Abstract
Because invasive species threaten the integrity of natural ecosystems, a major goal in ecology is to develop predictive models to determine which species may become widespread and where they may invade. Indeed, considerable progress has been made in understanding the factors that influence the local pattern of spread for specific invaders and the factors that are correlated with the number of introduced species that have become established in a given region. However, few studies have examined the relative importance of multiple drivers of invasion success for widespread species at global scales. Here, we use a dataset of >5,000 presence/absence records to examine the interplay between climatic suitability, biotic resistance by native taxa, human-aided dispersal, and human modification of habitats, in shaping the distribution of one of the world's most notorious invasive species, the Argentine ant (Linepithema humile). Climatic suitability and the extent of human modification of habitats are primarily responsible for the distribution of this global invader. However, we also found some evidence for biotic resistance by native communities. Somewhat surprisingly, and despite the often cited importance of propagule pressure as a crucial driver of invasions, metrics of the magnitude of international traded commodities among countries were not related to global distribution patterns. Together, our analyses on the global-scale distribution of this invasive species provide strong evidence for the interplay of biotic and abiotic determinants of spread and also highlight the challenges of limiting the spread and subsequent impact of highly invasive species.
Keywords: biological invasions, Formicidae, geography, human influence, prediction
Biological invasions can disrupt ecosystem functioning, homogenize biota, and threaten global diversity (1). To mitigate the often dramatic consequences of many invasive species on native ecosystems and the services they provide, a fundamental goal for conservation biology is to be able to predict which species will invade and which areas are most vulnerable to their invasion (2). Despite considerable efforts at both local and regional scales to elucidate the relative roles of biotic and abiotic conditions on the spread and impact of introduced species (e.g., refs. 3–6), understanding which factors limit the global distribution of species is still a largely unanswered question (7).
One approach that has been relatively successful is to relate the number of invasive species established in a given area to factors that describe the region. For example, Pyšek et al. recently used up-to-date information on the presence of alien species from a variety of taxa to identify general predictors of the level of invasion (e.g., number of established species) across Europe (8). They found an overwhelming influence of anthropogenic factors (i.e., wealth and demography) in determining the distribution of alien species. Few studies consider the influence of environmental and human-mediated factors in shaping the global distribution of invasive species (8, 9), particularly for single species with global (e.g., multicontinental) distributions. This paucity results, in part, from a lack of detailed information on (i) the distributions of widespread invasive species, (ii) the biotic and abiotic factors that may determine their establishment and spread, and (iii) how these factors covary with human commerce and habitat modification to contribute to invasion success at global scales.
In this study, we use a unique, global-scale dataset on the distribution of one of the most noxious and best studied invasive species, the Argentine ant (Linepithema humile) (10), to examine the relative roles of abiotic, biotic, and human-mediated factors in determining its global distribution. Many studies have examined the distribution and dispersal pathways of this invasive ant (11–22) and described the range limits at regional and global scales (20, 23–28). However, most of these studies relied on simple environmental niche models or described the current distribution of the species using only partial records. The work described here investigates the combined and relative roles of climatic suitability, biotic resistance by native ant fauna, human-aided dispersal, and human modification of habitats on the global distribution of this notorious invasive species, using the most complete information on the distribution of the Argentine ant (including 2,595 presences and, just as importantly, 2,867 absences) and environmental data available at a finer resolution than has previously been analyzed. Our goal is to describe the combined and relative influence of the key drivers of invasion success for a widespread invasive species at a global scale.
Results
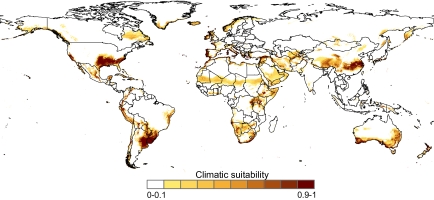
To assess the worldwide climatic suitability for the Argentine ant, we initially developed 1,000 replicate boosted regression trees (BRTs) combining occurrence data of the species with various climatic variables. The most influential variables were precipitation of wettest quarter, annual mean temperature, and maximum temperature of the warmest month (Table S1). The ability of these replicate models to predict testing localities where the Argentine ant was actually present and absent was high, as shown by the area under the curve (AUC) index (0.963 ± 0.004; mean ± SD) and the ratio of incorrectly classified data (0.091 ± 0.007; mean ± SD). This result suggests an overall good performance of model predictions. The resulting predicted climatic suitability based on averages of these models (Fig. 1) agrees with previous models of the distribution of this species (23, 24). Uncertain predictions are more noticeable in regions that have a low number of locality records (e.g., northern and central Africa and southern Asia) or where input climate data might be misleading (e.g., northernmost regions, where few weather stations were used to generate the variables) (29), and it would be preferable to include more data from these regions in the future (Fig. S1). Nevertheless, our models strongly suggest that the areas that are climatically suitable for L. humile are subtropical and Mediterranean regions and the species might eventually have a distribution that is more widespread than its current distribution.
