Abstract
Carney-Stratakis syndrome, an inherited condition predisposing affected individuals to gastrointestinal stromal tumor (GIST) and paraganglioma, is caused by germline mutations in succinate dehydrogenase (SDH) subunits B, C, or D, leading to dysfunction of complex II of the electron transport chain. We evaluated the role of defective cellular respiration in sporadic GIST lacking mutations in KIT or PDGFRA (WT). Thirty-four patients with WT GIST without a personal or family history of paraganglioma were tested for SDH germline mutations. WT GISTs lacking demonstrable SDH genetic inactivation were evaluated for SDHB expression by immunohistochemistry and Western blotting and for complex II activity. For comparison, SDHB expression was also determined in KIT mutant and neurofibromatosis-1–associated GIST, and complex II activity was also measured in SDH-deficient paraganglioma and KIT mutant GIST; 4 of 34 patients (12%) with WT GIST without a personal or family history of paraganglioma had germline mutations in SDHB or SDHC. WT GISTs lacking somatic mutations or deletions in SDH subunits had either complete loss of or substantial reduction in SDHB protein expression, whereas most KIT mutant GISTs had strong SDHB expression. Complex II activity was substantially decreased in WT GISTs. WT GISTs, particularly those in younger patients, have defects in SDH mitochondrial complex II, and in a subset of these patients, GIST seems to arise from germline-inactivating SDH mutations. Testing for germline mutations in SDH is recommended in patients with WT GIST. These findings highlight a potential central role of SDH dysregulation in WT GIST oncogenesis.
Keywords: genetic predisposition, sarcoma, pediatric
Gastrointestinal stromal tumor (GIST), the most common mesenchymal neoplasm of the gastrointestinal tract, is resistant to conventional cytotoxic chemotherapy (1). Oncogenic mutations in KIT or PDGFRA have been identified as central tumor-initiating events in many GISTs (2, 3). However, 85% of GISTs occurring in children and 15% of GISTs occurring in adults lack KIT or PDGFRA mutations (termed wild-type or WT GISTs) (4,5–6). The tumor-initiating event(s) in these WT GISTs is not known. Imatinib and sunitinib, small-molecule inhibitors of the mutant KIT and PDGFRA receptor tyrosine kinases, significantly prolong survival in patients with GIST (7, 8). However, imatinib is less effective against WT tumors (9, 10), and initial studies suggest that sunitinib only rarely results in objective responses in WT GIST (10, 11). Identification of the pathogenetic mechanism in WT GIST will facilitate the identification of drug targets in these tumors.
The succinate dehydrogenase (SDH)–ubiquinone complex II, a component of the Krebs cycle and the respiratory chain, is a heteroligomer composed of subunits A, B, C, and D. The familial paraganglioma syndromes 1, 3, and 4 are caused by germline-inactivating mutations in the genes coding for SDH subunits D (SDHD), C (SDHC), or B (SDHB), respectively. An additional 12–16% of patients with apparently sporadic paraganglioma carry germline-inactivating mutations in SDHB, -C, or -D (12, 13). Germline mutations in SDHB have also been associated with pheochromocytoma, especially malignant forms, and renal cell carcinoma. In familial paraganglioma, SDH acts as a classic tumor suppressor (14): germline-inactivating mutations in one allele combined with somatic inactivation of the remaining normal allele lead to tumor development. Inactivation of any one of the three commonly mutated SDH subunits results in destabilization of the SDH complex and loss of enzymatic function (15). An additional SDH–ubiquinone complex II component, SDHAF2, that interacts with and flavinates SDH subunit A (SDHA) was very recently described. Loss of function mutations in SDHAF2 also result in destabilization of the SDH complex and loss of complex II activity, and SDHAF2 germline mutation is a rare cause of familial paraganglioma (16–18).
Carney-Stratakis syndrome is an inherited predisposition to GIST and paraganglioma (19) that is caused by inactivating germline mutations in SDHB, -C, or -D (20, 21). Sporadic WT GIST occurring in patients without a personal or family history of paraganglioma is more common than Carney-Stratakis syndrome, but the causative oncogenic events in these WT GISTs remain unknown. We sought to evaluate the role of defective cellular respiration in sporadic WT GISTs.
Results
Subjects Were Identified Through the National Institutes of Health Pediatric and WT GIST Clinic.
