Abstract
Discodermolide is a microtubule-stabilizing agent that induces accelerated cell senescence. A discodermolide-resistant cell line, AD32, was generated from the human lung cancer cell line A549. We hypothesize that the major resistance mechanism in these cells is escape from accelerated senescence. AD32 cells have decreased levels of 4E-BP1 mRNA and protein, relative to the parental discodermolide-sensitive A549 cells. Lentiviral-mediated re-expression of wild-type 4E-BP1 in AD32 cells increased the proliferation rate and reverted resistance to discodermolide via restoration of discodermolide-induced accelerated senescence. Consistent with this, cell growth and response to discodermolide was confirmed in vivo using tumor xenograft models. Furthermore, reintroduction of a nonphosphorylatable mutant (Thr-37/46 Ala) of 4E-BP1 was able to partially restore sensitivity and enhance proliferation in AD32 cells, suggesting that these effects are independent of phosphorylation by mTORC1. Microarray profiling of AD32-resistant cells versus sensitive A549 cells, and subsequent unbiased gene ontology analysis, identified molecular pathways and functional groupings of differentially expressed mRNAs implicated in overcoming discodermolide-induced senescence. The most statistically significant classes of differentially expressed genes included p53 signaling, G2/M checkpoint regulation, and genes involved in the role of BRCA1 in the DNA damage response. Consistent with this, p53 protein expression was up-regulated and had increased nuclear localization in AD32 cells relative to parental A549 cells. Furthermore, the stability of p53 was enhanced in AD32 cells. Our studies propose a role for 4E-BP1 as a regulator of discodermolide-induced accelerated senescence.
Keywords: drug resistance, senescence reversion, TOR signaling
Microtubule-stabilizing agents bind to microtubules and abolish their dynamic behavior. The prototype for this class of chemotherapeutics is Taxol, an antitumor drug used extensively for the treatment of breast, ovarian, and lung carcinomas. The development of Taxol-resistant tumors in patients led to a search for new compounds with mechanisms of action similar to Taxol. One of the compounds identified, discodermolide (disco), which was isolated from the Carribbean sea sponge, Discodermia dissoluta, acts synergistically with Taxol, does not exhibit cross-resistance to Taxol-resistant cells, and is a potent inducer of accelerated senescence (1–3). Disco was originally identified as an immunosuppressant and was compared with other drugs in this class of compounds such as rapamycin (4, 5). Due to this characterization, we proceeded to investigate whether disco had an effect on mTORC1 (mammalian target of rapamycin complex 1) signaling.
“Accelerated cell senescence” (ACS), “premature senescence,” and “senescence-like growth arrest” are all terms that characterize the phenotype of tumor cells that have become growth arrested in response to treatment with a variety of chemotherapeutic agents (6). Many stimuli can trigger senescence, including telomere shortening (replicative senescence), oncogene activation, and DNA damage. The cellular program executing senescence is dependent on DNA damage checkpoints and mitogenic signaling networks including STAT1, RAS, and PI3K (7–9). Senescence is considered a tumor-suppressive mechanism in many malignancies (10), and the appreciation that many chemotherapeutic agents induce senescence has prompted a resurgence of interest in prosenescent anti-cancer therapies.
eIF4E, a translation initiation factor that recognizes the 7-methyl-guanosine cap structure, is overexpressed in many cancers and is known to induce cellular senescence (11) by regulating a specific subset of mRNAs involved in tumorigenesis. 4E-BPs (eIF4E-binding proteins) negatively regulate eIF4E and have been implicated in p53-mediated senescence (12). Here we describe a human tumor cell line, which has escaped ACS and has low 4E-BP1 expression. Upon reintroduction of 4E-BP1, these cells undergo ACS when challenged with disco. Our experiments support a role for 4E-BP1 as a regulator of ACS and provide evidence for reversal of chemotherapy-induced senescence.
Results
Isolation of a Discodermolide-Resistant Cell Line.
The ability of disco to induce ACS was discerned when several attempts to develop disco-resistant cell lines from many different tumor cell types proved unsuccessful. One resistant cell line was obtained from A549 lung carcinoma cells after prolonged stepwise selection with the drug and is the focus of this study. This cell line, AD32, was derived from A549 cells and is the only disco-resistant cell line that has been reported. Unlike other cell lines that are resistant to microtubule-stabilizing drugs, AD32 cells do not display any of the classic mechanisms of resistance such as β-tubulin mutations or overexpression of the multidrug resistance efflux pump, P-glycoprotein. In effect, these cells are resistant to ACS, the result of disco treatment.
Discodermolide-Resistant Cells Have Reduced 4E-BP1 Expression.
