Abstract
Upregulation of ADAM-12, a novel member of the multifunctional ADAM family of proteins is linked to cancer, arthritis and cardiac hypertrophy. Basal expression of ADAM-12 is very low in adult tissues but rises markedly in response to certain physiological cues, such as during pregnancy in the placenta, during development in neonatal skeletal muscle and bone and in regenerating muscle. Studies on ADAM-12 regulation have identified a highly conserved negative regulatory element (NRE) at the 5′-UTR of human ADAM-12 gene, which acts as a transcriptional repressor. The NRE contains a stretch of dinucleotide-repeat sequence that is able to adopt a Z-DNA conformation both in vitro and in vivo and interacts with hZαADAR1, a bona fide Z-DNA-binding protein. Substitution of the dinucleotide-repeat-element with a non-Z-DNA-forming sequence inhibited NRE function. We have detected a NRE DNA-binding protein activity in several tissues where ADAM-12 expression is low while no such activity was seen in the placenta where ADAM-12 expression is high. These observations suggest that interaction of these proteins with ADAM-12 NRE is critical for transcriptional repression of ADAM-12. We also show that the Z-DNA forming transcriptional repressor element, by interacting with these putative Z-DNA-binding proteins, is involved in the maintenance of constitutive low-level expression of human ADAM-12. Together these results provide a foundation for therapeutic down-regulation of ADAM-12 in cancer, arthritis and cardiac hypertrophy.
Keywords: gene regulation, repressor proteins, pathogenesis
ADAM-12 is a key member of a large (> 33 members) ADAM (a disintegrin and metalloprotease) family of proteins capable of performing a number of biological functions, including proteolysis, regulation of growth factor availability, cell–cell and cell–matrix adhesion, cell signaling, and ectodomain shedding (1). A multidomain structure composed of metalloproteinase, disintegrin, cysteine rich region, EGF-like and transmembrane domains, and a cytoplasmic tail allows the ADAM family of proteins to perform such various physiological tasks. ADAM-12 regulates growth factor bioavailability by cleaving and shedding heparin-binding EGF (HB-EGF) (2), insulin-like growth factor binding protein 3 (IGFBP-3), and IGFBP-5 from their membrane-anchored forms (3, 4). ADAM-12 cleaves various extracellular matrix proteins including gelatin, type IV collagen, and fibronectin and regulates cell–cell and cell–extracellular matrix contacts through interactions with cell surface receptors, integrins, and syndecans (5). ADAM-12 is implicated in myogenesis (6) and adipogenesis (7) through a linked developmental pathway (8). Although biological significance of ADAM-12 is yet to be fully understood, an increase in the level of ADAM-12 is detected during several pathological conditions, reviewed in ref. 9. In many tumor tissues, including breast, liver, gastric, brain, bone, and prostate cancer, ADAM-12 mRNA level is increased (10–15). ADAM-12 is being considered as a marker of breast cancer progression (5) and prostate tumor progression (15). Upregulation of ADAM-12 is also linked to osteoarthritis (16, 17) and cardiac hypertrophy (2).
Although normal expression of ADAM-12 in most adult tissues is very low (6, 7) during development and under some physiological conditions, its expression markedly rises. ADAM-12 level is high in placenta (7), neonatal skeletal muscle (18), regenerating muscle (19), and in neonatal bone (18). Although a link between ADAM-12 and various pathologies has been established, there is practically no information on the regulatory mechanisms controlling ADAM-12 expression during physiological and pathological conditions. In this study we report functional analysis of human ADAM-12 promoter and demonstrate the presence of a unique, highly conserved regulatory element within the 5′-UTR that acts as a transcriptional repressor. We have identified a stretch of dinucleotide-repeat sequences that acquires a left-handed Z-DNA conformation both in vitro and in vivo. We show a strong correlation between high level of DNA-binding protein activity to this Z-DNA and low-level expression of ADAM-12 and provide evidence that interaction of these proteins with the Z-DNA element in NRE is critical for transcriptional repression of ADAM-12.
Results
ADAM-12 mRNA Expression.
Among several tissues, placenta displayed markedly high level of ADAM-12 mRNA expression (Fig. 1). In contrast, ADAM-12 expression was very low, almost undetectable, in the lung, kidney, and liver tissues and could be detected only upon long exposure of the blot. These results correlated with previous observations that showed tissue-specific expression of ADAM-12 (6).
Fig. 1.
Expression of ADAM-12 in human tissues. Total RNA (50 μg) from each tissue was subjected to Northern blot analysis using an ADAM-12 cDNA probe. The upper panel shows a short exposure and the middle panel shows a longer exposure of the same blot. The blot was stripped and rehybridized with a β-actin cDNA probe.
A Transcriptional Repressor in the 5′-Untranslated Region Regulates ADAM-12 Expression.
The 5′-flanking region of human ADAM-12 promoter was cloned and sequenced (Fig. S1). The transcription start site of ADAM-12 in placenta-derived JEG-3 cells, which abundantly express ADAM-12, was determined by 5′-RACE analysis (Fig. S2). Human ADAM-12 promoter contains a TATA element, which is positioned at 24-nucleotide upstream of the major transcription start site (Fig. S1). One major and two minor transcription start sites were located in the JEG-3 cells (Fig. S2). In the low ADAM-12-expressing chondrocyte-derived HTB-94 cells a single start site, corresponding to the major start site in JEG-3 cells, was detected (Fig. S2).
