Abstract
Parathyroid hormone (PTH)-related protein (PTHrP), regulated by Indian hedgehog and acting through the PTH/PTHrP receptor (PPR), is crucial for normal cartilage development. These observations suggest a possible role of PPR signaling in the postnatal growth plate; however, the role of PPR signaling in postnatal chondrocytes is unknown. In this study, we have generated tamoxifen-inducible and cartilage-specific PPR KO mice to evaluate the physiological role of PPR signaling in postnatal chondrocytes. We found that inactivation of the PPR in chondrocytes postnatally leads to accelerated differentiation of chondrocytes, followed by disappearance of the growth plate. We also observed an increase of TUNEL-positive cells and activities of caspase-3 and caspase-9 in the growth plate, along with a decrease in phosphorylation of Bad at Ser155 in postnatal PPR KO mice. Administration of a low-phosphate diet, which prevents apoptosis of chondrocytes, prevented the disappearance of the growth plate. Taken together, these observations suggest that the major consequences of PPR activation are similar in both the fetal and postnatal growth plates. Moreover, chondrocyte apoptosis through the activation of a mitochondrial pathway may be involved in the process of premature disappearance of the growth plate by postnatal inactivation of the PPR in chondrocytes.
Endochondral ossification is an essential process for skeletal development in the fetal and neonatal periods and for subsequent bone growth (1). In the long bones of the limb, the formation of an epiphyseal secondary center of ossification leaves a disk of growth chondrocytes (the “growth plate”) that directs the lengthening of postnatal bone. In some species, notably humans and rabbits, growth plates close at the time of puberty through the actions of the estrogen receptor. In mice, growth slows progressively postnatally but most growth plates do not close.
A complex network of signaling pathways regulates epiphyseal chondrocyte development (1). Parathyroid hormone (PTH)-related protein (PTHrP), regulated by Indian hedgehog (Ihh) and acting through the PTH/PTHrP receptor (PPR), is crucial for normal bone growth. PTHrP is produced by periarticular chondrocytes and adjacent perichondrial cells in fetal life, whereas the PPR is produced by chondrocytes as they transition from the proliferative to postproliferative state. PTHrP regulates the length of the columnar region by allowing continued proliferation of columnar chondrocytes and suppressing their terminal differentiation into postmitotic hypertrophic chondrocytes in late fetal and perinatal growth plates.
Severe abnormalities in chondrocyte development are observed in genetic disorders in humans that disrupt signaling by the PPR through mutations in the PPR gene; the PTHrP gene; or the gene encoding the α-subunit of the stimulatory G-protein (Gsα), a mediator of actions of the PPR (2). PPR mutations that lead to constitutive activity occur in Jansen metaphyseal chondrodysplasia (3), and lethal homozygous or compound heterozygous mutations that lead to inactivation of PPRs cause short extremities and other defects (4). Albright hereditary osteodystrophy (AHO) is a result of a loss-of-function mutation in one copy of the GNAS1 gene, which encodes Gsα. Patients with AHO present with a skeletal phenotype that includes brachydactyly and premature closure of growth plates (5); this suggests that Gsα pathways, regulated by PTHrP, may be important regulators of the postnatal growth plate. Recently, two reports of families with inactivating, heterozygous mutations of the PTHrP gene show that such heterozygosity for PTHrP causes brachydactyly type E, which closely resembles the phenotype of AHO and was associated with premature cessation of growth in many cases (6, 7). Our results reveal a unique role for the PPR in that PPR expression in the postnatal chondrocytes, unlike in fetal life, is essential for maintenance of the growth plate in mice; growth plates disappear in the absence of continued PPR signaling.
Results
Postnatal Ablation of the PPR in Growth Plate Chondrocytes Causes Growth Plate Closure.
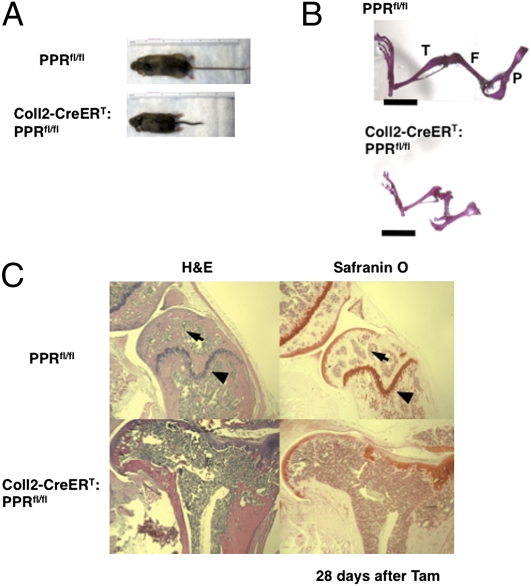
