Abstract
Dynamic signals linking the actin cytoskeleton and cell adhesion receptors are essential for morphogenesis during development and normal tissue homeostasis. Abi1 is a central regulator of actin polymerization through interactions with multiple protein complexes. However, the in vivo role of Abi1 remains to be defined. The α4 integrin adhesion receptor is associated with enhanced protrusive activity and regulation of directional cell migration. Among integrin subunits, α4 exhibits unique properties in that it predominantly accumulates at the leading edge of migrating cells; however, the pathways that link the actin-regulatory machinery to α4 at the leading edge have remained elusive. We generated Abi1 KO mice and found that loss of Abi1 phenocopies KO of α4. Mice lacking Abi1 or α4 exhibit midgestational lethality with abnormalities in placental and cardiovascular development. Notably, purified Abi1 protein binds directly to the α4 cytoplasmic tail and endogenous Abi1 colocalizes with phosphorylated α4 at the leading edge of spreading cells. Moreover, Abi1-deficient cells expressing α4 have impaired cell spreading, which is rescued by WT Abi1 but not an Abi1 mutant lacking the α4-binding site. These data reveal a direct link between the α4 integrin and actin polymerization and uncover a role for Abi1 in the regulation of morphogenesis in vivo. The Abi1–α4 interaction establishes a mechanistic paradigm for signaling between adhesion events and enhanced actin polymerization at the earliest stages of protrusion.
Keywords: WAVE complex, lamellipodia, chorio-allantoic fusion
Intercellular and cell-matrix interactions are essential for many diverse biological processes such as morphogenesis, immune responses, and cancer metastasis. These interactions are dependent on the dynamic regulation of the actin cytoskeleton downstream of adhesion receptors. Prominent among these receptors are the heterodimeric αβ integrin transmembrane receptors that bind to ECM proteins as well as a subset of cell adhesion molecules. Ligand-bound integrins promote not only adhesion to matrix or adjacent cells, but also initiate downstream signaling pathways through the recruitment of adaptor proteins to the integrin cytoplasmic domains (1). Bidirectional signals linking the actin cytoskeleton to integrin complexes provide cells with spatial cues to initiate directional cell migration, promote adhesion, and generate polarity. The protein complexes required for transducing integrin engagement to the regulation of actin cytoskeletal dynamics are beginning to be elucidated. Although numerous binding partners of the integrin β-subunit cytoplasmic tails have been identified, few interacting proteins for the integrin α-subunits have been uncovered (1). Among these is paxillin, a focal adhesion adaptor protein that binds directly to the α4 integrin cytoplasmic tail (2). This interaction is regulated by phosphorylation of α4 at S988 (3), as signals that initiate protrusive activity promote phosphorylation of α4 at the leading edge, resulting in dissociation of paxillin, thereby allowing for directional migration (4). Thus, paxillin binding to α4 negatively regulates protrusive activity. The α4 integrin exhibits unique properties among integrin subunits in that α4 predominantly accumulates at the leading edge of migrating cells, rather than at focal adhesions. Moreover, α4 expression is associated with protrusive activity and enhanced cell migration; however, the pathways that link α4 integrin to the actin-regulatory machinery at the leading edge have remained elusive. Here we identify Abi1 as a target of α4 integrin that positively regulates membrane protrusion by promoting actin polymerization at sites of integrin engagement.
The Abi family proteins, Abi1 and Abi2, were originally identified as binding partners and substrates of the Abl tyrosine kinase (5, 6). Subsequent in vitro studies have implicated Abi proteins in the regulation of cell motility, intercellular adhesion, endocytosis, cytokinesis, Golgi architecture, and transport carrier biogenesis, through their ability to interact with distinct protein complexes involved in the regulation of actin nucleation (7–14). Abi proteins localize to sites of actin polymerization at cell–cell contacts in epithelial adherens junctions (8, 9) and immune synapses (15). Abi proteins also localize to the leading edge of lamellipodia, and this localization correlates with positive regulation of directional migration (12, 16, 17). Regulation of protrusive activity by Abi proteins has been linked to the formation of protein complexes with the WASP/WAVE family of nucleation-promoting factors (18), leading to activation of the Arp2/3 complex (19). Abi1 has also been shown to interact with Diaphanous formins to promote actin polymerization-driven protrusions at the leading edge of motile cells and at sites of cell–cell contact (9, 10).
Genetic studies have shown that Abi family proteins are required for the regulation of cytoskeletal dynamics in vivo and morphogenetic abnormalities are observed in flies and mice lacking Abi proteins. We showed that homozygous deletion of mouse Abi2 results in abnormal phenotypes in the eye and brain—tissues with high Abi2 expression (8). Drosophila embryos lacking both maternal and zygotic abi exhibit embryonic lethality and collapse of axonal patterning (20). In contrast to Abi2, Abi1 exhibits widespread expression (8). To investigate Abi1 function during morphogenetic events in vivo, we disrupted the Abi1 gene in mice. Abi1-deficient mice exhibit defects in placental and cardiovascular development, leading to midgestational embryonic lethality. These phenotypes mirror those of mice deficient for the α4 integrin and its counter receptor VCAM1 (21–24). Integrin α4β1 binding to VCAM1 is essential not only for placental and cardiac development but also for extravasation of circulating lymphocytes into inflamed tissue by binding of α4β1-expressing lymphocytes to VCAM1 expressed on activated endothelia (25). Binding of α4β1 to VCAM1 also regulates tumor angiogenesis and homing of hematopoietic stem and progenitor cells (26, 27). Thus, blocking the interactions of α4 with downstream targets may be an effective therapy for the treatment of autoimmune diseases, pathological angiogenesis, and other disorders (25). Our findings reveal that Abi1 interacts with the α4 cytoplasmic tail and may mediate specific functions associated with α4-dependent processes in normal and pathological conditions.
