypertonic solute. J Clin Invest. 1972 Jan;51(1):118–126. doi: 10.1172/JCI106781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GOLDBERG B., GREEN H. The cytotoxic action of immune gamma globulin and complement on Krebs ascites tumor cells. I. Ultrastructural studies. J Exp Med. 1959 May 1;109(5):505–510. doi: 10.1084/jem.109.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HERDSON P. B., SOMMERS H. M., JENNINGS R. B. A COMPARATIVE STUDY OF THE FINE STRUCTURE OF NORMAL AND ISCHEMIC DOG MYOCARDIUM WITH SPECIAL REFERENCE TO EARLY CHANGES FOLLOWING TEMPORARY OCCLUSION OF A CORONARY ARTERY. Am J Pathol. 1965 Mar;46:367–386. [PMC free article] [PubMed] [Google Scholar]
- JENNINGS R. B., BAUM J. H., HERDSON P. B. FINE STRUCTURAL CHANGES IN MYOCARDIAL ISCHEMIC INJURY. Arch Pathol. 1965 Feb;79:135–143. [PubMed] [Google Scholar]
- Kloner R. A., Ganote C. E., Whalen D. A., Jr, Jennings R. B. Effect of a transient period of ischemia on myocardial cells. II. Fine structure during the first few minutes of reflow. Am J Pathol. 1974 Mar;74(3):399–422. [PMC free article] [PubMed] [Google Scholar]
- Kowada M., Ames A., 3rd, Majno G., Wright R. L. Cerebral ischemia. I. An improved experimental method for study; cardiovascular effects and demonstration of an early vascular lesion in the rabbit. J Neurosurg. 1968 Feb;28(2):150–157. doi: 10.3171/jns.1968.28.2.0150. [DOI] [PubMed] [Google Scholar]
- Krug A., Du Mesnil de Rochemont, Korb G. Blood supply of the myocardium after temporary coronary occlusion. Circ Res. 1966 Jul;19(1):57–62. doi: 10.1161/01.res.19.1.57. [DOI] [PubMed] [Google Scholar]
- Lang T. W., Corday E., Gold H., Meerbaum S., Rubins S., Costantini C., Hirose S., Osher J., Rosen V. Consequences of reperfusion after coronary occlusion. Effects on hemodynamic and regional myocardial metabolic function. Am J Cardiol. 1974 Jan;33(1):69–81. doi: 10.1016/0002-9149(74)90741-3. [DOI] [PubMed] [Google Scholar]
- Leaf A. Cell swelling. A factor in ischemic tissue injury. Circulation. 1973 Sep;48(3):455–458. doi: 10.1161/01.cir.48.3.455. [DOI] [PubMed] [Google Scholar]
- Leaf A. Regulation of intracellular fluid volume and disease. Am J Med. 1970 Sep;49(3):291–295. doi: 10.1016/s0002-9343(70)80019-5. [DOI] [PubMed] [Google Scholar]
- SCHLEGEL J. U. Demonstration of blood vessels and lymphatics with a fluorescent dye in ultraviolet light. Anat Rec. 1949 Nov;105(3):433-43, incl 2 pl. doi: 10.1002/ar.1091050305. [DOI] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Kinetics of calcium accumulation in acute myocardial ischemic injury. Am J Pathol. 1972 Jun;67(3):441–452. [PMC free article] [PubMed] [Google Scholar]
- Shen A. C., Jennings R. B. Myocardial calcium and magnesium in acute ischemic injury. Am J Pathol. 1972 Jun;67(3):417–440. [PMC free article] [PubMed] [Google Scholar]
- Summers W. K., Jamison R. L. The no reflow phenomenon in renal ischemia. Lab Invest. 1971 Dec;25(6):635–643. [PubMed] [Google Scholar]
- Whalen D. A., Jr, Hamilton D. G., Ganote C. E., Jennings R. B. Effect of a transient period of ischemia on myocardial cells. I. Effects on cell volume regulation. Am J Pathol. 1974 Mar;74(3):381–397. [PMC free article] [PubMed] [Google Scholar]
- Willerson J. T., Powell W. J., Jr, Guiney T. E., Stark J. J., Sanders C. A., Leaf A. Improvement in myocardial function and coronary blood flow in ischemic myocardium after mannitol. J Clin Invest. 1972 Dec;51(12):2989–2998. doi: 10.1172/JCI107126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willms-Kretschmer K., Majno G. Ischemia of the skin. Electron microscopic study of vascular injury. Am J Pathol. 1969 Mar;54(3):327–353. [PMC free article] [PubMed] [Google Scholar]
- ZOLLINGER H. U. Cytologic studies with the phase Microscope; the formation of blisters on cells in suspension, photocytosis, with observations on the nature of the cellular membrane. Am J Pathol. 1948 May;24(3):545–567. [PMC free article] [PubMed] [Google Scholar]