Abstract
Interpreting key ecological parameters, such as diet, of extinct organisms without the benefit of direct observation or explicit fossil evidence poses a formidable challenge for paleobiological studies. To date, dietary categorizations of extinct taxa are largely generated by means of modern analogs; however, for many species the method is subject to considerable ambiguity. Here we present a refined approach for assessing trophic habits in fossil taxa and apply the method to coelurosaurian dinosaurs—a clade for which diet is particularly controversial. Our findings detect 21 morphological features that exhibit statistically significant correlations with extrinsic fossil evidence of coelurosaurian herbivory, such as stomach contents and a gastric mill. These traits represent quantitative, extrinsically founded proxies for identifying herbivorous ecomorphology in fossils and are robust despite uncertainty in phylogenetic relationships among major coelurosaurian subclades. The distribution of these features suggests that herbivory was widespread among coelurosaurians, with six major subclades displaying morphological evidence of the diet, and that contrary to previous thought, hypercarnivory was relatively rare and potentially secondarily derived. Given the potential for repeated, independent evolution of herbivory in Coelurosauria, we also test for repetitive patterns in the appearance of herbivorous traits within sublineages using rank concordance analysis. We find evidence for a common succession of increasing specialization to herbivory in the subclades Ornithomimosauria and Oviraptorosauria, perhaps underlain by intrinsic functional and/or developmental constraints, as well as evidence indicating that the early evolution of a beak in coelurosaurians correlates with an herbivorous diet.
Keywords: bird, character correlation, fossil record, vertebrate, paleoecology
Many synthetic questions in paleobiology, such as coevolution of plant and herbivore communities (1, 2), discussions on feeding mechanics and metabolism (1, 3), and trophic models of competitive exclusion and extinction (4, 5), rely on inferring diet in extinct taxa. Unfortunately, fossil specimens rarely preserve extrinsic evidence of diet, and trophic interpretations of extinct taxa are generally derived from observing suites of skeletal traits with homologs or analogs in living species. Whereas functional considerations and/or explicit modern analogs leave little doubt regarding the diet of some fossil taxa (e.g., hypercarnivory in Tyrannosaurus rex or Smilodon; grazing in horses) (6), for numerous other fossil clades, including many archosaurian lineages, comparative morphological approaches present considerable challenges (7–10).
One archosaurian group for which diet has been particularly problematic to interpret is Coelurosauria—a speciose clade of feathered theropods encompassing modern birds and several closely related nonavian lineages. Comparative and microwear approaches to determining the diet of coelurosaurian species are negatively affected by one or more trophic shifts (8, 11), presumed dietary opportunism potentially including invertebrates and high-caloric plant materials (e.g., fruits, nuts, seeds) (12, 13), and a recurring trend toward edentulism (8, 11, 14). These factors have led to widely discrepant trophic interpretations, particularly among the predominantly toothless subclades Ornithomimosauria and Oviraptorosauria and the bizarre Therizinosauria, whose anatomical characteristics have been used to propose a myriad of dietary preferences with little consensus (8, 11, 15–20).
Recently, a burst of theropod dinosaur discoveries bearing an unexpected degree of ecomorphological disparity (particularly with respect to dental anatomy) (12, 20–24) has sparked a renewed interest in the diet of coelurosaurian species. Yet, to date, a large scale quantitative analysis on theropod ecomorphology has not been conducted. Serendipitously, an increasing number of theropod specimens preserve unequivocal evidence of diet in the form of fossilized gut contents (25–27), coprolites (28), or a gastric mill (21–23, 29–31), which are known to correlate with herbivory in extant theropod dinosaurs (32, 33). Such extrinsic dietary evidence offers the opportunity to evaluate ecomorphology in theropods in a rigorous, quantitative fashion. Here we apply character correlation tests within a phylogenetic framework to determine whether skeletal traits purported to indicate herbivory in published comparative studies (putatively herbivorous traits or PHTs; SI Appendix, Section A2) correlate with extrinsic evidence of herbivory (EEH) in coelurosaurian dinosaurs. Our findings present quantitative, species-level dietary interpretations for 72 coelurosaurian theropods and provide a robust proxy for inferring primary trophic level in problematic taxa (Tables S1–S7).
Other herbivorous archosaurs are characterized by common suites of PHTs, suggesting that the adaptive pathway to herbivory in terrestrial vertebrates may be constrained along consistent trajectories (9, 34–36). To date, these observations have not been tested statistically, and earlier studies have not attempted to identify recurring trends in the order of herbivorous trait accrual during the evolution of disparate archosaurian lineages. Given that multiple coelurosaurian subclades exhibit evidence of herbivory, we develop a unique approach based on the rank order of PHTs found to correlate with evidence of herbivory in theropods (correlated herbivorous traits, CHTs) to test for repetitive patterns in the refinement of herbivorous ecomorphology in Coelurosauria. Such a phenomenon would imply that intrinsic constraints are influencing the progressive morphological adaptation to plant eating in vertebrates and reveal unique specializations among herbivorous theropod lineages.
Results
Character Correlation Analyses.
To identify herbivorous ecomorphology in coelurosaurian theropods, we compiled 27 craniodental, axial, and pelvic PHTs considered to indicate an herbivorous diet in dinosaurs (Fig. 1, SI Appendix, Section A2), as well as taxon-specific information on EEH. PHTs and EEH were optimized onto a recent comprehensive phylogeny of 90 extinct coelurosaurians (24). Due to the rarity of extrinsic evidence of diet associated with fossil remains, only 22% of the taxa considered could be assigned to a trophic category a priori. Nonetheless, extrinsic evidence of diet was distributed across the phylogeny, occurring in all major subclades (except Therizinosauria and Alvarezsauroidea) and two outgroup taxa (Fig. 2A and Table S1).
