Abstract
Increasing evidence suggests that myeloid bone marrow-derived cells (BMDCs) play a critical role in lung metastasis. Blockade of VEGF receptor 1 (VEGFR1) has been proposed as a potential strategy to limit myeloid BMDC recruitment to tumors. However, preclinical evidence indicates that this strategy may not be effective in all tumors. Thus, establishing which molecular mechanisms are responsible for the “escape” of these BMDCs from VEGFR1 inhibition would facilitate development of strategies to control metastasis. Here, we report the complementary role of the chemokine (C-X-C motif) ligand 12/C-X-C chemokine receptor 4 (CXCR4) and VEGF/VEGFR1 pathways in promoting lung metastasis in mice via BMDC recruitment using chimeric mice with deficiency in CXCR4 and VEGFR1–tyrosine kinase in the BMDCs. We first demonstrate that CXCR4 activity is essential for recruitment of myeloid differentiation antigen (Gr-1)-positive BMDCs, whereas VEGFR1 activity is responsible for macrophage recruitment in established tumors. Inhibition of both VEGFR1 and CXCR4 signaling in myeloid BMDCs exerted greater effects on tumor vascular density, growth, and lung metastasis than inhibition of VEGFR1 alone. These effects were reproduced after pharmacologic inhibition of CXCR4 with AMD3100. VEGFR1 and CXCR4 independently exerted a promigratory effect in myeloid BMDCs by activating p38 mitogen-activating protein kinase. Thus, combining CXCR4 blockade with inhibition of VEGFR1 may induce greater tumor growth delay and prevent or inhibit metastasis.
To form distant metastases, cancer cells must have the ability to survive and reach the distal organ through circulation, extravasate, invade, and grow into a macroscopic tumor while evading the immune system (1). The efficiency of this multistep process is governed by interactions between the cancer cells and the host cells (1, 2).
Increasing evidence supports a critical role for the interaction between cancer cells and myeloid (CD11b+) bone marrow-derived cells (BMDCs) during tumor growth and metastasis. Myeloid BMDCs are mobilized into blood circulation and infiltrate the neoplastic tissues from early stages of tumor growth in response to tumor- and stroma-derived cytokines (3). Upon recruitment to the tumor, some of these inflammatory cells may facilitate new blood vessel formation either by directly incorporating into the vessel wall or by secreting factors that promote angiogenesis and activate stromal fibroblasts (4–6). For example, both tumor-associated macrophages (TAMs) and myeloid differentiation antigen (Gr-1)-positive myeloid BMDCs can promote angiogenesis and tumor progression (5). Of the cytokines that contribute to recruitment of myeloid BMDCs, VEGF, and placental growth factor (PlGF) may play important roles by activating their cognate tyrosine kinase (TK) receptor VEGF receptor 1 (VEGFR1) in TAMs (7). In particular, phosphorylation of p38 mitogen-activating protein kinase (p38 MAPK) is important for monocyte migration in response to VEGFR1 activation by VEGF or PlGF (8). However, recruitment by tumors of other myeloid BMDCs—such as Gr-1+ BMDCs—is independent of VEGF or VEGFR1 activity (9, 10). This may be critical for early metastatic growth because Gr-1+ myeloid BMDCs can promote tumor growth and mediate tumor resistance to anti-VEGF therapy (5, 10).
Because Gr-1+ myeloid BMDCs express C-X-C receptor type 4 (CXCR4 or fusin) in addition to VEGFR1, we hypothesized that the chemokine (C-X-C motif) ligand 12 (CXCL12)/CXCR4 pathway could compensate for inhibition of VEGFR1 activity in BMDCs and lead to tumor infiltration by these cells and promotion of metastasis. To test this hypothesis, we first evaluated the effect of genetic ablation of CXCR4—with or without inhibition of VEGFR1–TK activity—in BMDCs on primary tumor growth and metastasis in highly metastatic tumor models of breast and prostate cancer. Then we tested whether pharmacologic blockade of CXCR4 could overcome resistance to VEGFR1–TK inhibition in myeloid BMDCs and inhibit metastasis.
Results
Deletion of CXCR4 in BMDCs Has Moderate Effects on Primary Tumor Growth but Substantially Reduces Metastasis Independently of VEGFR1–TK Activity in BMDCs.
Because CXCR4−/− mice are embryonically lethal and because CXCR4 is a critical player in hematopoietic stem cell trafficking, we induced CXCR4 deficiency in BMDCs after restorative bone marrow transplantation (BMT) in C57BL/6 mice using MxCre-CXCR4flox/– mice as donors. We then induced Cre expression to generate CXCR4−/− BMDCs in the BMT mice (referred heretofore as BMT-CXCR4−/− mice; see Materials and Methods). To achieve inhibition of both CXCR4 and VEGFR–TK activity in BMDCs, we performed BMT from MxCre-CXCR4flox/–VEGFR1TK−/− donor mice to lethally irradiated C57BL/6 mice and then induced the CXCR4 null phenotype in BMDCs (referred heretofore as BMT-CXCR4−/−VEGFR1TK−/−). Next we evaluated the growth rate of TRAMP-C1 prostate tumors and E0771 mammary tumors in mice with BMDCs deficient for only CXCR4 or for both CXCR4 and VEGFR1–TK versus control (Materials and Methods). Both TRAMP-C1 and E0771 cells express detectable levels of CXCR4 transcripts by RT-PCR. However, BMDC-specific deficiency in CXCR4 and VEGFR1–TK induced a modest growth delay in both tumors, and BMDC-specific deficiency in CXCR4 alone induced a growth delay in the E0771 mammary carcinoma (Fig. 1A). More importantly, lung metastatic burden after primary tumor resection was reduced by more than twofold in BMT-CXCR4−/− mice and by more than threefold in BMT-CXCR4−/−VEGFR1TK−/− mice (Fig. 1 B and C). Thus, BMDC-specific inhibition of CXCR4 reduces metastasis—with or without VEGFR1–TK inhibition—despite the minimal delay in primary tumor growth. To dissect the mechanisms by which CXCR4 activity in BMDCs mediates metastasis, we next evaluated tumor angiogenesis and myeloid BMDC infiltration in the primary tumors.