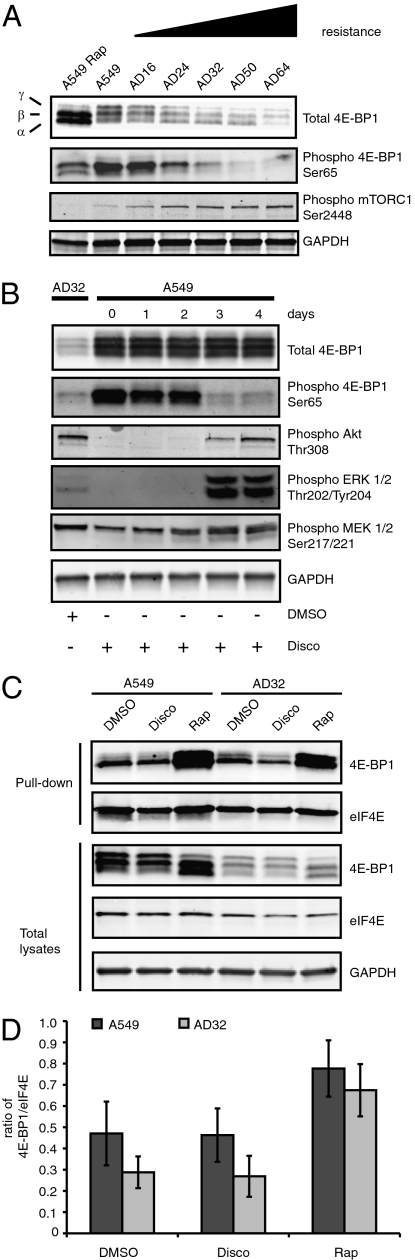
Disco-resistant clones, cultured in increasing concentrations of drug, were isolated. The increase in disco resistance corresponded to decreasing levels of 4E-BP1 (Fig. 1A). The AD32 cell line was selected for further study due to its ability to proliferate in the presence or absence of 32 nM disco, making it the most stable cell line available for use at the beginning of this study. The cells are approximately sevenfold resistant to disco (Table 1) and have threefold less 4E-BP1 protein than A549 cells (Fig. S1). A549 cells treated with disco for up to 1 h have no change in the expression or phosphorylation of 4E-BP1 (Fig. S2 A and B). Treatment of A549 with disco for up to 4 d, the time frame for development of an obviously senescent phenotype, did not alter levels of total 4E-BP1 (Fig. 1B). These data suggest that reduced expression of 4E-BP1 is not due to the induction and maintenance of senescence, but rather to escape from ACS.
Fig. 1.
Discodermolide-resistant cells (AD16, 24, 32, 50, 64) have diminished total 4E-BP1 protein. (A) Expression of 4E-BP1 is correlated with drug resistance. Immunoblot analysis of 4E-BP1, phospho 4E-BP1, and phospho mTORC1 in resistant clones relative to drug-sensitive A549 cells is shown. (B) Induction of senescence by disco does not alter total 4E-BP1 expression in A549 cells. Immunoblot of 4E-BP1, phospho 4E-BP1, phospho AKT, phsopho MEK, and phospho ERK1/2 after treatment of A549 with 32 nM disco for 0–4 d, the time for senescence onset. (C) m7-GTP-Sepharose chromatography and immunoblot of 4E-BP1 and eIF4E. Cells were treated with either DMSO, disco, or rapamycin (Rap) for 2 h. (D) Ratio of 4E-BP1/eIF4E values is representative of three independent experiments (±SEM).
Table 1.
Cytotoxicity to discodermolide and proliferation rate of cell lines
| Cell line | Doubling time (h) | Disco IC50 (nM) | Fold resistance |
| A549 | 22.1 ± 1.3 | 17.7 ± 7.8 | 1.0 |
| AD32 | 31.0 ± 0.7 | 125.9 ± 16.8 | 7.1 |
| A549EV | 23.8 ± 2.1 | 18.0 ± 11.3 | 1.0 |
| A5494E-BP1 | 15.2 ± 1.5 | 23.2 ± 0.2 | 1.3 |
| A549sh RNA | 20.9 ± 3.53 | 19.9 ± 4.4 | 1.1 |
| A549sh 4E-BP1 | 29.8 ± 1.1 | 21.0 ± 3.9 | 1.2 |
| AD32EV | 29.8 ± 2.1 | 136.7 ± 4.6 | 7.7 |
| AD32Ala-37/46 | 22.8 ± 0.9 | 43.3 ± 13.3 | 2.4 |
| AD324E-BP1 | 18.3 ± 0.8 | 18.3 ± 8.2 | 1.0 |
| AD32sh RNA | 30.2 ± 0.9 | 130.7 ± 2.3 | 7.3 |
| AD32sh p53 | 29.6 ± 0.4 | 117.9 ± 7.6 | 6.6 |
Cytotoxicity was determined by sulforhodamine B assay, and values are IC50 (±SEM). Proliferation rate was determined by population doublings, and values are in hours (±SEM). Fold resistance was calculated relative to A549 cells.
The 4E-BP protein family consists of 4E-BP1, 4E-BP2, and 4E-BP3 that act as translational repressors by binding to eIF4E and preventing its interaction with eIF4G. 4E-BP2 has high sequence similarity to 4E-BP1 (13); however, unlike 4E-BP1, levels of 4E-BP2 were not altered in the disco-resistant clones (Fig. S2C). 4E-BP3 is highly divergent and not well characterized, with only 57% identity to 4E-BP1 (14). 4E-BP1 is the most studied of the family members and a known substrate of mTORC1. Upon mTORC1 activation, 4E-BP1 is phosphorylated on Thr37 and Thr46, whereby it becomes primed for subsequent phosphorylation at Ser65 and Thr70 (15). Hyperphosphorylation of 4E-BP1 causes it to dissociate from eIF4E and results in enhanced translation initiation of defined subsets of mRNA (16). Conversely, mTORC1 inhibition by rapamycin leads to sequestration of eIF4E by 4E-BP1 and to decreased translation efficiency. Rapamycin treatment of A549 cells dephosphorylates 4E-BP1 as expected (Fig. 1A), indicating that 4E-BP1 is functioning normally in the parental cell line. Conversely, acute treatment of A549 cells with disco does not dephosphorylate 4E-BP1 or the other mTORC1 substrates p70S6k and rpS6 (Fig. S2 A, B, D, and E), suggesting that disco is not directly modulating the mTORC1 pathway. Furthermore, 4E-BP1 in AD32 cells binds eIF4E, as shown by m7GTP Sepharose affinity chromatography, and responds to rapamycin treatment normally by increasing association with eIF4E (Fig. 1C). These data suggest that, despite reduced expression in AD32 cells, 4E-BP1 is still functional as a translational repressor. However, AD32 cells are resistant to rapamycin (Table S1), and this may be related to the reduced level of eIF4E and 4E-BP1 in AD32 relative to A549 (Fig. S3) as suggested previously (17). Recent observations indicate that rapamycin has equal activity in both 4E-BP double-knockout and 4E-BP wild-type mouse embryonic fibroblasts (MEFs), demonstrating that 4E-BP expression does not predict response to rapamycin (18). Alternatively, cross-resistance to rapamycin may be due to inherent differences in DNA repair between A549 and AD32 cells. In support of this observation is the finding that colorectal cells with defects in mismatch repair are hypersensitive to rapamycin (19).