Fig. 1.
Climatic suitability of the Argentine ant based on boosted regression trees, calibrated using worldwide occurrences from areas where the species is known to persist outside human buildings and five climate variables. Areas with a mean annual temperature below the lower thermal limits of the species (−10.4 °C, according to ref. 32) were not considered.
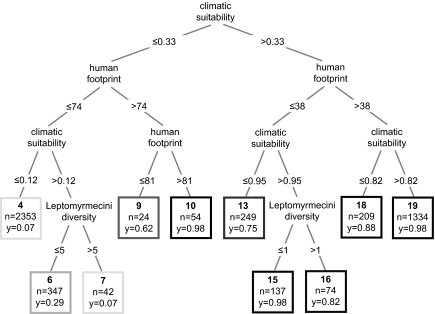
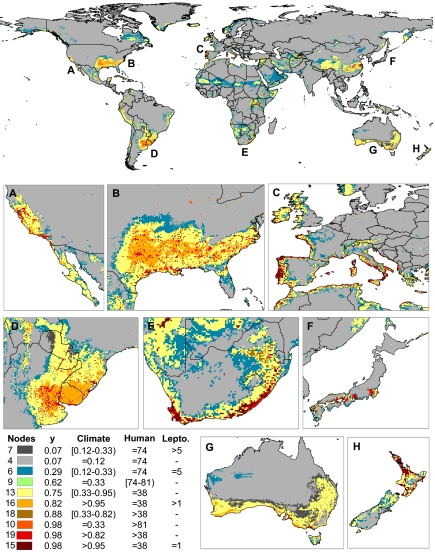
In a second step, we also calibrated 1,000 replicate classification trees (CTs) to determine the ranking of the climatic, biotic, and human-mediated factors as predictors of the global distribution of the Argentine ant. The variables that accounted for most of the distribution were climatic suitability and human modification of habitats, followed by ant generic diversity in the tribe Leptomyrmecini (Table 1). The probability of introduction of L. humile through human-aided dispersal was the lowest-ranked factor (Table 1). These three most relevant variables were then used to build a CT with a maximum depth of four node levels (Fig. 2). The average correct classification rate within each terminal node was ≈88% (Table S2), providing strong evidence that the final classification of regions worldwide based on the most influential climatic, biotic, and human-mediated factors is appropriate (Fig. 3). Climatic suitability emerged as the most relevant variable in shaping the worldwide distribution of the Argentine ant. Areas with a climatic suitability >0.33 (with 1 corresponding to highly suitable areas) promote establishment by the Argentine ant (Fig. 3, terminal nodes 13, 15, 16, 18, and 19), although in areas with climatic suitability <0.95 the species might be absent when the degree of human modification is low (Fig. 3, terminal node 13). Whereas areas with a climatic suitability ≤0.33 seem inappropriate for the species (Fig. 3, terminal nodes 4, 6, and 7), some populations are capable of establishing in these particular places when the degree of human modification is high (Fig. 3, terminal nodes 9 and 10). Generic diversity in the tribe Leptomyrmecini seems to play a role only in suboptimal climates with a degree of human modification ≤74 (Fig. 3, terminal nodes 6 and 7). In regions that present a high climatic suitability but with little human influence, biotic resistance plays, at best, a limited role (Fig. 3, terminal nodes 15 and 16). Under both circumstances, the occurrence of the Argentine ant decreases with increases in the generic diversity in the tribe Leptomyrmecini.
Table 1.
Proportion of variable occurrence at specific nodes in the classification trees used to determine the influence of abiotic, biotic, and human-mediated factors in determining the worldwide distribution of the Argentine ant
| Rank | Climatic suitability | Leptomyrmecini diversity | Bothriomyrmecini diversity | Dolichoderini diversity | Tapinomini diversity | Human footprint | Human trade |
| 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 |
| 2 | 0 | 0 | 0 | 0 | 0 | 1 | 0 |
| 3 | 0 | 1 | 0 | 0 | 0 | 0 | 0 |
| 4 | 0 | 0 | 0.12 | 0.76 | 0.12 | 0 | 0.01 |
| 5 | 0 | 0 | 0.67 | 0.16 | 0.15 | 0 | 0.01 |
| 6 | 0 | 0 | 0.18 | 0.07 | 0.70 | 0 | 0.04 |
| 7 | 0 | 0 | 0.03 | 0 | 0.03 | 0 | 0.94 |
The values correspond to the number of times that the variable first appeared at that specific rank order (or subdivision of the classification tree), on the basis of 1,000 replicate models that differ in the original subset of occurrence data used to calibrate them.