Results
Role for Abi1 in Chorio-Allantoic Fusion.
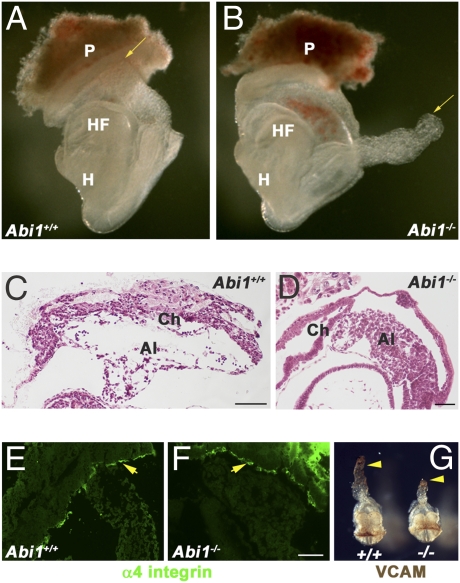
To examine the role of the Abi1 adaptor molecule in vivo, as well as to identify Abi1-dependent signaling pathways, we used homologous recombination to generate Abi1-deficient mice (Fig. 1 and Fig. S1). Loss of Abi1 resulted in embryonic lethality between embryonic day (E) 8.5 and E10.5, depending on the genetic background (Fig. S2). Mouse embryonic fibroblast (MEF) cells derived from these embryos showed absence of Abi1 protein expression, indicating that these embryos carry a null mutation (Fig. S3). At E7.5 to E8.0, there was no significant difference in appearance between WT and Abi1−/− embryos; however, as development proceeded, Abi1−/− embryos showed developmental delays and growth retardation. The onset of phenotypic abnormalities occurred at approxiately E8.5 for Abi1 KO mice in the C57BL/6 strain, and E9.5 for Abi1 loss in the CD1 strain. Notably, Abi1−/− mice bred on the C57BL/6 background showed failure of chorio-allantoic fusion (61 of 111; 55%), which was not associated with growth retardation (Fig. 1). The allantoises of these Abi1−/− embryos extended through the exocoelomic cavity, achieving a size comparable to that of WT littermates, but failed to initiate fusion (Fig. 1 A–D). The WT allantois undergoes de novo vasculogenesis as evidenced by the large hydropic spaces indicative of endothelial cell remodeling events occurring at the distal tip of the allantois before chorio-allantoic fusion (28) (Fig. 1C). In contrast, the allantoises of Abi1−/− embryos exhibited impaired vasculogenesis, appearing compact and lacking spaces at the distal tip (Fig. 1D). Chorio-allantoic fusion requires reciprocal interactions between α4 integrin and VCAM1 expressed on the surface of the chorion and allantois, respectively (21–23). Thus, we analyzed the expression of these proteins in Abi1−/− embryos at E8.5. We found that both α4 integrin and VCAM1 were expressed appropriately in these mutant embryos, indicating that loss of expression of either protein was not responsible for the observed lack of chorio-allantoic fusion (Fig. 1 E–G). These data implicate Abi1 in chorio-allantoic fusion, and suggest that placental failure is a contributing factor to the lethality of Abi1-deficient mice.
Fig. 1.
Defective chorio-allantoic fusion of Abi1 mutant embryos on the C57Bl6 background. (A) WT embryo at E8.5, dissected free from the uterus and attached to a portion of the maternal placenta (P), shows the allantois (arrow) attached to the chorion, which fuses at this stage. (B) Abi1 homozygous mutant embryo at E8.5 is shown at identical magnification to WT embryo in A. The distal tip of the allantois (arrow) floats freely, unattached to the chorion. (C and D) H&E sections of WT (C) and Abi1-null embryos (D) illustrate the lack of attachment of the allantois to the chorion in Abi1 mutants, although the allantois length is sufficient to allow contact. α4 integrin (E and F, arrows) and VCAM (G, arrowheads) are expressed appropriately in the chorion and allantois, respectively, of Abi1 mutant embryos. HF, headfold; H, heart; Ch, chorion. (Scale bars: 100 μm.)
Placental Abnormalities Are Associated with Chorionic Defects in Abi1−/− Embryos.
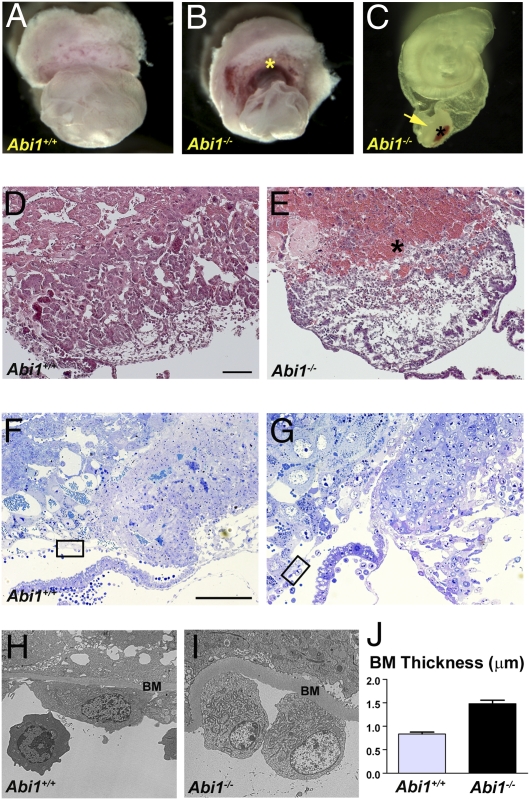
In contrast to the chorio-allantoic fusion abnormalities observed in the C57BL/6 background, Abi1 KO mice on the CD1 strain were able to initiate chorio-allantoic fusion, but displayed dramatic defects in chorionic development by E9.5 which were often accompanied by growth retardation (Fig. 2). In these Abi1−/− mice, the chorion appears compact and button-like, often with a prominent blood clot at the maternal interface (Fig. 2 B and C). In the absence of Abi1 the chorion appears densely packed, whereas at this stage the WT chorion has spread circumferentially throughout the surface of the ectoplacental cone (Fig. 2 D–G). Ultrastructurally, a thickened basement membrane associated with Reichart's membrane is apparent in Abi1−/− mice, suggestive of defects in integrin activation (Fig. 2 H–J) (29). Comparison of the phenotypes of Abi1−/− mice on the C57BL/6 and CD1 strains suggest that defects arising in chorionic trophoblast cells may underlie the aberrant placentation observed in both of these strains.