4E-BP1 is hyperphosphorylated by constitutive activation of the PI3K/AKT/mTOR pathway or MAPK pathway via its activation of mTORC1 (20). Disco-induced senescence onset in A549 is associated with phosphorylation of AKT Thr308 at days 3 and 4 coupled with dephosphorylation of 4E-BP1 Ser65 (Fig. 1B). This is likely due to the cessation of proliferation and increased cell survival signaling associated with senescence. Therefore, dephosphorylation of 4E-P1 at Ser65 indicates disco-induced ACS, although no changes were observed in total 4E-BP1 expression at days 3 and 4 when cells were senescent. Furthermore, increased phosphorylation of ERK Thr202/Tyr204 and MEK Ser217/221 was observed in senescent cells (Fig. 1B), consistent with previous observations (1, 21). Therefore, the data here support dissociation of PI3K/MAPK signaling from 4E-BP1 phosphorylation and expression during ACS.
AD32, which has escaped senescence, retains AKT Thr308, MEK Ser217/221, and ERK Thr202/Tyr204 phosphorylation (Fig. 1B). However, total 4E-BP1 expression was drastically reduced in AD32 cells relative to their senescent A549 precursor, whereas Ser65 phosphorylation is unchanged. This suggests that changes in total 4E-BP1 expression, rather than 4E-BP1 Ser65 phosphorylation, facilitate escape from ACS.
Disco-resistant clones show an increase in mTORC1 phosphorylation at Ser2448, indicating activation of mTORC1 with an increasing level of resistance (Fig. 1A). Importantly, phosphorylation at Ser65, relative to total 4E-BP1 expression, was unchanged (Fig. 1A), suggesting that the activity of 4E-BP1 is independent from mTORC1 in disco-resistant cell lines. From these experiments, we conclude that disco has a mechanism of action that is distinct from rapamycin, and 4E-BP1’s role in escaping senescence may be independent from its regulation by mTORC1.
4E-BP1 Overexpression in AD32 Cells Reverses Discodermolide Resistance and Resensitizes Cells to ACS.
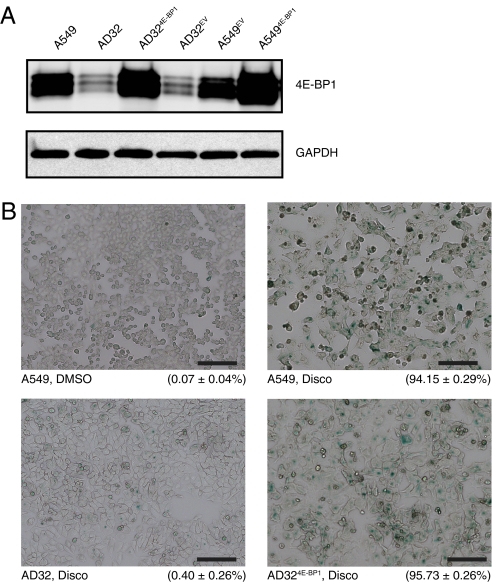
To determine whether the expression of 4E-BP1 contributes to disco resistance, AD32 cells were infected with lentivirus expressing 4E-BP1 or empty vector (AD324E-BP1 and AD32EV) (Fig. 2A). Overexpression of 4E-BP1 restored sensitivity to disco in AD32 cells (Table 1), suggesting that the resistance to disco is mediated via 4E-BP1. A549 cells overexpressing 4E-BP1 (A5494E-BP1), unlike AD32, demonstrated no change in sensitivity to disco, suggesting that, although 4E-BP1 plays a critical role in disco resistance, it probably functions with other proteins as part of a regulatory circuit that has been perturbed in AD32 cells.
Fig. 2.
4E-BP1 alters proliferation and sensitivity to discodermolide-induced senescence. (A) Immunoblot of 4E-BP1 expression in A549 and AD32 tranduced with lentivirus. (B) SA-β-galactosidase activity. Cells were treated with either DMSO (vehicle) or disco (32 nM) for 4 d and then fixed and stained. Percentage positive for β-galactosidase staining (±SEM) is indicated below the respective images.
Overexpression of a 4E-BP1 nonphosphorylatable (Thr37/46Ala) mutant that prevents 4E-BP1 dissociation from eIF4E (15) partially restored sensitivity to disco (Table 1), although the expression of total 4E-BP1 was ≈30% less than the level of overexpression achieved in AD324E-BP1 (Fig. S4). This difference in expression level may account for the inability to completely restore sensitivity to disco. Thus, these sites, which are considered to be primarily regulated by mTORC1 and are required for the ordered hierarchy of 4E-BP1 phosphorylation, are redundant in this system and do not appear to be involved in escape from senescence.