Fig. 2.
Classification tree used to identify the roles of abiotic, biotic, and human-mediated factors in explaining the worldwide distribution of the Argentine ant. Only the most influential variables were used to construct the tree: climatic suitability (ranging from 0 to 1, with higher values corresponding to areas with higher climatic suitability), degree of human modification of habitats (ranging from 0 to 100, with higher values corresponding to highly disturbed areas), and ant generic diversity of the Leptomyrmecini tribe at the country level (with a maximum number of nine genera) (Table 1). The length of the tree was limited to a maximum of four node levels, and the variables and conditions (i.e., threshold value) used to split the records are indicated at each split. For example, the terminal node 10 corresponds to localities with a climatic suitability ≤0.33 and a degree of human modification of the habitat >81. n values indicate the total number of observations assigned to the terminal nodes, and y values show the proportion of presences. Boxes with darker grays correspond to higher y values at the terminal node.
Fig. 3.
Classification of the world based on the classification tree used to identify the roles of abiotic, biotic, and human-mediated factors in explaining the worldwide distribution of the Argentine ant (Fig. 2), with (A–H, Lower) closer views of selected regions. The colors correspond to the classes assigned to each terminal node of the tree and indicate for each locality the relative influence of climatic suitability, degree of human modification of habitats, and generic diversity of the Leptomyrmecini tribe at the country-level variables. y values correspond to the proportion of presences in the terminal nodes (Fig. 2), and they can be seen as the probability of occurrence of the Argentine ant.
Discussion
This global analysis of factors often cited as key determinants of invasion success highlights the relative importance of climatic suitability and degree of human modification of habitats and the weaker influence of biotic interactions and probability of introduction through human-aided dispersal between countries at global scales. Climatic suitability sets the abiotic conditions that allow species to establish and spread, and climate matching has been identified as a key factor for invasion of many invasive species (9, 30). This is especially true for the Argentine ant, whose occurrence at regional scales is highly constrained by temperature and humidity (25, 26, 31, 32). At local scales, establishment by the Argentine ant in climatically favorable areas is also favored by anthropogenic factors (33, 34). Higher human pressure on the environment increases the frequency of new introductions (and hence the probability of establishment of new introduced propagules) but also may mitigate abiotic constraints by creating favorable microclimates. Argentine ants are thus capable of establishing viable populations in areas with climates that might otherwise not be suitable but are close to human habitations where microclimatic conditions are favorable and the negative influence of biotic interactions is negligible.
In its native range, the abundance and impact of the Argentine ant may be constrained by the presence of highly dominant native ant species in the same subfamily (Dolichoderinae) (18). The competitive dominance of the Argentine ant in most of its introduced range may result, in part, from the absence of other competitively dominant, closely related species. Along these lines, we found that sites with more native genera in the tribe Leptomyrmecini tended to have a lower probability of invasion by L. humile. Whereas the influence of this “biotic resistance” was generally weak in climatically optimal regions, it became important in less suitable climates with a moderate degree of human modification. These results are consistent with Argentine ants being capable of establishing viable populations in nonoptimal climates if the area includes a certain degree of human disturbance and if biotic resistance with related taxa is limited. The influence of biotic interactions at this scale of analysis was unexpected because we were not testing the effects of the number of species in local assemblages, but rather the number of genera in entire countries. However, although the presence of related taxa may hinder L. humile invasion during its initial stages, there is little evidence that native ants preclude the establishment of populations of the Argentine ant, particularly if the climate is suitable and human pressure on the environment is high (35–38).
Propagule pressure (i.e., probability of being introduced or the number of introductions) is considered to be one of the most important predictors for the spread of invasive species (39). Somewhat surprisingly, we found no evidence that the probability of introduction via human-aided dispersal among countries influenced the distribution of L. humile. This finding could be because these data are available at only the country level (40) and Argentine ants have been moved around for >100 y (41). Undoubtedly the influence of propagule pressure within countries is important, which our analyses could not detect. Jump dispersal from the native range (i.e., the primary source) played a major role at the initial stage of the expansion of the Argentine ant (16), but now the dispersion of propagules from secondary sources (i.e., established populations in the introduced range) accounts for much of the spread of the species worldwide (15, 16). Further information on dispersal pathways (sensu ref. 42) for the expansion of the Argentine ant at regional and local scales, not only among countries, is needed to assess the geographic dimensions of the invasion over time in fine detail. This type of information is crucial to anticipate the rate at which Argentine ants will spread and the areas most likely to be invaded (28, 43). Finer-resolution data on patterns of commerce, which incorporate a strong historical perspective, are needed to provide a more rigorous test of the role of propagule pressure at global scales.
