Cellular motors are fascinating machines that function by undergoing successive conformational changes that require joints in their structure. Where these are located is particularly critical for molecular motors that produce force with relatively rigid lever arms, such as myosins (1). A long-standing paradox in myosin function may finally be understood from structural insights provided by Cohen and colleagues in PNAS (2). Although highly flexible joints are necessary to turn off their activity, these molecular motors have evolved to control such compliance in active heads to produce force under various strain. The structure of the light chain-binding domain (LCD) of scallop catch muscle myosin II (2) describes a unique hinge in this lever arm that could be essential for the regulation of the motor and might also help explain how strain can promote the attachment of the second head of the myosin molecule to the actin filament in isometric contraction (3) and the efficient increase of the resistance of active muscle to stretch (4).
Myosin II forms the thick filaments of muscle that slide against the actin-containing thin filaments to allow muscle contraction. Nonmuscle myosins II are also critical for a number of functions in all eukaryotic cells, including cell migration and cytokinesis. To convert chemical energy into force production, these motors amplify the conformational changes of their motor domain, thanks to a lever arm composed of a converter subdomain followed by a C-terminal elongated region of the myosin head or LCD. The swing of the lever arm upon strong binding to actin is coupled with the release of the ATPase products and produces a stroke of approximately 10 nm (1) (Fig. 1A). The LCD of myosin II is a ternary complex composed of a long heavy chain α-helical segment (containing two special IQ motifs) stabilized by the recruitment of members of the calmodulin superfamily, namely an essential light chain (ELC) on IQ1 close to the converter and a distal regulatory light chain (RLC) on IQ2. The “WxW” motif in this IQ2 is characteristic and corresponds to the hook region where a rather acute bend occurs in the heavy chain. Myosins II are double-headed molecules, and the region that follows IQ2 corresponds to a dimeric heavy chain coiled-coil. Although the first part of the coiled-coil or S2 fragment allows the two myosin heads to be connected to the thick filament via an elongated rope, the remainder of the coiled-coil contains triggering sequences that allow assembly of the myosin molecules to form the helical thick (striated muscle) or side-polar filaments (smooth muscle).
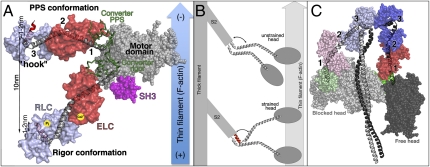
Fig. 1.
(A) Myosin powerstroke is illustrated with the motor domain (including SH3 domain in pink) bound to F-actin and with the lever arm (converter and LCD) in two positions: the primed (prepowerstroke, PPS) and down (Rigor) positions. Three hinges (1–3) are indicated. The newly identified “ankle” hinge involves the characteristic “hook” in the RLC binding region. The angle about the hook can be either obtuse (gray helix) or acute (red helix). (B) The unstrained myosin II dimer binds to F-actin using only one head. Strain applied to the dimer in combination with the structural change in the ankle region may promote binding of the second head to the actin filament. (C) The off state of myosin II promotes asymmetric interactions between the two heads (depicted using similar colors as in A) and the S2 coiled-coil region (gray) of the molecule.
The ATPase and actin binding potential of these powerful motors need to be controlled in the cell, and failure to do so can promote the development of cancers (5). Although thin filament regulation is predominant in striated muscles, myosin-linked (thick filament) regulation was discovered in the 1970s with molluscan catch muscle (6), in which direct Ca2+ binding on the ELC is required to switch on myosin activity. In smooth and nonmuscle myosin II, Ca2+ activates the myosin light chain kinase that phosphorylates the RLC at position S19 (7).
It is becoming clear that the sequence of these motors has evolved not only to optimize their motor activity but also to conserve the features necessary for the switch between inactivated (“off”) and activated (“on”) states of the motor. Regulatory sites within the LCD control the conformation of the hinges to promote intramolecular interactions between the two heads and the S2 region in the off state (dephosphorylated or Ca2+-free state) (8–11). Phosphorylated or Ca2+-bound LCD activates the motor because both heads become free and disordered and can interact with actin to produce force (12).
As an extension of the converter subdomain, the LCD is a major component of the lever arm and as such must be relatively rigid. Because flexibility in the LCD is necessary for myosin regulation, it is critical to identify the hinges in the LCD, how they operate depending on the state of the motor, and how they are controlled. Thus, the study published in PNAS (2) is remarkable in that it reveals that variation can occur in the angle of the hook. This provides insights regarding how the distal “ankle” joint of the myosin motor head may operate.