Primary fibroblasts that lack 4E-BP1 and 4E-BP2 but express wild-type p53 undergo premature senescence and display a decreased population doubling time (12). Both A549 and AD32 cells express wild-type p53 and do not express p16INK4a. To determine whether increased expression of 4E-BP1 affects proliferation, the doubling time was determined. Overexpression of 4E-BP1 in both A549 and AD32 cells increased proliferation rates (Table 1). Conversely, lentiviral transduction of A549 cells with an shRNA targeting 4E-BP1 had a reduced proliferation rate (Table 1), suggesting that 4E-BP1 modulates proliferation and that the slower growth of AD32 cells is due, at least in part, to a reduced level of 4E-BP1 protein expression.
Although it has been previously observed that p53−/− 4E-BP double-knockout MEFs proliferate more rapidly than wild-type MEFs under serum-starved conditions (18), 4E-BP1 overexpression in asynchronous A549 cells was found to increase proliferation (Table 1). In breast cancer, 4E-BP1 is overexpressed (22) and may cause enhanced tumorigenicity, thereby supporting a role for 4E-BP1 in accelerating proliferation. It is plausible that, because several signaling pathways are proposed to converge on 4E-BPs, the outcome, in terms of proliferation, depends upon the nature of the stimulus and the functional status of upstream regulators, including p53.
Senescent cells have a large, flat appearance accompanied by increased cytoplasmic area and granularity and have β-galactosidase activity at pH 6 (23). AD32 cells had a higher basal level of senescence-associated β-galactosidase (SA-β-gal)–positive cells than A549—0.40% and 0.07%, respectively (Fig. 2B). However, this change in basal senescence rate is not sufficient to compensate for the difference in doubling time observed between AD32 and A549 (Table 1). AD324E-BP1 cells, when challenged with 32 nM disco, had 95.7% SA-β-gal–positive cells (Fig. 2B), demonstrating that re-expression of 4E-BP1 in AD32 restores sensitivity to ACS in these cells and indicates that 4E-BP1 has a role in regulating senescence.
4E-BP1 Modulates Tumor Growth Rate in a Tumor Xenograft Model.
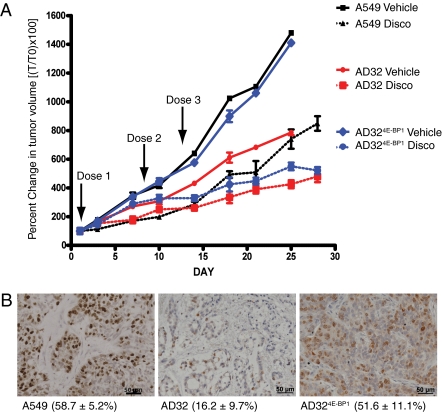
To determine if ectopic expression of 4E-BP1 in AD32 cells would reverse the proliferation rate and resistance to disco in vivo, the cells were grown as xenografts in nude mice. Tumor growth rates were determined by measuring the percentage of change in tumor volume compared with initial tumor volume. AD32 tumors grew slower than either A549 or AD324E-BP1, even though the mice were injected with twice as many cells. The growth of AD324E-BP1 tumors closely paralleled that of A549 cells (Fig. 3A). The fold decrease in tumor volume at day 25, relative to vehicle-only controls, indicated that AD324E-BP1 and A549 tumors decreased by 2.5- and 2.0-fold, whereas AD32 tumors decreased by 1.6-fold. Thus, the greatest response to drug treatment was observed in tumor models with higher levels of 4E-BP1 that proliferate more rapidly. Furthermore, immunohistochemical analysis of xenograft tumors using the proliferation marker Ki-67 indicated that AD324E-BP1 and A549 xenografts had a greater number of positive cells compared with AD32 tumors (Fig. 3B). Although AD32 cells are resistant to disco, they are sensitive to higher concentrations of drug, which rationalizes their response in vivo where high doses were used. These data validate our in vitro findings and provide strong evidence in support of a role for 4E-BP1 in modulating proliferation and sensitivity to disco-induced ACS.
Fig. 3.
Re-expression of 4E-BP1 increases proliferation rate and discodermolide sensitivity in a tumor xenograft model. (A) Mice bearing A549, AD32, or AD324E-BP1 xenografts were treated with three weekly doses (arrows) of disco (10 mg/kg i.v.), and tumor volume was calculated. Data are expressed as the percentage of change in tumor volume (T0) ± SEM of five mice per group. Mice treated with disco are indicated by dashed lines. (B) Immunohistochemistry for Ki-67 in formaldehyde-fixed tumor xenografts.
Identifying Molecular Pathways Involved in Discodermolide-Induced Senescence.
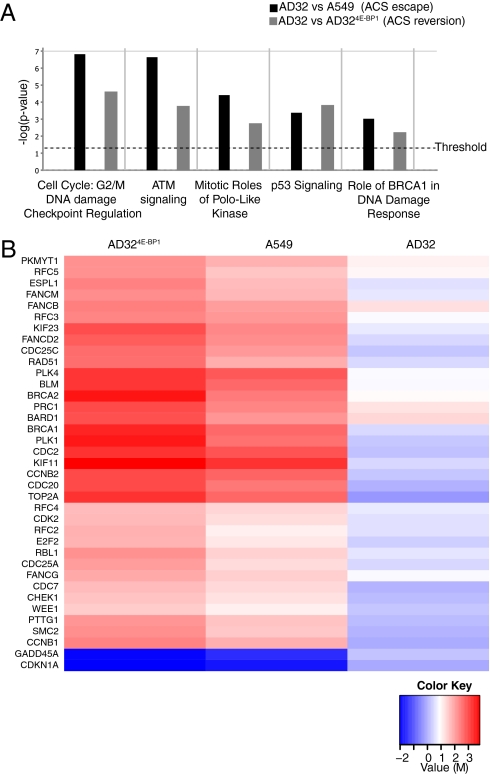
To determine which genes may be involved in the reversion and escape of disco-induced ACS, we performed gene expression profiling of AD32, A549, and AD324E-BP1. Two comparisons were made: (i) AD32 versus AD324E-BP1 to identify 4E-BP1 regulatory genes involved in escaping ACS and (ii) AD32 versus A549 to identify genes involved in ACS reversion. Subsequent unbiased gene ontology analysis of all transcripts was performed using Ingenuity Pathways Analysis (IPA) (Ingenuity Systems) for all comparisons with greater or less than a 1.5-log2 ratio change. IPA identifies the molecular networks and canonical signaling pathways that are most significantly perturbed in a filtered dataset. This analysis confirmed that genes regulated during ACS reversion and ACS escape were enriched for functions in cell proliferation, DNA damage response, and p53 signaling (Fig. 4A).