Fig. 1.
Specific inhibition of CXCR4 in BMDCs induces slight delays in tumor growth but potently inhibits lung metastasis. (A) Primary tumor growth is transient (i.e., at day 10) in BMT-CXCR4−/− mice (CXCR4-KO) in TRAMP-C1 and E0771 models. Both tumors grew significantly slower in BMT-CXCR4−/−VEGFR1TK−/− (CXCR4/TK-KO) mice compared with control [BMT-CXCR4flox/+VEGFR1TK+/+ mice: control BMT (Ctrl BMT)]. (B and C) Lung metastatic burden at 4 wk after primary tumor removal (i.e., number and volume of macrometastases) is substantially decreased by genetic deletion of CXCR4 in BMDCs in TRAMP-C1 (B) and E0771 (C) tumors in CXCR4-KO and CXCR4/TK-KO mice compared with Ctrl BMT mice. *P < 0.05 compared with control; error bars represent mean ± SEM.
CXCR4 Deletion in BMDCs Reduces Primary Tumor Angiogenesis Independently of VEGFR1–TK Activity in BMDCs.
We measured microvascular density (MVD) by quantitative immunostaining for MECA32 in sections from size-matched primary tumors (Materials and Methods). MVD was significantly decreased in both tumor models when grown in BMT-CXCR4−/− or BMT-CXCR4−/−VEGFR1TK−/− mice compared with control BMT mice (Fig. 2A). Thus, the effect of CXCR4 deletion in BMDCs on metastasis may be at least in part mediated by inhibition of angiogenesis.
Fig. 2.
Specific inhibition of CXCR4 in BMDCs decreases tumor angiogenesis and Gr-1+ myeloid BMDC recruitment. (A and B) Tumor vascular density (A) and myeloid BMDC infiltration (B) are decreased in both TRAMP-C1 and E0771 models in BMT-CXCR4−/− mice (CXCR4-KO) and in BMT-CXCR4−/−VEGFR1TK−/− (CXCR4/TK-KO) mice compared with control [BMT-CXCR4flox/+VEGFR1TK+/+ mice: control (Ctrl BMT)]. (C and D) Gr-1+ BMDC infiltration is decreased in both tumors in mice with CXCR4−/− BMDCs, irrespective of VEGFR1–TK status (C). Conversely, TAM infiltration is decreased only in mice with VEGFR1TK−/− BMDCs (D). *P < 0.05 compared with control; error bars represent mean ± SEM.
CXCR4 Deletion in BMDCs Reduces the Number of Tumor-Infiltrating Gr-1+ Myeloid BMDCs Independently of VEGFR1–TK Activity in BMDCs.
In addition, we evaluated the tumor-infiltrated myeloid BMDCs by quantitative immunostaining with myeloid markers in size-matched primary tumors (Materials and Methods). In both tumor models, the total number of tumor-infiltrating myeloid BMDCs was significantly decreased in mice deficient for CXCR4 in their BMDCs (Fig. 2B). However, further analysis of myeloid (CD11b+) BMDC subsets showed that, whereas the tumor infiltration by Gr-1+ BMDCs was decreased in both BMT-CXCR4−/− and BMT-CXCR4−/−VEGFR1TK−/− mice, the number of F4/80+ TAMs was reduced only in mice with BMDCs deficient in VEGFR1–TK (Fig. 2 C and D). Thus, the effect of CXCR4 inhibition in BMDCs on metastasis may be mediated by Gr-1+ myeloid BMDC recruitment to tumors. Next, to dissect the roles of CXCR4 and VEGFR1–TK in specific BMDC populations and to gain further insight into the kinetics of Gr-1+ myeloid BMDC recruitment to tumors, we used treatment with the CXCR4 inhibitor AMD3100 at different time points during tumor growth in BMT-VEGFR1TK−/− mice and C57BL/6 mice (Materials and Methods).
Pharmacologic Blockade of CXCR4 Efficiently Delays Tumor Growth and Reduces Metastasis Only in Mice with VEGFR1–TK-Deficient BMDCs.
