Abstract
CTLA-4, an Ig superfamily molecule with homology to CD28, is one of the most potent negative regulators of T-cell responses. In vivo blockade of CTLA-4 exacerbates autoimmunity, enhances tumor-specific T-cell responses, and may inhibit the induction of T-cell anergy. Clinical trials of CTLA-4–blocking antibodies to augment T-cell responses to malignant melanoma are at an advanced stage; however, little is known about the effects of CTLA-4 blockade on memory CD8+ T-cell responses and the formation and maintenance of long-term CD8+ T-cell memory. In our studies, we show that during in vivo memory CD8+ T-cell responses to Listeria monocytogenes infection, CTLA-4 blockade enhances bacterial clearance and increases memory CD8+ T-cell expansion. This is followed by an accumulation of memory cells that are capable of producing the effector cytokines IFN-γ and TNF-α. We also demonstrate that in a vaccination setting, blocking CTLA-4 during CD8+ T-cell priming leads to increased expansion and maintenance of antigen-specific memory CD8+ T cells without adversely affecting the overall T-cell repertoire. This leads to an increase in memory cell effector function and improved protective immunity against further bacterial challenges. These results indicate that transient blockade of CTLA-4 enhances memory CD8+ T-cell responses and support the possible use of CTLA-4–blocking antibodies during vaccination to augment memory formation and maintenance.
CTLA-4 is expressed 2 to 3 d after T-cell activation and plays a critical role in restricting cell cycle progression and inhibiting production of IL-2 (1, 2). Mice that are deficient for CTLA-4 amass large numbers of activated, proliferating T cells in lymph nodes and spleens, and these cells infiltrate multiple peripheral organs, causing severe myocarditis and death by 3 to 4 wk of age (3, 4). CTLA-4 is approximately 30% homologous to CD28 and binds with higher avidity to its ligands, B7-1 and B7-2, allowing CTLA-4 to promote termination of immune responses by preventing continued T-cell costimulation and activation (5). Crossing CTLA-4–deficient mice with B7-1 and -2 double-deficient animals prevents lymphoproliferation and tissue destruction in CTLA-4−/− mice (6). These studies indicate that CTLA-4 functions, at least in part, by halting CD28-enhanced proliferation and activation to avoid prolonged T-cell responses.
Because of its central role in restraining T-cell activation, modulation of CTLA-4–mediated T-cell inhibition holds great promise in several clinical applications. Two human anti–CTLA-4 mAbs are currently in development for the treatment of metastatic melanoma and other tumor types. Alone or in combination with other therapies, these mAbs have demonstrated impressive antitumor activity, with durable, objective responses and improved survival in melanoma patients (7, 8). Although it has not yet been directly examined, there is hope that this immunotherapy will also generate a memory T-cell response and increase long-term patient survival by inhibiting metastasis and preventing future recurrences.
CTLA-4 blockade has also been examined in the treatment of infectious diseases. Mice infected with Nippostrongylus brasiliensis and treated with CTLA-4 blockade exhibit enhanced T-cell cytokine production, leading to decreased parasite egg production and a profound reduction in intestinal worm burden (9). In a mouse model of pulmonary mycobacterial infection, however, injection of anti–CTLA-4 antibodies greatly enhances proliferation and cytokine production in the draining lymph nodes but has no effect on bacterial clearance in the lungs, liver, or spleen (10). Although these data suggest that in vivo CTLA-4 blockade can enhance T-cell responses to primary infections, little is known about the effects of blocking CTLA-4 on memory CD8+ T-cell responses.
To investigate how CTLA-4 affects CD8+ T-cell memory, we utilized the facultative intracellular pathogen Listeria monocytogenes. Clearance of these bacteria and long-term protective immunity are predominantly mediated by T cells, especially CD8+ T cells, and infection with Listeria elicits a robust memory CD8+ T-cell response. Listeria-specific memory CD8+ T cells produce IFN-γ and TNF-α, two cytokines that are essential for defense against this pathogen (11). In these studies, we used acute Listeria infection to characterize the effects of CTLA-4 blockade on in vivo antigen-specific memory CD8+ T-cell responses. Our data demonstrate that the presence of anti–CTLA-4 significantly improves CD8+ T-cell memory both in therapeutic and vaccination settings, leading to enhanced bacterial clearance. The ability to augment memory CD8+ T-cell responses by blocking CTLA-4 could have important implications for the current use of anti–CTLA-4 in the treatment of cancer and for possible future use in the prevention of infectious diseases.
Results
CTLA-4 Blockade During Memory CD8+ T-Cell Responses.