The National Institutes of Health (NIH) Pediatric and WT GIST Clinic, a biannual collaborative effort between clinicians, researchers, support groups, and patients, was established in 2008 to further the investigation of the clinical features and oncogenic mechanisms underlying WT GIST (www.pediatricgist.cancer.gov). After meeting with a geneticist and a genetic counselor, all patients attending the clinic were offered testing for germline mutations in SDHB, -C, and -D. At the time that this study was conducted, 37 patients had attended the NIH Pediatric and WT GIST Clinic. Thirty-four patients had confirmed WT GIST, had no family or personal history of paraganglioma, and consented to participation in genetic testing. Thirty of 34 tumors were confirmed to be WT in exons 9, 11, 13, and 17 of KIT and exons 12 and 18 of PDGFRA. Three of the remaining tumors were confirmed to be WT in at least four of the commonly mutated KIT and PDGFRA exons. One tumor was confirmed to be WT only in exons 9 and 11 of KIT. One patient had a diagnosis of neurofibromatosis type 1 (NF-1). In this group of patients, age at GIST diagnosis was 5–58 y (median = 22 y). The primary tumor site was gastric in 82% of patients, small intestine in 9%, and advanced in 9%. Fifty-six percent of primary tumors were multifocal at presentation, and 79% of the patients were female.
Germline SDH Mutations Are Present in 12% of Individuals With WT GIST Without a Personal or Family History of Paraganglioma.
SDHB, -C, and -D exons and exon–intron boundaries were sequenced from genomic DNA isolated from whole blood of the 34 patients with confirmed WT GIST. Four (12%) patients had germline mutations in SDHB or -C (Table 1 and Table S1). Three mutations were identified in SDHB in exons 3, 6, and 7. SDHB mutations were missense mutations resulting in changes in amino acids that are highly conserved across species. Two of the SDHB mutations have previously been reported in familial paraganglioma (22). The other SDHB mutation, S92T, resulted in a substitution at a highly conserved amino acid, which is expected—based on in silico analysis—to inactivate SDHB function [position-specific independent count (PSIC) score of 2.231]. One splice site mutation was identified in SDHC at position +1 of intron 5. A mutation at this site, previously reported in both paraganglioma and Carney-Stratakis syndrome, causes deletion of exon 5, and it results in a frame shift and premature termination (20, 23).
Table 1.
Germline SDH mutations identified in 34 patients with WT GIST without a family or personal history of paraganglioma
| Gene | Exon | Mutation | Amino acid change | Reference | Mutation type | Age at diagnosis (y) | Sex | SDHB IHC |
| SDHB | 3 | c. 274 T > A/T | p. Ser-92 Thr | No previous reports | Missense | 18 | M | Negative |
| SDHB | 6 | c. 600 G > G/T | p. Trp-200 Cys | 27 | Missense | 22 | M | Negative |
| SDHB | 7 | c. 725 G > A/G | p. Arg-242 His | 12 | Missense | 21 | F | NA |
| SDHC | Intron 5 | c. 405+1 G > A | 20 | Splice site | 16 | F | NA | |
| SDHD | 1 | c. 34 G > A | p. Gly-12 Ser | 24 | Polymorphism | 7 | F | NA |
| SDHD | 1 | c. 34 G > A | p. Gly-12 Ser | 24 | Polymorphism | 58 | F | 1+ |
Two patients (6%) had an SDHD germline sequence change with questionable pathogenicity (c. 34 G > A) that has previously been reported to be present in patients with pheochromocytoma (24), hereditary paraganglioma (25), and Cowden syndrome (26). In Cowden syndrome, the resulting amino acid change, G12S, was associated with a twofold increase in AKT and MAPK activity and an increase in reactive oxygen species. However, this SDHD sequence alteration has also been seen in control populations, with an incidence ranging from 0% to 2.5% (24, 26).
To confirm the functional impact of these germline mutations, we performed SDHB immunohistochemistry (IHC) on paraffin-embedded GIST tumor samples, when available, from patients with SDH subunit germline mutations. SDHB protein expression was evaluable in two of three patients with germline SDHB mutations, and in both, expression was absent (Fig. 1). SDHB protein expression was 1+ in the one patient with germline SDHD sequence change in which there was sufficient tumor for analysis.
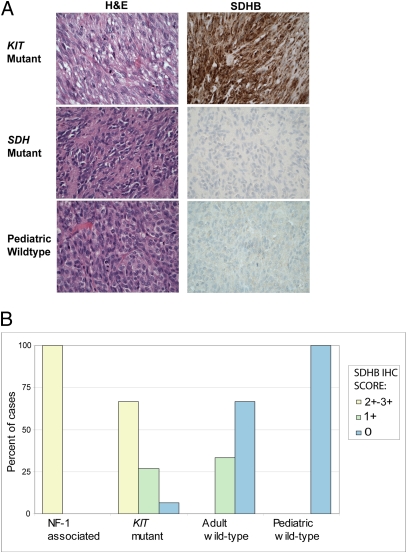
Fig. 1.