First, we tested the effect of CXCR4 inhibition alone by delivering AMD3100 using osmotic pumps from the time of tumor implantation (i.e., in a prevention setting). Consistent with the genetic deficiency model, AMD3100 treatment induced a minor and transient delay in the early growth of TRAMP-C1 and E0771 tumors (Fig. 3 A and B). On the other hand, whereas VEGFR1–TK inhibition in BMDCs induced a minor tumor growth delay in E0771 and TRAMP-C1 tumors, AMD3100 treatment substantially delayed tumor growth in BMT-VEGFR1TK−/− mice (Fig. 3 A and B). Next we evaluated the effect of AMD3100 treatment on the growth of established tumors (in an intervention setting, i.e., with the time for growth from 4 mm to ∼1 cm in diameter). Treatment with AMD3100 induced a less substantial growth delay in established TRAMP-C1 tumors, which was maintained after 4 wk of treatment only in BMT-VEGFR1TK−/− mice (Fig. 3C). Finally, we evaluated spontaneous lung metastasis after primary tumor resection. AMD3100 treatment significantly reduced metastatic burden in both tumor models only in BMT-VEGFR1TK −/− mice (Fig. 3 D and E). As seen in the genetic model of CXCR4 deficiency in BMDCs (Fig. 1 B and C), the inhibition of metastasis by AMD3100 treatment alone was significant only in the TRAMP-C1 model (Fig. 3 D and E). Thus, the effect of CXCR4 inhibition on metastasis is tumor-dependent, and inhibition of both CXCR4 and VEGFR1–TK in BMDCs is required to achieve a significant tumor growth delay and reduction of lung metastasis. We next evaluated the effects of AMD3100 treatment on tumor angiogenesis and myeloid BMDC infiltration in size-matched primary tumors.
Fig. 3.
Pharmacologic blockade of CXCR4 results in a substantial tumor growth delay only when combined with VEGFR1–TK inhibition and inhibits lung metastasis in a tumor-dependent manner. (A and B) Preventive setting: Early tumor growth is transiently inhibited by CXCR4 blockade with AMD3100 pumps (AMD) started from the time of tumor implantation in C57BL/6 mice (WT) and moderately delayed in BMT-VEGFR1TK−/− (TK) mice in both TRAMP-C1 (A) and E0771 (B) models. However, a more significant tumor growth delay is seen with AMD3100 in TK mice. (C) Intervention setting: after 28 d of AMD3100 treatment of established TRAMP-C1 tumors, a significant growth delay was seen only in TK mice. (D and E) AMD3100 treatment significantly decreases the number and volume of lung metastases at 4 wk after tumor resection only in TRAMP-C1 tumors (D). In E0771 tumors, a significant decrease in metastatic burden is seen after AMD3100 treatment only in TK mice (E). *P < 0.05 compared with control PBS (WT); error bars represent mean ± SEM.
CXCR4-Mediated Gr-1+ BMDC Infiltration Promotes Early Tumor Growth and Metastasis.
AMD3100 treatment alone decreased MVD when administered in a prevention setting in E0771 tumors but not in TRAMP-C1 tumors (Fig. 4A). However, AMD3100 treatment decreased MVD in both tumors when grown in BMT-VEGFR1TK−/− mice (Fig. 4A). AMD3100 treatment had no effect on MVD when administered in an interventional setting (to established tumors), irrespective of VEGFR1–TK status in BMDCs (Fig. S1).
Fig. 4.
Pharmacologic blockade of CXCR4 consistently decreases tumor angiogenesis and overall myeloid BMDC recruitment only in BMT-VEGFR1TK−/− mice. (A and B) AMD3100 treatment (AMD) decreases tumor vascular density (A) and myeloid BMDC infiltration (B) in both TRAMP-C1 and E0771 models only in BMT-VEGFR1TK−/− (TK) mice but not in C57BL/6 mice (WT). CXCR4 or VEGFR1–TK inhibition alone decreases vascular density and myeloid BMDC number only in E0771 tumors. (C) AMD3100 treatment decreases Gr-1+ BMDC infiltration irrespective of VEGFR1–TK status. (D) Conversely, TAM infiltration is decreased only in mice with VEGFR1TK−/− BMDCs. *P < 0.05 compared with control PBS (WT); error bars represent mean ± SEM.
Similarly, AMD3100 treatment alone decreased the number of total myeloid BMDCs only in E0771 tumors, but not in TRAMP-C1 tumors, and decreased the number of myeloid BMDCs in both tumors when grown in BMT-VEGFR1TK−/− mice (Fig. 4B). However, AMD3100 treatment decreased Gr-1+ myeloid BMDCs in both tumors irrespective of VEGFR1–TK status in BMDCs (Fig. 4C). Consistent with data from the genetic deficiency model, CXCR4 inhibition by AMD3100 treatment did not affect the TAM infiltration in the tumors. However, when combined with VEGFR1–TK deficiency in BMDCs, CXCR4 blockade showed significantly decreased TAM infiltration in both tumor models (Fig. 4D). Finally, to confirm the specific role of CXCR4 in Gr-1+ BMDC recruitment during early tumor growth, we evaluated the infiltration of Gr-1+ BMDCs in small (4-mm) tumors growing after AMD3100 treatment in a preventive setting. CXCR4 blockade significantly decreased the number of Gr-1+ BMDCs but not TAMs (Fig. S2). Thus, a substantial reduction in primary tumor growth and metastasis in these models required the combination of CXCR4 inhibition with AMD3100 with inhibition of VEGFR1–TK activity in BMDCs to reduce Gr-1+ myeloid BMDC as well as TAM infiltration and to inhibit angiogenesis.
CXCR4 and VEGFR1 Independently Mediate Gr-1+ BMDC and TAM Infiltration via p38 Activation.