Transgenic mice in which Cre recombinase fused to a mutated estrogen receptor ligand-binding domain [type 2 collagen (Coll2)-CreERT)] driven by the promoter of the collagen2α1 gene were generated as reported previously (8). To generate tamoxifen (Tam)-inducible and cartilage-specific KO of the PPR [Coll2-CreERT:floxed PPR (PPRfl/fl)], we crossed Coll2-CreERT:PPRfl/fl mice to PPRfl/fl mice (9). PPRfl/fl littermates were used as a control; their growth plates appeared identical to those of Coll2-CreERT:PPRfl/fl mice at all developmental stages. Coll2-CreERT:PPRfl/fl and PPRfl/fl control mice were treated with a single 0.5-mg dose of Tam per mouse at postnatal day (P) 3, and the effects on bone development were analyzed in P6, P10, and P31 mice. There was no change in body weight and size of Coll2-CreERT:PPRfl/fl mice compared with PPRfl/fl control mice until 7 d after administration of Tam. Their weights diverged from those of control mice by P17 and were 67.1% of controls after 28 d. As shown in Fig. 1A, the size of mutant mice was less than that of control littermates [body length (cm): 13.5 ± 0.2 vs. 9.1 ± 0.4; P < 0.05; n = 4 in each group], with a reduction in the length of the tails and limbs at 28 d after administration of Tam. Further, histological examination of the skeleton revealed shortening of the long bones and significant joint abnormalities in Coll2-CreERT:PPRfl/fl mice at 28 d after administration of Tam (Fig. 1B). To assess the efficiency of removal of the PPR in the growth plate, we performed in situ hybridization analysis using a PPR probe specific for the removed E1 exon (9). Expression of PPR mRNA was not seen in the growth plate chondrocytes of metatarsal bone in Coll2-CreERT:PPRfl/fl mice, whereas abundant expression was seen in the prehypertrophic and hypertrophic regions of the growth plate in PPRfl/fl mice at 3 d after Tam administration (Fig. S1A). We could not follow the disappearance of PPR protein because removal of the floxed exon E1 of the PPR did not change the expression of PPR immunoreactivity (Fig. S1B). Exon E1, although essential for PPR activity (10), has 105 bp; therefore, its removal does not change the protein's reading frame. Because we cannot be sure how long it takes for the PPR protein to disappear completely after Cre action, the phenotypes we describe here may reflect either complete absence of PPR protein or low levels of this protein. There was no difference in the body weight between vehicle-treated and Tam-treated PPRfl/fl mice [body weight (%): 100.0 ± 5.8 vs. 100.5 ± 2.4, n = 3 in each group; body length (cm): 13.7 ± 0.1 vs. 13.5 ± 0.2, n = 4 in each group], indicating that Tam does not affect bone development in our experiments (Fig. S1C). Furthermore, histological analysis revealed complete loss of growth plates (Fig. 1C, arrowheads) and secondary ossification centers (Fig. 1C, arrows) and distortion of the articular surfaces (Fig. S1D) in Coll2-CreERT:PPRfl/fl mice compared with PPRfl/fl control mice. Safranin O staining in Fig. 1C stains the matrix surrounding chondrocytes. The chondrocytes of the growth plate and their associated matrix are completely missing, and the cartilage matrix at the joint surface only irregularly stains with Safranin O. These observations suggest that postnatal ablation of the PPR in chondrocytes resulted in disappearance of the growth plates and permanent deformity of the epiphyses.
Fig. 1.
PPR signaling is required for normal skeletal development in postnatal life. (A) Photographs of P31 PPRfl/fl and Coll2-CreERT:PPRfl/fl littermates at 28 d after Tam administration. (B) Skeletal preparation of hind limbs from P31 PPRfl/fl and Coll2-CreERT:PPRfl/fl littermates. P, pelvis; F, femur; T, tibia. (Scale bar: 5 mm.) (C) Histological analysis of long bones of P31 mice revealed abnormalities of the skeleton in mutant mice. H&E (Left) and Safranin O staining (Right) of longitudinal cross-sections of femurs in P31 PPRfl/fl (Upper) and Coll2-CreERT:PPRfl/fl (Lower) mice at 28 d after Tam administration. Arrows indicate the secondary ossification centers, and arrowheads indicate the growth plates of femurs.
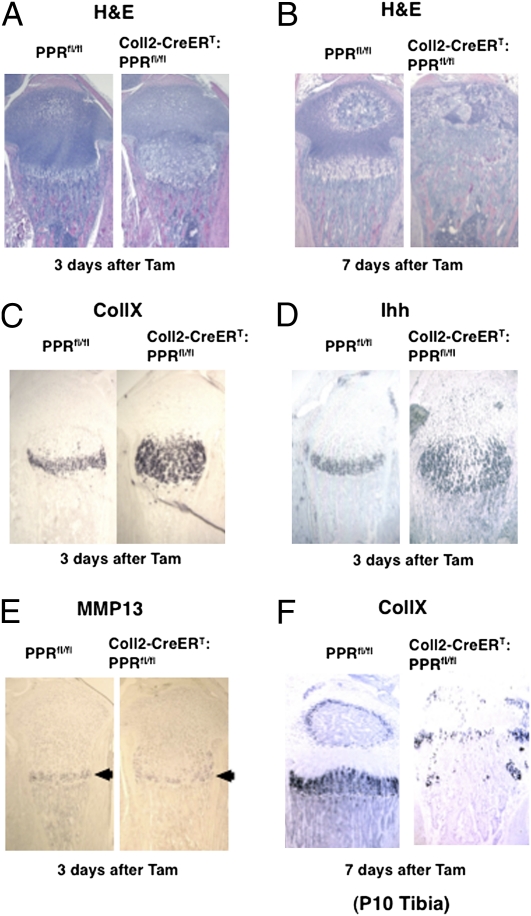