Development- or tissue-specific expression of many eukaryotic genes most often is regulated by cis-acting elements located near the transcription start site. We reasoned that deletion analysis of the promoter would enable us identify cis-regulatory elements that interact with trans-acting factors to regulate expression of ADAM-12 under different physiological conditions. Several factors were considered during the design of ADAM-12 reporter constructs. First, because typically the elements regulating transcription of a gene lie close to the transcription start site, for initial studies, the promoter DNA spanning 1,600 nucleotides upstream of the transcription start site was chosen. Second, because the 5′-untranslated region of ADAM-12 mRNA is highly conserved between ADAM-12 orthologs (Fig. S3), we reasoned that this region could also contribute to transcriptional control. Two ADAM-12-CAT reporter constructs (Fig. 2A) were transiently transfected into HepG2 liver, BEAS-2B lung, SW-156 kidney, HTB-94 chondrocyte, and JEG-3 placenta cells. Interestingly, expression of -1,600/+350ADAM12-CAT reporter was markedly higher in the JEG-3 cells compared to other cells (Fig. 2B). Compared to the control vector, pBLCAT3, there was about 17-fold higher level expression in JEG-3 and about fourfold higher expression in HepG2, BEAS-2B, SW-156, and HTB-94 cells. Remarkably, deletion of most of the 5′-UTR in -1,600/+20ADAM12-CAT construct resulted in almost threefold increase of expression in HepG2, BEAS-2B, SW-156, and HTB-94 cells with modest additional induction in JEG-3 cells. These results suggested that the sequences between +20 and +350 in human ADAM-12 may contain a negative regulatory element (NRE), deletion of which results in significant increase of CAT activity in the low-ADAM-12 expressing cells.
Fig. 2.
Identification of a cis-acting negative regulatory element (NRE) in the 5′-UTR of human ADAM-12 gene. (A) Schematic representation of two ADAM-12-CAT constructs. (B) A comparison of CAT expression of two ADAM-12-CAT constructs transiently transfected (0.5 μg of DNA for each) into HepG2, BEAS-2B, SW-156, HTB-94, and JEG-3 cells. Relative CAT activity was determined by comparing the activities of transfected plasmids with that of pBLCAT3 (0.5 μg) and correcting for transfection efficiency (β-gal). The results represent an average of three independent experiments (P < 0.05).
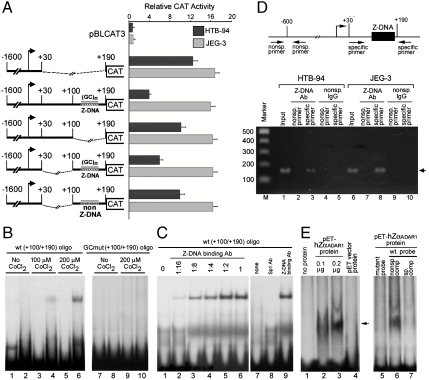
To delineate the boundaries of the NRE and identify specific sequences responsible for transcriptional repressor activity, in the context of large ADAM-12 promoter, additional 3′-end deletion constructs were prepared and transfected in HTB-94 cells in which NRE-mediated repression is active. Transcription from -1,600/+20ADAM12-CAT and -1600/+30ADAM12-CAT constructs was of similar level, but when sequences up to +70 at the 3′-end were included, the -1,600/+70ADAM12-CAT reporter exhibited some transcriptional repression (Fig. 3). These results suggested that the 5′-end boundary of the NRE is at +30. Likewise, the 3′-end boundary of the NRE was identified at +190. Together these results showed that a 160-nucleotide-long element located at the 5′-UTR, between +30 and +190 acts as a repressor of ADAM-12 expression in most cell types.
Fig. 3.
Analysis of segments of NRE that are important for repressor function. (A) Schematic representation of the NRE deletion mutants. (B) A comparison of CAT expression of various ADAM-12-CAT constructs (0.5 μg of DNA), transiently transfected into HTB-94 cells. Relative CAT activity was determined by following the method as described in Fig. 2. The results represent an average of three independent experiments (P < 0.05).
ADAM-12 Negative Regulatory Element Region Is Highly Conserved.
The NRE (+30/+190) is highly conserved in several ADAM-12 orthologs (Fig. 4A). Comparison of additional sequences, both upstream and downstream of the ADAM-12 NRE, has been provided in Fig. S3. To examine whether this conserved NRE can repress expression of a heterologous promoter, this sequence was placed downstream of the SV40 promoter, in both orientations, in the pSV2CAT reporter gene. Upon transfection in HTB-94 cells, the pSV2CAT and hybrid pSV2CAT reporters showed similar level of CAT activity, suggesting that the NRE has no gene silencing effect on a heterologous promoter (Fig. 4B). Presently, it remains to be determined whether the results of this experiment could be extended to other gene promoters as well to verify if ADAM-12 NRE has the ability to act as an efficient global transcriptional repressor or it acts in a more gene-specific manner.
Fig. 4.