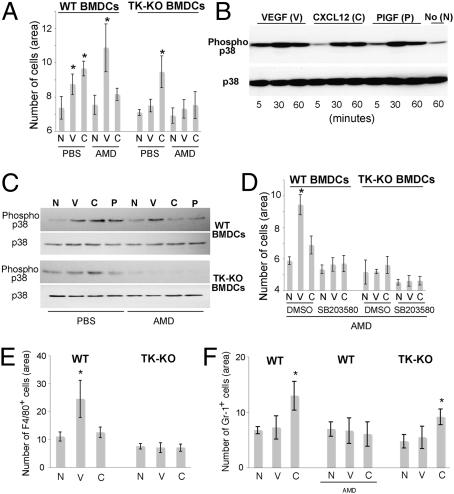
To reveal the mechanism of action of VEGFR1 and CXCR4 in myeloid BMDCs, we harvested bone marrow cells from C57BL/6 and VEGFR1TK−/− mice and purified mononuclear myeloid BMDCs using magnetic separation for CD11b+ cells. The migration of myeloid BMDCs in response to recombinant (r)VEGF (100 ng/mL) and rCXCL12 (100 ng/mL) was measured in a transwell assay with or without blockade of CXCR4 using 1 μg/mL of AMD3100. rVEGF and rCXCL12 significantly increased WT-myeloid BMDC migration, and the effect was reversed when using VEGFR1TK−/− myeloid BMDCs or AMD3100, respectively (Fig. 5A). Next we measured phosphorylated p38 MAPK—a key factor in cell migration—in myeloid BMDCs by Western blotting. rVEGF, rPlGF, and rCXCL12 rapidly increased p38 MAPK phosphorylation in myeloid BMDCs, with a peak level of activation after 30 min (Fig. 5B). Blockade of VEGFR1–TK (using VEGFR1TK−/− myeloid BMDCs) or CXCR4 (using AMD3100) selectively reversed the p38 MAPK phosphorylation induced in myeloid BMDC by rVEGF/rPlGF or by rCXCL12, respectively (Fig. 5C). Blockade of p38 MAPK using SB203580 (1 μM) significantly reduced the migration of myeloid BMDCs, irrespective of the chemotactic stimulus (Fig. 5D). To establish if CXCL12 and VEGF promote the migration of specific myeloid BMDC populations, we separated Gr-1+ and F4/80+ BMDCs and evaluated their migration in vitro. As expected, rVEGF increased the migration of F4/80+ BMDCs, and this effect was not seen when using VEGFR1TK−/− F4/80+ BMDCs (Fig. 5E). In contrast, rCXCL12 did not affect the migration of F4/80+ BMDCs, but significantly increased the migration of Gr-1+ BMDCs (Fig. 5 E and F). The effect of rCXCL12 was reversed by AMD3100, but not by the use of VEGFR1TK−/− Gr-1+ BMDCs (Fig. 5F).
Fig. 5.
Blocking CXCR4 and/or VEGFR1–TK activity significantly inhibits BMDC migration via inhibition of P38 MAPK activity. (A) Myeloid BMDC migration: VEGF and CXCL12 independently increase migration of CD11b+ BMDCs. (B and C) p38 MAPK activity: Exposure to recombinant VEGF, PlGF, or CXCL12 increased phospho-P38 MAPK in myeloid BMDCs (B); this increase was specifically abrogated by the inhibition of VEGFR1–TK (in TK-KO cells) or CXCR4 using AMD3100 (AMD), respectively (C). (D) Role of p38 MAPK in myeloid BMDC migration: The p38 MAPK inhibitor SB203580 decreased migration of myeloid BMDCs in response to either rVEGF or rCXCL12. (E and F) Migration of specific myeloid BMDC subsets: VEGF increased the migration of F4/80+ BMDCs (E) but not of Gr-1+ BMDCs (F), and this effect was abrogated by VEGFR1–TK inhibition (E). CXCL12 specifically increased the migration of Gr-1+ BMDCs, and this effect was reversed by CXCR4 but not by VEGFR1–TK inhibition. V, VEGF; P, PlGF, C, CXCL12; *P < 0.05 compared with control (N); error bars represent mean ± SEM.
Discussion
CXCR4 is a receptor specific for CXCL12 (also known as stromal-derived factor 1α) (11). Knockout of CXCR4 or CXCL12 is lethal due to the pleiotropic activity of this pathway, which is critical for hematopoietic, neural, vascular, and craniofacial organogenesis (12, 13). CXCR4 is constitutively expressed or induced by hypoxia in cancer cells and may mediate their migration and metastasis (14). However, CXCR4 is also a marker of myeloid BMDCs and is critical for BMDC retention in the bone marrow as well as for circulating BMDC infiltration in tissues (15–17). In brain tumors, hypoxia-inducible factor 1α (HIF-1α) activation—which can induce local expression of VEGF, PlGF, VEGFR1, and CXCL12—was shown to enhance the recruitment of multiple BMDC populations via the CXCL12/CXCR4 pathway (17). However, the effects of CXCR4 activity on angiogenesis may be independent of the VEGF pathway (18, 19). For example, in mice with brain tumors, treatment with a pan-VEGFR–TK inhibitor led to an increase in circulating CXCR4+ BMDCs and myeloid BMDC infiltration in the tumors growing in the face of treatment (20). Our goal was to elucidate the interplay between CXCR4 and VEGFR1 activity specifically in myeloid BMDC subsets and to determine its impact on tumor angiogenesis, growth, and metastasis in two highly metastatic tumor models.