To determine the mechanism causing disappearance of the growth plate chondrocytes, we analyzed bones at early times after administration of Tam. Three days after Tam administration, the region containing hypertrophic chondrocytes was expanded into the middle of the growth plate [hypertrophic region (%): 100.0 ± 3.5 vs. 196.9 ± 15.8, n = 4 in each group] (Fig. 2A). Seven days after Tam administration, at P10, premature closure of the growth plate had already occurred in Coll2-CreERT:PPRfl/fl mice (Fig. 2B). To clarify alterations in differentiation at P6, 3 d after Tam administration, we determined the expression of mRNA encoding markers of characteristic stages of differentiation of chondrocytes through in situ hybridization analysis. Corresponding to the expansion of the region of hypertrophic chondrocytes, the region of expression of type X collagen (CollX) mRNA was also expanded (Fig. 2C). The domain of expression of Ihh was also enlarged (Fig. 2D); many flat columnar chondrocytes expressed Ihh mRNA in these growth plates. In contrast, there was no expansion of the Mmp13 mRNA expression domain (arrowheads in Fig. 2E). The domain of expression of CollX mRNA had almost disappeared in the growth plates of Coll2-CreERT:PPRfl/fl mice by 7 d after Tam administration and abutted the CollX mRNA-expressing cells in what was left of the secondary ossification center (Fig. 2F). The lack of expansion of the group of cells expressing Mmp13 mRNA suggests that acceleration of hypertrophic differentiation, rather than reduction of cartilage resorption at the end of the growth plate, is responsible for the expansion of the hypertrophic region at P6. The disappearance of the growth plate in the Coll2-CreERT:PPRfl/fl mice soon after Tam administration might reflect abnormal processes outside the growth plate itself. The calcified matrix surrounding hypertrophic chondrocytes is degraded by chondroclasts/osteoclasts concomitant with vascular invasion at the primary ossification center. However, we found no difference in the number of tartrate-resistant acidic phosphatase (TRAP)-positive multinuclear cells in the primary spongiosa of the Coll2-CreERT:PPRfl/fl compared with PPRfl/fl control mice [TRAP-stained cells (%): 100.0 ± 3.8 vs. 102.6 ± 10.3, n = 3 in each group], however (Fig. S1E). In addition, we did not observe any abnormalities of vascularization (immunostaining for CD31) of the secondary ossification centers in the tibiae of P8 Coll2-CreERT:PPRfl/fl mice at 5 d after Tam administration (Fig. S1F). Thus, no morphological changes outside the growth plate suggest mechanisms for the disappearance of the growth plates.
Fig. 2.
Postnatal deletion of the PPR in chondrocytes resulted in premature closure of the growth plates. H&E staining of tibiae of PPRfl/fl (Left) and Coll2-CreERT:PPRfl/fl (Right) mice at 3 d (A) and 7 d (B) after Tam administration. In situ hybridization analysis of CollX (C), Ihh (D), and Mmp13 (E) mRNA expression in the proximal tibial growth plates of PPRfl/fl mice (Left) and Coll2-CreERT:PPRfl/fl mice (Right) at 3 d after Tam administration. (F) CollX mRNA expression in tibiae of PPRfl/fl (Left) and Coll2-CreERT:PPRfl/fl (Right) mice at 7 d after Tam administration. Data are representative of experiments performed on sections from three mice for each condition.
Inactivation of the PPR in Chondrocytes Leads to Increased Activation of the Mitochondrial Pathway of Apoptosis.
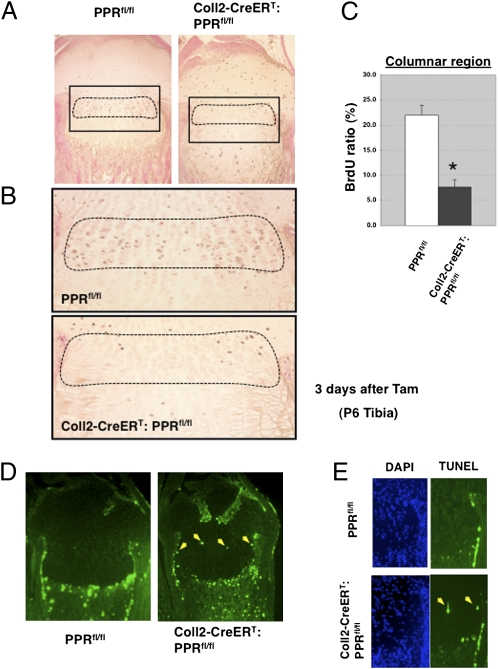
To assess how deletion of the PPR signal in postnatal chondrocytes triggers the disappearance of the growth plate, we next examined whether ablation of the PPR might affect chondrocyte proliferation and apoptosis. We focused on chondrocytes at P6, 4 d before the virtually complete disappearance of the growth plate, so that we could identify proliferating and dying cells. The expansion of the hypertrophic zone at P6 was accompanied by a dramatic decrease in the number of proliferating chondrocytes in the columnar region, as determined by BrdU labeling (Fig. 3 A–C). Normally, apoptosis is observed only in highly differentiated hypertrophic chondrocytes at the border of the primary spongiosa (11). We observed an increase in the number of TUNEL-positive cells at the expected location at the end of the hypertrophic layer of the Coll2-CreERT:PPRfl/fl mice at 4 d after administration of Tam [TUNEL-positive cells in the hypertrophic region (%): 4.11 ± 0.82 vs. 3.51 ± 0.78, n = 5 in each group], and we also observed TUNEL-positive cells in the region of columnar chondrocytes not seen in those cells in PPRfl/fl control mice (1.66 ± 0.53 vs. 0.04 ± 0.04; P < 0.05; n = 5 in each group) (Fig. 3 D and E). These findings were not observed in E18.5 Coll2-CreERT:PPRfl/fl mice given Tam at embryo day (E) 14.5 in fetal life (Fig. S2). These observations indicate that postnatal PPR signaling in chondrocytes, unlike fetal PPR signaling, may be required for survival of growth plate chondrocytes.