Given its current and potential clinical uses, we sought to explore whether CTLA-4 blockade affects long-term CD8+ T-cell memory. To generate antigen-specific memory CD8+ T cells that can be tracked in vivo, we used congenically marked OTI transgenic mice bearing CD8+ T cells with a T cell receptor (TCR) that recognizes the SIINFEKL peptide of ovalbumin (257–264) in the context of the Kb MHC class I allele. These OTI cells were adoptively transferred into naïve C57BL/6 (B6) mice, and these mice then received a low-titer infection with a strain of L. monocytogenes engineered to express ovalbumin (LM-OVA). Infected mice were left unperturbed for at least 45 d, allowing the transferred OTI cells to become activated and undergo clonal expansion, followed by contraction and memory differentiation. After at least 45 d, long-lived memory OTI cells were sorted according to expression of CD8α, the congenic marker CD45.1, and high expression of the activation/memory marker CD44. Sorted memory OTI cells were adoptively transferred into new naïve B6 recipients, creating an in vivo environment in which the only memory T cells specific for the LM-OVA pathogen were the memory cells that we transferred. These recipients then received a single i.p. injection of either anti–CTLA-4 blocking antibody or a hamster Ig isotype control just before i.v. infection with LM-OVA (Fig. S1A). Although these memory OTI cells did not express high levels of CTLA-4 at the time of transfer, CTLA-4 levels increased through the first 96 h of activation and expansion (Fig. S1B).
Blockade of CTLA-4 During Memory CD8+ T-Cell Responses Leads to Increased Expansion, Cytokine Production, and Effector Function.
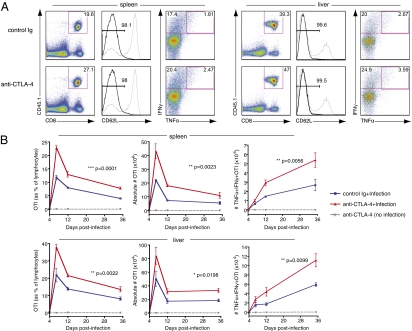
To examine how the presence of anti–CTLA-4 affects memory CD8+ T-cell responses in our system, we tracked the expansion and surface phenotype of memory OTI cells at days 4, 7, 12, and 35 after infection in the spleen and liver, the major reservoirs of systemic Listeria infection after i.v. inoculation (12, 13). At each time point, perfusion was performed to eliminate peripheral blood lymphocytes and allow isolation of tissue-infiltrating or -resident memory OTI CD8+ T cells. At day 7 after infection, the peak of memory cell expansion, we observed a significantly higher frequency of memory OTI CD8+ T cells in mice that received CTLA-4 blockade vs. mice that received control Ig (Fig. 1A). At this time point, memory OTI cells from both treatment groups continued to express similar high levels of several activation and memory markers and were mostly CD62L (L-selectin)-low in phenotype. Both IFN-γ and TNF-α are important mediators of Listeria bacterial clearance in vivo, as demonstrated by the increased susceptibility of mice that are deficient for these cytokines or their receptors (14, 15). When we restimulated these CD8+ T cells with their cognate peptide antigen, SIINFEKL, directly ex vivo, we found that memory OTI cells from mice that received CTLA-4–blocking antibodies harbored a higher frequency of IFN-γ–producing cells and a higher frequency of IFN-γ+TNF-α+ cytokine double-producers in comparison with controls. The expansion, contraction, and maintenance of OTI memory cells over the four time points measured in spleen and liver revealed that memory OTI expansion was significantly increased in mice that received CTLA-4–blocking antibody during the memory response. In addition, this enhanced expansion led to an increased number of memory OTI cells maintained in these organs at 35 d after infection (Fig. 1B, Left). We also observed a steady increase in the absolute number of effector cytokine-producing memory cells (with similar percentages in both treatment groups) in the first 35 d after infection. The increased memory OTI expansion in mice that received anti–CTLA-4 during memory activation thereby resulted in a pool of cytokine-producing memory OTI cells that was nearly double that of controls (Fig. 1B, Right). After observing increased memory OTI cell expansion and cytokine production after administration of a single dose of anti–CTLA-4, we wanted to determine how blockade of CTLA-4 affected the cytolytic function of memory CD8+ T cells and their ability to mediate protective immunity against subsequent Listeria challenges. We again adoptively transferred memory OTI cells into naïve mice and injected these recipients with anti–CTLA-4 or control Ig just before systemic infection with LM-OVA. At 24 h after infection, in animals that received both adoptive transfer of memory OTI cells and anti–CTLA-4, bacterial titer was significantly reduced in comparison with mice that received either memory cells and an isotype control or mice that did not receive any memory CD8+ T cells (Fig. S2), implying that CTLA-4 blockade enhances memory OTI cell activation and the consequent speed of bacterial clearance.
Fig. 1.
Blockade of CTLA-4 during memory responses increases memory CD8+ T-cell expansion and effector function. Memory OTI cells generated in vivo were sorted and adoptively transferred into new naïve recipients. Recipient mice then received either control Ig or anti–CTLA-4 and an i.v. infection with LM-OVA. (A) Flow cytometry of lymphocytes (left column) and gated CD8+CD45.1+ memory OTI cells (right two columns) in spleen and liver at 7 d after infection. Numbers in gates indicate frequency of OTI as a percentage of lymphocytes (left column) and frequency of CD62L-low and IFNγ+ and double-positive, TNFα+ IFNγ+, as a percentage of gated OTI (middle and right columns, respectively). Gray histograms represent expression levels in total, unfractionated lymphocytes for reference. Each plot is from one mouse (median for each group) of three. (B) Frequency and absolute number of OTI memory cells and effector cytokine-producing OTI memory cells at days 4, 7, 12, and 35 after infection. Data shown are of three mice per group for each time point, and error bars indicate means ± SEM. Two-way ANOVA was used to evaluate statistical significance. Data shown are representative of three independent experiments.