Recent reports indicate that VEGF pathway inhibition may not prevent metastasis formation on the basis of studies of anti-VEGF agents in experimental models in mice as well as trials in cancer patients treated in neoadjuvant or adjuvant settings (9, 21–24). Moreover, although blockade of VEGFR1 activity decreases the number of TAMs in certain tumors, it does not completely suppress myeloid BMDC infiltration in growing primary tumors or lung metastases (9, 25, 26). This may be due, at least in part, to the inability of VEGF/VEGFR1 inhibition to prevent Gr-1+ myeloid cell infiltration into tumors (9, 10). Because many recent studies have converged to make the finding that the CXCL12/CXCR4 pathway is activated after VEGF inhibition (20, 27–32) (Table S1), we tested the hypothesis that CXCR4 activation leads to myeloid BMDC recruitment in the face of VEGFR1 inhibition in BMDCs.
To gain definitive genetic evidence that CXCR4 activity in BMDCs is required for VEGFR1–TK-independent BMDC recruitment in tumors and facilitation of tumor growth, we established an animal model for conditional CXCR4 deficiency with and without VEGFR1–TK deletion. Using TRAMP-C1 prostate carcinoma and E0771 mammary carcinoma models in C57BL/6 mice (33, 34), we demonstrate that CXCR4 activity in BMDC specifically mediates the tumor infiltration of Gr-1+ BMDCs during early tumor growth and metastasis formation. Moreover, we show that CXCR4-driven Gr-1+ BMDC infiltration is independent of the VEGFR1–TK activity blockade, which primarily mediates TAM infiltration in established tumors, and their interplay modulates primary tumor growth and angiogenesis. Although CXCR4 inhibition had a minor effect on primary tumor growth, it significantly reduced metastasis.
We further dissected the effect of CXCR4 versus VEGFR1 activity using a pharmacologic blockade of CXCR4 with AMD3100 alone versus VEGFR1–TK genetic inhibition. As in the genetic model, the effect of CXCR4 inhibition was specific reduction in Gr-1+ BMDC infiltration in the primary tumor, but the effects on tumor growth and metastasis were tumor-dependent. CXCR4 inhibition alone delayed primary tumor growth and metastasis in the TRAMP-C1 model. In the E0771 model, a significant delay in primary tumor growth and metastasis was seen only after concomitant inhibition of CXCR4 and VEGFR1 in BMDCs. Collectively, these data indicate that a CXCR4 blockade can reduce the infiltration of Gr-1+ BMDCs—independently of VEGFR1–TK activity in BMDCs—and delay early tumor growth and metastasis formation. This mechanism may potentially explain the association between increased CXCL12 concentration and poor outcome in cancer patients after anti-VEGF therapy (35).
In vitro studies revealed that VEGF and CXCL12 independently affect the migration of myeloid BMDC subsets. This effect was pathway-specific as (i) the CXCR4 blockade did not affect myeloid BMDC migration in response to rVEGF, and (ii) VEGFR1TK−/− myeloid BMDCs show increased migration in response to rCXCL12. To identify the mechanisms responsible for this “escape” from VEGFR1 inhibition in myeloid BMDCs, we evaluated the role of p38 MAPK in cell migration. We found that p38 MAPK phosphorylation is a common mediator of myeloid BMDC migration—downstream of both CXCR4 in Gr-1+ BMDCs and VEGFR1–TK in F4/80+ TAMs.
In conclusion, inhibition of CXCR4 in myeloid BMDCs can suppress tumor growth, angiogenesis, and metastasis. This relies in a significant manner on selectively decreasing Gr-1+ BMDC infiltration in primary tumors. Activation of CXCR4 is particularly critical for Gr-1+ BMDC infiltration during tumor growth, whereas VEGFR1–TK activity selectively modulates TAM infiltration in established tumors. Our results indicate that inhibition of both CXCR4 and VEGFR1–TK activity may be required for a meaningful decrease in tumor angiogenesis, myeloid BMDC infiltration, and metastasis (Fig. 6). In addition, we show that p38 MAPK activation is a critical mediator downstream of both CXCR4 and VEGFR1–TK activation in BMDCs, which supports the development of p38 MAPK inhibitors for cancer (see clinicaltrials.gov). These results may also have important implications for therapeutics that are widely used in the clinic and suggest that combining the CXCR4 blockade with drugs that inhibit VEGFR1–TK activity may be a valid strategy to induce greater tumor growth delay and prevent or inhibit metastasis.
Fig. 6.
Model of BMDC contribution to tumors during metastasis. On the basis of our data, we propose a model in which the recruitment of tumor-promoting myeloid BMDCs is sequential. First, small tumors primarily recruit Gr-1+ myeloid BMDCs for their early growth using CXCL12/CXCR4 pathway to activate p38 MAPK in these cells. Next, established tumors recruit primarily macrophages by VEGFR1–TK and p38 MAPK activation in these BMDCs. This cycle may repeat during micrometastasis growth into a metastatic nodule: Initially the micrometastases may recruit Gr1+ myeloid BMDCs and, when established in a metastatic nodule, may recruit macrophages that are resident in the adjacent lung or from blood circulation. (Drawing courtesy of Lance L. Munn, Massachusetts General Hospital, Boston).
Materials and Methods
Reagents.