Fig. 3.
Chondrocyte apoptosis increases in the PPR-deficient growth plate postnatally. (A) Representative BrdU staining in P6 proximal tibiae in Coll2-CreERT:PPRfl/fl (Left) and PPRfl/fl (Right) growth plates at 3 d after Tam administration. (B) Magnified views of the boxed areas in A shown in PPRfl/fl (Upper) and Coll2-CreERT:PPRfl/fl (Lower) mice at 3 d after Tam administration. (C) BrdU labeling index was calculated in the columnar proliferating region of the proximal growth plate of the tibia in PPRfl/fl and Coll2-CreERT:PPRfl/fl mice at 3 d after Tam administration. The indicated values correspond to the average of cell counts from two sections from each mouse and from three animals of each genotype. Dashed lines indicate the columnar region. *P < 0.05, significantly different from PPRfl/fl control mice. (D) TUNEL assay shows ectopic TUNEL-positive chondrocytes. Shown are the TUNEL assays in PPRfl/fl (Left) and Coll2-CreERT:PPRfl/fl (Right) mice at 4 d after Tam administration. The yellow arrows point to the TUNEL-positive chondrocytes (identified morphologically in bright-field examination) in the mutant. Data are representative of experiments performed on sections from three mice for each condition. (E) Magnified views of DAPI (Left) and TUNEL (Right) staining are shown in PPRfl/fl (Upper) and Coll2-CreERT:PPRfl/fl (Lower) mice at 4 d after Tam administration. The yellow arrows point to the TUNEL-positive chondrocytes in the mutant.
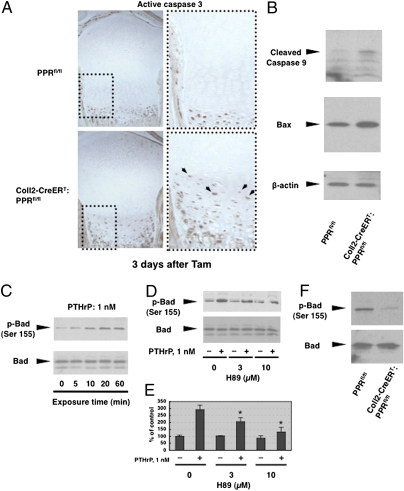
To confirm the abnormal pattern of chondrocyte apoptosis in the Tam-treated Coll2-CreERT:PPRfl/fl mice, we examined the activation of caspase-3 in these chondrocytes by performing immunohistochemical detection of cleaved caspase-3. As shown in Fig. 4A, active caspase 3-positive cells were seen in the middle of the growth plate in Coll2-CreERT:PPRfl/fl mice, whereas such ectopic activation was not seen in the growth plates in PPRfl/fl control mice at 3 d after administration of Tam. Apoptosis associated with activation of caspase 3 is triggered by two major mechanisms: plasma membrane death receptor-triggered activation of caspase-8 and caspase-10 (12) and a mitochondrial pathway regulated by Bcl family members that activates caspase-9 (13). We examined whether the mitochondrial pathway is responsible for the activation of caspase-3 by assessing the activation of caspase-9. We found increased active caspase-9 in homogenates prepared from the distal femur and proximal tibia of P6 Coll2-CreERT:PPRfl/fl mice at 3 d after Tam administration (Fig. 4B). Further, we examined the activation of caspase-8 to assess the contribution of the death receptor-triggered mechanisms in chondrocyte apoptosis. The activation of caspase-8 was decreased in the growth plates of Coll2-CreERT:PPRfl/fl mice given Tam when compared with similarly treated PPRfl/fl mice (Fig. S3A). To investigate mediators of apoptosis in the Coll2-CreERT:PPRfl/fl growth plate, we isolated RNA from Coll2-CreERT:PPRfl/fl and PPRfl/fl mice at 3 d after Tam administration and used a commercially available PCR array for apoptosis-related genes to identify differences in expression after PPR ablation. Results demonstrate that deletion of PPR signaling in chondrocytes induced little change in expression of most of these genes but did cause substantial changes in mRNA levels in 13 of them (Table S1). We found increased expression of mRNA encoding Bax, a proapoptotic member of the Bcl-2 family in the Coll2-CreERT:PPRfl/fl growth plate. We also observed increased expression of Bax protein in the distal femur and proximal tibia of P6 Coll2-CreERT:PPRfl/fl mice at 3 d after Tam administration (Fig. 4B and Fig. S3B). Some of this increased expression simply reflects the increased number of hypertrophic chondrocytes in these mice. Nevertheless, some of the high levels of Bax expression was seen in cells that were morphologically flat chondrocytes; these cells normally express only very low levels of Bax (14) (Fig. S3B). Further, although semiquantitative, the expression levels of Bax were consistently higher than normal in the hypertrophic chondrocytes of the Coll2-CreERT:PPRfl/fl growth plate after Tam administration. These findings are consistent with the idea that inactivation of the PPR leads to activation of the mitochondrial pathway of apoptosis. Bad, a proapoptotic BH3-only member of the Bcl-2 family, binds to and inactivates Bcl2 and Bcl-xL, which are antiapoptotic proteins located on the outer mitochondrial membrane. Phosphorylation by protein kinase A (PKA) at Ser155 of Bad blocks association of Bad with Bcl-xL and favors association with cytoplasmic 14-3-3 proteins (15, 16). We examined whether PTHrP (1-36) regulates the phosphorylation of Bad in primary cultured chondrocytes. As shown in Fig. 4C, phosphorylation of endogenous Bad at Ser155 was up-regulated by PTHrP at 5 min and was more extensive at 60 min. Furthermore, Bad phosphorylation by PTHrP was sensitive to H89, a PKA inhibitor, suggesting that PKA mediates Bad phosphorylation after PTHrP stimulation (Fig. 4 D and E). Finally, homogenates prepared from the distal femur and proximal tibia of Tam-treated P6 Coll2-CreERT:PPRfl/fl mice showed reduced phosphorylation of endogenous Bad at Ser155 compared with PPRfl/fl control mice (Fig. 4F). We could not perform immunohistochemistry using the antibody to phospho-Bad; thus, we cannot identify the specific chondrocyte type expressing the epitope. Nevertheless, the up-regulation of activity of proapoptic Bad caused by suppression of Bad phosphorylation at the Ser155 site after inactivation of PPR signaling in chondrocytes may contribute to this process.