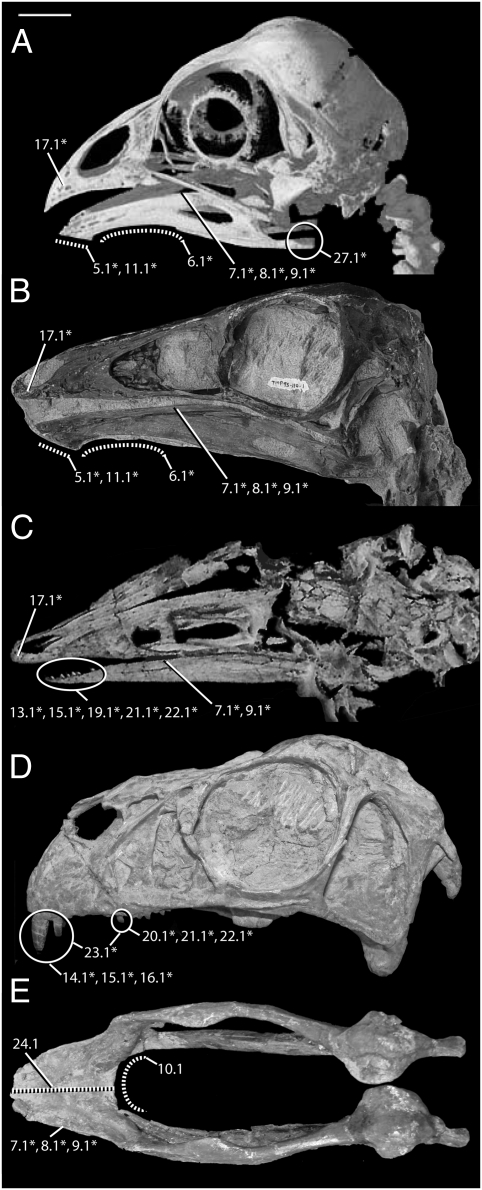
Fig. 1.
Select cranial traits appearing in multiple lineages of coelurosaurian theropod dinosaurs tested herein as PHTs. Ecomorphological indicators statistically correlated with extrinsic evidence of herbivory (first- and second-order CHTs) are marked with an asterisk. Traits featured on six species representing four coelurosaurian theropod subclades, including the only predominantly folivorous modern bird. (A) Opisthocomus hoazin skull, left lateral view (extant Aves), adapted from http://digimorph.org/. Extinct theropods: (B) Ornithomimus edmontonicus (RTMP 95.110.1) skull, left lateral view (derived Ornithomimosauria); (C) Shenzhousaurus orientalis (NGMC 977–4-002) skull, left oblique dorsolateral view (primitive Ornithomimosauria), adapted from ref. 21; (D) Incisivosaurus gauthieri (IVPP V13326) skull left lateral view (primitive Oviraptorosauria); and (E) Caenagnathus collinsi (CMN 8776) fused lower mandibles, dorsal view (derived Oviraptorosauria; Table S7, and see SI Appendix, Section A3 for trait descriptions.
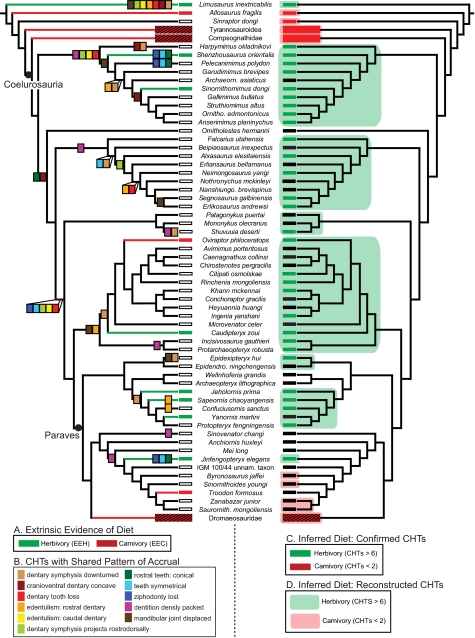
Fig. 2.
Phylogeny of coelurosaurian theropods used in correlation and correspondence analyses showing: (A) Distribution of EEH and EEC (Left; for explanation, see Table S1); (B) Ancestral state reconstruction optimizations using maximum parsimony (ASR-MP) of the 11 CHTs detected as evolving in a statistically significant order in the clades Ornithomimosauria and Oviraptorosauria (Left); and (C and D) Quantitative dietary interpretations at the species level based on the total number of actual CHTs (C Right) and interpretations estimated using ASR-MP (D Right), respectively (Table S3). Black bars on phylogeny at right represent taxa with intermediate numbers of CHTs for which diet is ambiguous. Only ancestral (not autapomorphic) optimizations were used for rank correspondence analysis although both are shown here. For alternate tree topologies used in analyses, see Fig. S1. Species collapsed into higher-level OTUs for Dromaeosauridae, Compsognathidae, and Tyrannosauroidea. Black/red stripe indicates that some species in higher-level OTUs possess evidence of diet. Actual CHT number ≤1 for all dromaeosaurs except Microraptor. Reconstructed CHT number ≤3 for all dromaeosaurs except Rahonavis, Buitreraptor, and Unenlagia. For specific data on CHT number per species, see Table S3. See SI Appendix, Section A3 for genus abbreviations.