(A) Representative H&E stains (Left) and corresponding SDHB IHC (Right) in KIT mutant (Top), SDH mutant (Middle), and WT GIST (Bottom), highlighting strong SDHB immunoexpression in the KIT mutant GIST and absence of SDHB expression in the SDH mutant GIST and the WT GIST. (B) Summary of SDHB IHC scores in pediatric (n = 14) and adult (n = 12) WT GIST, KIT mutant GIST (n = 15), and NF-1–associated GIST (n = 5).
Patients with SDHB mutations were all young adults, diagnosed at 18, 21, and 22 y of age. The patient with the SDHC mutation was 16 y at diagnosis. The sex distribution of patients with SDH mutations was 50% male and 50% female. All patients with SDH mutations had multifocal GIST, but 50% of the patients without SDH mutations also had multifocal GIST.
WT GISTs Have Either Complete Loss or Substantial Reduction in SDHB Protein Expression.
To determine whether loss of SDHB protein expression was a general feature of WT GISTs, 30 WT GIST tumors without associated SDH mutations were evaluated for SDHB protein expression by IHC (n = 22), Western blotting (n = 4), or both Western blotting and IHC (n = 4). Eighteen of the WT GISTs used in these studies were classified as pediatric (age of diagnosis ≤ 18 y); 12 were classified as adult. In 25 of 30 WT GISTs, absence of an associated SDH mutation was confirmed by sequence analysis using germline or tumor DNA. For the remaining five WT GISTs, there was neither germline DNA nor tumor DNA available to confirm a lack of an associated SDH mutation (Table S1). Furthermore, 250,000 SNP analyses, performed in 7 of 31 GISTs, showed absence of SDHB, SDHC, or SDHD deletions in 6 GISTs (Fig. S1), whereas one tumor had a loss of most of 1p, a common abnormality in KIT mutant GISTs, resulting in an SDHB deletion.
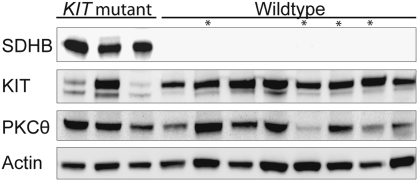
SDHB protein expression was absent in 18 of 18 (100%) pediatric WT GISTs evaluated for SDHB expression by IHC (Fig. 1) or Western blotting (Fig. 2), including four cases that were negative by both methods (Fig. 2, cases marked with an asterisk had an SDHB IHC score of 0). SDHB protein expression was absent in 8 of 12 (67%) and was weak (SDHB IHC score of 1+) in 4 of 12 (33%) of the adult WT GISTs. By comparison, only 1 of 18 (6%) of the KIT mutant GISTs and 0 of 5 NF-1–associated GISTs lacked SDHB expression.
Fig. 2.
Western blot of SDHB expression in KIT mutant and WT GIST. Cases marked by an asterisk had an SDHB IHC score of 0.
WT GIST Has Markedly Decreased SDH (Complex II) Activity.
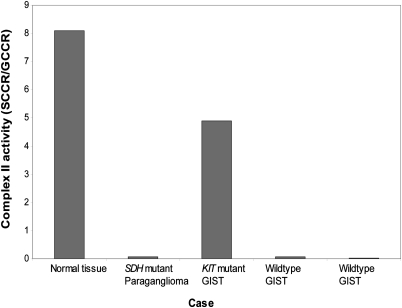
Loss of SDHB expression has previously been shown to be highly correlated with SDH or complex II inactivation in paraganglioma (15). However, we did not know whether this would also be true in GIST. Therefore, a detailed spectrophotometric study of the activity of mitochondrial respiratory chain complexes rotenone-sensitive NADH-quinone reductase (NQR; complex I), malonate-sensitive succinate-cytochrome c reductase (SCCR; complexes II and III), glycerol-3-phosphate cytochrome c reductase (GCCR; glycerol-3-phosphate dehydrogenase + complex III), antimycin-sensitive decylubiquinol-cytochrome c reductase (QCCR; complex III), cyanide-sensitive cytochrome c oxidase (COX; complex IV), and oligomycin-sensitive ATPase (complex V) was performed in two WT GISTs lacking somatic mutations or deletions in SDH subunit genes, a KIT mutant GIST, and an SDH mutant paraganglioma. Both absolute and relative (to GCCR) SCCR activity, in which the limiting activity is the SDH complex, were markedly reduced in the WT GISTs (Fig. 3). The extent of reduction in SCCR activity seen in the WT GISTs was equal to that seen in an SDHB mutant paraganglioma. In KIT mutant GIST, SCCR activity was comparable with that seen in normal abdominal tissue.
Fig. 3.
Assessment of complex II activity in a paraganglioma arising in a patient with a germline SDH mutation, in two WT GISTs, and in a KIT mutant GIST. Cytochrome c oxidase (complex IV), quinol cytochrome c reductase (complex III), NADH quinone reducatse (complex I), and ATPase (complex V) activities were normal in these tumors.