Fig. 4.
Microarray analysis reveals classes of genes involved in 4E-BP1–directed discodermolide-accelerated cell senescence. (A) Ingenuity Pathway Analysis was performed with the complete data set filtered for significance (threshold of P < 0.05), and regulation of canonical pathways (n = 350) was classified according to P value. (B) Heat map of log2-ratio (M) values of genes that are regulated in significant pathways (M > 1.5). Senescent A549 cells are used as a common reference.
A set of 39 genes from these classes was used to generate a heat map, using senescent A549 as a common reference, because the precursor cells from which AD32 was derived were senescent cells (Fig. 4B). This gene set was highly enriched for p53-inducible transcripts, highlighting the importance of this pathway in the reversion of ACS. A subset of the 39 genes identified was validated by quantitative RT-PCR (Table S2). A similar analysis using A549 cells as a reference identified many of the same genes (Fig. S5). Although not a major pathway implicated in our analysis, but still statistically significant, the acute phase response or inflammation response was identified in a comparison between AD32 cells and senescent A549 cells. This class of genes included those involved in extracellular matrix remodeling such as fibronectin, serpinA3, and peptidases (Table S3). These specific genes have not been previously identified as being involved in ACS; however, this pattern of gene expression is consistent with previous studies that document the role of the DNA damage response in initiating an inflammatory response associated with senescence (8, 24). Thus, genome-wide analyses strongly indicates a significant role for the DNA damage response in disco-induced ACS, reversion, and escape.
4E-BP1 Modulates the Expression and Stability of p53.
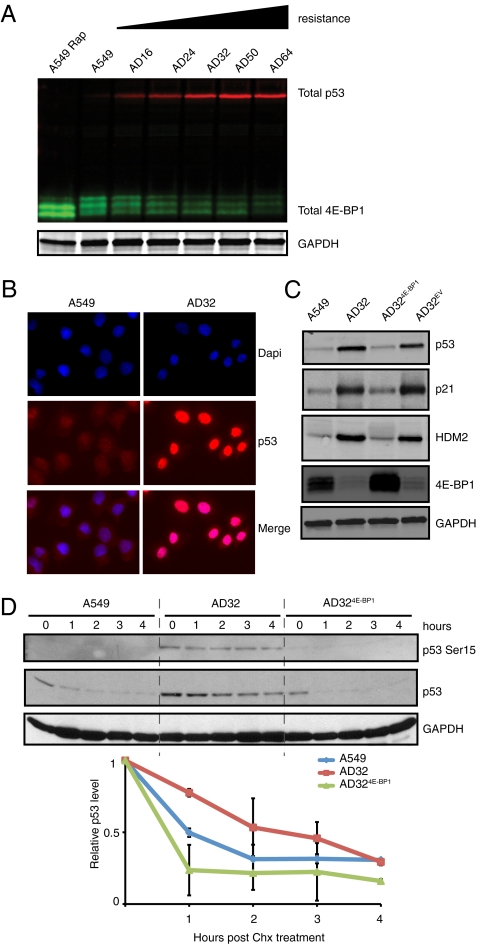
To probe the relationship between p53 and 4E-BP1 further, we determined the expression of both in disco-resistant clones and found an inverse relationship (Fig. 5A). Basal levels of p53 protein in AD32 were significantly increased compared with A549 cells, with increased nuclear staining of p53 in AD32 cells by immunofluorescence (Fig. 5B). Conversely, AD324E-BP1 cells had decreased levels of p53 protein, indicating that p53 levels may be decreased by overexpression of 4E-BP1 in AD32 cells (Fig. 5C). Levels of p53 decreased with overexpression of 4E-BP1, as did levels of p21, which regulates senescence onset, as well as human MDM2 (HDM2), which affects the stability of p53 (Fig. 5C). Western blot analysis of cycloheximide-treated A549, AD32, and AD324E-BP1 indicates that p53 is more stable in AD32 (Fig. 5D) and that overexpression of 4E-BP1 reverts the stability of p53 to A549 levels. Phosphorylation of p53 at Ser15, a marker of the DNA damage response (25), was absent in A549 cells and strongly evident in AD32 cells (Fig. 5D). This site became partially dephosphorylated upon re-expression of 4E-BP1, providing evidence for 4E-BP1 regulation of the p53-mediated DNA damage response in AD32 cells.
Fig. 5.
Inverse relationship between 4E-BP1 and p53 protein levels in discodermolide-resistant cells. (A) Total 4E-BP1 (green) decreases, whereas total p53 (red) increases, with resistance to disco. (B) Immunofluorescence of p53 (red) and HDM2 (green) in A549 and AD32 cells. (C) Immunoblot of total p53, p21, HDM2, and 4E-BP1. (D) Immunoblot of p53 and phosphorylated p53 Ser15 expression in A549, AD32, and AD324E-BP1 treated with 50 μg/mL of cycloheximide (Chx) for the indicated times. Graph of the relative level of total p53 protein in indicated cell lines during Chx treatment. The p53 protein level at 0 h was normalized to GAPDH and set to a relative value of “1.” Values are representative of three independent experiments (±SEM).