Documenting the relative roles of these factors for invasive species addresses one of the long-standing questions in invasion biology: What determines the distribution of invasive species? Taken together, our results show that climate and human modification of habitats are the main drivers of invasion by the Argentine ant at global scales. Human activity clearly influences many biological invasions, at least during the initial stage of the invasion process. However, over time, the stochastic nature of human-mediated dispersal and establishment events gives way to a clear signal of climatic suitability as the potential climate envelope begins to be filled. Our results paint a sobering picture for human attempts to limit the spread of the Argentine ant through border protection measures or through reliance on biotic resistance. This outcome is especially true under a context of global change, where the climatic range of the species is expected to change (24) and the interactions of climate with other biotic and human-mediated components are largely unknown (44). Understanding how these factors interact to shape the global distributions of other invasive species (be they insects or otherwise) and ultimately mediate their impacts on biodiversity and the services it provides is a daunting, but important challenge.
Methods
Occurrence Data.
Occurrence data on the Argentine ant (in the form of longitude/latitude coordinates) were extracted from numerous datasets (21, 23–25, 45, 46), whereas most absences were obtained from field survey of ant communities (45) (dataset available upon request from N.R.-P.). Because we were not able to check the source of all records and duplicates could induce errors, we decided to consider presence localities separated by <300 m as duplicates and to include them only once in the model. Although some of the “absence” records might correspond to false absences due to insufficient sampling effort, we decided to use them because they provide a more reliable set of absences than pseudoabsences created at random from areas where the species is not known to occur—many of which may never have been surveyed. The final dataset comprised 5,462 localities, corresponding to 2,595 presences (85% from localities where the species is able to persist outside human buildings according to expert knowledge) and 2,867 absences (Fig. S2).
Explanatory Variables.
Three major types of explanatory factors were considered: climatic, biotic, and human-mediated factors, each consisting of a number of variables, although not all of them were used in all of the analyses (Fig. S3). Climate data were used to derive a worldwide climatic suitability index for the Argentine ant. The additional biotic and human-mediated factors, together with the climatic suitability index, were used to determine the roles of abiotic, biotic, and human-mediated factors explaining the distribution of the Argentine ant. We assessed the role of biotic resistance (47, 48) by tallying the number of ant genera belonging to different tribes of the Dolichoderinae subfamily—according to ref. 49—in each country (50). We also considered two human-mediated factors: probability of introduction of the species through human-aided dispersal at the country level [derived from applying a metapopulation model to the value of traded commodities between countries (51)] and degree of human modification of habitats (extracted from ref. 52). The probability of introduction through human-aided dispersal can be a proxy for propagule pressure promoting the introduction of invading populations in a country (39), whereas the degree of human modification includes both finer-scale propagule pressure within countries and environmental factors like disturbance (see SI Methods for details).
Classification and Regression Trees.
We examined the relative importance of variables by means of classification and regression trees (53–55). Trees are used to explore the variation of a single response variable by several explanatory variables. The response variable is either categorical (classification tree) or numeric (regression trees), and the explanatory variables can be in turn categorical and/or numeric (55). Trees are constructed by iteratively splitting the occurrence data into binary partitions, by using a simple rule based on a single explanatory variable. At each split the data are partitioned into two groups as internally homogeneous as possible, which are in turn split again up to a certain reasonable size. Each group or terminal node is characterized by either the distribution (categorical response) or the mean value (numerical response) of the response variable, the number of occurrences assigned to the group, and the thresholds of the explanatory variables that define it (54).
Classical classification and regression trees are, however, known to be unstable, giving different trees each time they are repeated on a single dataset. To overcome the inaccuracies of single regression trees, BRTs use an iterative method (the boosting algorithm) to fit a large number of relatively simple regression trees whose predictions are then combined into a final ensemble prediction (53, 56). Boosting is a general method of producing accurate prediction rules by combining rough and moderately inaccurate “rules of thumb,” making possible the modeling of complex response surfaces (57). Although a high number of repetitions can compensate for the variability of regression trees, there is still some difficultly combining CTs for a final conclusion. Possible methods [e.g., cluster ensembles (58)] are theoretically feasible, but they do not handle data of high dimension. Hence, we preferred to use classification inference trees within the ctree function (available in the party R package), which bases its node splitting on statistical tests (59). This robust method produces an identical tree every time it is repeated and provides a P value for the significance of its splitting.
Methodological Approach.
We adopted a two-step modeling approach using BRTs to assess the areas climatically suitable for the species and then CTs to combine the resulting climate index with the biotic and human-mediated factors (Fig. S4).