Discussion
The SDHB and SDHC germline mutations identified in 12% of patients with WT GIST in this study are highly likely to be pathogenic, and to have predisposed these patients to the development of GIST. These germline mutations in the SDH subunit genes were found in individuals with GIST without a personal or family history of paraganglioma. Three of the four SDHB and SDHC germline mutations identified in these patients with GIST have previously been reported to occur in individuals with paragangliomas (12, 20, 27, 28). Like the majority of SDHB mutations associated with paraganglioma, the identified SDHB mutations in these patients with WT GIST are missense mutations in highly conserved amino acids (12, 22). The SDHC mutation identified here has previously been shown to result in an inactivating frame shift (20). GIST tumor specimens from two of the patients with SDHB germline mutations lacked SDHB protein expression, and the other patient was not evaluable. Absence of SDHB protein expression, as determined by IHC, has recently been shown to have a sensitivity of 100% for the presence of SDHB, SDHC, or SDHD mutations in paragangliomas and pheochromocytomas (15). We have not been able to determine the penetration of the clinical phenotype associated with these mutations, because not all first-degree relatives have undergone germline testing. The SDHD base pair change identified here in two patients is likely to be a polymorphism, despite the previously reported associations with pheochromocytoma, paraganglioma, and Cowden syndrome; this is because the c.34A > G nt change has been reported in up to 2.5% of normal controls (24), and the base pair change alters an amino acid that is not conserved across species. Furthermore, a GIST tumor specimen from one of the patients with this SDHD sequence change had 1+ SDHB protein expression.
Based on the 12% incidence of SDH subunit germline mutations in this series of patients with WT GIST, testing for germline mutations in SDHB, SDHC, and SDHD in all patients diagnosed with WT GIST is recommended, particularly in younger individuals. The incidence of germline mutations in apparently sporadic pheochromocytoma or functional paraganglioma is similar to that seen in GIST (12–16%) (12, 13), and germline testing has been recommended (29) for these patients. The identification of a germline mutation in a patient with WT GIST has the potential for clinical benefit by alerting the treating physician to a presumed increased risk of paragangliomas and additional GISTs. In addition, because SDHB-associated paragangliomas and GIST share several features such as PET positivity and intraabdominal location, it is possible for a functional paraganglioma to be mistaken for recurrent GIST. Knowledge of a germline mutation in one of the SDH subunit genes could prevent the potentially life-threatening complication of resection of a functional paraganglioma mistaken for a GIST.
This series is not sufficiently large to definitively identify clinical features associated with the presence of SDH germline mutations in patients with WT GIST. However, the sex distribution of those patients with germline mutations was 50% male, which is different from the female predominance typical of WT GIST in general and the female predominance of patients seen in the NIH Pediatric and WT GIST Clinic. In fact, two of seven males tested (29%) were found to have germline mutations in SDH subunit genes.
The association of germline SDHB and SDHC mutations and WT GIST suggested that abnormalities of cellular respiration might exist in WT GISTs generally, even in patients without germline mutations in one of the SDH subunits. To investigate this possibility, we evaluated SDHB expression and function in WT GISTs without associated SDH mutations. SDHB expression is absent in all pediatric WT GISTs and absent or weak in adult WT GISTs, whereas most KIT mutant and all NF-1–associated GISTs had strong SDHB expression. The observed lack of SDHB expression is not likely to be explained by somatic mutations in SDHB, -C, or -D in GIST tumors, because SDH mutation analysis was performed from tumor in 13 of the cases lacking SDH protein expression on IHC or Western blot. There has been one prior study of SDHB IHC in GIST (30). It is somewhat difficult to compare our results with this previously published study, because in the published study, KIT, PDGFRA, and SDH subunit genotype were available for only a limited number of cases. In that study, 97% of sporadic GISTs had positive SDHB IHC. The nine GISTs lacking SDHB expression occurred in patients with either Carney Triad or clinical features suggestive of WT GIST. Therefore, our results are not inconsistent with this previously published study.
KIT and PDGFRA sequencing is recommended in suspected WT GIST, because response to standard GIST therapies, imatinib and sunitinib, and natural history differs in WT tumors (9, 11, 31). However, molecular analysis is frequently not performed because of cost. Given the association between SDHB IHC results and genotype, an SDHB IHC score of less than 2+ could be used to identify tumors that are likely to be WT.