Highly conserved ADAM-12 NRE represses transcription. (A) Nucleotide sequence alignment between the human ADAM-12 (GenBank accession no. NT030059.13), mouse ADAM-12 (GenBank accession no. NT039433.7 ), rat ADAM-12 (GenBank accession no. NW047544.2 ), and bovine ADAM-12 (GenBank accession no. AB164436) promoter demonstrates a high level of sequence homology (indicated by gray highlighting). The Z-DNA forming dinucleotide repeat region in human ADAM-12 sequence has a Z score (Z-DNA forming potential) of 30126431.65 (Z score minimum is 700), as determined with the ZHunt algorithm and is identified by double overline and by bold lettering. The dinucleotide repeat element is 15, 12, 12, and 25 copy-long in human, mouse, rat, and bovine ADAM-12 gene, respectively. (B) Schematic map of ADAM-12 NRE constructs and promoter function analysis. The pSV2NRECAT and pSV2revNRECAT constructs contain the NRE fragment (+30 to +190) cloned in both orientations, downstream of the SV40 promoter in the pSV2CAT vector. The reporter plasmid DNAs were transfected into HTB-94 cells by following the method as described in Fig. 2. The results represent an average of three independent experiments (P < 0.05). (C) Expression of CAT mRNA in HTB-94 cells transfected with equal DNA (2.0 μg) of constructs pSV2CAT (lane 1), -1,600/+350ADAM-12CAT (lane 2) and -1,600/+30ADAM-12CAT (lane 3), respectively. Total RNA (50 μg) prepared from transfected cells was hybridized with a CAT cDNA probe. The same blot was stripped and rehybridized with a β-actin cDNA probe.
Because the NRE is present at the 5′-untranslated region of ADAM-12 mRNA, the possibility of its involvement in regulating translation cannot be ruled out. We therefore compared the level of CAT reporter gene mRNA in cells transfected with -1,600/+350ADAM12-CAT and -1,600/+30ADAM12-CAT plasmids (Fig. 4C). This assay showed that deletion of the NRE results in about threefold higher level (as determined by densitometric analysis) of CAT mRNA. In the same cells, the level of β-actin mRNA, an internal control, remained unchanged. The pSV2CAT plasmid transfected cells were used as a positive control for CAT mRNA hybridization. Together these results suggested that ADAM-12 NRE functions by repressing transcription.
Negative Regulatory Element of ADAM-12 Contains a Z-DNA Forming Sequence.
To further characterize NRE, several internal-deletion constructs were prepared. In JEG-3 cells, all of these reporters were similarly active (Fig. 5A). However, these same reporters functioned differently in HTB-94 cells because deletion of sequences from +30 to +99 or from +100 to +190 compromised the NRE activity of the wild-type construct with complete sequences up to +190 (Fig. 5A). Comparison of the data revealed that the repressor function of +100/+190 region is more than that of +30 to +99 region. This data suggested that the NRE is possibly composed of two subunits, and because none of the subunits displayed complete NRE function, we reasoned that both subunits need to function in cooperation for complete transcriptional repression. In the +100/+190 element, a stretch of dinucleotide repeats, consisting mostly of CGs and some CAs, flanked by CGG and GGG sequences are found. Long stretches of dinucleotide repeats (n≥12), especially CG repeats, are capable of forming higher energy left-handed Z-DNA (20). By using ZHunt algorithm, the Z score (Z-DNA-forming potential) of this region was determined to be 30126431.65 (Z score minimum is 700) (21). Substitution of the dinucleotide repeat sequences with a non-Z-DNA forming sequence totally blocked NRE function of the reporter gene in HTB-94 cells but showed no effect in JEG-3 cells (Fig. 5A). All internal deletion reporters, when transfected in HepG2, SW-156, and BEAS-2B cells, showed similar types of results as those obtained from the transfection in HTB-94 cells. We verified the Z-DNA forming ability of the dinucleotide repeat sequences by using a monoclonal antibody, Z22 that specifically recognizes a Z-DNA structure (22, 23). The Z22 antibody interacted with the wild-type NRE ( +100/+190) only in the presence of hexamine CoCl2 which is known to stabilize the Z-DNA structure in a dose-dependent manner (Fig. 5B, lanes 1–6). There was no further improvement of interaction of the probe at higher than 200 μM hexamine CoCl2. No interaction with the mutant NRE, GCmut ( +100/+190) was seen (Fig. 5B, lanes 7–10). Further, the wild-type NRE (+100/+190) interacted with the Z22 antibody in a dose-dependent manner (Fig. 5C, lanes 2–6) but did not interact with Sp1 antibody that was used as a control (Fig. 5C, lane 8). Together, these results suggested that a Z-DNA forming element holds a critical role in regulating NRE-mediated ADAM-12 transcription.
Fig. 5.