Fig. 2.
Placental defects in Abi1-null mice on the CD1 genetic background. (A) WT embryo surrounded by yolk sac with placenta. (B) Abi1 mutant embryo, also surrounded by yolk sac with hemorrhagic placenta (asterisk). (C) Abi1 mutant embryo, with a portion of the yolk sac, and attached “chorionic button” (arrow) and blood clot at the maternal surface (asterisk). (D and E) H&E sections of chorion and labyrinth regions of the placentas of WT (D) and Abi1 mutant (E) embryos. Note the blood clot adjacent to the maternal placenta in Abi−/− (asterisk). (F and G) Thin sections of the chorion and labyrinth regions of the placentas of WT (F) and Abi1 mutant (G) embryos stained with toluidine blue to show cellular detail and provide orientation for ultrathin section transmission EM analysis. Boxed areas correspond to regions shown in H and I, respectively. (H and I) Transmission EM micrographs of ultrathin sections of WT (H) and Abi1 mutant (I) embryos. Note the thickened basement membrane (BM) of Reichart membrane that surrounds the yolk sac of the embryo. (J) Quantification of basement membrane thickness in WT and Abi1 mutant embryos (P < 0.0001). (Scale bars: 100 μm.)
Loss of Abi1 Affects Cardiovascular Development.
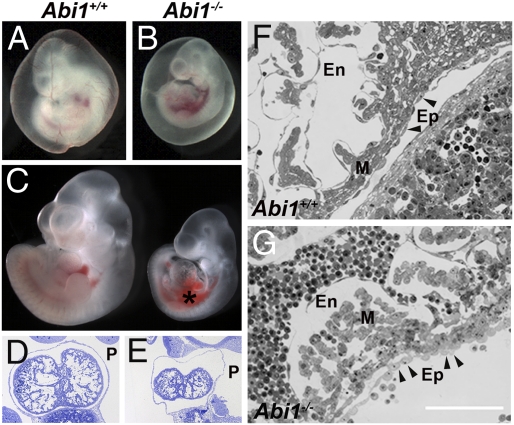
In addition to the placental and allantoic vasculogenic defects in the Abi1−/− mice, loss of Abi1 resulted in cardiovascular abnormalities (Fig. 3). Abi1−/− mice on the CD1 strain displayed reduced angiogenesis in the yolk sac, edema and hemorrhage around the heart, as well as disruption of the heart layers (Fig. 3 A–G). The epicardium of Abi1−/− mice appears disorganized (Fig. 3G), a phenotype reminiscent of the α4 integrin KO mice (21). In the absence of Abi1, the myocardium is also disorganized, and the endocardium is discontinuous (Fig. 3G). These data demonstrate a requirement for Abi1 in cardiovascular development and suggest that cardiovascular defects in the Abi1−/− mice may contribute to their embryonic lethality.
Fig. 3.
Loss of Abi1 results in cardiovascular abnormalities. (A) WT embryo with surrounding vascularized yolk sac at E10.5. (B) Abi1−/− littermate shows defective vascularization in the yolk sac. Embryos in A and B were photographed at the same magnification. (C) WT (Left) and Abi1−/− (Right) embryos with yolk sac removed were photographed together; the Abi1−/− embryo displays edema and hemorrhage around the heart. (D–G) In thin sections, the Abi1−/− heart (E) is smaller in size with a swollen pericardium and associated edema, whereas the WT heart (D) fills the pericardial cavity. At higher magnification, the epicardial (Ep), myocardial (M), and endocardial (En) layers of the heart are disorganized in Abi1−/− embryos (G) in contrast to WT embryo (F). Of note is the ruffled appearance of the epicardial cells (arrowheads in F and G) adjacent to the myocardium in Abi mutants. This phenotype is reminiscent of that of the α4 integrin KO mouse. (Scale bar: 100 μm.)
Abi1−/− MEF Cells Display Cell Spreading Defects Downstream of α4 Integrin.