Discussion
We have demonstrated that disco-resistant AD32 cells have decreased levels of 4E-BP1 and that re-expression in these cells restores sensitivity to disco and increases proliferation rate. Since disco is a potent inducer of ACS, resensitization to the drug is accompanied by restoration of senescence. Finally, we show that restored sensitivity to disco can be recapitulated in vivo using a tumor xenograft model. Taken together, our data demonstrate that 4E-BP1 has an important role in disco-induced ACS.
4E-BP1 is a key factor that integrates several upstream signals that drive proliferation and subsequently malignant progression (18, 26). The 4E-BP proteins, like S6, act as integrators for the various components of both PI3K and RAS signaling, although they are primarily characterized as downstream effectors of mTORC1. In support of this, we have previously demonstrated that multitargeted suppression of the RAS–PI3K signaling network by MEK inhibition in combination with rapamycin is synergistic in numerous cancer cell models. This mechanism is via potent suppression of cap-dependent translation and dephosphorylation of S6 and 4E-BP1 (27).
Transcriptome profiling indicates that p53 signaling is involved in disco-induced senescence. Unlike other studies that have shown a relationship between increased p53 levels and cell senescence (28), we describe a cell line that, despite expressing increased basal levels of p53 and its targets p21 and HDM2, is able to escape ACS. This raises the intriguing possibility that loss of 4E-BP1 mediates escape from this proliferative arrest despite increased levels of p53, arguing that disco-induced ACS is more influenced by 4E-BP1 than by p53. Previous studies have shown that 4E-BP1 can modulate expression of p21 and HDM2 independently of p53 transcriptional regulation (29, 30). An alternative explanation to rationalize the survival of AD32 cells with high expression of p53 may be that p53 acts as a repressor of senescence, as has been recently proposed in a p21-driven model system (31). In another study, it was shown that p53 can repress the senescence-associated secretory phenotype and that ablation of p53 function actually accelerates ACS (24). Thus, it is possible that p53, although a hallmark of ACS, may not have a causative role in driving disco-induced ACS. This is in agreement with previous studies from our lab and others (1, 32) demonstrating that senescence can be induced in cells with knocked-out or mutant p53. Furthermore, p53 knockdown in A549 cells did not prevent disco-induced ACS, and knockdown of p53 in AD32 cells did not alter the proliferation rate or have an effect on disco sensitivity (Table 1).
Using microarray analysis, we identified many mRNAs involved in disco-induced ACS. Interestingly, the transcripts identified are mostly involved in the DNA damage response, supporting a role for disco as a senescence inducer that is able to invoke the DNA damage response. We propose that AD32 cells survive by maintaining a system primed for DNA damage repair that is facilitated by the translation of specific subsets of mRNAs coupled with enhanced transcriptional activity of genes that regulate repair, with the overall net effect of permitting re-entry into the cell cycle. In support of this, it has been shown elsewhere that cells undergoing a DNA damage response after ionizing radiation translate specific subsets of mRNAs that enable repair (33). When 4E-BP1 levels were ectopically restored in AD32, the proliferation rate increased, which is consistent with its recently proposed function as a regulator of cell proliferation and not cell size (18), and rechallenging cells with disco caused senescence to recur. It seems likely that 4E-BP1, similarly to p53, integrates a milieu of physiological signals that control cell fate, suggesting multiple mechanisms that interface between translation and transcription are used by 4E-BP1 during cellular stress.
Although it has been firmly established that 4E-BP1 is an inhibitor of cap-dependent translation that is regulated via phosphorylation by mTORC1, there is still much more to learn about 4E-BP1. It has seven phosphorylation sites and may be regulated by various kinases in addition to mTORC1, such as Cdk1, PI3K, ERK1/2, and ATM/ATR (34–37). In addition, the transcription of 4E-BP1 is controlled by various transcription factors, such as SMAD4 and ATF (38, 39). Our data indicate a possible mTORC1-independent role for 4E-BP1 in regulating senescence.
Finally, these data and that of others challenge the dogma that senescence is an irreversible process (21, 40). Although we cannot exclude that our AD32 cells originated from a population that bypassed disco-induced senescence, we consider this highly unlikely as cells were cloned at multiple steps throughout selection and the period required for AD32 cells to emerge was extremely long. We favor a model of escape from ACS that was brought about by additional genetic or epigenetic changes to a senescent cell that permitted re-entry to the cell cycle. This is supported by previous studies describing neosis, the emergence of drug-resistant neoplastic cells from a senescent population (41). Our experimental data confirm a regulatory role for 4E-BP1 in overseeing restoration of proliferation. Consequently, senescence may be regarded only as a permanent state in the absence of further additional genetic alterations, which in the context of human tumors, is unlikely. This may rationalize why many human tumors that have activating mutations in oncogenes that cause senescence, such as RAF and RAS, continue to proliferate due to the accumulation of additional genetic lesions that permit escape from senescence.
Materials and Methods
Detailed methods are available in SI Materials and Methods.
A549 cells were purchased from the American Type Culture Collection. A549 and AD32 were cultured in RPMI medium 1640 (Invitrogen) containing 10% FBS. Stable overexpression of 4E-BP1 by lentiviral transduction was done by subcloning 4E-BP1 of pACTAG-2–4E-BP1 and pACTAG-2–4E-BP1 Thr37/46 Ala (gift of Nahum Sonenberg, Department of Biochemistry, McGill University, Montreal, QC, Canada) into pHAGE UbcRIG. Stable cell lines were FACS-sorted for GFP-positive cells. Stable sh4E-BP1, shControl, and shp53 knockdown cell lines were selected with 10 μg/mL puromycin after transduction.