To detect correlation between PHTs and EEH, we used three different statistical techniques for examining character correlations on phylogenies: concentrated changes test (37), pairwise comparisons (38), and Discrete (39) (Materials and Methods and SI Appendix, Section A6). We tested over 2,600 individual trait pairings, encompassing multiple iterations reflecting current uncertainties in coelurosaurian subclade relationships and character codes for incipiently developed states (SI Appendix, Section B2) (3). Each test produced variable clusters of correlative PHTs. We then contrasted results between the three tests and compiled a common suite of PHTs that were significantly correlated (P < 0.05) in all three tests (CHTs). Traits with significant correlations in concentrated changes tests and/or Discrete, yet not achieving significance using pairwise comparisons were evaluated to determine whether the discrepancy could be attributed to the inability of the pairwise test to achieve statistical significance with low trait-change frequencies (Materials and Methods). Insignificant pairwise comparisons were not used to reject CHTs if the number of independent transformations fell below the threshold for significance and results included iterations in which all possible pairings showed a coincident change in both traits.
We consider CHTs as first-order correlates if they achieve significance when tested directly against EEH. However, given the limited occurrence of EEH in our study, we also used first-order correlates as proxy data to test for additional correlations between remaining PHTs. PHTs possessing correlations with first-order traits are considered as second-order CHTs. Although we detected third- through fifth-order correlations in our analyses, and these patterns are valuable in identifying trait correlations in theropods, we deem these features as too many steps removed from primary EEH observations to be reliable herbivorous indicators.
Despite the low incidence of EEH across the sampled diversity, we detected significant first- and second-order correlations between EEH and 21 of 27 PHTs purported as ecomorphological indicators of herbivory in dinosaurs (Fig. 1 and Table S7; see SI Appendix, Section B1, for results by test). The evolution of dentary concavity, conical-subconical teeth, tooth symmetry, and loss of ziphodonty (Fig. 1) possess the strongest correlation with EEH under multiple methods (P values 0.0006–0.01). Rostral projection of the dentary symphysis, ventral deflection of the dentary symphysis producing a rostral gap, and progressive tooth loss are also highly correlative (P values 0.008–0.05).
Rank Concordance Analysis.
To investigate common patterns of herbivorous trait accrual during the evolution of theropods, we ranked the order of appearance of CHTs with independent originations in the herbivorous coelurosaurian subclades Ornithomimosauria, Therizinosauria, and Oviraptorosauria under multiple optimization techniques (Fig. 2B and Materials and Methods). We excluded birds because few CHTs were found to optimize at ancestral avian nodes. Of the 21 CHTs recovered in our study, a maximum of eight were consistent with this parameter and could be tested for congruence. Here again we tested alternate tree topologies to investigate multiple phylogenetic hypotheses (Materials and Methods).
We found no statistical significance in the order of CHTs acquisition among all three nonavian herbivorous lineages under any of our optimization methods and tree topologies. However, when we compared ornithomimosaurians and oviraptorosaurians alone we found the pattern of trait accrual among 5–8 CHTs to be significant (P value 0.05–0.005) under iterations involving all three optimization techniques (Fig. 1B; see SI Appendix, Section B4, for CHT descriptions). The lack of significance between the sequences of CHT accrual in all three theropod lineages may relate to methodological problems such as missing data, causing widely discrepant character rankings and the low number of traits appearing with independent origins in all three subclades (three to four in some optimizations), or genuine differences in the refinement of ecomorphological traits that indicate unique patterns of specialization, as discussed in the following section.
Discussion
Dietary Inference.
Previous authors have proposed that coelurosaurian dinosaurs diverged from ancestral faunivory (7, 8, 11–13, 29, 40, 41); however, our analysis represents a quantified attempt to base trophic interpretations of theropod species upon extrinsic evidence rather than analogous comparisons or an apparent deviation from typical carnivorous anatomy. Here we find quantitative evidence for herbivory in 44 coelurosaurian species across six major theropod subclades (29 with seven or more confirmed CHTs—the minimum number present in all terminal taxa exhibiting positive EEH evidence; Fig. 2 and Table S3). Herbivory is inferred for Ornithomimosauria, Therizinosauria, Oviraptorosauria, and Alvarezsauroidea, the single troodontid Jinfengopteryx, and seven avialans, as well as the ceratosaurian Limusaurus (Fig. 2 C and D; see Materials and Methods for dietary terminology). However, alvarezsauroids (except Shuvuuia) and several oviraptorosaurians, including Chirostenotes, Microvenator, Conchoraptor, Avimimus, and Heyuannia possess lower numbers of confirmed CHTs possibly due to missing data (Table S3). Herbivory in these taxa is inferred based on reconstructed CHTs and the discovery of new materials could change this interpretation. In addition, we infer carnivory in 24 coelurosaurians (19 with one or fewer confirmed CHTs) representing all of Tyrannosauroidea and Compsognathidae, the derived troodontids Zanabazar, Sinornithoides, Byronosaurus, and Sinovenator, as well as the majority of Dromaeosauridae. Such a preponderance of evidence for herbivory in coelurosaurian theropods runs counter to earlier ideas regarding their ecology in that it suggests the hypercarnivorous diet of tyrannosaurs, compsognathids, and some paravians is best regarded as an exception to what is otherwise extensive dietary opportunism in the clade.
Among basal members of several subclades, CHTs numbers are indefinite. Basal troodontids (other than Jinfengopteryx) exhibit at least two CHTs and ancestral state reconstructions allow for up to six in some taxa. For unenlagiine dromaeosaurids and the basal maniraptoran Ornitholestes an intermediate number of estimated and confirmed CHTs also precludes trophic assignment and dietary habits remain inconclusive (Fig. 2 C and D and Table S3). Intermediate numbers of CHTs in these taxa may indicate omnivory or dietary specializations not manifest widely in other coelurosaurians (e.g., insectivory). Given the diet of basal paravians does not conform to predominant carnivory and may reflect omnivory, this pattern supports the hypothesis that hypercarnivory in derived paravians is a secondary dietary specialization and that the primitive diet for paravians includes an herbivorous component (11).