Step 1. With the optimal set of parameters (see SI Methods and Table S3 for details), the model was trained (with a 0.75 training fraction) and tested 1,000 times. Presence localities were limited to areas where the species is known to persist outside human buildings (Fig. S2, red dots) to avoid the inclusion of false presences due to the influence of humans, which favor the survival of the species in unsuitable climatic conditions inside buildings or highly modified environments. As output for the training step, the mean and SD of the 1,000 predicted suitabilities were calculated and the performance of the models was evaluated using receiver operating characteristic (ROC) plots (60). The mean predicted suitability was projected onto a geographic space to produce a map of the species’ climatic suitability, as well as the SD across the replicate models to assess the accuracy of this averaged prediction. Areas with a mean annual temperature below the lower thermal limits of the species (−10.4 °C, according to ref. 32) were considered nonsuitable and excluded from the analysis. The predicted climatic suitability (ranging from 0 to 1, with higher values corresponding to more suitable climates) was subsequently used as one of seven input coverages to assess the relative importance of different factors in determining the distribution of the Argentine ant.
Step 2. This analysis comprised two simultaneous processes: one similar to the analysis conducted in the previous step (step 1) to derive an index of climatic suitability for the species using BRTs, and another process using CTs to identify the influence of the various climatic, biotic, and human-mediated factors. Because the objective was to understand the way in which different variables interact to determine the distribution of the Argentine ant, here we used presences from where the species is known to persist outside human buildings (as in the previous step) but also localities from areas where climatic conditions restrict the occurrence of the species inside human habitations or in highly transformed habitats with particular microclimates. The use of all occurrences together allows us to identify the set of conditions that explain the establishment success or failure of the species under different circumstances. As an initial step, we randomly split the presence/absence data into independent halves: sets A and A′. After excluding presences from areas where the species does not persist outside human buildings, set A was used for training a BRT with training fraction 1, using a similar approach to the one detailed in the previous step 1. The resulting model was used to predict a climatic suitability index for the localities included in set A′, set aside from model development. This climatic suitability index was then combined with the other variables related to biotic resistance by native ant fauna, human-aided dispersal, and human modification of habitats to construct a classification tree using only set A′ to disentangle the complex interactions between the variables. This process was repeated 1,000 times to grow 1,000 sets of CTs. From each of the 1,000 CTs, variable rankings were extracted (because one CT takes much memory to store) and aggregated to obtain a table of proportions of the rankings for each of the variables on the basis of all repetitions. The top-ranked variables from the aggregated ranking (i.e., those with a clear certainty in their rankings) were used to create one final CT, along with the mean climatic suitability derived from step 1 under the condition that it was chosen as one of the top-ranked variables. A fully grown tree consists of various terminal nodes, but the final tree was, however, reduced to a certain depth depending on the number of variables chosen and the correct classification of observations within each terminal node. This final tree was then projected into a geographic space. A terminal node was assigned to each locality (i.e., pixel) in the map on the basis of the abiotic, biotic, and human-mediated characteristics of the site and the interactions between the variables that define each terminal node. This classification process allowed us to explain (using a certain combination of explanatory variables) why one area was more susceptible to host the species than any other.
Computational Constraints and Software.
The preparation and presentation of the geospatial data were done using ESRI ArcGIS v9.2. The analyses were conducted at ∼1 km2 resolution (30 arc s), which corresponded to the resolution of the variables related to climate and degree of human modification and allowed us to represent the small-scale divergences on the distribution of the species. However, a coarser resolution (10 arc min, ∼18 km2) was used to visualize the model predictions worldwide due to computational limitations. All statistical analyses were done with R (61), using the packages: gbm, party, ROCR, and sp (59, 62–64).
Supplementary Material
Acknowledgments
We thank James Wetterer for sharing occurrence data and knowledge on the Argentine ant distribution and two anonymous reviewers for their comments on the manuscript. We acknowledge financial support from the Centre of Excellence for Invasion Biology (N.R.-P. and D.M.R.), from the Catalan Agency for Management of University and Research Grants (Generalitat de Catalunya) through Beatriu de Pinós Postdoctoral Grants 2006 BP-A 10124 and 2008 BP-B 00042 (to N.R.-P.), from the Hans Sigrist Foundation (to D.M.R.), from the Spanish Ministry of Science and Innovation Grant CGL2007-64080-C02-02/BOS (to C.G. and N.R.-P.), from the Spanish Ministry of Science and Innovation and the European Regional Development Fund Grant CGL2007-64080-C02-01/BOS (to X.E.), from the Blue Skies Programme of the National Research Foundation (to C.H.), from the Danish National Research Foundation (to J.S.P.), from Department of Energy–Program for Ecosystem Research Grant DE-FG02-08ER64510 (to N.J.S.), from National Science Foundation Grant DEB 0716966 (to A.V.S.), and from the New Zealand Foundation for Research, Science, and Technology Grant C09X0507 (to D.W.).