Blockade of CTLA-4 During Primary Infection also Leads to Increased OTI Memory.
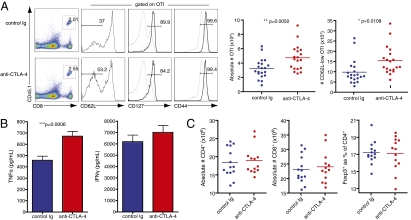
Although it has been previously shown that CTLA-4 deficiency improves secondary but not primary CD8+ T-cell responses to peptide (16), the effects of transient blockade of CTLA-4 during CD8+ T-cell priming have not been explored in the context of CD8+ T-cell memory. We therefore wanted to investigate the in vivo effects of anti–CTLA-4 during primary activation on long-term memory CD8+ T-cell formation and phenotype. Congenically marked, naïve OTI CD8+ T cells were adoptively transferred into B6 recipients, as described earlier, and recipient mice then received control Ig or anti–CTLA-4 just before low-titer primary infection (vaccination) with LM-OVA. At least 45 d after infection, with no further CTLA-4 blockade or antigen exposure, splenocytes were examined for frequency and phenotype of resting memory OTI cells. Although OTI cells recovered from both treatment groups exhibited similar memory marker CD44 and CD127 expression, the frequency of antigen-specific OTI cells was elevated in mice that received CTLA-4 blockade during priming (Fig. 2A). In addition, an increased number of these splenic memory OTI cells were CD62L-low, a phenotype associated with effector-memory T cells. This memory T-cell subset is reported to more readily produce effector cytokines and have enhanced cytotoxic function compared with its CD62L-high counterpart, central-memory T cells (17). When memory OTI cells from these mice were restimulated with their cognate peptide antigen, supernatants from anti–CTLA-4–treated OTI cells contained similar levels of IFN-γ and significantly elevated levels of TNF-α when compared with cells from control Ig-treated mice (Fig. 2B). These data strongly suggest that the transient blockade of CTLA-4 during priming results in an increased frequency of antigen-specific effector-memory CD8+ T cells that are capable of producing effector cytokines upon restimulation. To ensure that blocking this inhibitor of T-cell proliferation and activation does not lead to an aberrant overabundance of T cells in treated mice, we also examined the polyclonal CD4+ and CD8+ T-cell compartments of these animals. The single dose of anti–CTLA-4 present during priming did not significantly affect absolute numbers of polyclonal CD4+ or CD8+ T cells at this postinfection time point, and CD4+Foxp3+ T regulatory cells (Tregs) were present in similar frequencies in both treatment groups (Fig. 2C).
Fig. 2.
Presence of anti–CTLA-4 during priming increases memory cells and their effector function without adversely affecting overall T-cell repertoire. Naïve OTI cells were adoptively transferred into B6 recipients that were then injected with either control Ig or anti–CTLA-4 just before infection with LM-OVA. Spleens were analyzed at least 45 d after infection, with no further treatment or antigen exposure. (A) Left: Flow cytometry of lymphocytes (left column) and gated CD8+CD45.1+ memory OTI cells (right three columns) from spleens of mice that received either control Ig or anti–CTLA-4 during priming. Numbers in gates indicate frequency of OTI as a percentage of lymphocytes (left column) and frequency of CD62L-low, CD127+, and CD44-high as percentage of gated OTI (right three columns). Gray histograms represent expression levels in total, unfractionated lymphocytes for reference. Each plot is from one mouse (median for each group) of seven. Right: Graphs of quantified data pooled from three independent experiments with six to seven mice per group. (B) Cells were restimulated with SIINFEKL for 20 h, and supernatants were analyzed for effector cytokine secretion using cytometric bead arrays. Pooled data from three independent experiments are shown, and error bars indicate means ± SEM. (C) Absolute numbers of CD4+ and CD8+ T cells and frequencies of Tregs from spleens of treated mice were quantified from flow cytometry. Data shown are pooled from two independent experiments with six to seven mice per group. Statistical significance was determined with t tests.
Anti–CTLA-4 During Priming Enhances Endogenous CD8+ T-Cell Memory.