Herbivorous Ecomorphology in Theropods.
The 21 skeletal traits identified as CHTs and their distribution provide solid criteria for establishing herbivorous ecomorphology and for investigating patterns of evolutionary change correlated with the trophic shift from hypercarnivory to herbivory/omnivory within the clade (Fig. 2B). We find multiple tooth types as herbivorous correlates in coelurosaurians. For example, primitive herbivorous ornithomimosaurians and avialans exhibit relatively homogeneous conical teeth in denticulate regions of the mandibles. In contrast, conical/subconical incisiform teeth, when present, are restricted to the rostral mandibles in primitive members of the herbivorous clades Oviraptorosauria and Therizinosauria and cheek teeth are lanceolate. Among the latter two clades, ecomorphological tooth alteration of rostral teeth also includes relative elongation (also in Scansoriopterygidae) and procumbency (Oviraptorosauria only), which contributes further to the dental heterogeneity. Heterogeneity in the form of markedly different tooth types is also characteristic of other dinosaurian taxa thought to span a trophic shift and/or for which omnivory/herbivory is proposed (e.g., basal sauropodomorphs, basal ornithischians) (7).
We find it interesting that conical/incisiform teeth are present in areas of subsequent tooth loss in these four herbivorous lineages, suggesting a potential form/function association between the tooth type and rhamphotheca. In fact, among therizinosaurians, progressive tooth loss within the clade is restricted exclusively to mandibular regions previously exhibiting conical/subconical teeth in more basal taxa. We also find dental modifications that are functional analogs to a beak, such as loss of pronounced replacement waves and gaps between teeth, producing a continuous horizontal cutting surface (42), to be ecomorphological indicators of herbivory. Traits representing ultimate stages in beak evolution in nonavian subclades, such as mandibular ventral concavity and ventral displacement, as well as rostral projection of the mandibular symphysis, also correlate with EEH in coelurosaurians. Such patterns indicate that the repeated evolution and refinement of a beak in nonavian theropods initially correlates with the adoption of plant fodder into the diet.
With regard to postcranial morphology, we detect an elongate neck (numerical increase of cervical vertebrae) as an herbivorous ecomorphological indicator. Lengthening of the neck is widely manifest among multiple independent lineages of putative dinosaurian herbivores, including sauropodomorphs (43) and stegosaurians (44), and is speculated to increase browsing range (45, 46). Although in some clades the feature has been attributed to sexual selection (44), our study supports the interpretation that the trend is correlated with herbivory in theropods.
In sum, our findings suggest that in Coelurosauria: (i) multiple tooth morphotypes (e.g., lanceolate, conical, and/or incisiform tooth types with rostral elongation and/or procumbency) correlate with plant consumption, (ii) such modification in tooth form (whether homo- or heterogeneous) is a primary indicator of the trophic shift from carnivory, (iii) region-specific tooth alteration universally precedes tooth loss, (iv) conical/subconical tooth morphology is likely a functional precursor of the theropod rhamphotheca, (v) the initial evolution of a beak correlates with the shift from faunivory to herbivory early in the evolutionary history of multiple coelurosaurian lineages, and (vi) an elongate neck by virtue of increased vertebral count is an herbivorous ecomorphological indicator in coelurosaurian dinosaurs.
Although we did not include extant taxa in our study, we find support for our interpretations in the modern realm. Specifically, all cranial CHTs except dental traits that are found on extinct theropods are also present on Opisthocomus hoazin (Fig. 1A)—one of the few predominant folivores among modern birds (47, 48). Opisthocomus is the only avian known to have active foregut fermentation, as is found in ruminant mammals, and is arguably the most specialized avian folivore, with a diet comprised of 80% leaves (48). Although several features (e.g., rostrodorsal trending mandibular symphysis and dentary convexity) achieve a widespread distribution in modern birds (49), their presence in O. hoazin demonstrates that these traits are consistent with a plant-based diet in theropod dinosaurs.
Common Adaptive Pathways and Innovations to Herbivory.
Many of the traits supported as ecomorphological indicators of herbivory herein appear repeatedly and independently in multiple lineages, exhibit highly significant correlations with suites of other CHTs, and display repetitive sequences of acquisition and refinement. Although our rank analyses are restricted by missing data, we find that a significant degree of commonality characterizes the evolution of select CHTs within the subclades Ornithomimosauria and Oviraptorosauria. The CHTs: ventral deflection and rostrodorsal trending of the mandibular symphysis, concavity of the ventral margin of the dentary, and tooth loss are remarkably congruent (Discrete P < 0.01) and appear to be coupled, exhibiting only slight plasticity in their order of appearance. In ornithomimosaurians and oviraptorosaurians, tooth loss and rostral projection of the dentary symphysis precede ventral symphyseal deflection and dentary concavity, whereas in Therizinosauria, a downturned and convex dentary and rostrally projecting symphysis are primary adaptations to herbivory, and tooth loss is manifested subsequently. Despite these differences, these CHTs appear within two to three nodes of each other during the evolution of all three lineages tested. This finding, together with statistical support for a pattern of progression in at least two coelurosaurian subclades, suggests the evolution of select herbivorous features may be guided by intrinsic developmental or functional constraints regardless of apparent flexibility in which trait marks the initial step in the evolution of the full suite of CHTs within sublineages. Moreover, plasticity in order of appearance indicates that though correlated, these characters do exhibit a degree of underlying independence and can correctly be regarded as discrete adaptations to herbivory, as opposed to a single invariant character system.