Loss of SDHB expression and lack of complex II activity in WT GIST without an associated SDH mutation or deletion implicate defects in cellular respiration as a potential central oncogenic mechanism in WT GIST. One possible mechanism for the observed loss of SDHB expression and complex II function in the WT GISTs samples analyzed in this study is epigenetic modification resulting in decreased mRNA expression of one of the components of the SDH complex. However, mRNA expression of SDHB, SDHC, and SDHD did not differ significantly between WT and KIT mutant GISTs, as evaluated by quantitative RT-PCR (Fig. S2). Another possible explanation is loss of function mutations in SDHA or SDHAF2, each of which has recently been described to occur in an individual patient and an individual family, respectively, with paraganglioma (16, 17, 32). However, SDHAF2 mutation analysis was conducted in 42 of the WT GIST cases from this study and an additional 48 WT GISTs, and no mutations were identified. SDHA mutation analysis was conducted in four of the WT GIST cases from this study and one additional WT GIST, and no mutations were identified (Table S1). We sequenced SDHA in only a small group of WT GISTs because of availability of appropriate material for sequencing, and further investigation of SDHA mutations in WT GIST is warranted. Another consideration warranting further study is alterations in other components of cellular respiration such as isocitrate dehydrogenase (33, 34) or yet to be identified tricarboxylic acid cycle proteins interacting with SDH.
Materials and Methods
Patients and Tumor Samples.
Patients were either self-referred or referred by their treating physician to the NIH Pediatric and WT GIST Clinic (www.pediatricgist.cancer.gov). Patients were accepted into the clinic only if they had GIST diagnosed at age 18 y or less (pediatric GIST), prior molecular analysis of their tumor with results consistent with WT GIST, or clinical features highly suggestive of WT GIST. Patients participated in research protocols that were approved by the institutional review boards at the relevant institutions. All participants gave consent or when relevant, assent for participation in the clinic and associated studies, including genetic testing.
For each participant in the NIH Pediatric and WT GIST Clinic, primary medical data, including clinic notes, radiographic studies, surgical reports, and pathology reports, were reviewed by NIH GIST team members. Over a 2.5-d period, participants in the NIH Pediatric and WT GIST Clinic underwent a history, physical examination, consultation with a geneticist, and a session with a genetic counselor. In addition, participants met with physician members of the Consortium for Pediatric and WT GIST Research (CPGR), a consortium of clinicians, researchers, and patient advocates who share the goal of defining the natural history and underlying biology of WT GIST in an effort to develop effective and novel treatment regimens.
Patients’ GIST tumors were confirmed to be WT by obtaining the report describing the results of mutation testing. When mutation analysis had not previously been performed, genomic DNA was extracted from the paraffin-embedded tumor, and exons 9, 11, 13, and 17 of KIT and exons 12 and 18 of PDGFRA were sequenced as previously described (6). Additional tumor samples, not from participants in the NIH Pediatric and WT GIST Clinic used in this study, have been described previously (4, 35, 36). Ten additional pediatric GIST cases were collected from the archives and referral cases of one of the authors (M.O.) for inclusion in the immunohistochemistry portion of this study.
Mutation Analysis.
Genomic DNA was isolated from blood or cryopreserved tumor. All exons and exon–intron boundaries of SDHB, SDHC, and SDHD were PCR-amplified and screened for mutation by standard methods at Beckman Coulter Genomics (for cryopreserved tumor; formerly Agencourt) or GeneDx (for germline DNA; www.geneDx.com) or as previously described (for paraffin-embedded tumor) (13, 37, 38). Sequence analysis was performed using the Mutation Surveyor software (SoftGenetics) and based on RefSeq [National Center for Biotechnology Information (NCBI)] for the appropriate gene (for cryopreserved tumor) or as previously described [for germline DNA (20, 21) and paraffin-embedded tumor (13, 37–40)]. Homology was determined based on homologene (NCBI).
Immunohistochemistry.
Immunohistochemistry analysis was performed on 4-μm sections of formalin-fixed tumor as previously described (15, 41). Immunoreactivity was graded semiquantitatively using the following scale: 0, no staining; 1+, less than 5% of tumor cells reactive; 2+, 6–50% of tumor cells reactive; 3+, over 51% of tumor cells reactive.
Western Blotting.
Whole-cell lysates of cryopreserved tumors were prepared as previously described (42). Lysates were separated by gel electrophoresis using NuPAGE 4–12% Bis·Tris gels (Invitrogen) and blotted to nitrocellulose membranes. Immunostains were detected using enhanced chemiluminescence (ECL; Amersham Pharmacia Biotech) and captured with the Fuji LAS1000-plus imaging system. Blots were stained with antibodies to SDHB, PKCθ (Santa Cruz Biotechnology), KIT (DAKO), and actin (Sigma).
SNP Array.