To examine the utility of our findings in more physiological conditions, we next observed the effects of CTLA-4 blockade during priming on CD8+ T-cell memory in mice with normal, endogenous T-cell repertoires and precursor frequencies. Without the adoptive transfer of exogenous antigen-specific T cells, we administered either anti–CTLA-4 or control Ig to wild-type B6 mice just before primary, low-dose infection with LM-OVA. At least 45 d after infection, we examined the polyclonal CD8+ T-cell populations in treated mice. Mice in which CTLA-4 was blocked during initial T-cell priming and expansion exhibited a similar overall frequency of memory CD8+CD44-high T cells but had elevated frequencies of CD62L-low effector-memory CD8+ T cells compared with controls (Fig. S3). To precisely examine the incidence of antigen-specific memory CD8+ T cells in anti–CTLA-4–treated mice, we used SIINFEKL tetramers to enumerate SIINFEKL-specific CD8+ T cells. In mice that received anti–CTLA-4 during primary infection, the frequency and absolute number of tetramer-positive CD8+ T cells were significantly increased in comparison with mice that received only control Ig during priming. These tetramer-positive cells also exhibited a CD44-high, CD62L-low effector-memory phenotype (Fig. S4A). When splenocytes from these mice were restimulated with SIINFEKL peptide, the absolute numbers of antigen-specific IFN-γ–producing and TNF-α/IFN-γ double-producing CD8+ T cells were increased in mice that received CTLA-4 blockade during priming compared with controls (Fig. S4B).
CTLA-4 Blockade During Priming Enhances Memory CD8+ T-Cell Function.
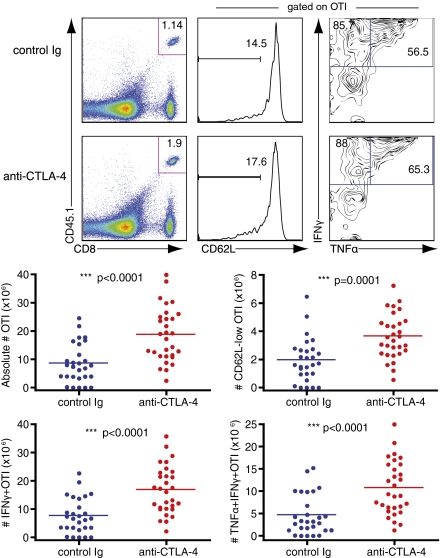
We next hypothesized that the increased frequency and cytokine production of memory CD8+ T cells seen in mice treated with anti–CTLA-4 during primary infection could lead to better protective immunity against subsequent infections. To investigate how blockade of CTLA-4 during CD8+ T-cell priming affects the protective capacity of long-term memory CD8+ T cells, mice that received OTI cells and either control Ig or anti–CTLA-4 during priming were reinfected with a high titer of LM-OVA at least 60 d after primary infection. Although mice from both treatment groups developed efficient protective memory responses, mice treated with anti–CTLA-4 during priming exhibited significantly decreased bacterial burdens in spleen at 24 h after reinfection (Fig. S5). This enhanced bacterial clearance corresponded to an increased presence of cytokine-producing memory CD8+ T cells in these organs. Antigen-specific memory OTI cells analyzed in the spleens of these mice occurred at nearly double the frequency as that of control-treated mice, and many exhibited a CD62L-low, effector-memory phenotype. These memory CD8+ T cells also produced both IFN-γ and TNF-α effector cytokines in response to restimulation with SIINFEKL (Fig. 3). To ascertain whether administration of anti–CTLA-4 during priming leads to enhanced long-term protective immunity in mice with endogenous CD8+ T-cell repertoires, we again administered either anti–CTLA-4 or control Ig to wild-type mice in the absence of any adoptive transfer of exogenous antigen-specific T cells just before primary infection with LM-OVA. When we reinfected previously treated mice with a lethal dose of LM-OVA and assessed bacterial clearance in spleens at 24 h after infection, we observed that the bacterial burden was significantly reduced in mice that received anti–CTLA-4 during priming in comparison with both mice that received control Ig during primary infection and naïve control mice that lacked any previous antigen experience (Fig. S6). To assess whether antigen-specific memory cells primed in the presence of anti–CTLA-4 are more effective on a per-cell basis, we also examined bacterial clearance in mice that received transferred sorted memory OTI cells from donors that received either control Ig or anti–CTLA-4 during primary infection. Recipient mice, which harbored no memory for our pathogen except for the transferred memory OTI cells, were examined at 24 h after infection for bacterial burden and memory OTI cell content. Mice that received memory OTI cells from mice previously treated with anti–CTLA-4 showed reduced bacterial burden in comparison with recipients of cells from control Ig-treated mice (Fig. S7A). Despite the equal number of cells transferred, a higher number of memory OTI cells were recovered from the spleens of mice that received anti–CTLA-4–treated cells, and once again, many of these cells displayed a CD62L-low, effector-memory phenotype. These antigen-specific memory CD8+ T cells also produced IFN-γ and TNF-α effector cytokines upon restimulation with their cognate peptide (Fig. S7B).
Fig. 3.