Although results of concordance analyses detect consistencies in the acquisition of some CHTs, it is clear that individual coelurosaurian subclades met the challenge of an herbivorous diet not only by means of convergent adaptations and constrained patterns, but also by unique anatomical innovations and subtle variations in herbivorous trait accumulation. As a case in point, although both ornithomimosaurians and oviraptorosaurians evolve a completely edentulous beak, premaxillary edentulism precedes dentary edentulism in ornithomimosaurians, but is not manifest in oviraptorosaurians until all teeth have been lost from the dentary. Moreover, dentary tooth loss is initiated caudally in ornithomimosaurians and oviraptorosaurians and progresses to complete edentulism, whereas dentary edentulism begins rostrally in therizinosaurians, and complete tooth loss was apparently never achieved.
The latter pattern is exhibited by a plethora of other dinosaurian lineages possessing extrinsic evidence of herbivory or inferred to be herbivorous via ecomorphological analogy (e.g., hadrosaurids, ceratopsians, sauropodomorphs, stegosaurians). In these putative herbivores a rostral beak exists in tandem with cheek teeth. The majority of the species within these clades are similar to therizinosaurians in that they are not known to have possessed a gastric mill (33). Therefore, sufficient oral processing of vegetative matter may have been a factor acting against complete tooth loss (33) and prompting the evolution of complex dental batteries and chewing strokes in these clades (1, 35). In contrast, basal members of the ultimately toothless subclades Ornithomimosauria and Oviraptorosauria, extinct and extant avian species, and the single known toothless ceratosaurian (Limusaurus) (23) all possess a gastric mill, which likely loosened constraints on oral processing and may have enabled the evolution of a complete beak in these taxa.
Ultimately, the repeated evolution of a completely edentulous beak in coelurosaurians was an evolutionary innovation. Although our findings suggest that the appearance of the trait is correlated with herbivory, once evolved, an edentulous beak could be coopted for multiple functions. Specialization of the beak may eventually have led to increased plasticity in food capture, processing, and dietary composition, opened new ecological niches for exploitation, and, secondarily, reduced the reliance on plant eating in derived members of some coelurosaurian subclades (e.g., avialans, oviraptorosaurians). This speculation is supported by the lack of evidence for a gastric mill in derived edentulous members of Ornithomimosauria (Table S1) and Oviraptorosauria (Table S1), as well as extrinsic evidence of carnivory in the latter (50), and also by the ecology of extant theropods (modern birds) and other living species (e.g., turtles), which have coopted the beak into a stunning array of forms, reflecting a high degree of dietary diversity, including hypercarnivory (14, 49). Therizinosaurians, by contrast, retain lanceolate cheek teeth along with a rostral rhamphotheca throughout their evolution—a pattern exhibited by most other dinosaurian herbivores. This finding suggests that retention of a high-fiber folivorous diet may preclude evolution of a complete rhamphotheca in therizinosaurians and other archosaurian lineages.
Only a handful of living arboreal birds are high-fiber herbivores, presumably because the digestive modifications needed to sustain the diet are generally incompatible with powered flight (47). Extinct flightless coelurosaurs would not have suffered from such physiological constraints, and high-fiber folivory may have been a more readily evolving diet in nonavian theropods. Our findings suggest that herbivory was indeed widespread among coelurosaurian theropods and that plant materials were already a dietary component of the basal-most members of several lineages hypothesized to have split before the origin of avialans. We therefore conclude that herbivory likely preceded the origin of avialans and speculate that dietary diversification played an important role in the early evolution of Avialae.
Finally, the ecomorphological indicators of herbivory in theropods identified in this study also achieve broad distributions in other putative vertebrate herbivores (1, 35, 51). Expansion of this method to the whole of Archosauria will provide quantitative insights into the evolution of herbivorous ecomorphology in the dominant megafauna of the Mesozoic and reveal additional commonalities in the adaptation to herbivory among terrestrial vertebrates. Indeed many of the traits under study here are observed widely in extinct amniotes (e.g., beaks in anomodonts, heterodonty in crocodyliforms) (10). Ultimately, such an approach will contribute to a better understanding of the patterns and processes underlying the evolution of amniote herbivory.
Materials and Methods
Diet as a Trait.
We considered five lines of extrinsic evidence in assigning presence/absence states for EEH (SI Appendix, Section A1, and Table S1. Species were coded EEH present if exhibiting fossilized herbaceous gut content and/or evidence of a gastric mill (32), and EEH absent if exhibiting vertebrate gut contents or coprolites with ingested bone and/or other evidence of predation (Table S1). This differs from earlier character correlation studies (11, 51), which assigned the character herbivorous based on analogous morphological evidence and thereby invoked a degree of circularity, because the same traits being tested for correlation may have been used to infer diet. We used a conservative approach toward coding EEH, but applied different thresholds to herbivory and carnivory in consideration of taphonomic biases (SI Appendix, Section A4). We do not recognize all gastroliths as evidence for an avian-style gastric mill. To be coded as possessing a gastric mill, a taxon must preserve a definitive mass of stomach stones that lack a high polish, conform to the predicted ratio of body mass to gastrolith mass, and be erratics with regard to entombing sediment (32). Theropods for which a relatively few rounded stones have been found spread out in the gut region (e.g., Baryonyx) do not meet this criteria (see SI Appendix, Section A1, for further explanation).
Character Matrix.
Our morphological dataset comprised 27 PHTs (11 dental, 12 cranial, 4 postcranial) derived from specimen observation and from multiple sources citing these features as morphological evidence for herbivory (see Table S2, for references). Character correlation methods dictate that all characters be represented as binary (presence/absence) traits. Two characters (7 and 15) are nonindependent variations of other traits. Pairwise comparisons cannot process missing data; therefore, nonapplicable characters are considered absent (state 0).
Trees.