We evaluated SDH subunit gene deletions in WT GIST using SNP arrays. Genomic DNA isolated from cryopreserved GISTs and four normal control samples was digested with the StyI restriction enzyme. Digested DNA was then ligated to an adaptor before subsequent PCR amplification using AmpliTaq Gold (Applied Biosystems). PCR products were pooled, concentrated, and fragmented with DNase I to a size range of 200–1,100 bp. Fragmented PCR products were then labeled, denatured, and hybridized to Affymetrix 250K Sty SNP arrays interrogating ∼238,000 SNPs. After hybridization, the arrays were washed on Affymetrix fluidics stations, stained, scanned using Gene Chip Scanner 3000 7G, and interpreted using genotyping software Affymetrix Genotyping Tools Version 2.0. Data analysis was performed as previously described (4).
Mitochondrial Respiratory Chain Activity.
The activities of the respiratory chain complexes, rotenone-sensitive NQR (complex I), malonate-sensitive SCCR (complexes II and III), GCCR (glycerol-3-phosphate dehydrogenase + complex III), antimycin-sensitive QCCR (complex III), cyanide-sensitive COX (complex IV), and oligomycin-sensitive ATPase (complex V) were spectrophotometrically measured on homogenates prepared from frozen tumors as previously described (43). Normal abdominal tissue was used as a positive control, and paraganglioma from a patient with a pathogenic SDH mutation was used as a negative control. Results are expressed as a ratio of SCCR to GCCR activity.
Supplementary Material
Acknowledgments
This work was supported by the GIST Cancer Research Fund (to K.A.J., C.R.A., and J.A.F.), the St. Baldrick's Foundation (to K.A.J.), the Leduq Foundation (to P.R.), the Agence Nationale de la Recherche Mitoxy (to P.R.), the Voelcker Fund Investigator Award (to P.L.M.D.), a grant from the Health Research Board of Ireland through the Medical Research Charities Group (to M.O.), American Cancer Society Mentored Research Scholar Grant CCE-106841 (to C.R.A.), National Institutes of Health (NIH) P01CA47179 (to C.R.A.), Life Raft Group (to C.R.A.), Shuman Family Fund for GIST Research (to C.R.A.), the Intramural Program of The Eunice Kennedy Shriver National Institute of Child Health and Human Development (NICHD), the Intramural Program of the National Cancer Institute, and a grant from the Office of Rare Diseases of the National Institutes of Health.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
3The National Institutes of Health Pediatric GIST Clinic participants, in addition to K.A.J., S.Y.K., M.L., M.R., G.D.D., C.R.A., L.H., and C.A.S., are Suzanne George, Michael LaQuaglia, Alberto Pappo, Jonathan Trent, and Margaret von Mehren.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1009199108/-/DCSupplemental.
References
- 1.Gold JS, et al. Outcome of metastatic GIST in the era before tyrosine kinase inhibitors. Ann Surg Oncol. 2007;14:134–142. doi: 10.1245/s10434-006-9177-7. [DOI] [PubMed] [Google Scholar]
- 2.Hirota S, et al. Gain-of-function mutations of c-kit in human gastrointestinal stromal tumors. Science. 1998;279:577–580. doi: 10.1126/science.279.5350.577. [DOI] [PubMed] [Google Scholar]
- 3.Heinrich MC, et al. PDGFRA activating mutations in gastrointestinal stromal tumors. Science. 2003;299:708–710. doi: 10.1126/science.1079666. [DOI] [PubMed] [Google Scholar]
- 4.Janeway KA, et al. Pediatric KIT WT and platelet-derived growth factor receptor alpha-WT gastrointestinal stromal tumors share KIT activation but not mechanisms of genetic progression with adult gastrointestinal stromal tumors. Cancer Res. 2007;67:9084–9088. doi: 10.1158/0008-5472.CAN-07-1938. [DOI] [PubMed] [Google Scholar]
- 5.Corless CL, Fletcher JA, Heinrich MC. Biology of gastrointestinal stromal tumors. J Clin Oncol. 2004;22:3813–3825. doi: 10.1200/JCO.2004.05.140. [DOI] [PubMed] [Google Scholar]
- 6.Agaram NP, et al. Molecular characterization of pediatric gastrointestinal stromal tumors. Clin Cancer Res. 2008;14:3204–3215. doi: 10.1158/1078-0432.CCR-07-1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Demetri GD, et al. Efficacy and safety of sunitinib in patients with advanced gastrointestinal stromal tumour after failure of imatinib: A randomised controlled trial. Lancet. 2006;368:1329–1338. doi: 10.1016/S0140-6736(06)69446-4. [DOI] [PubMed] [Google Scholar]