Supplementary Material
Acknowledgments
We thank L. Klein, M.-E. Legrier, and J. Chao for helpful discussions. This work was supported by National Cancer Institute Grant CA077263, the Breast Cancer Research Foundation, the National Foundation for Cancer Research, National Institutes of Health National Institute of General Medical Sciences Training Grant T32 GM007491 (to S.K.C.), and National Institutes of Health Grant K12CA132783-01A1 (to H.M.M.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The microarray reported in this paper have been deposited in the Gene Expression Omnibus (GEO) database, www.ncbi.nlm.nih.gov/geo (accession no.GSE25904).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016962108/-/DCSupplemental.
References
- 1.Klein LE, Freeze BS, Smith AB, III, Horwitz SB. The microtubule stabilizing agent discodermolide is a potent inducer of accelerated cell senescence. Cell Cycle. 2005;4:501–507. doi: 10.4161/cc.4.3.1550. [DOI] [PubMed] [Google Scholar]
- 2.Huang GS, et al. Potentiation of taxol efficacy and by discodermolide in ovarian carcinoma xenograft-bearing mice. Clin Cancer Res. 2006;12:298–304. doi: 10.1158/1078-0432.CCR-05-0229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Martello LA, et al. Taxol and discodermolide represent a synergistic drug combination in human carcinoma cell lines. Clin Cancer Res. 2000;6:1978–1987. [PubMed] [Google Scholar]
- 4.Longley RE, Caddigan D, Harmody D, Gunasekera M, Gunasekera SP. Discodermolide: A new, marine-derived immunosuppressive compound. I. In vitro studies. Transplantation. 1991;52:650–656. doi: 10.1097/00007890-199110000-00014. [DOI] [PubMed] [Google Scholar]
- 5.Schreiber SL. Chemistry and biology of the immunophilins and their immunosuppressive ligands. Science. 1991;251:283–287. doi: 10.1126/science.1702904. [DOI] [PubMed] [Google Scholar]
- 6.Gewirtz DA, Holt SE, Elmore LW. Accelerated senescence: An emerging role in tumor cell response to chemotherapy and radiation. Biochem Pharmacol. 2008;76:947–957. doi: 10.1016/j.bcp.2008.06.024. [DOI] [PubMed] [Google Scholar]
- 7.Novakova Z, et al. Cytokine expression and signaling in drug-induced cellular senescence. Oncogene. 2010;29:273–284. doi: 10.1038/onc.2009.318. [DOI] [PubMed] [Google Scholar]
- 8.Rodier F, et al. Persistent DNA damage signalling triggers senescence-associated inflammatory cytokine secretion. Nat Cell Biol. 2009;11:973–979. doi: 10.1038/ncb1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Courtois-Cox S, et al. A negative feedback signaling network underlies oncogene-induced senescence. Cancer Cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Campisi J. Cellular senescence as a tumor-suppressor mechanism. Trends Cell Biol. 2001;11:S27–S31. doi: 10.1016/s0962-8924(01)02151-1. [DOI] [PubMed] [Google Scholar]
- 11.Ruggero D, et al. The translation factor eIF-4E promotes tumor formation and cooperates with c-Myc in lymphomagenesis. Nat Med. 2004;10:484–486. doi: 10.1038/nm1042. [DOI] [PubMed] [Google Scholar]
- 12.Petroulakis E, et al. p53-dependent translational control of senescence and transformation via 4E-BPs. Cancer Cell. 2009;16:439–446. doi: 10.1016/j.ccr.2009.09.025. [DOI] [PubMed] [Google Scholar]
- 13.Pause A, et al. Insulin-dependent stimulation of protein synthesis by phosphorylation of a regulator of 5′-cap function. Nature. 1994;371:762–767. doi: 10.1038/371762a0. [DOI] [PubMed] [Google Scholar]
- 14.Poulin F, Gingras AC, Olsen H, Chevalier S, Sonenberg N. 4E-BP3, a new member of the eukaryotic initiation factor 4E-binding protein family. J Biol Chem. 1998;273:14002–14007. doi: 10.1074/jbc.273.22.14002. [DOI] [PubMed] [Google Scholar]
- 15.Gingras AC, et al. Regulation of 4E-BP1 phosphorylation: A novel two-step mechanism. Genes Dev. 1999;13:1422–1437. doi: 10.1101/gad.13.11.1422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rhoads RE. eIF4E: New family members, new binding partners, new roles. J Biol Chem. 2009;284:16711–16715. doi: 10.1074/jbc.R900002200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dilling MB, et al. 4E-binding proteins, the suppressors of eukaryotic initiation factor 4E, are down-regulated in cells with acquired or intrinsic resistance to rapamycin. J Biol Chem. 2002;277:13907–13917. doi: 10.1074/jbc.M110782200. [DOI] [PubMed] [Google Scholar]
- 18.Dowling RJ, et al. mTORC1-mediated cell proliferation, but not cell growth, controlled by the 4E-BPs. Science. 2010;328:1172–1176. doi: 10.1126/science.1187532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Vilar E, et al. Gene expression patterns in mismatch repair-deficient colorectal cancers highlight the potential therapeutic role of inhibitors of the phosphatidylinositol 3-kinase-AKT-mammalian target of rapamycin pathway. Clin Cancer Res. 2009;15:2829–2839. doi: 10.1158/1078-0432.CCR-08-2432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shaw RJ, Cantley LC. Ras, PI(3)K and mTOR signalling controls tumour cell growth. Nature. 2006;441:424–430. doi: 10.1038/nature04869. [DOI] [PubMed] [Google Scholar]