Fig. 4.
Mitochondrial pathway contribution to the increased apoptosis in the PPR-deficient growth plate. (A) Immunohistochemical detection of cleaved caspase-3 of tibias in PPRfl/fl (Upper) and Coll2-CreERT:PPRfl/fl (Lower) mice at 3 d after Tam administration. (Right) Magnified views of the growth plate chondrocytes are shown. Data are representative of experiments performed on sections from three mice for each condition. (B) Activities of caspase-9 and the expression of Bax in growth plates from the distal femur and proximal tibia were analyzed by Western blotting. The same blot was stripped and reprobed with a β-actin antibody. Data are representative of independent experiments performed on each of three mice. (C) Cultured primary chondrocytes were treated with PTHrP (1 nM) for various times as indicated. Phosphorylation of Bad was analyzed by Western blotting using phosphospecific Ser155 antibody. The same blot was stripped and reprobed with an anti-Bad antibody. Data are representative of experiments performed in three independent experiments. (D) Cultured primary chondrocytes were pretreated with H89 for 30 min and then stimulated with PTHrP (1 nM) for 20 min. (E) Quantitative analysis of D. Western blots were quantified using ImageJ 1.43 software (National Institutes of Health) after densitometric scanning of the films. *P < 0.05, significantly different from the value obtained after PTHrP administration in the absence of H89. Data are representative of three independent experiments. (F) Homogenates were prepared from growth plate cartilage of the distal femur and proximal tibia of P6 Coll2-CreERT:PPRfl/fl and PPRfl/fl mice at 3 d after Tam administration. Phosphorylation of Bad was analyzed by Western blotting using phosphospecific Ser155 antibody. Data are representative of three independent experiments.
Effects of Phosphate Deprivation on the Growth Plate Abnormalities Induced by Inactivation of the PPR.
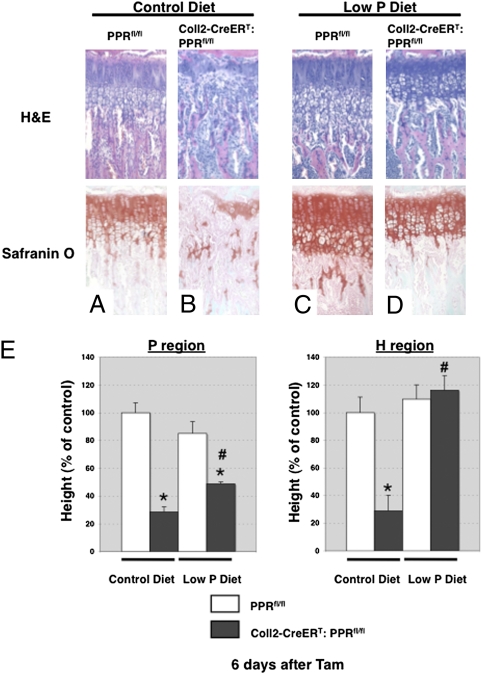
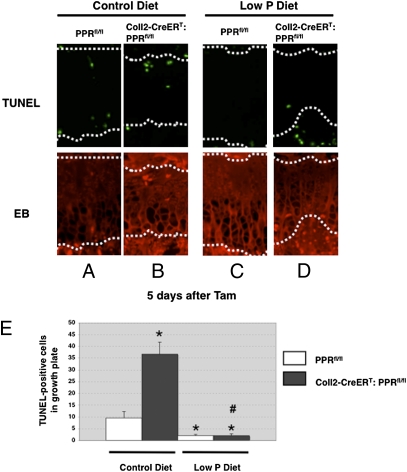
To examine the relationship between apoptosis and growth plate disappearance, we used an in vivo model known to suppress apoptosis of late hypertrophic chondrocytes. Sabbagh et al. (11) showed that low levels of inorganic phosphate in the blood lead to suppression of apoptosis of late hypertrophic chondrocytes and expansion of the hypertrophic chondrocyte layer (rickets). To determine whether hypophosphatemia could suppress apoptosis in the absence of PPR signaling and, if so, whether this suppression mitigated the phenotype of the PPR KO mice, we subjected Coll2-CreERT:PPRfl/fl and PPRfl/fl control mice to a low-phosphate diet. At 4 wk of age, Coll2-CreERT:PPRfl/fl and PPRfl/fl mice were treated with Tam (0.1 mg/g of body weight every 3 d), and the effects on development of the growth plate were analyzed at 3–10 d after Tam administration. Mice were fed a low-phosphate diet throughout the experiment, starting the day before the first injection of Tam (Fig. S4). As we had observed with younger mice without a secondary ossification center (Fig. 2), we again observed premature hypertrophic differentiation in the growth plates of mice fed a normal diet at 4 d after Tam administration. The expansion of the hypertrophic region was accompanied by a shortening of the region of proliferating chondrocytes and a concomitant decrease in the fraction of chondrocytes in this region that were synthesizing DNA, as determined by BrdU labeling [BrdU labeling index (%): 18.6 ± 1.7 vs. 8.0 ± 1.2; P < 0.05; n = 3 in each group]. Histological analysis showed that premature closure of the growth plate had occurred in the tibia of Coll2-CreERT:PPRfl/fl mice at 6–10 d after Tam administration. The phenotype in the Coll2-CreERT:PPRfl/fl mice in vehicle-treated