We used human recombinant (r)VEGF (National Cancer Institute), mouse rCXCL12 and rPlGF (R&D Systems), AMD3100 (Sigma), Alzet osmotic pumps (DURECT), p38 MAPK and phospho-p38 MAPK antibodies (Cell Signaling), and a p38 inhibitor (Invitrogen) (SI Materials and Methods). TRAMP-C1 prostate cancer cells were purchased from ATCC. The E0771 breast cancer cell line was originally established by F. M. Sirotnak and kindly provided by E. Mihich (Roswell Park Memorial Institute) (34). Both cell lines are derived from tumors from C57BL/6 mice.
Animals.
All animal procedures were performed following the guidelines of the Public Health Service Policy on Humane Care of Laboratory Animals and approved by the Institutional Animal Care and Use Committee of the Massachusetts General Hospital. VEGFR1TK−/− mice were kindly provided by M. Shibuya at the University of Tokyo (36). To obtain double knockout mice, CXCR4+/− mice (The Jackson Laboratory; #004341, B6.129×-Cxcr4tm1Qma/J) were mated with VEGFR1TK−/− mice to generate CXCR4+/−VEGFR1TK−/− mice. Next, CXCR4+/−VEGFR1TK−/− mice were cross-bred with MxCre-CXCR4flox/flox mice (37) to generate MxCre-CXCR4flox/–VEGFR1TK−/− mice. C57BL/6 (#000664) and Actb-GFP [#003291, C57BL/6-Tg(CAG-EGFP)1Osb/J, constitutively expressing EGFP] mice were obtained from the The Jackson Laboratory. All mice were backcrossed to 99.9% C57BL/6 strain background (N10 equivalent). Strain background was verified by the The Jackson Laboratory Speed Congenic Development Service.
Bone Marrow Transplantation.
C57BL/6 mice (8 wk old) were lethally irradiated (137Cs Irradiator; Atomic Energy of Canada) using one 12-Gy fraction delivered to the whole body. Irradiated mice were rescued 24 h later by a bone marrow transplant isolated from Actb-GFP/C57BL/6. Eight weeks after BMT, we confirmed over 90–95% reconstruction from GFP-bone marrow cells using flow cytometry analyses. After we set up the irradiation condition for C57BL/6 mice, we carried out BMT using several genotyped mice. We used the following as bone marrow donors: WT C57BL/6 mice, VEGFR1TK−/− mice, MxCre-CXCR4flox/–VEGFR1TK−/− mice, MxCre-CXCR4flox/–VEGFR1TK+/+ mice, or MxCre-CXCR4flox/+VEGFR1TK+/+ in the control group. Cre expression (and hence CXCR4 deficiency in BMDCs) was induced by seven weekly i.p. injections of poly (I) poly (C) (250 μg per body; Invitrogen) in the BMT mice, as described previously (37). Tumor implantations were performed after 8 wk (1 wk after the last i.p. injection).
Tumor Implantation and Metastasis Assay.
TRAMP-C1 tumors were implanted s.c. in male C57BL/6 mice, and E0771 tumors were implanted s.c. in female C57BL/6 mice after BMT and recovery and/or after BMT and Mx-Cre recombination (n = 5–6 mice) (SI Materials and Methods). Primary tumors were resected when they reached a diameter of 10 mm (TRAMP-C1) or 13 mm (E0771), sizes that have been shown to reproducibly induce lung metastasis in pilot studies. Lung tissue was isolated from mice 2–4 wk after removal of the primary tumor.
Pharmacologic CXCR4 Inhibition Using AMD3100.
AMD3100 was delivered by s.c. Alzet pumps containing 10 mg/mL AMD3100 in PBS at a dose of 60 μg/day, and pumps with PBS only were used as a control. The pumps were replaced every 2 wk.
Immunohistochemistry and Western Blot Analysis.
Primary tumor tissues were fixed and frozen (SI Materials and Methods). Sections were stained with rat antibodies against mouse CD11b, MECA 32, Gr-1 (BD Pharmingen), and F4/80 (Serotec). Cy3- or FITC-conjugated secondary antibodies were used for the detection of signals by confocal microscopy. Slides were counterstained with DAPI for nuclear staining. The number of cells was quantified by measuring the area occupied by immunostained mononuclear cells normalized by the area of DAPI-stained nuclei (i.e., unitless). Phopho-p38 MAPK level was measured by Western blotting (SI Materials and Methods).
Isolation of Mononuclear Cells and CD11b+, F4/80+, and Gr-1+ BMDCs.
Bone marrow cells were collected in heparin-mixed PBS. The cellular filtrate was washed and a layer containing mononuclear BM cells was separated using Histopaque-1083 (Sigma). For the migration assay, BM cells were separated by CD11b, F4/80, or Gr-1 magnetic microbeads (MACS-beads; Miltenyi Biotech) from VEGFR1TK+/+ and VEGFR1TK−/− mice as per manufacturer's protocol. These cells were incubated with recombinant proteins for migration assays (see SI Materials and Methods).
Statistical Analysis.
All data are expressed as mean ± SEM. The Student t test was used for all analyses. We considered a p value of less than 0.05 to be statistically significant.
Supplementary Material
Acknowledgments
We thank P. Huang for transgenic mouse support, M. Ancukiewicz for statistical support, K. Cohen for useful discussions, and S. Kozin for assistance with whole-body irradiation studies. This work was supported by National Institutes of Health Grant R01-CA115767 (to R.K.J.) and in part by National Institutes of Health Grants P01-CA080124, R01-CA085140, and R01-CA126642 (to R.K.J.) and Federal Share/National Cancer Institute Proton Beam Program Income (to R.K.J. and D.G.D.); National Institutes of Health Grant R21-CA139168 and a Spiro Award (to D.G.D.); and National Institutes of Health Grant R01-CA096915 (to D.F.). R.K.J. is the recipient of Department of Defense Breast Cancer Research Innovator Award W81XWH-10-1-0016.