Blockade of CTLA-4 during priming enhances memory CD8+ T-cell effector function. Naïve OTI cells were adoptively transferred into B6 recipients and injected with either control Ig or anti–CTLA-4 just before infection with LM-OVA. After at least 60 d, mice received a rechallenge infection with LM-OVA. Upper: Flow cytometry of lymphocytes (left column) and gated CD8+CD45.1+ memory OTI cells (right two columns) in spleens at 24 h after infection. Numbers in gates indicate frequency of OTI as a percentage of lymphocytes (left column) and frequency of CD62L-low and IFNγ+ and double-positive, TNFα+ IFNγ+ as a percentage of gated OTI (middle and right columns, respectively). Each plot is from 1 mouse (median) of 10. Lower: Graphs of quantified data pooled from three independent experiments with 9 to 10 mice per group. Statistical significance was determined with t tests.
Anti–CTLA-4–Mediated Increases in Long-Term CD8+ T-Cell Memory Require Cell-Extrinsic CTLA-4 Blockade.
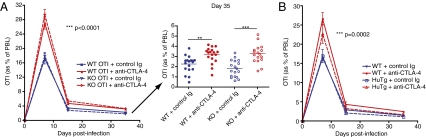
Although the effects of anti–CTLA-4 on antigen-specific memory CD8+ T-cell proliferation were apparent, it was not clear whether this was a result of direct blockade of CTLA-4 on the antigen-specific memory CD8+ T cells themselves or whether blockade of CTLA-4 on helper CD4+ T cells or CD4+ T regulatory cells might be indirectly affecting memory CD8+ T-cell expansion. To explore these possibilities, we used two different experimental approaches. First, we generated CTLA-4–deficient OTI CD8+ T cells to determine whether cell-intrinsic CTLA-4 deficiency mirrors the effects of transient CTLA-4 blockade on the memory expansion and function of these cells. CTLA-4–deficient OTI cells did not, however, exhibit increased expansion in the absence of CTLA-4 blockade of the endogenous T-cell compartment (Fig. S8). In addition, unless anti–CTLA-4 was administered systemically, by day 35 after infection CTLA-4−/− OTI cells were not present in greater numbers than wild-type OTI cells treated with control Ig during priming (Fig. 4A). To compare this cell-intrinsic CTLA-4 deficiency with the cell-specific effects of transient CTLA-4 blockade, we used mice that have been engineered to only express human CTLA-4 on their T cells (CTLA-4 HuTg). Anti–mouse-CTLA-4 antibodies are unable to block this molecule on T cells in these HuTg animals (18). Wild-type OTI cells were adoptively transferred into either wild-type B6 or CTLA-4 HuTg recipients, and anti–CTLA-4 or control Ig were injected before primary infection, allowing blockade of CTLA-4 on only the transferred OTI cells, in HuTg recipients, or on all T cells, in wild-type recipients. Although blocking CTLA-4 on OTI cells alone during priming did result in significantly increased expansion of these cells in comparison with control Ig-treated mice, this increase was not maintained by 35 d after infection. Only mice in which CTLA-4 was blocked on the entire T-cell compartment exhibited a larger pool of long-term memory OTI cells (Fig. 4B).
Fig. 4.
Anti–CTLA-4 enhancement of memory CD8+ T cells requires cell-extrinsic blockade of CTLA-4. (A) Naïve OTI cells from either wild-type (WT) or CTLA-4–deficient (KO) mice were adoptively transferred into new naïve wild-type recipients. Recipient mice then received either control Ig or anti–CTLA-4 and an i.v. infection with LM-OVA. Left: Frequency of antigen-specific OTI cells collected from peripheral blood at days 7, 15, and 35 after infection. Right: Dot plots of OTI frequency in individual mice at day 35. Data shown are pooled from three independent experiments with five to six mice per group. Two-way ANOVA was used to evaluate statistical significance for all time points, and error bars indicate means ± SEM. One-way ANOVA was used for day-35 comparisons. **P < 0.01; ***P < 0.001. (B) Naïve OTI cells were adoptively transferred into naïve recipients, either wild-type or transgenic mice engineered to express only human CTLA-4 (HuTg). Recipient mice then received either control Ig or anti–CTLA-4 and an i.v. infection with LM-OVA, allowing for blockade of CTLA-4 only on cells expressing mouse CTLA-4. Plot shows frequency of OTI cells collected from peripheral blood at days 7, 15, and 35 after infection. Data shown are pooled from two independent experiments with five mice per group and analyzed by two-way ANOVA.
Discussion
On the basis of our studies, we conclude that administration of CTLA-4–blocking antibodies during a memory response can both increase expansion and enhance effector function of memory CD8+ T cells. This results in greater accumulation of functional memory CD8+ T cells and improved ability to clear subsequent infections with the same pathogen. Furthermore, administration of anti–CTLA-4 during primary antigen exposure leads to an increased frequency of functional, cytokine-producing memory CD8+ T cells that are capable of mediating efficient bacterial clearance, even in the context of endogenous T-cell repertoires and precursor frequencies. This could have important implications for the clinical applications of anti–CTLA-4, both in its current use in tumor immunotherapy and in the possible prevention of infectious diseases.