controls could not be distinguished from normal (Fig. S4). Further, there was no difference in the morphology of the growth plate, chondrocyte proliferation, or apoptosis between vehicle-treated and Tam-treated PPRfl/fl mice [BrdU labeling index (%): 17.3 ± 2.3 vs. 17.2 ± 1.1, n = 3; TUNEL-positive cells per section: 14.4 ± 3.0 vs. 11.5 ± 3.7, n = 5], indicating that Tam does not affect chondrocyte proliferation or apoptosis in our experiments. As expected, the mice quickly became hypophosphatemic and mildly hypercalcemic, with low PTH levels in the blood (Fig. S5). Interestingly, we observed the continued presence of the growth plate in Coll2-CreERT:PPRfl/fl mice fed a low-phosphate diet 6 d after administration of Tam, a time at which chondrocytes were almost absent from the growth plates in Coll2-CreERT:PPRfl/fl mice fed a control diet (Fig. 5). Twenty days after beginning Tam, the growth plate was entirely closed in the Coll2-CreERT:PPRfl/fl mice fed a control diet but remained in similar mice fed the low-phosphate diet (Fig. S6). Finally, to test the possibility that the disappearance of the growth plate involved suppression of chondrocyte apoptosis, we performed the TUNEL assay at 5 d after Tam administration. We observed significantly increased TUNEL-positive cells in the growth plate chondrocytes of Coll2-CreERT:PPRfl/fl mice compared with those of their WT littermates at 5 d after Tam administration. Hypophosphatemia was effective in suppressing the apoptosis, even in cells undergoing apoptosis in the columnar chondrocyte region, a site not normally associated with chondrocyte apoptosis (Fig. 6). These observations demonstrate that PPR signaling is required for continued survival and suppression of apoptosis of chondrocytes in the postnatal growth plate.
Fig. 5.
Concurrent administration of a low-phosphate diet prevents disappearance of the growth plate. H&E (Upper) and Safranin O (Lower) staining of longitudinal cross-sections of tibiae in PPRfl/fl (A and C) and Coll2-CreERT:PPRfl/fl (B and D) mice at 4 wk of age at 6 d after Tam administration. PPRfl/fl (A and C) and Coll2-CreERT: PPRfl/fl (B and D) mice were fed a control diet (A and B) or a low-phosphate (P) diet (C and D) from 28 to 35 d of age. (E) Statistical analysis of the length of the columnar proliferating (Left) and hypertrophic (Right) regions of the proximal growth plate of the tibiae in PPRfl/fl and Coll2-CreERT:PPRfl/fl mice fed a control or P diet. White bars represent PPRfl/fl mice, and black bars represent Coll2-CreERT:PPRfl/fl mice. *P < 0.05, significantly different from PPRfl/fl mice fed a control diet; #P < 0.05, significantly different from the value obtained in Coll2-CreERT:PPRfl/fl mice fed a control diet. Data are representative of experiments performed on sections from three or more mice for each condition.
Fig. 6.
Chondrocyte apoptosis is involved in the premature disappearance of the growth plate by postnatal deletion of PPR signaling in chondrocytes. Shown are TUNEL assays of PPRfl/fl (A and C) and Coll2-CreERT:PPRfl/fl (B and D) mice fed a control diet (A and B) or a low-phosphate (P) diet (C and D). The region outlined by dotted lines indicates the growth plate. Data are representative of experiments performed on sections from four mice for each condition. EB, Evans blue. (E) Statistical analysis of the number of TUNEL-positive cells in the proximal growth plate of the tibiae. White bars represent PPRfl/fl mice, and black bars represent Coll2-CreERT:PPRfl/fl mice. *P < 0.05, significantly different from PPRfl/fl mice fed a control diet; #P < 0.05, significantly different from the value obtained in Coll2-CreERT:PPRfl/fl mice fed a control diet. Data are representative of experiments performed on sections from three mice for each condition.