Footnotes
Conflict of interest: R.K.J. received commercial research grants from Dyax, AstraZeneca, and MedImmune; consultant fees from AstraZeneca/MedImmune, Dyax, Astellas-Fibrogen, Regeneron, Genzyme, Morphosys, and Noxxon Pharma; and a speaker honorarium from Genzyme. R.K.J. owns stock in SynDevRx. No reagents or funding from these companies was used in these studies. There is no significant financial or other competing interest in the work.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016917108/-/DCSupplemental.
References
- 1.Talmadge JE, Fidler IJ. AACR centennial series. The biology of cancer metastasis: Historical perspective. Cancer Res. 2010;70:5649–5669. doi: 10.1158/0008-5472.CAN-10-1040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McAllister SS, Weinberg RA. Tumor-host interactions: A far-reaching relationship. J Clin Oncol. 2010;28:4022–4028. doi: 10.1200/JCO.2010.28.4257. [DOI] [PubMed] [Google Scholar]
- 3.Mantovani A, Allavena P, Sica A, Balkwill F. Cancer-related inflammation. Nature. 2008;454:436–444. doi: 10.1038/nature07205. [DOI] [PubMed] [Google Scholar]
- 4.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8:211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Murdoch C, Muthana M, Coffelt SB, Lewis CE. The role of myeloid cells in the promotion of tumour angiogenesis. Nat Rev Cancer. 2008;8:618–631. doi: 10.1038/nrc2444. [DOI] [PubMed] [Google Scholar]
- 6.Yan L, Borregaard N, Kjeldsen L, Moses MA. The high molecular weight urinary matrix metalloproteinase (MMP) activity is a complex of gelatinase B/MMP-9 and neutrophil gelatinase-associated lipocalin (NGAL). Modulation of MMP-9 activity by NGAL. J Biol Chem. 2001;276:37258–37265. doi: 10.1074/jbc.M106089200. [DOI] [PubMed] [Google Scholar]
- 7.Fischer C, Mazzone M, Jonckx B, Carmeliet P. FLT1 and its ligands VEGFB and PlGF: Drug targets for anti-angiogenic therapy? Nat Rev Cancer. 2008;8:942–956. doi: 10.1038/nrc2524. [DOI] [PubMed] [Google Scholar]
- 8.Tchaikovski V, Fellbrich G, Waltenberger J. The molecular basis of VEGFR-1 signal transduction pathways in primary human monocytes. Arterioscler Thromb Vasc Biol. 2008;28:322–328. doi: 10.1161/ATVBAHA.107.158022. [DOI] [PubMed] [Google Scholar]
- 9.Dawson MR, Duda DG, Chae SS, Fukumura D, Jain RK. VEGFR1 activity modulates myeloid cell infiltration in growing lung metastases but is not required for spontaneous metastasis formation. PLoS ONE. 2009;4:e6525. doi: 10.1371/journal.pone.0006525. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Shojaei F, et al. Tumor refractoriness to anti-VEGF treatment is mediated by CD11b+Gr1+ myeloid cells. Nat Biotechnol. 2007;25:911–920. doi: 10.1038/nbt1323. [DOI] [PubMed] [Google Scholar]
- 11.Teicher BA, Fricker SP. CXCL12 (SDF-1)/CXCR4 pathway in cancer. Clin Cancer Res. 2010;16:2927–2931. doi: 10.1158/1078-0432.CCR-09-2329. [DOI] [PubMed] [Google Scholar]
- 12.Nagasawa T, et al. Defects of B-cell lymphopoiesis and bone-marrow myelopoiesis in mice lacking the CXC chemokine PBSF/SDF-1. Nature. 1996;382:635–638. doi: 10.1038/382635a0. [DOI] [PubMed] [Google Scholar]
- 13.Tachibana K, et al. The chemokine receptor CXCR4 is essential for vascularization of the gastrointestinal tract. Nature. 1998;393:591–594. doi: 10.1038/31261. [DOI] [PubMed] [Google Scholar]
- 14.Staller P, et al. Chemokine receptor CXCR4 downregulated by von Hippel-Lindau tumour suppressor pVHL. Nature. 2003;425:307–311. doi: 10.1038/nature01874. [DOI] [PubMed] [Google Scholar]
- 15.Grunewald M, et al. VEGF-induced adult neovascularization: Recruitment, retention, and role of accessory cells. Cell. 2006;124:175–189. doi: 10.1016/j.cell.2005.10.036. [DOI] [PubMed] [Google Scholar]
- 16.Jin DK, et al. Cytokine-mediated deployment of SDF-1 induces revascularization through recruitment of CXCR4+ hemangiocytes. Nat Med. 2006;12:557–567. doi: 10.1038/nm1400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Du R, et al. HIF1alpha induces the recruitment of bone marrow-derived vascular modulatory cells to regulate tumor angiogenesis and invasion. Cancer Cell. 2008;13:206–220. doi: 10.1016/j.ccr.2008.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kryczek I, et al. CXCL12 and vascular endothelial growth factor synergistically induce neoangiogenesis in human ovarian cancers. Cancer Res. 2005;65:465–472. [PubMed] [Google Scholar]
- 19.Kryczek I, Wei S, Keller E, Liu R, Zou W. Stroma-derived factor (SDF-1/CXCL12) and human tumor pathogenesis. Am J Physiol Cell Physiol. 2007;292:C987–C995. doi: 10.1152/ajpcell.00406.2006. [DOI] [PubMed] [Google Scholar]