Given the effects of anti–CTLA-4 demonstrated here, CTLA-4 blockade may serve as a valuable means of preventing infectious diseases in which a robust memory CD8+ T-cell response is not efficiently induced by infection but is necessary for effective clearance of the pathogen. Treatment of chronic lymphocytic choriomeningitis virus (LCMV) infection with CTLA-4 blockade has been shown to have no significant effect on either antigen-specific T-cell responses or viral control (19). This could be due to constant TCR stimulation or a dominant function for other negative regulators like PD-1 during chronic infection. However, we observed that mice treated with anti–CTLA-4 during an acute primary infection harbor an increased frequency and absolute number of antigen-specific memory CD8+ T cells. This indicates that coadministration of a single dose of CTLA-4 blockade with a vaccine might be used to augment CD8+ T-cell–mediated long-term, protective immunity in situations in which vaccine alone does not elicit adequate memory CD8+ T-cell formation and maintenance. These findings could also have important implications for the current use of anti–CTLA-4 in the treatment of cancer. At the time of anti–CTLA-4 administration, cancer patients likely harbor a heterogeneous pool of tumor-specific CD8+ T cells that include naïve, effector, and memory T cells. In clinical trials, CTLA-4 blockade has resulted in improved survival in patients with metastatic melanoma (20, 21), and our data now suggest that this treatment could function to improve tumor-specific CD8+ T-cell memory and promote durable antitumor responses.
Blocking CTLA-4 on helper CD4+ T cells is known to increase production of IL-2 by these cells (1), and IL-2 signaling during primary CD8+ T-cell responses has been shown to have a programming effect in driving the complete differentiation of memory CD8+ T cells (22). In addition, enhancement of IL-2 signals through the use of IL-2/anti–IL-2 complexes has been shown to increase the proliferation of memory CD8+ T cells and natural killer cells (23, 24). It is therefore possible that anti–CTLA-4 treatment enhances CD8+ T-cell memory by increasing production of IL-2 by helper CD4+ T cells. Because both CTLA-4 blockade and cell-intrinsic CTLA-4 deficiency have been shown to decrease the suppressive function of CD4+ Tregs (18, 25, 26), it also possible that the increased expansion and function of memory CD8+ T cells apparent in mice treated with CTLA-4 blockade is due to decreased CD4+ Treg-mediated suppression. In addition, blockade of CTLA-4 on the endogenous CD8+ T-cell population may contribute to the enhancement of OTI memory that we observe. Although we do see that cell-extrinsic blockade of CTLA-4 is necessary for enhancing long-term CD8+ T-cell memory (Fig. 4), further studies are required to determine what cell types are responsible and the specific mechanisms involved.
It will also be valuable to examine the possible mechanisms of CTLA-4-blockade–mediated memory CD8+ T-cell enhancement. When equal numbers of memory OTI cells that were exposed to anti–CTLA-4 during primary infection are adoptively transferred to new naïve recipients, these recipients still exhibit an increased frequency of memory OTI cells at 24 h after infection in comparison with recipients of cells from mice previously treated with control Ig (Fig. S7B). This implies that the increased number of long-term memory CD8+ T cells observed in CTLA-4–treated mice is not strictly a product of enhanced primary expansion but that CD8+ T cells may be programmed or imprinted for better survival and/or proliferation when CTLA-4 is blocked during priming. Further investigation is necessary to determine why administration of CTLA-4–blocking antibodies during priming of naïve CD8+ T cells might lead to improved survival and/or proliferation of these cells at the memory stage.
The enhancement of memory CD8+ T-cell responses by CTLA-4 blockade that we report here further supports its utility in the treatment of cancer and supports the possible use of anti–CTLA-4 in the context of infectious disease. Anti–CTLA-4 may prove useful as an adjuvant in vaccination settings to augment formation of long-term CD8+ T-cell memory, improving protective immunity against infection and promoting durable antitumor immune responses.
Materials and Methods
Mice.
Mouse work was performed in accordance with institutional guidelines and animal protocols approved by the Institutional Animal Care and Use Committee at Memorial Sloan-Kettering. All mice were maintained in microisolator cages in a pathogen-free facility according to National Institutes of Health Animal Care guidelines. C57BL/6J (B6) mice, congenic CD45.1+ B6.SJL mice, and OVA-specific OTI TCR transgenic mice were purchased from the Jackson Laboratory. Immunodeficient Rag2−/− mice were purchased from Taconic Farms. OTI mice were crossed to Rag2−/− and B6.SJL mice to generate mice harboring only congenically marked antigen-specific OTI T cells. These mice were then crossed to CTLA-4–deficient mice previously generated in our laboratory (27). Mice expressing only CTLA-4 with a transgenic human extracellular domain were also previously generated in our laboratory (18).
Cell Sorting and Adoptive Transfer.