Discussion
We have found that ablation of the PPR in chondrocytes postnatally leads to accelerated differentiation of chondrocytes, followed by disappearance of the growth plate, in association with an increase in chondrocyte apoptosis. Hypophosphatemia greatly diminished the apoptosis and slowed the disappearance of the growth plate. We cannot eliminate the possibility that hypophosphatemia affects the growth plate by additional unknown mechanisms as well, which could act to preserve the growth plate independent of its effect on apoptosis. For example, mice in which the PTH gene is ablated have an expansion of the layer of hypertrophic chondrocytes (17). No change in apoptosis was noted in the chondrocytes of those mice. Thus, low levels of PTH, in addition to the direct effects of hypophosphatemia, might contribute to the transient expansion of the hypertrophic layer and the suppression of apoptosis in the PPR KO mice.
The explanation for the accelerated and ectopic apoptosis is unclear. We found an increase in the activated form of caspase-9 in the growth plates of the Coll2-CreERT:PPRfl/fl mice given Tam postnatally (Fig. 4B); caspase-9 activation implicates the mitochondrial pathway in chondrocyte apoptosis. Bax and Bak are multidomain transmembrane proteins that trigger mitochondrial-driven apoptosis (13). Bcl2 and Bcl-xl are antiapoptotic multidomain transmembrane proteins that oppose the actions of Bax and Bak. BH3-only proteins respond to a variety of stimuli to interact with Bax, Bak, and the Bcl2/Bcl-xl antiapoptotic proteins to activate apoptosis. We surveyed expression of several members of the Bcl family using a PCR array and found no change in expression of most of them in Coll2-CreERT:PPRfl/fl mice after administration of Tam, although mRNA encoding the apoptosis-inducing family member Bax was increased. This increase was associated with an increase in Bax protein levels, as assessed by Western blot analysis (Fig. 4B), which is partly explained by the expansion of the hypertrophic layer of chondrocytes. Amling et al. (14) found that Bcl2 expression is increased in late proliferative and prehypertrophic chondrocytes in transgenic mice overexpressing PTHrP in chondrocytes, but we found no change in Bcl2 mRNA levels in the growth plates of Coll2-CreERT:PPRfl/fl mice after administration of Tam. We focused on the BH3-only protein Bad because Bad is regulated by PKA, and therefore is a candidate regulator of apoptosis downstream of activation of the PPR. Bad binds to Bcl-xL and Bcl2, thereby opposing their antiapoptotic actions (15, 16). When phosphorylated at serine 112, 136, or 155, Bad binds to cytoplasmic 14-3-3 proteins, and thus is not available to trigger apoptosis (13, 15). We found that PTHrP rapidly led to phosphorylation of Bad in chondrocytes in primary culture (Fig. 4C). Further, levels of phosphorylated Bad fell in the Coll2-CreERT:PPRfl/fl mice after Tam administration (Fig. 4E). The resultant increase in active Bad might contribute to the apoptosis exhibited by the growth plates of these mice.
Other processes may contribute to the increased apoptosis as well. For example, Nkx3.2, a transcription factor expressed at high levels in proliferating chondrocytes, has been shown to inhibit apoptosis in a chondrocyte cell line (18). Because PPR activation increases Nkx3.2 expression in chick and mouse growth plates (19), this pathway may contribute to the antiapoptotic actions of the PPR.
The growth plate disappearance described here may be relevant to certain forms of human growth plate closure. Early growth plate closure, contributing to brachydactyly and short stature (5), occurs in children with AHO. The bone disease is caused by haploinsufficiency of Gsα, attributable to a variety of mutations in the coding region of Gsα. In similar fashion, people heterozygous for inactivating mutations of the gene encoding PTHrP exhibit short metacarpals, metatarsals, and phalanges, along with premature cessation of growth leading to short stature (6, 7). Because PTHrP activates the PPR and then Gsα, and because the KO of Gsα in the growth plate of mice leads to accelerated chondrocyte differentiation resembling that of the PPR KO (4, 20), it is plausible that the growth plate closure in AHO and human heterozygosity for the PTHrP gene result from processes like those demonstrated here. Growth plate closure is also seen when Ihh is knocked out postnatally in the growth plate (21) or when hedgehog antagonists are administered to mice (22). Because Ihh stimulates PTHrP production in chondrocytes (1), this closure may involve mechanisms similar to those described here. Hedgehog antagonists are now in clinical trials for conditions such as medulloblastoma (23); growth plate closure may limit the use of these drugs in growing children.
Materials and Methods
Descriptions of mice, low phosphate diet, skeletal preparation and histological analysis, primary chondrocyte culture, and statistics are provided in SI Materials and Methods.
Tam Administration.
Tam (Sigma) was dissolved in corn oil (Sigma) at a concentration of 10 mg/mL and administered as described in SI Materials and Methods.
In Situ Hybridization, BrdU Labeling and Detection, and Evaluation of Apoptosis.
These studies were performed in routine fashion as described in SI Materials and Methods.
Immunohistochemistry.
Standard use of antibodies to cleaved caspase-3 (diluted 1:100; Cell Signaling) is described in SI Materials and Methods.
Western Blotting.
Western blot analysis was performed with cell homogenates from growth plate chondrocytes using standard methods described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Marie B. Demay, Shigeki Nishimori, and Makoto Okazaki for helpful suggestions. This work was funded by National Institutes of Health Grant DK56246.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1005011108/-/DCSupplemental.