- 20.Kamoun WS, et al. Edema control by cediranib, a vascular endothelial growth factor receptor-targeted kinase inhibitor, prolongs survival despite persistent brain tumor growth in mice. J Clin Oncol. 2009;27:2542–2552. doi: 10.1200/JCO.2008.19.9356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Padera TP, et al. Differential response of primary tumor versus lymphatic metastasis to VEGFR-2 and VEGFR-3 kinase inhibitors cediranib and vandetanib. Mol Cancer Ther. 2008;7:2272–2279. doi: 10.1158/1535-7163.MCT-08-0182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dawson MR, Duda DG, Fukumura D, Jain RK. VEGFR1-activity-independent metastasis formation. Nature. 2009;461:E4–E5; discussion E5. doi: 10.1038/nature08254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Allegra CJ, et al. Phase III trial assessing bevacizumab in stages II and III carcinoma of the colon: Results of NSABP protocol C-08. J Clin Oncol. 2010 doi: 10.1200/JCO.2010.30.0855. 10.1200/JCO.2010.30.0855. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Willett CG, et al. A safety and survival analysis of neoadjuvant bevacizumab with standard chemoradiation in a phase I/II study compared with standard chemoradiation in locally advanced rectal cancer. Oncologist. 2010;15:845–851. doi: 10.1634/theoncologist.2010-0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Casanovas O, Hicklin DJ, Bergers G, Hanahan D. Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell. 2005;8:299–309. doi: 10.1016/j.ccr.2005.09.005. [DOI] [PubMed] [Google Scholar]
- 26.Kerber M, et al. Flt-1 signaling in macrophages promotes glioma growth in vivo. Cancer Res. 2008;68:7342–7351. doi: 10.1158/0008-5472.CAN-07-6241. [DOI] [PubMed] [Google Scholar]
- 27.Batchelor TT, et al. AZD2171, a pan-VEGF receptor tyrosine kinase inhibitor, normalizes tumor vasculature and alleviates edema in glioblastoma patients. Cancer Cell. 2007;11:83–95. doi: 10.1016/j.ccr.2006.11.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ebos JM, Lee CR, Christensen JG, Mutsaers AJ, Kerbel RS. Multiple circulating proangiogenic factors induced by sunitinib malate are tumor-independent and correlate with antitumor efficacy. Proc Natl Acad Sci USA. 2007;104:17069–17074. doi: 10.1073/pnas.0708148104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Willett CG, et al. Efficacy, safety, and biomarkers of neoadjuvant bevacizumab, radiation therapy, and fluorouracil in rectal cancer: A multidisciplinary phase II study. J Clin Oncol. 2009;27:3020–3026. doi: 10.1200/JCO.2008.21.1771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhu AX, et al. Efficacy, safety, and potential biomarkers of sunitinib monotherapy in advanced hepatocellular carcinoma: A phase II study. J Clin Oncol. 2009;27:3027–3035. doi: 10.1200/JCO.2008.20.9908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Xu L, et al. Direct evidence that bevacizumab, an anti-VEGF antibody, up-regulates SDF1alpha, CXCR4, CXCL6, and neuropilin 1 in tumors from patients with rectal cancer. Cancer Res. 2009;69:7905–7910. doi: 10.1158/0008-5472.CAN-09-2099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Raut CP, et al. Measurement of interstitial fluid pressure (IFP) and circulating biomarkers in soft tissue sarcoma (STS): An exploratory phase II clinical and correlative study of sorafenib (SOR) in patients with refractory STS (NCI Protocol 6948) J Clin Oncol. 2010;28(Suppl; abstr 10091) [Google Scholar]
- 33.Somers KD, et al. Orthotopic treatment model of prostate cancer and metastasis in the immunocompetent mouse: Efficacy of flt3 ligand immunotherapy. Int J Cancer. 2003;107:773–780. doi: 10.1002/ijc.11464. [DOI] [PubMed] [Google Scholar]
- 34.Ewens A, Mihich E, Ehrke MJ. Distant metastasis from subcutaneously grown E0771 medullary breast adenocarcinoma. Anticancer Res. 2005;25(6B):3905–3915. [PubMed] [Google Scholar]
- 35.Jain RK, et al. Biomarkers of response and resistance to antiangiogenic therapy. Nat Rev Clin Oncol. 2009;6:327–338. doi: 10.1038/nrclinonc.2009.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Hiratsuka S, Minowa O, Kuno J, Noda T, Shibuya M. Flt-1 lacking the tyrosine kinase domain is sufficient for normal development and angiogenesis in mice. Proc Natl Acad Sci USA. 1998;95:9349–9354. doi: 10.1073/pnas.95.16.9349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Sugiyama T, Kohara H, Noda M, Nagasawa T. Maintenance of the hematopoietic stem cell pool by CXCL12-CXCR4 chemokine signaling in bone marrow stromal cell niches. Immunity. 2006;25:977–988. doi: 10.1016/j.immuni.2006.10.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.