In addition to the compliance within the motor domain, three hinges have now been identified in the myosin II lever arm (Fig. 1). A pliant region between the converter and the ELC (13, 14) can operate in the primed ADP.Pi state of the motor and allows exploration of various lateral orientations of the heads that likely contribute to efficiency in rebinding to the actin filament. In states strongly bound to actin, the presence of the N-terminal and SH3 subdomains of the motor next to the ELC limits the compliance at this point for heads in which the lever arm has swung to a down position (Fig. 1A). The second joint in the LCD corresponds to the ELC/RLC interface, but it is likely relatively rigid in active heads. Binding of Ca2+ at this interface in molluscan myosins requires formation of very specific bonds that restrict the pliancy in this region (15). In contrast, this joint could play a critical role in allowing the Ca2+-free heads to adopt an off state and thus shut down their activity (16).
The third joint is described in atomic details by Cohen and colleagues in PNAS (2). Two distinct conformations of the ankle joint are observed for the same unphosphorylated myosin LCD, and interestingly only one seems appropriate for anchoring the N-terminal extension of the RLC within the N-lobe (when the hook region is most obtuse and the D helix of the RLC stays straight). Note that fluorescence resonance energy transfer experiments have also identified two states both for unphosphorylated and phosphorylated heads (17). Additional structural studies are, however, necessary to depict whether the phosphorylated serine of this RLC extension could fit within the RLC N-lobe or whether it would in fact disfavor the obtuse hook conformation.
In the context of an active myosin head, it is interesting that the change in this joint would correspond to a ≈1- to 2-nm axial compliance for heads both before and after force generation (Fig. 1A). It is thus likely that this ankle joint is sensitive to strain and is part of the 1.7-nm compliance that resides in the cross-bridges when the muscle undergoes isometric contraction (18). Upon stretching, strain also likely changes the ankle of the attached head, promoting binding of the second head (4) (Fig. 1B). The RLC phosphorylation modulates the contractile performance in striated skeletal muscle by promoting tension development at low calcium levels (19, 20). These effects are due to an increase in the apparent cross-bridge attachment rate. Although the mechanism is still unknown, phosphorylation-dependent modulation of the ankle conformation may provide an important clue because differences in the hook angles for the two heads of the myosin molecule could greatly favor the recruitment of the second head on actin filaments (Fig. 1B).
The off state of different myosins II has been visualized by a number of EM studies (9–11) that agree on an evolutionarily conserved asymmetric disposition of the two heads that interact with the S2 region and create a number of asymmetric interactions between the motor domains and the two RLCs of the myosin molecules (Fig. 1C). The most important hinge to allow such asymmetric interactions seems to reside at the motor domain/LCD (converter/ELC) interface, with a major difference in the pliant region of the two molecules. From a symmetric coiled-coil, it is unclear, however, whether differences in the head/S2 junction and in the pliant region are sufficient to promote the asymmetric intramolecular interactions characteristic of the off state. The unique scallop structures (2) indicate that different hook conformations might be used in the off state to promote the asymmetry between the heads. The current resolution of the EM studies is, however, too low to reveal whether the two LCD adopt different conformations either at the ELC/RLC interface or in the hook/RLC region of the molecule. Higher-resolution structures of the off state and of the phosphorylated LCD are required to reveal the detailed mechanism of these switches. However, the current structures provide a framework for the design of exciting experiments with doubly labeled RLC that should give a more precise picture of the dynamics of these joints in on and off states of the motor. Thus, the strain dependence of these hinges depending on RLC phosphorylation can now be precisely investigated. This should reveal how the ankle contributes to optimizing the number of heads and the amount of time heads stay bound to actin in isometric contraction. It will also be interesting to study how this hinge operates when a dimeric processive nonmuscle myosin IIb motor cycles along an actin filament (21). Importantly, mutations in either the RLC or in the myosin light chain kinase have been reported to cause human myopathies and cardiac hypertrophy (22, 23). The next challenge is thus to understand how the myosin ankle functions upon phosphorylation of the RLC. This is essential because it is likely exploited for a number of cellular roles, such as proper function of the heart, which requires a spatial gradient of the myosin RLC phosphorylation (23).
Footnotes
The authors declare no conflict of interest.
See companion article on page 114.