CD8+ T cells from TCR transgenic mice were isolated for adoptive transfer using a MACS CD8a+ T Cell Isolation Kit for mouse (Miltenyi). Memory OTI CD8+ T cells were sorted electronically by FACS after staining with antibodies against CD8α, CD45.1, and CD44 (eBioscience). Purity of isolated cells was confirmed by flow cytometry, and sorted cells were counted using a hemacytometer or Guava PCA/ViaCount cell counting system (Millipore) before i.v. injection.
Bacteria.
Recombinant L. monocytogenes strain expressing ovalbumin (LM-OVA) was provided by Dr. H. Shen (University of Pennsylvania, Philadelphia, PA). Bacteria were cultured in Bacto BHI broth (BD Bioscience). Bacteria were grown to log phase (A6oo of 0.08–0.13) and resuspended in PBS before i.v. injection into lateral tail veins. At 24 h after infection, spleens were harvested, weighed, and dissociated in PBS containing 0.1% Triton X-100 (Roche). Numbers of bacterial colony-forming units were determined by plating serial dilutions on BHI agar plates containing 2 μg/mL erythromycin (Sigma).
Cardiac Perfusion and Isolation of Liver Lymphocytes.
At indicated time points after infection, animals were injected i.p with 4 mg ketamine HCl and 400 μg xylazine HCl. Anesthetized mice were perfused with PBS, and liver and spleen were harvested from perfused animals for isolation of lymphocytes. Livers were incubated with DNase and Liberase (both from Roche) at 37 °C and dissociated. Liver lymphocytes were then separated using a discontinuous Percoll density gradient (GE Healthcare).
Tetramer Staining.
SIINFEKL peptide (OVA257–264) was synthesized and HPLC purified by the Molecular Biology Proteomics Facility of the University of Oklahoma Health Sciences Center. Peptide/MHC tetramers were produced by I. Leiner in the Memorial Sloan-Kettering Cancer Center Tetramer Core Facility (New York, NY). Cells were stained with tetramer-phycoerythrin (PE) in the presence of anti–CD8α-Pacific Blue (clone 53-6.7, eBioscience) and other surface marker antibodies for 1 h on ice and analyzed by flow cytometry.
Cytokine Production.
T cells were restimulated ex vivo with 1 μg/mL SIINFEKL peptide at 37 °C. For intracellular cytokine staining, cells were restimulated for 4–6 h in the presence of monensin. Cells were stained for surface markers, fixed and permeabilized, and antibody-stained for intracellular IFN-γ and TNF-α (eBioscience) before analysis by flow cytometry. For quantification of cytokine secretion, cells were restimulated for 20 h. Supernatants were harvested for analysis with BD Cytometric Bead Arrays for detection of IFN-γ and TNF-α.
Blockade of CTLA-4.
Approximately 2 h before infection, mice were injected i.p. with 200 μg of anti–mouse-CTLA-4 (clone 9H10) or hamster IgG isotype control (both from BioXCell) diluted in sterile PBS.
Supplementary Material
Acknowledgments
We thank Dr. E. G. Pamer for advice and constructive discussion of this work; J. Lu, R. Waitz, J. I. Wei, L. Lipuma, and J. Geddes for technical assistance; and M. Curran, J. I. Wei, E. Alonzo, M. L. Miller, and P. Gregor for critical reading and discussion of the manuscript. V.A.P. was supported in part by a predoctoral fellowship from the Cancer Research Institute.
Footnotes
Conflict of interest statement: J.P.A. is a paid consultant for Bristol-Myers Squibb and is the primary inventor on the patent “Blockade of T lymphocyte down-regulation associated with CTLA-4 signaling.”
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1016791108/-/DCSupplemental.