The NRE of the human ADAM-12 promoter has a Z-DNA forming propensity. (A) Various ADAM-12-CAT constructs (0.5 μg of DNA) were transiently transfected into HTB-94 and JEG-3 cells. Relative CAT activity was determined by comparing the activities of transfected plasmids with that of pBLCAT3 (0.5 μg) and correcting for transfection efficiency (β-gal). The results represent an average of three independent experiments (P < 0.05). (B) Z-DNA-binding assay. A radiolabeled wild-type ADAM-12 DNA containing sequences from +100 to +190 [WT(+100/+190)] was first treated with various concentrations of hexamine CoCl2 and incubated without (lanes 1, 3, and 5) and with 1.0 μl of undiluted anti-Z-DNA Z22 antibody (lanes 2, 4, and 6), as indicated. A radiolabeled mutant ADAM-12 DNA containing sequences from 100 to +190, GCmut (+100/+190), with mutated nucleotide sequence in the GC-rich domain of ADAM-12 DNA as described in Materials and Methods was treated without or with 200 μM concentration of hexamine CoCl2 and incubated without (7 and 9) and with 1.0 μl of undiluted anti-Z-DNA Z22 antibody (lanes 8 and 10), as indicated. (C) Radiolabeled wild-type (+100/+190) probe as used in B was first treated with 200 μM hexamine CoCl2 and incubated without any protein (lanes 1 and 7), with 1.0 μl of various dilutions of the anti-Z-DNA Z22 antibody (lanes 2–5) or with1.0 μl of undiluted Z22 antibody (lanes 6 and 9), as indicated. In lane 8, 1.0 μl of undiluted Sp1 antibody was added. (D) Semiquantitative ChIP assay. Formaldehyde cross-linked HTB-94 and JEG-3 cells were immunoprecipitated with anti-Z-DNA Z22 antibody or nonspecific IgG. Immunoprecipitated DNA was used for PCR amplification of ADAM-12 promoter using primers encompassing the Z-DNA-binding NRE segment (denoted as specific primer) or an upstream region that does form a Z-DNA structure (denoted as nonspecific primer). The arrow indicates specific PCR amplified product. (E) The radiolabeled wild-type (+100/+190) probe, first treated with 200 μM hexamine CoCl2, was incubated without any protein (lanes 1 and 5), with increasing concentrations of purified pET-hZαADAR1 protein (lanes 2 and 3) or with 0.2 μg of purified protein isolated from pET vector transfected cells (lane 4), as indicated. The arrow denotes DNA–protein complex formation with pETZα protein. Some DNA-binding reactions with 0.2 μg of pET-hZαADAR1 protein contained molar excess of nonspecific oligonucleotide (lane 6) and specific oligonucleotide (lane 7) as competitor.
ChIP analysis with Z22 antibody indicated that indeed the NRE (+100/+190) can adopt a Z-DNA conformation, in vivo (Fig. 5D). Both HTB-94 and JEG-3 cells exhibited quite similar level of Z-DNA forming activity. It is worthy of mention that a quantitative analysis of in vivo Z-DNA formation is not possible with semiquantitative ChIP analysis. To determine whether the Z-DNA-forming sequences of the NRE can interact with a bona fide Z-DNA-binding protein, we used recombinant hZαADAR1 protein. The hZαADAR1 protein contains the Z-DNA-binding domain (133–209 amino acids) of the human RNA editing enzyme ADAR1 protein and is capable of interacting with a DNA sequence that adopts Z-DNA conformation (24). The pET-hZαADAR1 protein avidly interacted in a dose-dependent and specific manner with the wild-type ADAM-12 NRE (Fig. 5E, lanes 1–7).
Identification of Z-DNA-Binding Proteins in Low-ADAM-12 Expressing Tissues.
To identify the proteins interacting with Z-DNA in ADAM-12 NRE, nuclear extracts from different tissues were incubated with a radiolabeled wild-type NRE probe. Four DNA-protein complexes (complexes I–IV) were formed with lung, kidney, and liver tissue nuclear extracts while almost no complex was visible with equal protein amount of placenta tissue nuclear extract (Fig. 6A, lanes 1–15). A mutant (GCmut +100/+190) probe with a non Z-DNA-forming sequence showed no DNA-protein interaction (Fig. 6B). All four DNA-protein complexes were efficiently competed out by molar excess of homologous oligonucleotide but not by an unrelated DNA (Fig. 6C). Although these initial experiments are suggestive, further experiments will be necessary to determine whether these are Z-DNA-binding proteins.
Fig. 6.
Electrophoretic mobility shift assay provides a correlation between NRE-binding proteins and transcriptional repression of ADAM-12. (A) A radiolabeled ADAM-12 DNA (+100/+190) was incubated with equal protein amount of nuclear extracts (10 μg) from different tissues, as indicated. Lane 1 contains no protein. The DNA–protein complexes are identified as complexes I–IV. (B) A radiolabeled probe containing mutant Z-DNA element, as described in Materials and Methods was incubated with equal protein amount of nuclear extracts (10 μg) from different tissues, as indicated. Lane 1 contains no protein. (C) Lung tissue nuclear extract was incubated with a radiolabeled probe as used in A. In addition, a 100-fold molar excess of homologous oligonucleotide (lane 2) or nonspecific oligonucleotide (lane 3) was included.
Discussion
Expression of ADAM-12 gene is low in adult tissues, but under some physiological and developmental conditions as well as under several pathological conditions, this gene is expressed at a markedly higher level. Until now mechanisms that control ADAM-12 gene expression during physiological or pathological conditions remained elusive. The present study identifies a mechanism that delineates regulation of ADAM-12 during normal physiological condition. A highly conserved transcriptional repressor element with the ability to form Z-DNA structure is found to be essential in regulating basal ADAM-12 gene expression.
Because regulated transcription plays a significant role in the control of basal mRNA level of ADAM-12, we searched for cis-acting elements that mediate this regulation. Characterization of human ADAM-12 revealed a highly conserved negative repressor element (NRE) at the 5′-untranslated region (Fig. 4A), whose primary mode of action is repression of transcription of ADAM-12 (Fig. 4C). Interestingly, NRE-mediated regulation of ADAM-12 expression is found to be similar in four different low-ADAM-12 expressing cell lines that were examined (Fig. 2).