References
- 1.Geeves MA, Holmes KC. Structural mechanism of muscle contraction. Annu Rev Biochem. 1999;68:687–728. doi: 10.1146/annurev.biochem.68.1.687. [DOI] [PubMed] [Google Scholar]
- 2.Brown JH, Kumar VSS, O'Neall-Hennessey E, Reshetnikova L, Robinson H, Nguyen-McCarty M, Szent-Györgyi AG, Cohen C. Visualizing key hinges and a potential major source of compliance in the lever arm of myosin. Proc Natl Acad Sci USA. 2011;108:114–119. doi: 10.1073/pnas.1016288107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wu S, et al. Electron tomography of cryofixed, isometrically contracting insect flight muscle reveals novel actin-myosin interactions. PLoS ONE. 2010;5:5. doi: 10.1371/journal.pone.0012643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Fusi L, et al. The mechanism of the resistance to stretch of isometrically contracting single muscle fibres. J Physiol. 2010;588:495–510. doi: 10.1113/jphysiol.2009.178137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alhopuro P, et al. Unregulated smooth-muscle myosin in human intestinal neoplasia. Proc Natl Acad Sci USA. 2008;105:5513–5518. doi: 10.1073/pnas.0801213105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Kendrick-Jones J, Lehman W, Szent-Györgyi AG. Regulation in molluscan muscles. J Mol Biol. 1970;54:313–326. doi: 10.1016/0022-2836(70)90432-8. [DOI] [PubMed] [Google Scholar]
- 7.Sellers JR. Regulation of cytoplasmic and smooth muscle myosin. Curr Opin Cell Biol. 1991;3:98–104. doi: 10.1016/0955-0674(91)90171-t. [DOI] [PubMed] [Google Scholar]
- 8.Wendt T, Taylor D, Trybus KM, Taylor K. 3-D image reconstruction of dephosphorylated smooth muscle heavy meromyosin reveals asymmetry in the interaction between myosin heads and placement of subfragment 2. Proc Natl Acad Sci USA. 2000;98:4361–4366. doi: 10.1073/pnas.071051098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Woodhead JL, et al. Atomic model of a myosin filament in the relaxed state. Nature. 2005;436:1195–1199. doi: 10.1038/nature03920. [DOI] [PubMed] [Google Scholar]
- 10.Jung HS, et al. Conservation of the regulated structure of folded myosin 2 in species separated by at least 600 million years of independent evolution. Proc Natl Acad Sci USA. 2008;105:6022–6026. doi: 10.1073/pnas.0707846105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Alamo L, et al. Three-dimensional reconstruction of tarantula myosin filaments suggests how phosphorylation may regulate myosin activity. J Mol Biol. 2008;384:780–797. doi: 10.1016/j.jmb.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Taylor KA, et al. Tomographic 3D reconstruction of quick-frozen, Ca2+-activated contracting insect flight muscle. Cell. 1999;99:421–431. doi: 10.1016/s0092-8674(00)81528-7. [DOI] [PubMed] [Google Scholar]
- 13.Houdusse A, Szent-Gyorgyi AG, Cohen C. Three conformational states of scallop myosin S1. Proc Natl Acad Sci USA. 2000;97:11238–11243. doi: 10.1073/pnas.200376897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Thomas DD, Kast D, Korman VL. Site-directed spectroscopic probes of actomyosin structural dynamics. Annu Rev Biophys. 2009;38:347–369. doi: 10.1146/annurev.biophys.35.040405.102118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Houdusse A, Cohen C. Structure of the regulatory domain of scallop myosin at 2 A resolution: Implications for regulation. Structure. 1996;4:21–32. doi: 10.1016/s0969-2126(96)00006-8. [DOI] [PubMed] [Google Scholar]
- 16.Himmel DM, Mui S, O'Neall-Hennessey E, Szent-Györgyi AG, Cohen C. The on-off switch in regulated myosins: Different triggers but related mechanisms. J Mol Biol. 2009;394:496–505. doi: 10.1016/j.jmb.2009.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kast D, Espinoza-Fonseca LM, Yi C, Thomas DD. Phosphorylation-induced structural changes in smooth muscle myosin regulatory light chain. Proc Natl Acad Sci USA. 2010;107:8207–8212. doi: 10.1073/pnas.1001941107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Piazzesi G, et al. Skeletal muscle performance determined by modulation of number of myosin motors rather than motor force or stroke size. Cell. 2007;131:784–795. doi: 10.1016/j.cell.2007.09.045. [DOI] [PubMed] [Google Scholar]
- 19.Metzger JM, Greaser ML, Moss RL. Variations in cross-bridge attachment rate and tension with phosphorylation of myosin in mammalian skinned skeletal muscle fibers. Implications for twitch potentiation in intact muscle. J Gen Physiol. 1989;93:855–883. doi: 10.1085/jgp.93.5.855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Sweeney HL, Stull JT. Alteration of cross-bridge kinetics by myosin light chain phosphorylation in rabbit skeletal muscle: Implications for regulation of actin-myosin interaction. Proc Natl Acad Sci USA. 1990;87:414–418. doi: 10.1073/pnas.87.1.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Norstrom MF, Smithback PA, Rock RS. Unconventional processive mechanics of non-muscle myosin IIB. J Biol Chem. 2010;285:26326–26334. doi: 10.1074/jbc.M110.123851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Poetter K, et al. Mutations in either the essential or regulatory light chains of myosin are associated with a rare myopathy in human heart and skeletal muscle. Nat Genet. 1996;13:63–69. doi: 10.1038/ng0596-63. [DOI] [PubMed] [Google Scholar]
- 23.Davis JS, et al. The overall pattern of cardiac contraction depends on a spatial gradient of myosin regulatory light chain phosphorylation. Cell. 2001;107:631–641. doi: 10.1016/s0092-8674(01)00586-4. [DOI] [PubMed] [Google Scholar]