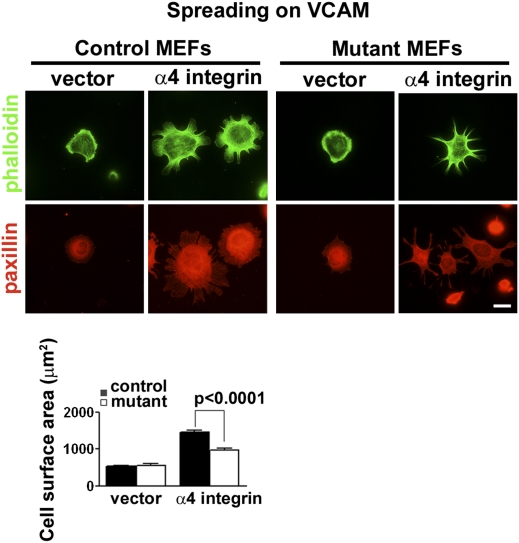
The phenotypes of the Abi1-null mice suggested that Abi1 may play a role in α4 integrin-mediated morphogenetic events, and may regulate α4-mediated signaling (21). To examine this possibility, we derived control (Abi1+/−/Abi2+/−) and Abi1 mutant (Abi1−/−/Abi2+/−) MEFs, a cell type that does not endogenously express the α4 integrin, and engineered them to exogenously express α4 integrin. Mutant MEFs were derived from embryos that were null for Abi1, and additionally heterozygous for Abi2, as we found up-regulation of Abi2 expression in MEFs that were null for Abi1 on an Abi2 WT background (Fig. S3B). Control MEFs were derived from a littermate that was heterozygous for both Abi1 and Abi2. Control and mutant MEFs were assayed for adhesion-dependent cell spreading on VCAM1, a ligand that specifically interacts with α4 integrin. As expected, in the absence of α4 expression both control and mutant cells failed to spread on VCAM1 (Fig. 4). Expression of α4 integrin in control cells allowed for significantly enhanced spreading on VCAM1 (Fig. 4). In contrast, Abi1 mutant cells expressing equivalent cell surface levels of α4 integrin were severely impaired in their ability to spread on VCAM1 (Fig. 4). These data implicate Abi1 in α4 integrin-mediated cellular behaviors, but do not address the specificity of this interaction. We tested the specificity for Abi1 in α4-mediated cell spreading by analyzing the spreading of control and mutant MEFs on a fibronectin substratum (Fig. S4). MEFs endogenously express the α5β1 integrin heterodimer, which similar to other integrins, including α4β1, interacts with the ECM protein fibronectin (30). Abi1 mutant cells exhibit only a modest decrease in cell spreading on fibronectin compared with their control counterparts, indicating Abi1 is not required for engagement downstream of the classical fibronectin receptor, α5β1 (Fig. S4). However, when engineered to express the α4 integrin, Abi1 mutant cells demonstrate a significant reduction in cell spreading on fibronectin compared with their control counterparts (Fig. S4). Together, these data support a role for Abi1 in α4 integrin-mediated signaling in vitro, and suggest that aberrant α4 signaling may underlie the embryonic lethality of Abi1−/− mice.
Fig. 4.
Abi1 mutant MEFs expressing α4 integrin show diminished cell spreading on VCAM1. Control (Abi1+/−/Abi2+/−) or mutant (Abi1−/−/Abi2+/−) MEFs were transduced with vector alone or vector containing WT full-length α4 integrin. Cells were sorted by FACS to obtain cell populations expressing equivalent amounts of α4, and these cells were plated immediately on coverslips coated with VCAM1. After 2 h, cells were fixed and processed for indirect immunofluorescence for detection of paxillin (red). Actin staining with phalloidin is shown (green). Quantification of cell spreading was analyzed by measuring cell surface area of phalloidin stained cells using Zeiss software (Bottom). Abi1 mutant cells expressing α4 integrin exhibit significantly reduced spreading compared with their control counterparts on VCAM1 substratum. (Scale bar: 10 μm.)
Abi1 Interacts with the α4 Tail.
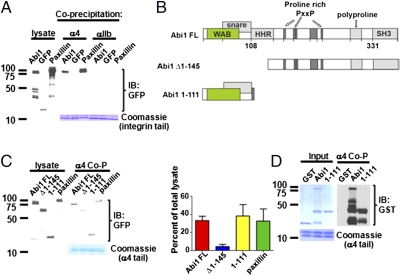
Paxillin was identified as a major binding partner of the α4 integrin, an interaction thought to regulate Rac activation and protrusive activity (2, 3). Phosphorylation of α4 at the leading edge of migrating cells disrupts the inhibitory adhesive interaction with paxillin, thereby allowing for directional migration (4, 31, 32). However, this model does not provide a mechanistic explanation for how phosphorylated α4 promotes actin-dependent lamellipodial protrusions at the leading edge. Thus, we asked whether Abi1 might function to directly link α4 to positive regulation of actin dynamics at the leading edge of lamellipodial protrusions and analyzed whether these proteins could interact physically and spatially. We found that Abi1 bound to purified α4 cytoplasmic tail (Fig. 5A). Abi1 binding to the α4 tail was selective, as no binding was detected between Abi1 and αIIb, a distinct α subunit (Fig. 5A).
Fig. 5.
Abi1 protein binds to α4 integrin. (A) Bacterially expressed α4 or αIIb integrin cytoplasmic tails were incubated with lysates from 293T cells expressing GFP-Abi1, GFP-paxillin, or GFP alone. Detection of integrin tail-bound proteins by Western blotting for GFP showed that Abi1 bound to α4 but not to αIIb (Top). Coomassie staining showed equal loading of integrin cytoplasmic tails in the pull-down assays (Bottom). (B) Structural diagram of Abi1 full-length (FL) and indicated fragments. Abi1 contains proline-rich and SH3 domains in its C terminus, and the WAVE binding (WAB), SNARE, and homeodomain homology region (HHR) in its N terminus. (C) Identification of α4-binding site on Abi1 was carried out as described in A. Deletion of the N-terminal region of Abi1 decreases binding to the α4 cytoplasmic tail, whereas the N-terminal Abi1 (1–111) fragment is sufficient for binding to the α4 tail. (D) Direct binding of purified α4 tail to purified GST-Abi1 full-length and GST-Abi1-1-111 N-terminal fragment, but not to GST alone. Coomassie staining of GST fusion proteins (Upper) and α4 integrin cytoplasmic tails is shown (Bottom). Protein molecular weight markers are indicated (A, C, and D, Left). Co-P, coprecipitation.