Footnotes
*This Direct Submission article had a prearranged editor.
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1011723108/-/DCSupplemental.
References
- 1.Mack RN, et al. Biotic invasions: Causes, epidemiology, global consequences, and control. Ecol Appl. 2000;10:689–710. [Google Scholar]
- 2.Ehrlich PR. In: Biological Invasions: A Global Perspective. Drake JA, et al., editors. Chichester: Wiley; 1989. pp. 315–328. [Google Scholar]
- 3.Higgins SI, Richardson DM, Cowling RM. Modeling invasive plant spread: The role of plant-environment interactions and model structure. Ecology. 1996;77:2043–2054. [Google Scholar]
- 4.Boulant N, Garnier A, Curt T, Lepart J. Disentangling the effects of land use, shrub cover and climate on the invasion speed of native and introduced pines in grasslands. Divers Distrib. 2009;15:1047–1059. [Google Scholar]
- 5.Hastings A, et al. The spatial spread of invasions: New developments in theory and evidence. Ecol Lett. 2005;8:91–101. [Google Scholar]
- 6.Holway DA. Factors governing rate of invasion: A natural experiment using Argentine ants. Oecologia. 1998;115:206–212. doi: 10.1007/s004420050509. [DOI] [PubMed] [Google Scholar]
- 7.Puth LM, Post DM. Studying invasion: Have we missed the boat? Ecol Lett. 2005;8:715–721. [Google Scholar]
- 8.Pysek P, et al. Disentangling the role of environmental and human pressures on biological invasions across Europe. Proc Natl Acad Sci USA. 2010;107:12157–12162. doi: 10.1073/pnas.1002314107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Thuiller W, et al. Niche-based modelling as a tool for predicting the risk of alien plant invasions at a global scale. Glob Change Biol. 2005;11:2234–2250. doi: 10.1111/j.1365-2486.2005.001018.x. [DOI] [PubMed] [Google Scholar]
- 10.Mayr G. Formicidae novae Americanae collectae a Prof. P. de Strobel. Annu Soc Nat Mat Modena. 1868;3:161–178. [Google Scholar]
- 11.McGlynn TP. The worldwide transfer of ants: Geographical distribution and ecological invasions. J Biogeogr. 1999;26:535–548. [Google Scholar]
- 12.Suarez AV, Holway DA, Ward PS. The role of opportunity in the unintentional introduction of nonnative ants. Proc Natl Acad Sci USA. 2005;102:17032–17035. doi: 10.1073/pnas.0506119102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ward DF, Beggs JR, Clout MN, Harris RJ, O'Connor S. The diversity and origin of exotic ants arriving in New Zealand via human-mediated dispersal. Divers Distrib. 2006;12:601–609. [Google Scholar]
- 14.Lester PJ. Determinants for the successful establishment of exotic ants in New Zealand. Divers Distrib. 2005;11:279–288. [Google Scholar]
- 15.Vogel V, Pedersen JS, Giraud T, Krieger MJB, Keller L. The worldwide expansion of the Argentine ant. Divers Distrib. 2010;16:170–186. [Google Scholar]
- 16.Suarez AV, Holway DA, Case TJ. Patterns of spread in biological invasions dominated by long-distance jump dispersal: Insights from Argentine ants. Proc Natl Acad Sci USA. 2001;98:1095–1100. doi: 10.1073/pnas.98.3.1095. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Espadaler X, Gómez C. The Argentine ant, Linepithema humile, in the Iberian Peninsula. Sociobiology. 2003;42:187–192. [Google Scholar]
- 18.Wild AL. Taxonomy and distribution of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae) Ann Entomol Soc Am. 2004;97:1204–1215. [Google Scholar]
- 19.Ward DF, Harris RJ, Stanley MC. Human-mediated range expansion of Argentine ants Linepithema humile (Hymenoptera: Formicidae) in New Zealand. Sociobiology. 2005;45:401–407. [Google Scholar]
- 20.Harris R, Ward DF, Sutherland MA. A Survey of the Current Distribution of Argentine Ants, Linepithema humile, in Native Habitats in New Zealand, and Assessment of Future Risk of Establishment. Landcare Research, Lincoln: Landcare Research Report LC0102/105; 2002. [Google Scholar]
- 21.Wetterer JK, Wild AL, Suarez AV, Roura-Pascual N, Espadaler X. Worldwide spread of the Argentine ant, Linepithema humile (Hymenoptera: Formicidae) Myrmecol News. 2009;12:187–197. [Google Scholar]