- 8.Demetri GD, et al. Efficacy and safety of imatinib mesylate in advanced gastrointestinal stromal tumors. N Engl J Med. 2002;347:472–480. doi: 10.1056/NEJMoa020461. [DOI] [PubMed] [Google Scholar]
- 9.Heinrich MC, et al. Correlation of kinase genotype and clinical outcome in the North American Intergroup Phase III Trial of imatinib mesylate for treatment of advanced gastrointestinal stromal tumor: CALGB 150105 Study by Cancer and Leukemia Group B and Southwest Oncology Group. J Clin Oncol. 2008;26:5360–5367. doi: 10.1200/JCO.2008.17.4284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Janeway KA, et al. Sunitinib treatment in pediatric patients with advanced GIST following failure of imatinib. Pediatr Blood Cancer. 2009;52:767–771. doi: 10.1002/pbc.21909. [DOI] [PubMed] [Google Scholar]
- 11.Heinrich MC, et al. Primary and secondary kinase genotypes correlate with the biological and clinical activity of sunitinib in imatinib-resistant gastrointestinal stromal tumor. J Clin Oncol. 2008;26:5352–5359. doi: 10.1200/JCO.2007.15.7461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Burnichon N, et al. The succinate dehydrogenase genetic testing in a large prospective series of patients with paragangliomas. J Clin Endocrinol Metab. 2009;94:2817–2827. doi: 10.1210/jc.2008-2504. [DOI] [PubMed] [Google Scholar]
- 13.Amar L, et al. Genetic testing in pheochromocytoma or functional paraganglioma. J Clin Oncol. 2005;23:8812–8818. doi: 10.1200/JCO.2005.03.1484. [DOI] [PubMed] [Google Scholar]
- 14.Gottlieb E, Tomlinson IP. Mitochondrial tumour suppressors: A genetic and biochemical update. Natl Rev Cancer. 2005;5:857–866. doi: 10.1038/nrc1737. [DOI] [PubMed] [Google Scholar]
- 15.van Nederveen FH, et al. An immunohistochemical procedure to detect patients with paraganglioma and phaeochromocytoma with germline SDHB, SDHC, or SDHD gene mutations: A retrospective and prospective analysis. Lancet Oncol. 2009;10:764–771. doi: 10.1016/S1470-2045(09)70164-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hao HX, et al. SDH5, a gene required for flavination of succinate dehydrogenase, is mutated in paraganglioma. Science. 2009;325:1139–1142. doi: 10.1126/science.1175689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao L, et al. Mutations of the metabolic genes IDH1, IDH2, and SDHAF2 are not major determinants of the pseudohypoxic phenotype of sporadic pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2010;95:1469–1472. doi: 10.1210/jc.2009-2245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bayley JP, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–372. doi: 10.1016/S1470-2045(10)70007-3. [DOI] [PubMed] [Google Scholar]
- 19.Carney JA, Stratakis CA. Familial paraganglioma and gastric stromal sarcoma: A new syndrome distinct from the Carney triad. Am J Med Genet. 2002;108:132–139. doi: 10.1002/ajmg.10235. [DOI] [PubMed] [Google Scholar]
- 20.Pasini B, et al. Clinical and molecular genetics of patients with the Carney-Stratakis syndrome and germline mutations of the genes coding for the succinate dehydrogenase subunits SDHB, SDHC, and SDHD. Eur J Hum Genet. 2008;16:79–88. doi: 10.1038/sj.ejhg.5201904. [DOI] [PubMed] [Google Scholar]
- 21.McWhinney SR, Pasini B, Stratakis CA International Carney Triad and Carney-Stratakis Syndrome Consortium. Familial gastrointestinal stromal tumors and germ-line mutations. N Engl J Med. 2007;357:1054–1056. doi: 10.1056/NEJMc071191. [DOI] [PubMed] [Google Scholar]
- 22.Bayley JP, Devilee P, Taschner PE. The SDH mutation database: An online resource for succinate dehydrogenase sequence variants involved in pheochromocytoma, paraganglioma and mitochondrial complex II deficiency. BMC Med Genet. 2005;6:39. doi: 10.1186/1471-2350-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Niemann S, Müller U, Engelhardt D, Lohse P. Autosomal dominant malignant and catecholamine-producing paraganglioma caused by a splice donor site mutation in SDHC. Hum Genet. 2003;113:92–94. doi: 10.1007/s00439-003-0938-0. [DOI] [PubMed] [Google Scholar]
- 24.Cascón A, et al. SDHB mutation analysis in familial and sporadic phaeochromocytoma identifies a novel mutation. J Med Genet. 2002;39:E64. doi: 10.1136/jmg.39.10.e64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Taschner PE, et al. Nearly all hereditary paragangliomas in the Netherlands are caused by two founder mutations in the SDHD gene. Genes Chromosomes Cancer. 2001;31:274–281. doi: 10.1002/gcc.1144. [DOI] [PubMed] [Google Scholar]