Abi1 is a multisubunit adaptor protein with distinct protein–protein interacting domains, including a proline-rich region and SH3 domain (Fig. 5B) (16). We found that the Abi1 N-terminal amino acids (1–145) were required for α4 binding (Fig. 5C). In contrast, the C-terminal region, containing both the proline-rich region and SH3 domain, was dispensable for interaction with α4, as a deletion mutant of Abi1 containing only the first 111 amino acids bound robustly to α4 (Fig. 5C). Moreover, by using purified GST-Abi1 full length or GST-Abi1-1-111 proteins, we showed that the interaction of Abi1 with α4 is direct (Fig. 5D). Thus, the Abi1 N-terminal sequences are both necessary and sufficient for binding to α4.
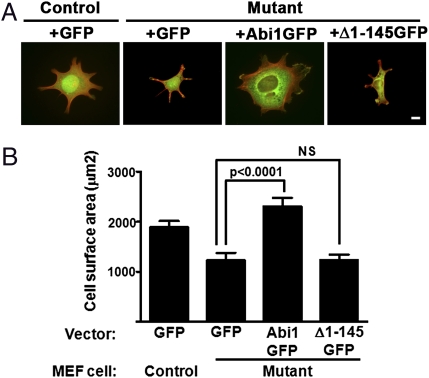
The N-Terminal Region of Abi1 Is Required for Rescue of α4-Mediated Cell Spreading on VCAM1.
Biochemical analysis demonstrated that the N-terminal region of Abi1 was required for binding to the cytoplasmic tail of the α4 integrin; thus, we wished to assess the role of this region of Abi1 in α4-mediated cell spreading. For these experiments, α4-integrin expressing control MEFs (Abi1+/−/Abi2+/−) reconstituted with GFP vector or α4-expressing mutant MEFs (Abi1−/−/Abi2+/−) reconstituted with GFP vector, WT Abi1-GFP, or the Δ1–145 Abi1-GFP were plated on VCAM1 (Fig. 6). We found that defective spreading of the α4-expressing Abi1-deficient (mutant) cells was rescued by expression of exogenous WT GFP-tagged Abi1, but not the N-terminal deletion mutant, Δ1–145, which lacks the ability to bind to the α4 integrin (Fig. 6). These data demonstrate that the N terminus of Abi1 couples α4-integrin receptor engagement to actin cytoskeleton-mediated cell spreading.
Fig. 6.
The N-terminal region of Abi1 is required for rescue of α4-mediated cell spreading on VCAM1. The α4-expressing control (Abi1+/−/Abi2+/−) MEFs were reconstituted with GFP vector and α4-expressing mutant (Abi1−/−/Abi2+/−) MEFs were reconstituted with GFP vector, GFP-tagged WT Abi1 or the GFP-tagged Δ1–145 Abi1 deletion mutant. Cells were sorted by FACS to obtain cell populations expressing equivalent amounts of α4 as well as GFP, and these cells were plated immediately on coverslips coated with VCAM1. (A) Representative images of each cell type are shown. Merged images reveal GFP or the GFP fusion proteins (green) with phalloidin-stained actin (red). (B) Quantification of cell spreading was analyzed by measuring cell surface area of phalloidin stained cells using Zeiss software. Abi1 mutant cells expressing α4 integrin and GFP displayed significantly reduced spreading compared with their control counterparts. This defect was rescued by adding back GFP-tagged WT Abi1 (compare GFP with GFP-Abi1; P < 0.0001, two-tailed Student t test), whereas GFP-Abi1 Δ1–145 was unable to rescue [GFP vs. GFP-Δ1–145; not significant (NS)]. (Scale bar: 10 μm.)
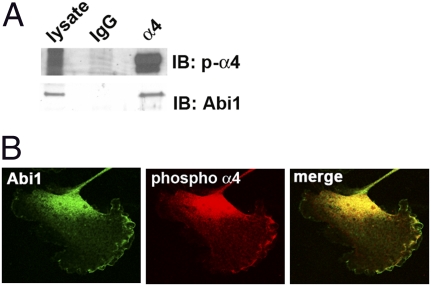
Endogenous Abi1 and Phosphorylated α4 Colocalize at the Leading Edge.
To test whether endogenous α4 and Abi1 could interact, we used A7r5 cells, a rat smooth muscle cell line that expresses α4 as well as Abi1, and responds to scratch wound stimuli by accumulation of S988-phosphorylated α4 at the leading edge (33). Indeed, endogenous Abi1 coimmunoprecipitated with endogenous α4 integrin, and these immunoprecipitates contain S988-phosphorylated α4 (Fig. 7A). In cells plated on VCAM1, Abi1 and S988-phosphorylated α4 colocalized at the leading edge as detected by confocal microscopy (Fig. 7B). Thus, Abi1 binding to the α4 integrin at the leading edge of migrating cells or in polarized epithelium provides a direct link to the regulation of actin dynamics during tissue morphogenesis.
Fig. 7.