- 22.Ward DF, Rees J, Harris RJ, Stanley MC. New Zealand Ant Distribution Database. v2.0. 2009 Available at http://www.landcareresearch.co.nz/research/biocons/invertebrates/ants/distribution/. Accessed January, 2009. [Google Scholar]
- 23.Hartley S, Harris R, Lester PJ. Quantifying uncertainty in the potential distribution of an invasive species: Climate and the Argentine ant. Ecol Lett. 2006;9:1068–1079. doi: 10.1111/j.1461-0248.2006.00954.x. [DOI] [PubMed] [Google Scholar]
- 24.Roura-Pascual N, et al. Geographical potential of Argentine ants (Linepithema humile Mayr) in the face of global climate change. Proc Biol Sci. 2004;271:2527–2535. doi: 10.1098/rspb.2004.2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Menke SB, Holway DA, Fisher RN, Jetz W. Characterizing and predicting species distributions across environments and scales: Argentine ant occurrences in the eye of the beholder. Glob Ecol Biogeogr. 2009;18:50–63. [Google Scholar]
- 26.Hartley S, Krushelnycky PD, Lester PJ. Integrating physiology, population dynamics and climate to make multi-scale predictions for the spread of an invasive insect: The Argentine ant at Haleakala National Park, Hawaii. Ecography. 2010;33:83–94. [Google Scholar]
- 27.Abril S, Roura-Pascual N, Oliveras J, Gómez C. Assessing the distribution of the Argentine ant using physiological data. Acta Oecol. 2009;35:739–745. [Google Scholar]
- 28.Pitt JPW, Worner SP, Suarez AV. Predicting Argentine ant spread over the heterogeneous landscape using a spatially explicit stochastic model. Ecol Appl. 2009;19:1176–1186. doi: 10.1890/08-1777.1. [DOI] [PubMed] [Google Scholar]
- 29.Hijmans RJ, Cameron SE, Parra JL, Jones PG, Jarvis A. Very high resolution interpolated climate surfaces for global land areas. Int J Climatol. 2005;25:1965–1978. [Google Scholar]
- 30.Ulrichs C, Hopper KR. Predicting insect distributions from climate and habitat data. Biocontrol Sci Technol. 2009;53:881–894. [Google Scholar]
- 31.Abril S, Oliveras J, Gómez C. Effect of temperature on the oviposition rate of Argentine ant queens (Linepithema humile Mayr) under monogynous and polygynous experimental conditions. J Insect Physiol. 2008;54:265–272. doi: 10.1016/j.jinsphys.2007.09.009. [DOI] [PubMed] [Google Scholar]
- 32.Jumbam KR, Jackson S, Terblanche JS, McGeoch MA, Chown SL. Acclimation effects on critical and lethal thermal limits of workers of the Argentine ant, Linepithema humile. J Insect Physiol. 2008;54:1008–1014. doi: 10.1016/j.jinsphys.2008.03.011. [DOI] [PubMed] [Google Scholar]
- 33.Menke SB, Holway DA. Abiotic factors control invasion by Argentine ants at the community scale. J Anim Ecol. 2006;75:368–376. doi: 10.1111/j.1365-2656.2006.01056.x. [DOI] [PubMed] [Google Scholar]
- 34.Holway DA, Suarez AV. Homogenization of ant communities in mediterranean California: The effects of urbanization and invasion. Biol Conserv. 2006;127:319–326. [Google Scholar]
- 35.Carpintero S, Reyes-Lopez J. The role of competitive dominance in the invasive ability of the Argentine ant (Linepithema humile) Biol Invasions. 2008;10:25–35. [Google Scholar]
- 36.Holway DA. Competitive mechanisms underlying the displacement of native ants by the invasive Argentine ant. Ecology. 1999;80:238–251. [Google Scholar]
- 37.Menke SB, Fisher RN, Jetz W, Holway DA. Biotic and abiotic controls of Argentine ant invasion success at local and landscape scales. Ecology. 2007;88:3164–3173. doi: 10.1890/07-0122.1. [DOI] [PubMed] [Google Scholar]
- 38.Human KG, Gordon DM. Exploitation and interference competition between the invasive Argentine ant, Linepithema humile, and native ant species. Oecologia. 1996;105:405–412. doi: 10.1007/BF00328744. [DOI] [PubMed] [Google Scholar]
- 39.Simberloff D. The role of propagule pressure in biological invasions. Annu Rev Ecol Syst. 2009;40:81–102. [Google Scholar]
- 40.Lennon JJ. Red-shifts and red herrings in geographical ecology. Ecography. 2000;23:101–113. [Google Scholar]