We have determined that a Z-DNA forming dinucleotide repeat element acts as the principal constituent of the ADAM-12 NRE. Z-DNA is a left-handed conformation that can be formed by double-stranded DNA and RNA containing a small segment of alternating purine/pyrimidine dinucleotide repeats (n≥12 units) (20). Z-DNA represents a higher energy state with a short half life unless it is stabilized by factors such as negative (−) DNA supercoiling, chemical modification, and proteins that can interact with Z-DNA (25–29). Expression of rat nucleolin gene (30), voltage-gated potassium channel Kv1.5 gene (31), human colony-stimulating factor 1(CSF-1) gene (32), hsp26 gene of Drosophila melanogaster (33), and macrophage immune response gene SLC11A1 (34) has been shown to be regulated by Z-DNA forming cis-acting elements. Z-DNA forming sequences have been shown to promote gene expression by acting as an enhancer of transcription (32, 35) while in some cases they are shown to suppress gene expression (30, 31, 36). A recent study indicates that genetic instability and rapid evolution of human centromeres might, in part, be driven by Z-DNA segments (37).
For the exercise of Z-DNA mediated regulatory function, the Z-DNA-binding proteins (ZBP) play an equally important role. These proteins contain one or more domains that can interact with a Z-DNA structure and thereby specifically stabilize and induce Z-DNA and Z-RNA conformation. At present only a handful of such proteins have been identified, and these include the RNA editing enzyme ADAR1 (38), DNA-dependent activator of IFN-regulatory factor (DAI/ZBP1/DLM-1) (27, 39, 40), vaccinia virus Z-DNA-binding protein E3L (28, 41), and zebrafish PKR-like protein kinase (42). Some of the Z-DNA-binding proteins are inducible in nature and have been shown to play a critical role in the host response against tumors and in the activation of innate immune response (39, 40, 43). We show that the tissues that express a low level of ADAM-12 contain a high level of ADAM-12 Z-DNA-interacting protein activity (Fig. 6). Identity of these proteins as Z-DNA-binding proteins is yet to be determined. Interestingly, however, no such putative Z-DNA-binding protein activity was detected in high ADAM-12 expressing placenta tissue nuclear extract. These results suggested that a major function of the putative Z-DNA-binding proteins in low-expressing tissues could be to stabilize and maintain the Z-DNA structure of the NRE of ADAM-12. In this light, it is likely that ADAM-12 NRE and the NRE-binding proteins together might be functioning physiologically as a constitutive repressor, reducing basal transcription levels in tissues, and also under conditions when ADAM-12 expression is undesirable. The lack of a detectable level of Z-DNA-binding protein activity in the placenta could be a result of either low concentration of the candidate proteins or paucity of necessary posttranslational modifications or both. Presently, tissue-specific Z-DNA-binding proteins are unknown while inducible Z-DNA-binding proteins, including ADAR1, zebrafish PKR-like kinase, and DAI/DLM/ZBP1 have been reported. EMSA indicated that ADAM-12 NRE-binding proteins are indistinguishable in lung, kidney, and liver tissues, because similar migrating four DNA–protein complexes appeared consistently (Fig. 6). It is not presently clear whether different Z-DNA-binding proteins are involved in the formation of these DNA–protein complexes as a homodimeric complex of the same protein or a heterodimeric complex with ancillary proteins or if all of the above can give rise to multiple DNA–protein complexes. Alternatively, multiple DNA–protein complexes could arise from interaction of the full-length or small fragments containing the Z-DNA-binding domains of the same Z-DNA-binding protein.
In conclusion, our study provides insights into human ADAM-12 gene expression and documents the existence of a unique regulatory mechanism that controls its basal expression via a Z-DNA forming repressor element present at the 5′-UTR. Because ADAM-12 is synthesized at a high level in several cancers (5, 10–15), it is tempting to speculate that alteration of Z-DNA-mediated transcriptional regulation of ADAM-12 might be responsible, at least in part, for such an increase. Further understanding of the molecular basis of ADAM-12 regulation will have functional implications for suppressing ADAM-12 linked pathologies and ultimately lead to design of new therapeutics.
Materials and Methods
Cell Lines, Tissues, and Transfection Analysis.
Human lung, kidney, liver, and placenta tissues were obtained from Integrated Laboratory Services-Biotech (ILSbio). Human HepG2 liver, BEAS-2B lung, SW-156 kidney, HTB-94 chondrocyte, and JEG-3 placenta cells (ATCC) were transfected by adding reporter plasmid DNA together with pSVβ-gal (Promega Corporation) plasmid DNA as described (44).
RNA Isolation and Northern Blot Analysis.
Total RNA was isolated by using guanidinium thiocyanate method (45). Fifty mg of total RNA was used for Northern blot analysis and hybridized to ADAM-12 cDNA probe or chloramphenicol acetyltransferase (CAT) cDNA probe, as indicated.
Preparation of Progressive Deletion and Mutant Promoter Constructs.