Trees were derived from a recent comprehensive phylogenetic study of Coelurosauria containing 90 taxa and 363 characters (24) with the putatively herbivorous Jurassic ceratosaur Limusaurus inextricabilis posited as basal to all OTUs (23). Eight taxa, that could not be scored for PHTs were pruned uniformly to maintain consistency among tests. Concentrated changes tests and Pairwise Comparisons necessitate dichotomous trees and are either incapable of incorporating or heavily impacted by missing data, therefore we used a combination of pruning and positing taxa in various iterations to minimize assumptions and maximize informative data (SI Appendix, Section A5).
Branch Lengths.
Branch lengths for Discrete were calibrated chronostratigraphically (Table S2). For taxa from radiometrically dated formations, we adopted dates of closest stratigraphic proximity to fossil localities. For taxa from strata of uncertain age, we used midstage or midrange estimates based on published age ranges (e.g., 159 Ma for the Oxfordian) using the 2009 geologic timescale (52). We used a 1-Ma branch-length adjustment following (53). We also tested the impact of scaling branch lengths by a factor of 0.001 (54), which resulted in a <0.01% change in the likelihood ratio and was therefore of null effect.
Character Correlation.
Concentrated changes tests (37) were implemented in MacClade 4.05 (55) using four optimization techniques (SI Appendix, Section A6). For PHTs with high numbers of gains/losses, exact calculations are too computationally intensive; therefore, simulations using sample sizes ranging from 10,000 to 20,000 were used (37). The ancestral node state was considered ambiguous in all cases. Distinguished branches were treated as having state 1 of the independent variable. However, we also counted simultaneous instances of gains/losses to capture instances of tight character coupling (37). Pairwise comparisons (38) were conducted in Mesquite version 2.72 (56). The states of both binary characters were considered. No limit on pair numbers was imposed. We used the Discrete module of the BayesTraits OSX V1-1.0 software package to run a maximum-likelihood-based trait correlation test (Discrete) (39). We ran maximum-likelihood analysis using 10 attempts per tree, four rates, and no restrictions. Significance was tested using the Freedman χ2 statistic with four degrees of freedom (57).
Trait Progression.
To test for common trait progression we used optimization methods to establish the rank order of CHTs (characters 5–9, 11, 13–23, 25–27, 31). We omitted all but one logically nonindependent character if several were ranked (e.g., omitted character 7 when 7 and 8 were testable; SI Appendix, Section A4). We posited character change at the basal-most occurrence of unambiguous state 1 optimization using three optimization techniques: ACTRAN and DELTRAN optimizations in MacClade 4.05 (55) and unambiguous ancestral state reconstructions using maximum parsimony in Mesquite version 2.72 (56) to investigate the variable effect of missing data on ranks. In cases where a CHT optimized at a node ancestral to more than one of the investigated theropod subclades (i.e., shared through common ancestry rather than convergence), it was omitted from the analysis to ensure independence among samples. CHTs not common to all subclades tested were also omitted, as the test cannot accommodate missing rank data. To determine whether the rank order of appearance of CHTs in nonavian theropod lineages is concordant, we used a modified version of Kendall's coefficient of concordance W with a correction for tied ranks (i.e., PHTs that appear at the same node) (57). This coefficient (value 0–1) can be converted to a Freedman χr2 statistic for nonparametric significance testing with n/M degrees of freedom or if n or M is larger approximated by χ2 with n − 1 degrees of freedom (57).
Dietary Interpretation and Terminology.
To interpret diet we used a combination of confirmed and estimated numbers of CHTs (Fig. 2 and Table S3). Estimated CHT number is derived from ancestral state reconstructions conducted in Mesquite, version 2.72 (56). Ancestral state reconstructions were used to supplement confirmed numbers of CHTs for dietary interpretations because confirmed CHT count is highly influenced by missing data; low confirmed numbers of CHTs can mean that a taxon lacks the CHT or lacks the skeletal elements on which a CHT would be manifest. The use of seven CHTs to infer herbivory was not intended as a hard boundary, rather it reflects a degree of confidence based on the number of CHTs in taxa with EEH in this sample. There is a fluid boundary between herbivory and omnivory among extant vertebrates. Moreover, the evolution of amniote herbivory is thought to originate via omnivory (7, 35). In consideration of this, we do not draw a hard distinction between the two dietary habits. Here the term herbivore is used to denote species obtaining a significant percentage of their diet from herbaceous fodder (including fruits, nuts, seeds, bark, roots, leaves, and shoots). It is not intended to reflect exclusive herbivory. A larger number of CHTs may denote a higher degree of herbivory, may indicate folivory rather than other forms of herbivory (e.g., frugivory, seminivory), or may reflect different herbivorous specializations.
Supplementary Material
Acknowledgments
We thank C. McGarrity for assistance with data collection, and K. Angielczyk, J. Fröbisch, T. Gates, B. Kilbourne, and four anonymous reviewers for constructive critiques. Support for this work was provided by a J. Caldwell-Meeker Fellowship (to L.E.Z.). Data collection for this study was partially supported by National Science Foundation Earth Sciences Assembling the Tree of Life Grant 0228607 (to P.J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. R.I. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011924108/-/DCSupplemental.