- 21.Kuilman T, et al. Oncogene-induced senescence relayed by an interleukin-dependent inflammatory network. Cell. 2008;133:1019–1031. doi: 10.1016/j.cell.2008.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Braunstein S, et al. A hypoxia-controlled cap-dependent to cap-independent translation switch in breast cancer. Mol Cell. 2007;28:501–512. doi: 10.1016/j.molcel.2007.10.019. [DOI] [PubMed] [Google Scholar]
- 23.Kurz DJ, Decary S, Hong Y, Erusalimsky JD. Senescence-associated (beta)-galactosidase reflects an increase in lysosomal mass during replicative ageing of human endothelial cells. J Cell Sci. 2000;113:3613–3622. doi: 10.1242/jcs.113.20.3613. [DOI] [PubMed] [Google Scholar]
- 24.Coppé JP, et al. Senescence-associated secretory phenotypes reveal cell-nonautonomous functions of oncogenic RAS and the p53 tumor suppressor. PLoS Biol. 2008;6:2853–2868. doi: 10.1371/journal.pbio.0060301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Shieh SY, Ikeda M, Taya Y, Prives C. DNA damage-induced phosphorylation of p53 alleviates inhibition by MDM2. Cell. 1997;91:325–334. doi: 10.1016/s0092-8674(00)80416-x. [DOI] [PubMed] [Google Scholar]
- 26.Armengol G, et al. 4E-binding protein 1: A key molecular “funnel factor” in human cancer with clinical implications. Cancer Res. 2007;67:7551–7555. doi: 10.1158/0008-5472.CAN-07-0881. [DOI] [PubMed] [Google Scholar]
- 27.Legrier ME, et al. Targeting protein translation in human non small cell lung cancer via combined MEK and mammalian target of rapamycin suppression. Cancer Res. 2007;67:11300–11308. doi: 10.1158/0008-5472.CAN-07-0702. [DOI] [PubMed] [Google Scholar]
- 28.Chen Z, et al. Crucial role of p53-dependent cellular senescence in suppression of Pten-deficient tumorigenesis. Nature. 2005;436:725–730. doi: 10.1038/nature03918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kannan-Thulasiraman P, et al. Role of the translational repressor 4E-BP1 in the regulation of p21(Waf1/Cip1) expression by retinoids. Biochem Biophys Res Commun. 2008;368:983–989. doi: 10.1016/j.bbrc.2008.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Phillips A, Blaydes JP. MNK1 and EIF4E are downstream effectors of MEKs in the regulation of the nuclear export of HDM2 mRNA. Oncogene. 2008;27:1645–1649. doi: 10.1038/sj.onc.1210785. [DOI] [PubMed] [Google Scholar]
- 31.Demidenko ZN, Korotchkina LG, Gudkov AV, Blagosklonny MV. Paradoxical suppression of cellular senescence by p53. Proc Natl Acad Sci USA. 2010;107:9660–9664. doi: 10.1073/pnas.1002298107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chang BD, et al. Molecular determinants of terminal growth arrest induced in tumor cells by a chemotherapeutic agent. Proc Natl Acad Sci USA. 2002;99:389–394. doi: 10.1073/pnas.012602599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Braunstein S, Badura ML, Xi Q, Formenti SC, Schneider RJ. Regulation of protein synthesis by ionizing radiation. Mol Cell Biol. 2009;29:5645–5656. doi: 10.1128/MCB.00711-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yang DQ, Kastan MB. Participation of ATM in insulin signalling through phosphorylation of eIF-4E-binding protein 1. Nat Cell Biol. 2000;2:893–898. doi: 10.1038/35046542. [DOI] [PubMed] [Google Scholar]
- 35.Heesom KJ, Gampel A, Mellor H, Denton RM. Cell cycle-dependent phosphorylation of the translational repressor eIF-4E binding protein-1 (4E-BP1) Curr Biol. 2001;11:1374–1379. doi: 10.1016/s0960-9822(01)00422-5. [DOI] [PubMed] [Google Scholar]
- 36.Gingras AC, Kennedy SG, O'Leary MA, Sonenberg N, Hay N. 4E-BP1, a repressor of mRNA translation, is phosphorylated and inactivated by the Akt(PKB) signaling pathway. Genes Dev. 1998;12:502–513. doi: 10.1101/gad.12.4.502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Herbert TP, Tee AR, Proud CG. The extracellular signal-regulated kinase pathway regulates the phosphorylation of 4E-BP1 at multiple sites. J Biol Chem. 2002;277:11591–11596. doi: 10.1074/jbc.M110367200. [DOI] [PubMed] [Google Scholar]
- 38.Azar R, Alard A, Susini C, Bousquet C, Pyronnet S. 4E-BP1 is a target of Smad4 essential for TGFbeta-mediated inhibition of cell proliferation. EMBO J. 2009;28:3514–3522. doi: 10.1038/emboj.2009.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Yamaguchi S, et al. ATF4-mediated induction of 4E-BP1 contributes to pancreatic beta cell survival under endoplasmic reticulum stress. Cell Metab. 2008;7:269–276. doi: 10.1016/j.cmet.2008.01.008. [DOI] [PubMed] [Google Scholar]
- 40.Elmore LW, Di X, Dumur C, Holt SE, Gewirtz DA. Evasion of a single-step, chemotherapy-induced senescence in breast cancer cells: Implications for treatment response. Clin Cancer Res. 2005;11:2637–2643. doi: 10.1158/1078-0432.CCR-04-1462. [DOI] [PubMed] [Google Scholar]
- 41.Rajaraman R, Guernsey DL, Rajaraman MM, Rajaraman SR. Stem cells, senescence, neosis and self-renewal in cancer. Cancer Cell Int. 2006;6:25. doi: 10.1186/1475-2867-6-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.