References
- 1.Krummel MF, Allison JP. CTLA-4 engagement inhibits IL-2 accumulation and cell cycle progression upon activation of resting T cells. J Exp Med. 1996;183:2533–2540. doi: 10.1084/jem.183.6.2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Walunas TL, et al. CTLA-4 can function as a negative regulator of T cell activation. Immunity. 1994;1:405–413. [PubMed] [Google Scholar]
- 3.Tivol EA, et al. Loss of CTLA-4 leads to massive lymphoproliferation and fatal multiorgan tissue destruction, revealing a critical negative regulatory role of CTLA-4. Immunity. 1995;3:541–547. doi: 10.1016/1074-7613(95)90125-6. [DOI] [PubMed] [Google Scholar]
- 4.Waterhouse P, et al. Lymphoproliferative disorders with early lethality in mice deficient in Ctla-4. Science. 1995;270:985–988. doi: 10.1126/science.270.5238.985. [DOI] [PubMed] [Google Scholar]
- 5.Alegre ML, Frauwirth KA, Thompson CB. T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol. 2001;1:220–228. doi: 10.1038/35105024. [DOI] [PubMed] [Google Scholar]
- 6.Mandelbrot DA, McAdam AJ, Sharpe AH. B7-1 or B7-2 is required to produce the lymphoproliferative phenotype in mice lacking cytotoxic T lymphocyte-associated antigen 4 (CTLA-4) J Exp Med. 1999;189:435–440. doi: 10.1084/jem.189.2.435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hodi FS, et al. Improved survival with ipilimumab in patients with metastatic melanoma. N Engl J Med. 2010;363:711–723. doi: 10.1056/NEJMoa1003466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Robert C, Ghiringhelli F. What is the role of cytotoxic T lymphocyte-associated antigen 4 blockade in patients with metastatic melanoma? Oncologist. 2009;14:848–861. doi: 10.1634/theoncologist.2009-0028. [DOI] [PubMed] [Google Scholar]
- 9.McCoy K, Camberis M, Gros GL. Protective immunity to nematode infection is induced by CTLA-4 blockade. J Exp Med. 1997;186:183–187. doi: 10.1084/jem.186.2.183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Kirman J, et al. CTLA-4 blockade enhances the immune response induced by mycobacterial infection but does not lead to increased protection. Infect Immun. 1999;67:3786–3792. doi: 10.1128/iai.67.8.3786-3792.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pamer EG. Immune responses to Listeria monocytogenes. Nat Rev Immunol. 2004;4:812–823. doi: 10.1038/nri1461. [DOI] [PubMed] [Google Scholar]
- 12.Conlan JW, North RJ. Neutrophils are essential for early anti-Listeria defense in the liver, but not in the spleen or peritoneal cavity, as revealed by a granulocyte-depleting monoclonal antibody. J Exp Med. 1994;179:259–268. doi: 10.1084/jem.179.1.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.MacKaness GB. Cellular resistance to infection. J Exp Med. 1962;116:381–406. [PubMed] [Google Scholar]
- 14.Rothe J, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798–802. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 15.Harty JT, Bevan MJ. Specific immunity to Listeria monocytogenes in the absence of IFN gamma. Immunity. 1995;3:109–117. doi: 10.1016/1074-7613(95)90163-9. [DOI] [PubMed] [Google Scholar]
- 16.Chambers CA, Sullivan TJ, Truong T, Allison JP. Secondary but not primary T cell responses are enhanced in CTLA-4-deficient CD8+ T cells. Eur J Immunol. 1998;28:3137–3143. doi: 10.1002/(SICI)1521-4141(199810)28:10<3137::AID-IMMU3137>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 17.Sallusto F, Lenig D, Förster R, Lipp M, Lanzavecchia A. Two subsets of memory T lymphocytes with distinct homing potentials and effector functions. Nature. 1999;401:708–712. doi: 10.1038/44385. [DOI] [PubMed] [Google Scholar]
- 18.Peggs KS, Quezada SA, Chambers CA, Korman AJ, Allison JP. Blockade of CTLA-4 on both effector and regulatory T cell compartments contributes to the antitumor activity of anti-CTLA-4 antibodies. J Exp Med. 2009;206:1717–1725. doi: 10.1084/jem.20082492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Barber DL, et al. Restoring function in exhausted CD8 T cells during chronic viral infection. Nature. 2006;439:682–687. doi: 10.1038/nature04444. [DOI] [PubMed] [Google Scholar]
- 20.Hersh EM, et al. A phase II multicenter study of ipilimumab with or without dacarbazine in chemotherapy-naive patients with advanced melanoma. Invest New Drugs. 2010 doi: 10.1007/s10637-009-9376-8. in press. [DOI] [PubMed] [Google Scholar]
- 21.Sarnaik AA, Weber JS. Recent advances using anti-CTLA-4 for the treatment of melanoma. Cancer J. 2009;15:169–173. doi: 10.1097/PPO.0b013e3181a7450f. [DOI] [PubMed] [Google Scholar]
- 22.Williams MA, Tyznik AJ, Bevan MJ. Interleukin-2 signals during priming are required for secondary expansion of CD8+ memory T cells. Nature. 2006;441:890–893. doi: 10.1038/nature04790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kamimura D, Bevan MJ. Naive CD8+ T cells differentiate into protective memory-like cells after IL-2 anti IL-2 complex treatment in vivo. J Exp Med. 2007;204:1803–1812. doi: 10.1084/jem.20070543. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Boyman O, Kovar M, Rubinstein MP, Surh CD, Sprent J. Selective stimulation of T cell subsets with antibody-cytokine immune complexes. Science. 2006;311:1924–1927. doi: 10.1126/science.1122927. [DOI] [PubMed] [Google Scholar]
- 25.Read S, et al. Blockade of CTLA-4 on CD4+CD25+ regulatory T cells abrogates their function in vivo. J Immunol. 2006;177:4376–4383. doi: 10.4049/jimmunol.177.7.4376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wing K, et al. CTLA-4 control over Foxp3+ regulatory T cell function. Science. 2008;322:271–275. doi: 10.1126/science.1160062. [DOI] [PubMed] [Google Scholar]
- 27.Chambers CA, Cado D, Truong T, Allison JP. Thymocyte development is normal in CTLA-4-deficient mice. Proc Natl Acad Sci USA. 1997;94:9296–9301. doi: 10.1073/pnas.94.17.9296. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.