Endogenous Abi1 interacts with endogenous α4 integrin. (A) Lysates from A7r5 cells were subjected to immunoprecipitation with the HP2/1 antibody specific for α4 integrin. Coprecipitation of endogenous Abi1 with α4 integrin containing S988-phosphorylated α4 integrin was detected by using a phospho-specific antibody. Specificity was shown by incubation of lysates with isotype IgG control. (B) Indirect immunofluorescence of endogenous Abi1 and S988-phosphorylated α4 integrin proteins revealed colocalization at the leading edge of cells spread on VCAM1 by using confocal microscopy.
Discussion
Analysis of Abi1 mutant mice uncovered an unexpected mechanistic link between Abi1-regulated cytoskeletal dynamics and α4 integrin-dependent morphogenetic events. Mice knockout for α4 or Abi1 exhibit defective chorio-allantoic fusion and cardiovascular defects. Chorio-allantoic fusion is regulated by the binding of α4 integrin on the surface of the chorion to VCAM1 on the surface of the allantois (21–23). The defects in placental development observed in the absence of Abi1 suggest that loss of Abi1 in the chorionic mesothelium impairs α4-mediated signaling, ultimately leading to chorionic abnormalities and placental failure in the C57BL/6 or CD1 genetic background. Abi1-null mice, similar to α4 integrin and VCAM1 KO mice, display defects in cardiovascular development with impaired vessel maturation in the yolk sac, defective adhesion of epicardial cells to the adjacent myocardium, and general edema around the heart. Interestingly, mice knocked out for the Abi-binding protein WAVE2 also exhibit cardiovascular abnormalities and die midgestationally (24). WAVE2-deficient mice exhibit impaired vascular remodeling in response to growth factor stimulation (24). Further, mouse mutants of Nap1, a direct binding partner of Abi1 in the WAVE complex, also exhibit defective chorio-allantoic fusion, and other developmental phenotypes associated with defects in cell migration and adhesion (34). Interestingly, heterozygous insertional mutants of Nap1, which display neural tube closure defects, also have dramatic reductions in vessel diameter (35). Thus, the Abi1–WAVE2–Nap1 complex may regulate a subset of signals downstream of integrin receptors during cardiovascular development.
In contrast to other integrin subunits, α4 integrin signaling occurs primarily in the cell periphery, rather than in focal adhesions or focal complexes (4, 33, 36). Indeed, expression of α4 in CHO cells promotes protrusion of broad lamellipodia in response to scratch wounding (36). Furthermore, phosphorylated α4 integrin preferentially localizes to the leading edge of migrating cells and this localization is linked to positive regulation of lamellipodial extensions and enhanced cell migration (37, 38). Unexpectedly, we found that Abi1 interacts with the purified α4 cytoplasmic tail and colocalizes with the endogenous phosphorylated α4 integrin at the leading edge of spreading cells. To date, the model for the regulation of α4-mediated directional migration hinges on the interaction of α4 with paxillin, which negatively regulates Rac activity, and the termination of this association at the leading edge by phosphorylation of the α4 cytoplasmic tail. Paxillin and α4 remain associated at the sides and rear of the cell, where paxillin recruits the ADP ribosylation factor GTPase activating protein (Arf-GAP), leading to the inhibition of Rac (32). Directional cell migration was thereby proposed to occur by relieving an inhibitory interaction at the leading edge of the cell; however, the signals that link α4 to enhanced actin polymerization and protrusive activity at the leading edge have remained poorly understood. We have identified Abi1 as a key mediator of α4-mediated signaling at the leading edge. Abi1 interacts and colocalizes in discrete puncta at the leading edge of lamellipodia with phosphorylated α4. Moreover, Abi1 interacts with the purified cytoplasmic tail of α4 in vitro and KO of Abi1 dramatically diminishes cell spreading of fibroblasts engineered to express α4 on both VCAM1 and fibronectin.
The α4β1 integrin heterodimer plays an important role in diseases of the cardiovascular and immune systems (39). Antagonists of α4 have shown promise in the treatment of autoimmune disorders such as multiple sclerosis and Crohn's disease, but the associated toxicities have led to the design of alternative approaches other than those directed at blocking integrin-ligand binding (40, 41). Blocking α4 signaling may have important implications with respect to ablation of tumor vasculature, as it has been recently demonstrated that homing of progenitor cells to tumor areas undergoing active angiogenesis uses the α4 integrin/VCAM1 counter receptor pair (42, 43). Moreover, exciting new findings have shown that suppression of α4β1 function in the endothelium inhibits tumor lymphangiogenesis and metastasis in mouse models (44). Current approaches to antagonize α4 function have focused on development of drugs that disrupt the interaction of α4 with paxillin (41). Our data suggest that development of inhibitors of the Abi1–α4 interaction may be an alternative therapeutic strategy for the treatment of metastatic tumors, inflammatory immune disorders and other α4-dependent pathologic processes.
Materials and Methods
Generation of Abi1−/− Mice.
The mouse Abi1 locus was targeted by homologous recombination using standard methods and confirmed by Southern blotting and PCR analysis as described in Fig. S1. Additional experimental details are provided in SI Materials and Methods. Included in this information are reagents and protocols with respect to coprecipitation and in vitro binding assays, cell culture, cell spreading assays and immunofluorescence microscopy as well as statistical analysis performed.
Supplementary Material
Acknowledgments
The authors thank Cheryl Bock of the Duke Transgenic and Knockout Mouse Facility for generation of Abi1 KO mice and Matthew Grove for preparation of the targeting vector and identification of positive embryonic stem cell clones. We also thank Sam Johnson of the Duke Light Microscopy Facility for excellent advice, Rob Wechsler-Reya for use of the stereoscope, Phillip Christopher of Duke Electron Microscopy Services, Donna Webb for insights on evaluation of focal adhesion dynamics, and James Cross for insights into the placental phenotypes. This study was supported by National Institutes of Health Grants CA070940 (to A.M.P.), HL084102 (to A.M.P.), and AR27214 (to M.H.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012316108/-/DCSupplemental.