The 5′-flanking region of human ADAM-12 was generated by PCR amplification of human genomic DNA using the primers, forward, 5′-CCACCAGGTCCCTCCCACAACACATG-3′, and reverse, 5′-TAGTTCGGCGACTTA GGTCGGGC-3′. The amplified DNA was cloned into pBLCAT3 plasmid vector (46) to construct -1,600/+350ADAM-12 CAT reporter. Other ADAM-12 deletion constructs were generated by PCR amplification using different primers at the indicated sites. The pSV2NRECAT and pSV2revNRECAT constructs were generated by ligation of ADAM-12 (+30/+190) sequences, in both orientations, in the pSV2CAT vector. The sequence of non Z-DNA forming oligonucleotide used to replace the Z-DNA forming element from position +124 to +159 was: 5′- GCATGCATTCAGGAACCATCGAACTTAGTCAATCGG-3′.
Nuclear Extract Preparation and Electrophoretic Mobility Shift Assay.
Nuclear extract from different tissues was prepared as described earlier (47). Equal protein amounts of nuclear extracts were incubated with a radiolabeled double-stranded ADAM-12 DNA fragment (+100/+190). To determine Z-DNA forming propensity, a radiolabeled probe was incubated for 3 h at 37°C in 10 μl of EMSA buffer and various concentrations of hexamine CoCl2 to induce Z-DNA formation. Aliquots (1 pmol) were incubated with undiluted or various dilutions of monoclonal anti-Z22 antibody (a gift from B. David Stollar, Tufts University) in the EMSA buffer for 30 min at room temperature. In some assays, 1.0 μl of Sp1 antibody (Santa Cruz Biotechnology) or purified Z-DNA-binding protein (pET28a-hZαADAR1, a gift from Alexander Rich and Ky Lowenhaupt, Massachusetts Institute of Technology) were used. Sequence of the mutant ADAM-12 Z-DNA probe, GCmut (+100/+190), containing sequences from +100 to +190 with underlined region representing mutated bases was 5′- GGTCAAGGCTGGCTTGTGCCAGAAGCATGCATTCAGGAACCATCGAACTTAGTCAATCGGGGAAACTTTTTTAAAAATGAAAGGCTAGACG-3′.
Chromatin Immunoprecipitation (ChIP) Assay.
Formaldehyde crosslinked (1% final concentration) HTB-94 and JEG-3 cells were processed using a ChIP assay kit (Upstate Biotechnology) and following manufacturer’s protocol. The lysed cell solutions were precleared with salmon sperm DNA/protein G agarose slurry, incubated with Z22 antibody or control IgG (Santa Cruz Biotechnology) at 4 °C. DNA in the immune complexes were amplified by PCR. Primers used for amplification of the Z-DNA-binding region were: sense, 5′-CAACTCGGACAGTTTGCTCA-3′, and antisense, 5′-AGCTCTTCTAGCCTTTCATTTTT-3′; the size of the amplified product corresponded to 126 bp. As control, an upstream region of ADAM-12 promoter that cannot form Z-DNA structure (deduced by Zhunt algorithm) was amplified using following primers: sense, 5′-GAGGGCGCCCGGGAATCTTTTT-3′, and antisense, 5′-CTCGGAGTGCGGGTCAGGTCT-3′. Size of the amplified product correspond to 292 bp.
Supplementary Material
Acknowledgments.
We are grateful to B. David Stollar, Tufts University, Boston, for anti-Z22 antibody and Alexander Rich and Ky Lowenhaupt, Massachusetts Institute of Technology, Cambridge, MA, for a generous gift of pET28a-hZαADAR1 plasmid. We also thank Drs. Rich and Lowenhaupt for critical reading of the manuscript. This work was supported by grants from the U.S. Army Medical Research and Material Command, University of Missouri Research Board, and University of Missouri College of Veterinary Medicine.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1008831108/-/DCSupplemental.
References
- 1.White JM. ADAMs: Modulators of cell–cell and cell–matrix interactions. Curr Opin Cell Biol. 2003;15:598–606. doi: 10.1016/j.ceb.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 2.Asakura M, et al. Cardiac hypertrophy is inhibited by antagonism of ADAM12 processing of HB-EGF: Metalloproteinase inhibitors as a new therapy. Nat Med. 2002;8:35–40. doi: 10.1038/nm0102-35. [DOI] [PubMed] [Google Scholar]
- 3.Shi Z, Xu W, Loechel F, Wewer UM, Murphy LJ. ADAM 12, a disintegrin metalloprotease, interacts with insulin-like growth factor-binding protein-3. J Biol Chem. 2000;275:18574–18580. doi: 10.1074/jbc.M002172200. [DOI] [PubMed] [Google Scholar]
- 4.Loechel F, Fox JW, Murphy G, Albrechtsen R, Wewer UM. ADAM 12-S cleaves IGFBP-3 and IGFBP-5 and is inhibited by TIMP-3. . Biochem Biophys Res Commun. 2000;278:511–515. doi: 10.1006/bbrc.2000.3835. and erratum (2001) 280:421. [DOI] [PubMed] [Google Scholar]
- 5.Roy R, Wewer UM, Zurakowski D, Pories SE, Moses MA. ADAM 12 cleaves extracellular matrix proteins and correlates with cancer status and stage. J Biol Chem. 2004;279:51323–51330. doi: 10.1074/jbc.M409565200. [DOI] [PubMed] [Google Scholar]
- 6.Gilpin BJ, et al. A novel, secreted form of human ADAM 12 (meltrin alpha) provokes myogenesis in vivo. J Biol Chem. 1998;273:157–166. doi: 10.1074/jbc.273.1.157. [DOI] [PubMed] [Google Scholar]