References
- 1.Kronenberg HM. Developmental regulation of the growth plate. Nature. 2003;423:332–336. doi: 10.1038/nature01657. [DOI] [PubMed] [Google Scholar]
- 2.Bastepe M, et al. Stimulatory G protein directly regulates hypertrophic differentiation of growth plate cartilage in vivo. Proc Natl Acad Sci USA. 2004;101:14794–14799. doi: 10.1073/pnas.0405091101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schipani E, et al. Constitutively activated receptors for parathyroid hormone and parathyroid hormone-related peptide in Jansen's metaphyseal chondrodysplasia. N Engl J Med. 1996;335:708–714. doi: 10.1056/NEJM199609053351004. [DOI] [PubMed] [Google Scholar]
- 4.Jobert AS, et al. Absence of functional receptors for parathyroid hormone and parathyroid hormone-related peptide in Blomstrand chondrodysplasia. J Clin Invest. 1998;102:34–40. doi: 10.1172/JCI2918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Steinbach HL, Rudhe U, Jonsson M, Young DA. Evolution of skeletal lesions in pseudohypoparathyroidism. Radiology. 1965;85:670–676. doi: 10.1148/85.4.670. [DOI] [PubMed] [Google Scholar]
- 6.Klopocki E, et al. Deletion and point mutations of PTHLH cause brachydactyly type E. Am J Hum Genet. 2010;86:434–439. doi: 10.1016/j.ajhg.2010.01.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Maass PG, et al. A cis-regulatory site downregulates PTHLH in translocation t(8;12)(q13;p11.2) and leads to Brachydactyly Type E. Hum Mol Genet. 2010;19:848–860. doi: 10.1093/hmg/ddp553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Nakamura E, Nguyen MT, Mackem S. Kinetics of tamoxifen-regulated Cre activity in mice using a cartilage-specific CreER(T) to assay temporal activity windows along the proximodistal limb skeleton. Dev Dyn. 2006;235:2603–2612. doi: 10.1002/dvdy.20892. [DOI] [PubMed] [Google Scholar]
- 9.Kobayashi T, et al. PTHrP and Indian hedgehog control differentiation of growth plate chondrocytes at multiple steps. Development. 2002;129:2977–2986. doi: 10.1242/dev.129.12.2977. [DOI] [PubMed] [Google Scholar]
- 10.Lee C, et al. Role of the extracellular regions of the parathyroid hormone (PTH)/PTH-related peptide receptor in hormone binding. Endocrinology. 1994;135:1488–1495. doi: 10.1210/endo.135.4.7523099. [DOI] [PubMed] [Google Scholar]
- 11.Sabbagh Y, Carpenter TO, Demay MB. Hypophosphatemia leads to rickets by impairing caspase-mediated apoptosis of hypertrophic chondrocytes. Proc Natl Acad Sci USA. 2005;102:9637–9642. doi: 10.1073/pnas.0502249102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Nagata S. Apoptosis by death factor. Cell. 1997;88:355–365. doi: 10.1016/s0092-8674(00)81874-7. [DOI] [PubMed] [Google Scholar]
- 13.Green DR, Kroemer G. The pathophysiology of mitochondrial cell death. Science. 2004;305:626–629. doi: 10.1126/science.1099320. [DOI] [PubMed] [Google Scholar]
- 14.Amling M, et al. Bcl-2 lies downstream of parathyroid hormone-related peptide in a signaling pathway that regulates chondrocyte maturation during skeletal development. J Cell Biol. 1997;136:205–213. doi: 10.1083/jcb.136.1.205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zhou XM, Liu Y, Payne G, Lutz RJ, Chittenden T. Growth factors inactivate the cell death promoter BAD by phosphorylation of its BH3 domain on Ser155. J Biol Chem. 2000;275:25046–25051. doi: 10.1074/jbc.M002526200. [DOI] [PubMed] [Google Scholar]
- 16.Datta SR, et al. 14-3-3 proteins and survival kinases cooperate to inactivate BAD by BH3 domain phosphorylation. Mol Cell. 2000;6:41–51. [PubMed] [Google Scholar]
- 17.Miao D, He B, Karaplis AC, Goltzman D. Parathyroid hormone is essential for normal fetal bone formation. J Clin Invest. 2002;109:1173–1182. doi: 10.1172/JCI14817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Park M, et al. Constitutive RelA activation mediated by Nkx3.2 controls chondrocyte viability. Nat Cell Biol. 2007;9:287–298. doi: 10.1038/ncb1538. [DOI] [PubMed] [Google Scholar]
- 19.Provot S, et al. Nkx3.2/Bapx1 acts as a negative regulator of chondrocyte maturation. Development. 2006;133:651–662. doi: 10.1242/dev.02258. [DOI] [PubMed] [Google Scholar]
- 20.Sakamoto A, Chen M, Kobayashi T, Kronenberg HM, Weinstein LS. Chondrocyte-specific knockout of the G protein G(s)alpha leads to epiphyseal and growth plate abnormalities and ectopic chondrocyte formation. J Bone Miner Res. 2005;20:663–671. doi: 10.1359/JBMR.041210. [DOI] [PubMed] [Google Scholar]
- 21.Maeda Y, et al. Indian Hedgehog produced by postnatal chondrocytes is essential for maintaining a growth plate and trabecular bone. Proc Natl Acad Sci USA. 2007;104:6382–6387. doi: 10.1073/pnas.0608449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kimura H, Ng JM, Curran T. Transient inhibition of the Hedgehog pathway in young mice causes permanent defects in bone structure. Cancer Cell. 2008;13:249–260. doi: 10.1016/j.ccr.2008.01.027. [DOI] [PubMed] [Google Scholar]
- 23.Garber K. Hedgehog drugs begin to show results. J Natl Cancer Inst. 2008;100:692–697. doi: 10.1093/jnci/djn169. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.