References
- 1.Legate KR, Fässler R. Mechanisms that regulate adaptor binding to β-integrin cytoplasmic tails. J Cell Sci. 2009;122:187–198. doi: 10.1242/jcs.041624. [DOI] [PubMed] [Google Scholar]
- 2.Liu S, et al. Binding of paxillin to α4 integrins modifies integrin-dependent biological responses. Nature. 1999;402:676–681. doi: 10.1038/45264. [DOI] [PubMed] [Google Scholar]
- 3.Han J, et al. Phosphorylation of the integrin α 4 cytoplasmic domain regulates paxillin binding. J Biol Chem. 2001;276:40903–40909. doi: 10.1074/jbc.M102665200. [DOI] [PubMed] [Google Scholar]
- 4.Goldfinger LE, Han J, Kiosses WB, Howe AK, Ginsberg MH. Spatial restriction of α4 integrin phosphorylation regulates lamellipodial stability and α4β1-dependent cell migration. J Cell Biol. 2003;162:731–741. doi: 10.1083/jcb.200304031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dai Z, Pendergast AM. Abi-2, a novel SH3-containing protein interacts with the c-Abl tyrosine kinase and modulates c-Abl transforming activity. Genes Dev. 1995;9:2569–2582. doi: 10.1101/gad.9.21.2569. [DOI] [PubMed] [Google Scholar]
- 6.Shi Y, Alin K, Goff SP. Abl-interactor-1, a novel SH3 protein binding to the carboxy-terminal portion of the Abl protein, suppresses v-abl transforming activity. Genes Dev. 1995;9:2583–2597. doi: 10.1101/gad.9.21.2583. [DOI] [PubMed] [Google Scholar]
- 7.Stradal TE, Scita G. Protein complexes regulating Arp2/3-mediated actin assembly. Curr Opin Cell Biol. 2006;18:4–10. doi: 10.1016/j.ceb.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 8.Grove M, et al. ABI2-deficient mice exhibit defective cell migration, aberrant dendritic spine morphogenesis, and deficits in learning and memory. Mol Cell Biol. 2004;24:10905–10922. doi: 10.1128/MCB.24.24.10905-10922.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ryu JR, Echarri A, Li R, Pendergast AM. Regulation of cell-cell adhesion by Abi/Diaphanous complexes. Mol Cell Biol. 2009;29:1735–1748. doi: 10.1128/MCB.01483-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yang C, et al. Novel roles of formin mDia2 in lamellipodia and filopodia formation in motile cells. PLoS Biol. 2007;5:e317. doi: 10.1371/journal.pbio.0050317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pollitt AY, Insall RH. Abi mutants in Dictyostelium reveal specific roles for the SCAR/WAVE complex in cytokinesis. Curr Biol. 2008;18:203–210. doi: 10.1016/j.cub.2008.01.026. [DOI] [PubMed] [Google Scholar]
- 12.Innocenti M, et al. Abi1 regulates the activity of N-WASP and WAVE in distinct actin-based processes. Nat Cell Biol. 2005;7:969–976. doi: 10.1038/ncb1304. [DOI] [PubMed] [Google Scholar]
- 13.Kondylis V, van Nispen tot Pannerden HE, Herpers B, Friggi-Grelin F, Rabouille C. The Golgi comprises a paired stack that is separated at G2 by modulation of the actin cytoskeleton through Abi and Scar/WAVE. Dev Cell. 2007;12:901–915. doi: 10.1016/j.devcel.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 14.Anitei M, et al. Protein complexes containing CYFIP/Sra/PIR121 coordinate Arf1 and Rac1 signalling during clathrin-AP-1-coated carrier biogenesis at the TGN. Nat Cell Biol. 2010;12:330–340. doi: 10.1038/ncb2034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zipfel PA, et al. Role for the Abi/wave protein complex in T cell receptor-mediated proliferation and cytoskeletal remodeling. Curr Biol. 2006;16:35–46. doi: 10.1016/j.cub.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 16.Echarri A, Lai MJ, Robinson MR, Pendergast AM. Abl interactor 1 (Abi-1) wave-binding and SNARE domains regulate its nucleocytoplasmic shuttling, lamellipodium localization, and wave-1 levels. Mol Cell Biol. 2004;24:4979–4993. doi: 10.1128/MCB.24.11.4979-4993.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Stradal T, et al. The Abl interactor proteins localize to sites of actin polymerization at the tips of lamellipodia and filopodia. Curr Biol. 2001;11:891–895. doi: 10.1016/s0960-9822(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 18.Takenawa T, Suetsugu S. The WASP-WAVE protein network: connecting the membrane to the cytoskeleton. Nat Rev Mol Cell Biol. 2007;8:37–48. doi: 10.1038/nrm2069. [DOI] [PubMed] [Google Scholar]
- 19.Goley ED, Welch MD. The ARP2/3 complex: An actin nucleator comes of age. Nat Rev Mol Cell Biol. 2006;7:713–726. doi: 10.1038/nrm2026. [DOI] [PubMed] [Google Scholar]
- 20.Lin TY, et al. Abi plays an opposing role to Abl in Drosophila axonogenesis and synaptogenesis. Development. 2009;136:3099–3107. doi: 10.1242/dev.033324. [DOI] [PubMed] [Google Scholar]
- 21.Yang JT, Rayburn H, Hynes RO. Cell adhesion events mediated by α 4 integrins are essential in placental and cardiac development. Development. 1995;121:549–560. doi: 10.1242/dev.121.2.549. [DOI] [PubMed] [Google Scholar]