- 7.Kawaguchi N, et al. ADAM 12 protease induces adipogenesis in transgenic mice. Am J Pathol. 2002;160:1895–1903. doi: 10.1016/S0002-9440(10)61136-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kawaguchi N, et al. ADAM 12 induces actin cytoskeleton and extracellular matrix reorganization during early adipocyte differentiation by regulating beta1 integrin fusion. J Cell Sci. 2003;116:3893–3904. doi: 10.1242/jcs.00699. [DOI] [PubMed] [Google Scholar]
- 9.Kveiborg M, Albrechtsen R, Couchman JR, Wewer UM. Cellular roles of ADAM12 in health and disease. Int J Biochem Cell Biol. 2008;40:1685–1702. doi: 10.1016/j.biocel.2008.01.025. [DOI] [PubMed] [Google Scholar]
- 10.Lendeckel U, et al. Increased expression of ADAM family members in human breast cancer and breast cancer cell lines. J Cancer Res Clin. 2005;131:41–48. doi: 10.1007/s00432-004-0619-y. [DOI] [PubMed] [Google Scholar]
- 11.Le Pabic H, et al. ADAM12 in human liver cancers: TGF-beta-regulated expression in stellate cells is associated with matrix remodeling. Hepatology. 2003;37:1056–1066. doi: 10.1053/jhep.2003.50205. [DOI] [PubMed] [Google Scholar]
- 12.Carl-McGrath S, Lendeckel U, Ebert M, Roessner A, Rocken C. The disintegrin-metalloproteinases ADAM9, ADAM12, and ADAM15 are upregulated in gastric cancer. Int J Oncol. 2005;26:17–24. [PubMed] [Google Scholar]
- 13.Kodama T, et al. ADAM12 is selectively overexpressed in human glioblastomas and is associated with glioblastoma cell proliferation and shedding of heparin-binding epidermal growth factor. Am J Pathol. 2004;165:1743–1753. doi: 10.1016/S0002-9440(10)63429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tian BL, et al. The expression of ADAM12 (meltrin alpha) in human giant cell tumours of bone. Mol Path. 2002;55:394–397. doi: 10.1136/mp.55.6.394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Peduto L, et al. ADAM12 is highly expressed in carcinoma-associated stroma and is required for mouse prostate tumor progression. Oncogene. 2006;25:5462–5466. doi: 10.1038/sj.onc.1209536. [DOI] [PubMed] [Google Scholar]
- 16.Valdes AM, et al. Reproducible genetic associations between candidate genes and clinical knee osteoarthritis in men and women. Arthritis Rheum. 2006;54:533–539. doi: 10.1002/art.21621. [DOI] [PubMed] [Google Scholar]
- 17.Okada A, et al. ADAM-12 (meltrin alpha) is involved in chondrocyte proliferation via cleavage of insulin-like growth factor binding protein 5 in osteoarthritic cartilage. Arthritis Rheum. 2008;58:778–789. doi: 10.1002/art.23262. [DOI] [PubMed] [Google Scholar]
- 18.Yagami-Hiromasa T, et al. A metalloprotease-disintegrin participating in myoblast fusion. Nature. 1995;377:652–656. doi: 10.1038/377652a0. [DOI] [PubMed] [Google Scholar]
- 19.Galliano MF, et al. Binding of ADAM12, a marker of skeletal muscle regeneration, to the muscle-specific actin-binding protein, alpha -actinin-2, is required for myoblast fusion. J Biol Chem. 2000;275:13933–13939. doi: 10.1074/jbc.275.18.13933. [DOI] [PubMed] [Google Scholar]
- 20.Rich A, Zhang S. Timeline: Z-DNA: The long road to biological function. Nat Rev Genet. 2003;4:566–572. doi: 10.1038/nrg1115. [DOI] [PubMed] [Google Scholar]
- 21.Ho PS, Ellison MJ, Quigley GJ, Rich A. A computer aided thermodynamic approach for predicting the formation of Z-DNA in naturally occurring sequences. EMBO J. 1986;5:2737–2744. doi: 10.1002/j.1460-2075.1986.tb04558.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Brigido MM, Stollar BD. Two induced anti-Z-DNA monoclonal antibodies use VH gene segments related to those of anti-DNA autoantibodies. J Immunol. 1991;146:2005–2009. [PubMed] [Google Scholar]
- 23.Lafer EM, Moller A, Nordheim A, Stollar BD, Rich A. Antibodies specific for left-handed Z-DNA. Proc Natl Acad Sci USA. 1981;78:3546–3550. doi: 10.1073/pnas.78.6.3546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oh DB, Kim YG, Rich A. Z-DNA-binding proteins can act as potent effectors of gene expression in vivo. Proc Natl Acad Sci USA. 2002;99:16666–16671. doi: 10.1073/pnas.262672699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rich A, Nordheim A, Wang AH. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz T, Rould MA, Lowenhaupt K, Herbert A, Rich A. Crystal structure of the Zalpha domain of the human editing enzyme ADAR1 bound to left-handed Z-DNA. Science. 1999;284:1841–1845. doi: 10.1126/science.284.5421.1841. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz T, Behlke J, Lowenhaupt K, Heinemann U, and Rich A. Structure of the DLM-1-Z-DNA complex reveals a conserved family of Z-DNA-binding proteins. Nat Struct Biol. 2001;8:761–765. doi: 10.1038/nsb0901-761. [DOI] [PubMed] [Google Scholar]