- 26.Ni Y, et al. Germline mutations and variants in the succinate dehydrogenase genes in Cowden and Cowden-like syndromes. Am J Hum Genet. 2008;83:261–268. doi: 10.1016/j.ajhg.2008.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Timmers HJ, et al. Clinical presentations, biochemical phenotypes, and genotype-phenotype correlations in patients with succinate dehydrogenase subunit B-associated pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2007;92:779–786. doi: 10.1210/jc.2006-2315. [DOI] [PubMed] [Google Scholar]
- 28.Schiavi F, et al. Predictors and prevalence of paraganglioma syndrome associated with mutations of the SDHC gene. JAMA. 2005;294:2057–2063. doi: 10.1001/jama.294.16.2057. [DOI] [PubMed] [Google Scholar]
- 29.Jiménez C, Cote G, Arnold A, Gagel RF. Review: Should patients with apparently sporadic pheochromocytomas or paragangliomas be screened for hereditary syndromes? J Clin Endocrinol Metab. 2006;91:2851–2858. doi: 10.1210/jc.2005-2178. [DOI] [PubMed] [Google Scholar]
- 30.Gill AJ, et al. Immunohistochemistry for SDHB divides gastrointestinal stromal tumors (GISTs) into 2 distinct types. Am J Surg Pathol. 2010;34:636–644. doi: 10.1097/PAS.0b013e3181d6150d. [DOI] [PubMed] [Google Scholar]
- 31.Miettinen M, Lasota J, Sobin LH. Gastrointestinal stromal tumors of the stomach in children and young adults: A clinicopathologic, immunohistochemical, and molecular genetic study of 44 cases with long-term follow-up and review of the literature. Am J Surg Pathol. 2005;29:1373–1381. doi: 10.1097/01.pas.0000172190.79552.8b. [DOI] [PubMed] [Google Scholar]
- 32.Burnichon N, et al. SDHA is a tumor suppressor gene causing paraganglioma. Hum Mol Genet. 2010;19:3011–3020. doi: 10.1093/hmg/ddq206. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bayley JP, et al. SDHAF2 mutations in familial and sporadic paraganglioma and phaeochromocytoma. Lancet Oncol. 2010;11:366–372. doi: 10.1016/S1470-2045(10)70007-3. [DOI] [PubMed] [Google Scholar]
- 34.Gaal J, et al. Isocitrate dehydrogenase mutations are rare in pheochromocytomas and paragangliomas. J Clin Endocrinol Metab. 2010;95:1274–1278. doi: 10.1210/jc.2009-2170. [DOI] [PubMed] [Google Scholar]
- 35.Liegl B, Hornick JL, Corless CL, Fletcher CD. Monoclonal antibody DOG1.1 shows higher sensitivity than KIT in the diagnosis of gastrointestinal stromal tumors, including unusual subtypes. Am J Surg Pathol. 2009;33:437–446. doi: 10.1097/PAS.0b013e318186b158. [DOI] [PubMed] [Google Scholar]
- 36.Liegl B, et al. Heterogeneity of kinase inhibitor resistance mechanisms in GIST. J Pathol. 2008;216:64–74. doi: 10.1002/path. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Niemann S, Müller U. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat Genet. 2000;26:268–270. doi: 10.1038/81551. [DOI] [PubMed] [Google Scholar]
- 38.Gimenez-Roqueplo AP, et al. The R22X mutation of the SDHD gene in hereditary paraganglioma abolishes the enzymatic activity of complex II in the mitochondrial respiratory chain and activates the hypoxia pathway. Am J Hum Genet. 2001;69:1186–1197. doi: 10.1086/324413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Gimenez-Roqueplo AP, et al. Functional consequences of a SDHB gene mutation in an apparently sporadic pheochromocytoma. J Clin Endocrinol Metab. 2002;87:4771–4774. doi: 10.1210/jc.2002-020525. [DOI] [PubMed] [Google Scholar]
- 40.Korpershoek E, et al. Genetic analyses of apparently sporadic pheochromocytomas: The Rotterdam experience. Ann N Y Acad Sci. 2006;1073:138–148. doi: 10.1196/annals.1353.014. [DOI] [PubMed] [Google Scholar]
- 41.Dahia PL, et al. A HIF1alpha regulatory loop links hypoxia and mitochondrial signals in pheochromocytomas. PLoS Genet. 2005;1:72–80. doi: 10.1371/journal.pgen.0010008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duensing A, et al. Mechanisms of oncogenic KIT signal transduction in primary gastrointestinal stromal tumors (GISTs) Oncogene. 2004;23:3999–4006. doi: 10.1038/sj.onc.1207525. [DOI] [PubMed] [Google Scholar]
- 43.Bénit P, et al. Three spectrophotometric assays for the measurement of the five respiratory chain complexes in minuscule biological samples. Clin Chim Acta. 2006;374:81–86. doi: 10.1016/j.cca.2006.05.034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.