- 41.Sanders NJ, Suarez AV. In: Fifty Years of Invasion Ecology: The Legacy of Charles Elton. Richardson DM, editor. Oxford: Wiley-Blackwell; 2011. pp. 239–251. [Google Scholar]
- 42.Wilson JRU, Dormontt EE, Prentis PJ, Lowe AJ, Richardson DM. Something in the way you move: Dispersal pathways affect invasion success. Trends Ecol Evol. 2009;24:136–144. doi: 10.1016/j.tree.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 43.Roura-Pascual N, et al. From introduction to equilibrium: Reconstructing the invasive pathways of the Argentine ant in a Mediterranean region. Glob Change Biol. 2009;15:2101–2115. [Google Scholar]
- 44.Hellmann JJ, Byers JE, Bierwagen BG, Dukes JS. Five potential consequences of climate change for invasive species. Conserv Biol. 2008;22:534–543. doi: 10.1111/j.1523-1739.2008.00951.x. [DOI] [PubMed] [Google Scholar]
- 45.Dunn RR, et al. Global ant (Hymenoptera: Formicidae) biodiversity and biogeography—a new database and its possibilities. Myrmecol News. 2007;10:77–83. [Google Scholar]
- 46.Luruli NM. MSc thesis. Stellenbosch, South Africa: University of Stellenbosch; 2007. Distribution and impact of the Argentine ant, Linepithema humile (Mayer), in South Africa. [Google Scholar]
- 47.Elton C. The Ecology of Invasions by Animals and Plants. London: Methuen; 1958. [Google Scholar]
- 48.Fridley JD, et al. The invasion paradox: Reconciling pattern and process in species invasions. Ecology. 2007;88:3–17. doi: 10.1890/0012-9658(2007)88[3:tiprpa]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 49.Ward PS, Brady SG, Fisher BL, Schultz TR. Phylogeny and biogeography of dolichoderine ants: Effects of data partitioning and relict taxa on historical inference. Syst Biol. 2010;59:342–362. doi: 10.1093/sysbio/syq012. [DOI] [PubMed] [Google Scholar]
- 50.Guénard B, Weiser MD, Dunn RR. Ant Genera of the World. 2010. Available at http://www.antmacroecology.org/ant_genera/index.html. Accessed January, 2010.
- 51.United-Nations. UN Commodity Trade Statistics Database. 2007 Available at http://comtrade.un.org/pb/CountryPages.aspx?y=2007. Accessed October, 2008. [Google Scholar]
- 52.Sanderson EW, et al. The human footprint and the last of the wild. Bioscience. 2002;52:891–904. [Google Scholar]
- 53.Ridgeway G. The state of boosting. Comput Sci Stat. 1999;31:172–181. [Google Scholar]
- 54.Breiman L, Friedman JH, Olshen RA, Stone CJ. Classification and Regression Trees. New York: Chapman & Hall; 1984. [Google Scholar]
- 55.De'ath G, Fabricius KE. Classification and regression trees: A powerful yet simple technique for ecological data analysis. Ecology. 2000;81:3178–3192. [Google Scholar]
- 56.Friedman JH. Greedy function approximation: A gradient boosting machine. Ann Stat. 2001;29:1189–1232. [Google Scholar]
- 57.Elith J, et al. Novel methods improve prediction of species' distributions from occurrence data. Ecography. 2006;29:129–151. [Google Scholar]
- 58.Strehl A, Ghosh J. Cluster ensembles: A knowledge reuse framework for combining multiple partitions. J Mach Learn Res. 2002;3:583–617. [Google Scholar]
- 59.Hothorn T, Hornik K, Zeileis C. Unbiased recursive partitioning: A conditional inference framework. J Comput Graph Stat. 2006;15:651–674. [Google Scholar]
- 60.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]
- 61.R Development Core Team. R: A Language and Environment for Statistical Computing. Vienna: R Foundation for Statistical Computing; 2008. [Google Scholar]
- 62.Sing T, Sander O, Beerenwinkel N, Lengauer T. ROCR: Visualizing the Performance of Scoring Classifiers. R Package Version 1.0-4. 2009 Available at http://CRAN.R-project.org/package=ROCR. Accessed January, 2010. [Google Scholar]
- 63.Ridgeway G. gbm: Generalized Boosted Regression Models. R Package Version 1.6-3.1. 2010 Available at http://CRAN.R-project.org/package=gbm. Accessed January, 2010. [Google Scholar]
- 64.Bivand RS, Pebesma EJ, Gomez-Rubio B. Applied Spatial Data Analysis with R. New York: Springer; 2008. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.