References
- 1.Weishampel DB, Norman DB. In: Paleobiology of the Dinosaurs. Farlow JO, editor. Boulder, CO: Geol Soc Am; 1989. pp. 87–100. [Google Scholar]
- 2.Barrett PM, Willis KJ. Did dinosaurs invent flowers? Dinosaur-angiosperm coevolution revisited. Biol Rev Camb Philos Soc. 2001;76:411–447. doi: 10.1017/s1464793101005735. [DOI] [PubMed] [Google Scholar]
- 3.Farlow JO. Speculations about the diet and digestive physiology of herbivorous dinosaurs. Paleobiology. 1987;13:60–72. [Google Scholar]
- 4.Benton MJ. Dinosaur success in the Triassic: A noncompetitive ecological model. Q Rev Biol. 1983;58:29–55. [Google Scholar]
- 5.Brusatte SL, Benton MJ, Ruta M, Lloyd GT. Superiority, competition, and opportunism in the evolutionary radiation of dinosaurs. Science. 2008;321:1485–1488. doi: 10.1126/science.1161833. [DOI] [PubMed] [Google Scholar]
- 6.Janis CM. In: Functional Morphology in Vertebrate Paleontology. Thomasson JJ, editor. Cambridge: Cambridge Univ Press; 1995. pp. 76–98. [Google Scholar]
- 7.Barrett PM. In: Evolution of Herbivory in Terrestrial Vertebrates. Sues HD, editor. Cambridge: Cambridge Univ Press; 2000. pp. 42–78. [Google Scholar]
- 8.Barrett PM. The diet of ostrich dinosaurs (Theropoda: Ornithomimosauria) Palaeontol. 2005;48:347–358. [Google Scholar]
- 9.Irmis RB, Parker WG, Nesbitt SJ, Liu J. Early ornithischian dinosaurs: The Triassic record. Hist Biol. 2007;19:3–22. [Google Scholar]
- 10.O'Connor PM, et al. The evolution of mammal-like crocodyliforms in the Cretaceous Period of Gondwana. Nature. 2010;466:748–751. doi: 10.1038/nature09061. [DOI] [PubMed] [Google Scholar]
- 11.Zanno LE, Gillette DD, Albright LB, Titus AL. A new North American therizinosaurid and the role of herbivory in ‘predatory’ dinosaur evolution. Proc Biol Sci. 2009;276:3505–3511. doi: 10.1098/rspb.2009.1029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Xu X, Cheng YN, Wang XL, Chang CH. An unusual oviraptorosaurian dinosaur from China. Nature. 2002;419:291–293. doi: 10.1038/nature00966. [DOI] [PubMed] [Google Scholar]
- 13.Senter P. Function in the stunted forelimbs of Mononykus olecranus (Theropoda), a dinosaurian anteater. Paleobiology. 2005;31:373–381. [Google Scholar]
- 14.Hieronymus TL, Witmer LM. Homology and evolution of the avian compound rhamphothecae. Auk. 2010;127:590–604. [Google Scholar]
- 15.Osborn HF. Skeletal adaptations of Ornitholestes, Struthiomimus, Tyrannosaurus. Bull Am Mus Nat Hist. 1917;35:733–771. [Google Scholar]
- 16.Norell MA, Makovicky PJ, Currie PJ. Palaeontology. The beaks of ostrich dinosaurs. Nature. 2001;412:873–874. doi: 10.1038/35091139. [DOI] [PubMed] [Google Scholar]
- 17.Barsbold R. Carnivorous dinosaurs from the Cretaceous of Mongolia (Translated from Russian) Trans Jt Sov-Mongolian Paleontol Exped. 1983;19:1–116. [Google Scholar]
- 18.Russell DA. Ostrich dinosaurs from the Late Cretaceous of western Canada. Can J Earth Sci. 1972;9:375–402. [Google Scholar]
- 19.Currie PJ, Godfrey SJ, Nessov L. Results of the Sino-Canadian Dinosaur Project. Can J Earth Sci. 1993;30:2255–2272. [Google Scholar]
- 20.Zanno LE. Osteology of Falcarius utahensis: Characterizing the anatomy of basal therizinosaurs. Zool J Linn Soc. 2010;158:196–230. [Google Scholar]
- 21.Ji Q, Currie PJ, Norell MA, Ji S-A. Two feathered dinosaurs from northeastern China. Nature. 1998;393:753–761. [Google Scholar]
- 22.Ji Q, et al. An early ostrich dinosaur and implications for ornithomimosaur phylogeny. Am Mus Novit. 2003;3420:1–19. [Google Scholar]
- 23.Xu X, et al. A Jurassic ceratosaur from China helps clarify avian digital homologies. Nature. 2009;459:940–944. doi: 10.1038/nature08124. [DOI] [PubMed] [Google Scholar]
- 24.Hu D, Hou L, Zhang L, Xu X. A pre-Archaeopteryx troodontid theropod from China with long feathers on the metatarsus. Nature. 2009;461:640–643. doi: 10.1038/nature08322. [DOI] [PubMed] [Google Scholar]
- 25.Zhou Z, Zhang F. A long-tailed, seed-eating bird from the Early Cretaceous of China. Nature. 2002;418:405–409. doi: 10.1038/nature00930. [DOI] [PubMed] [Google Scholar]
- 26.Zhou Z, Clarke JA, Zhang F. Archaeoraptor’s better half. Nature. 2002;420:285. doi: 10.1038/420285a. [DOI] [PubMed] [Google Scholar]
- 27.Ji Q, et al. First avialan bird from China (Jinfengopteryx elegans gen. et sp. nov.) Geol Bull China. 2005;24:197–205. [Google Scholar]
- 28.Chin K, Tokaryk TT, Erickson GM, Calk LC. A king-sized theropod coprolite. Nature. 1998;93:680–682. [Google Scholar]
- 29.Kobayashi Y, et al. Herbivorous diet in an ornithomimid dinosaur. Nature. 1999;402:480–481. [Google Scholar]
- 30.Zhou Z, Zhang F. Anatomy of the primitive bird Sapeornis chaoyangensis from the Early Cretaceous of Liaoning, China. Can J Earth Sci. 2003;40:731–737. [Google Scholar]