- 28.Kim YG, et al. A role for Z-DNA binding in vaccinia virus pathogenesis. Proc Natl Acad Sci USA. 2003;100:6974–6979. doi: 10.1073/pnas.0431131100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Droge P. Protein tracking-induced supercoiling of DNA: a tool to regulate DNA transactions in vivo? Bioessays. 1994;16:91–99. doi: 10.1002/bies.950160205. [DOI] [PubMed] [Google Scholar]
- 30.Rothenburg S, Koch-Nolte F, Rich A, Haag F. A polymorphic dinucleotide repeat in the rat nucleolin gene forms Z-DNA and inhibits promoter activity. Proc Natl Acad Sci USA. 2001;98:8985–8990. doi: 10.1073/pnas.121176998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mori Y, Folco E, Koren G. GH3 cell-specific expression of Kv1.5 gene. Regulation by a silencer containing a dinucleotide repetitive element. J Biol Chem. 1995;270:27788–27796. doi: 10.1074/jbc.270.46.27788. [DOI] [PubMed] [Google Scholar]
- 32.Liu R, et al. Regulation of CSF1 promoter by the SWI/SNF-like BAF complex. Cell. 2001;106:309–318. doi: 10.1016/s0092-8674(01)00446-9. [DOI] [PubMed] [Google Scholar]
- 33.Lu Q, Wallrath LL, Granok H, Elgin SC. (CT)n (GA)n repeats and heat shock elements have distinct roles in chromatin structure and transcriptional activation of the Drosophila hsp26 gene. Mol Cell Biol. 1993;13:2802–2814. doi: 10.1128/mcb.13.5.2802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Bayele HK, et al. HIF-1 regulates heritable variation and allele expression phenotypes of the macrophage immune response gene SLC11A1 from a Z-DNA forming microsatellite. Blood. 2007;110:3039–3048. doi: 10.1182/blood-2006-12-063289. [DOI] [PubMed] [Google Scholar]
- 35.Nordheim A, Rich A. Negatively supercoiled simian virus 40 DNA contains Z-DNA segments within transcriptional enhancer sequences. Nature. 1983;303:674–679. doi: 10.1038/303674a0. [DOI] [PubMed] [Google Scholar]
- 36.Tae HJ, Luo X, Kim KH. Roles of CCAAT/enhancer-binding protein and its binding site on repression and derepression of acetyl-CoA carboxylase gene. J Biol Chem. 1994;269:10475–10484. [PubMed] [Google Scholar]
- 37.Li H, et al. Human genomic Z-DNA segments probed by the Z alpha domain of ADAR1. Nucleic Acids Res. 2009;37:2737–2746. doi: 10.1093/nar/gkp124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Herbert A, et al. A Z-DNA binding domain present in the human editing enzyme, double-stranded RNA adenosine deaminase. Proc Natl Acad Sci USA. 1997;94:8421–8426. doi: 10.1073/pnas.94.16.8421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Takaoka A, et al. DAI (DLM-1/ZBP1) is a cytosolic DNA sensor and an activator of innate immune response. Nature. 2007;448:501–505. doi: 10.1038/nature06013. [DOI] [PubMed] [Google Scholar]
- 40.Fu Y, et al. Cloning of DLM-1, a novel gene that is up-regulated in activated macrophages, using RNA differential display. Gene. 1999;240:157–163. doi: 10.1016/s0378-1119(99)00419-9. [DOI] [PubMed] [Google Scholar]
- 41.Kwon JA, Rich A. Biological function of the vaccinia virus Z-DNA-binding protein E3L: gene transactivation and antiapoptotic activity in HeLa cells. Proc Natl Acad Sci USA. 2005;102:12759–12764. doi: 10.1073/pnas.0506011102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rothenburg S, et al. A PKR-like eukaryotic initiation factor 2alpha kinase from zebrafish contains Z-DNA binding domains instead of dsRNA binding domains. Proc Natl Acad Sci USA. 2005;102:1602–1607. doi: 10.1073/pnas.0408714102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.George CX, Samuel CE. Human RNA-specific adenosine deaminase ADAR1 transcripts possess alternative exon 1 structures that initiate from different promoters, one constitutively active and the other interferon inducible. Proc Natl Acad Sci USA. 1999;96:4621–4626. doi: 10.1073/pnas.96.8.4621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Sambrook J, Russell DW. Cold Spring Harbor Laboratory. 3rd Ed. Cold Spring Harbor, NY: Cold Spring Harbor Lab Press; 2001. Molecular cloning: A laboratory manual. [Google Scholar]
- 45.Chomczynski P, Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987;162:156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- 46.Luckow B, Schutz G. CAT constructions with multiple unique restriction sites for the functional analysis of eukaryotic promoters and regulatory elements. Nucleic Acids Res. 1987;15:5490–5490. doi: 10.1093/nar/15.13.5490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ray A, Ray BK. Isolation and functional characterization of cDNA of serum amyloid A-activating factor that binds to the serum amyloid A promoter. Mol Cell Biol. 1998;18:7327–7335. doi: 10.1128/mcb.18.12.7327. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.