- 22.Kwee L, et al. Defective development of the embryonic and extraembryonic circulatory systems in vascular cell adhesion molecule (VCAM-1) deficient mice. Development. 1995;121:489–503. doi: 10.1242/dev.121.2.489. [DOI] [PubMed] [Google Scholar]
- 23.Gurtner GC, et al. Targeted disruption of the murine VCAM1 gene: essential role of VCAM-1 in chorioallantoic fusion and placentation. Genes Dev. 1995;9:1–14. doi: 10.1101/gad.9.1.1. [DOI] [PubMed] [Google Scholar]
- 24.Yamazaki D, et al. WAVE2 is required for directed cell migration and cardiovascular development. Nature. 2003;424:452–456. doi: 10.1038/nature01770. [DOI] [PubMed] [Google Scholar]
- 25.Kummer C, Ginsberg MH. New approaches to blockade of α4-integrins, proven therapeutic targets in chronic inflammation. Biochem Pharmacol. 2006;72:1460–1468. doi: 10.1016/j.bcp.2006.06.014. [DOI] [PubMed] [Google Scholar]
- 26.Qian H, et al. Distinct roles of integrins α6 and α4 in homing of fetal liver hematopoietic stem and progenitor cells. Blood. 2007;110:2399–2407. doi: 10.1182/blood-2006-10-051276. [DOI] [PubMed] [Google Scholar]
- 27.Garmy-Susini B, et al. Integrin α4β1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–1551. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Downs KM, Gifford S, Blahnik M, Gardner RL. Vascularization in the murine allantois occurs by vasculogenesis without accompanying erythropoiesis. Development. 1998;125:4507–4520. doi: 10.1242/dev.125.22.4507. [DOI] [PubMed] [Google Scholar]
- 29.van der Flier A, et al. Endothelial α5 and alphav integrins cooperate in remodeling of the vasculature during development. Development. 2010;137:2439–2449. doi: 10.1242/dev.049551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Geiger B, Bershadsky A, Pankov R, Yamada KM. Transmembrane extracellular matrix–cytoskeleton crosstalk. Nat Rev Mol Cell Biol. 2001;2:793–805. doi: 10.1038/35099066. [DOI] [PubMed] [Google Scholar]
- 31.Liu S, et al. A fragment of paxillin binds the α 4 integrin cytoplasmic domain (tail) and selectively inhibits α 4-mediated cell migration. J Biol Chem. 2002;277:20887–20894. doi: 10.1074/jbc.M110928200. [DOI] [PubMed] [Google Scholar]
- 32.Nishiya N, Kiosses WB, Han J, Ginsberg MH. An α4 integrin-paxillin-Arf-GAP complex restricts Rac activation to the leading edge of migrating cells. Nat Cell Biol. 2005;7:343–352. doi: 10.1038/ncb1234. [DOI] [PubMed] [Google Scholar]
- 33.Howe AK, Baldor LC, Hogan BP. Spatial regulation of the cAMP-dependent protein kinase during chemotactic cell migration. Proc Natl Acad Sci USA. 2005;102:14320–14325. doi: 10.1073/pnas.0507072102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rakeman AS, Anderson KV. Axis specification and morphogenesis in the mouse embryo require Nap1, a regulator of WAVE-mediated actin branching. Development. 2006;133:3075–3083. doi: 10.1242/dev.02473. [DOI] [PubMed] [Google Scholar]
- 35.Yokota Y, Ring C, Cheung R, Pevny L, Anton ES. Nap1-regulated neuronal cytoskeletal dynamics is essential for the final differentiation of neurons in cerebral cortex. Neuron. 2007;54:429–445. doi: 10.1016/j.neuron.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pinco KA, He W, Yang JT. α4β1 integrin regulates lamellipodia protrusion via a focal complex/focal adhesion-independent mechanism. Mol Biol Cell. 2002;13:3203–3217. doi: 10.1091/mbc.02-05-0086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Féral CC, et al. Blocking the α 4 integrin-paxillin interaction selectively impairs mononuclear leukocyte recruitment to an inflammatory site. J Clin Invest. 2006;116:715–723. doi: 10.1172/JCI26091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chan JR, Hyduk SJ, Cybulsky MI. Chemoattractants induce a rapid and transient upregulation of monocyte α4 integrin affinity for vascular cell adhesion molecule 1 which mediates arrest: an early step in the process of emigration. J Exp Med. 2001;193:1149–1158. doi: 10.1084/jem.193.10.1149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Liu S, Rose DM, Han J, Ginsberg MH. α4 integrins in cardiovascular development and diseases. Trends Cardiovasc Med. 2000;10:253–257. doi: 10.1016/s1050-1738(00)00073-6. [DOI] [PubMed] [Google Scholar]
- 40.Cantor JM, Ginsberg MH, Rose DM. Integrin-associated proteins as potential therapeutic targets. Immunol Rev. 2008;223:236–251. doi: 10.1111/j.1600-065X.2008.00640.x. [DOI] [PubMed] [Google Scholar]
- 41.Lee J, et al. A small molecule inhibitor of α4 integrin-dependent cell migration. Bioorg Med Chem. 2009;17:977–980. doi: 10.1016/j.bmc.2008.02.067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jin H, et al. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;116:652–662. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–617. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Garmy-Susini B, et al. Integrin α4β1 signaling is required for lymphangiogenesis and tumor metastasis. Cancer Res. 2010;70:3042–3051. doi: 10.1158/0008-5472.CAN-09-3761. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.