- 31.Zhou Z, Clarke JA, Zhang F, Wings O. Gastroliths in Yanornis: An indication of the earliest radical diet-switching and gizzard plasticity in the lineage leading to living birds? Naturwissenschaften. 2004;91:571–574. doi: 10.1007/s00114-004-0567-z. [DOI] [PubMed] [Google Scholar]
- 32.Gionfriddo JP, Best LB. Grit use by birds-a review. Curr Ornithol. 1996;15:89–148. [Google Scholar]
- 33.Wings O, Sander PM. No gastric mill in sauropod dinosaurs: New evidence from analysis of gastrolith mass and function in ostriches. Proc Biol Sci. 2007;274:635–640. doi: 10.1098/rspb.2006.3763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weishampel DB. Hadrosaurid jaw mechanics. Acta Palaeontol Pol. 1983;28:271–280. [Google Scholar]
- 35.Reisz RR, Sues HD. In: Evolution of Herbivory in Terrestrial Vertebrates. Sues HD, editor. Cambridge: Cambridge Univ Press; 2000. pp. 9–41. [Google Scholar]
- 36.Upchurch P, Barrett PM. In: Evolution of Herbivory in Terrestrial Vertebrates. Sues HD, editor. Cambridge: Cambridge Univ Press; 2000. pp. 79–122. [Google Scholar]
- 37.Maddison WP. A method for testing the correlated evolution of two binary characters: Are gains or losses concentrated on certain branches of a phylogenetic tree? Evol. 1990;44:539–557. doi: 10.1111/j.1558-5646.1990.tb05937.x. [DOI] [PubMed] [Google Scholar]
- 38.Maddison WP. Testing character correlation using pairwise comparisons on a phylogeny. J Theor Biol. 2000;202:195–204. doi: 10.1006/jtbi.1999.1050. [DOI] [PubMed] [Google Scholar]
- 39.Pagel M. Detecting correlated evolution on phylogenies: A general method for the comparative analysis of Discrete characters. Proc Biol Sci. 1994;255:37–45. [Google Scholar]
- 40.Holtz TR, Jr, Brinkman DL, Chandler CL. Denticle morphometrics and a possibly omnivorous feeding habit for the theropod dinosaur Troodon. Gaia. 1998;15:159–166. [Google Scholar]
- 41.Barrett PM, Rayfield EJ. Ecological and evolutionary implications of dinosaur feeding behaviour. Trends Ecol Evol. 2006;21:217–224. doi: 10.1016/j.tree.2006.01.002. [DOI] [PubMed] [Google Scholar]
- 42.Perez-Moreno BP, Sanz JL, Buscalloni AD, Moratalla FO, Rasskin-Gutman D. A unique multitoothed ornithomimosaur dinosaur from the lower Cretaceous of Spain. Nature. 1994;370:363–367. [Google Scholar]
- 43.Upchurch P. Sauropod phylogeny and palaeoecology. Gaia. 1994;10:249–260. [Google Scholar]
- 44.Mateus O, Maidment SCR, Christiansen NA. A new long-necked ‘sauropod-mimic’ stegosaur and the evolution of the plated dinosaurs. Proc Biol Sci. 2009;276:1815–1821. doi: 10.1098/rspb.2008.1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stevens KA, Parrish JM. In: Thunder-Lizards: The Sauropodomorph Dinosaurs. Tidwell V, Carpenter K, editors. Bloomington, IN: Indiana Univ Press; 2005. pp. 212–232. [Google Scholar]
- 46.Sander M. Biology of the sauropod dinosaurs: The evolution of gigantism. Biol Rev. 2010 doi: 10.1111/j.1469-185X.2010.00137.x. 10.1111/j.1469-185X.2010.00137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Morton ES. In: The Ecology of Arboreal Folivores. Montgomery GG, editor. Washington, DC: Smithsonian Inst; 1978. pp. 123–130. [Google Scholar]
- 48.Grajal A, Strahl SD, Parra R, Gloria Dominguez M, Neher A. Foregut fermentation in the hoatzin, a neotropical leaf-eating bird. Science. 1989;245:1236–1238. doi: 10.1126/science.245.4923.1236. [DOI] [PubMed] [Google Scholar]
- 49.Gill FB. Ornithology. 2nd Ed. New York: Freeman; 1995. [Google Scholar]
- 50.Norell MA, et al. A theropod dinosaur embryo and the affinities of the flaming cliffs dinosaur eggs. Science. 1994;266:779–782. doi: 10.1126/science.266.5186.779. [DOI] [PubMed] [Google Scholar]
- 51.Nesbitt SJ, et al. Ecologically distinct dinosaurian sister group shows early diversification of Ornithodira. Nature. 2010;464:95–98. doi: 10.1038/nature08718. [DOI] [PubMed] [Google Scholar]
- 52.Walker JD, Geissman JW compilers. Geologic Time Scale. Boulder, CO: Geol Soc Am; 2009. 10.1130/2009.CTS004R2C. [Google Scholar]
- 53.Kilbourne BM, Makovicky PJ. Limb bone allometry during postnatal ontogeny in non-avian dinosaurs. J Anat. 2010;217:135–152. doi: 10.1111/j.1469-7580.2010.01253.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Organ CL, Janes DE, Meade A, Pagel M. Genotypic sex determination enabled adaptive radiations of extinct marine reptiles. Nature. 2009;461:389–392. doi: 10.1038/nature08350. [DOI] [PubMed] [Google Scholar]
- 55.Maddison WP, Maddison DR. MacClade 4.0: Analysis of Phylogeny and Character Evolution. Sunderland: Sinauer; 2000. [DOI] [PubMed] [Google Scholar]
- 56.Maddison WP, Maddison DR. Mesquite: A Modular System for Evolutionary Analysis, Version 2.5. 2008. Available at http://mesquiteproject.org/mesquite/mesquite.html. Accessed March 1, 2010.
- 57.Zar JH. Biostatistical Analysis. 4th Ed. Upper Saddle River, NY: Prentice Hall; 1999. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.