Abstract
Brown fat is specialized for energy expenditure and has therefore been proposed to function as a defense against obesity. Despite recent advances in delineating the transcriptional regulation of brown adipocyte differentiation, cellular lineage specification and developmental cues specifying brown-fat cell fate remain poorly understood. In this study, we identify and isolate a subpopulation of adipogenic progenitors (Sca-1+/CD45−/Mac1−; referred to as Sca-1+ progenitor cells, ScaPCs) residing in murine brown fat, white fat, and skeletal muscle. ScaPCs derived from different tissues possess unique molecular expression signatures and adipogenic capacities. Importantly, although the ScaPCs from interscapular brown adipose tissue (BAT) are constitutively committed brown-fat progenitors, Sca-1+ cells from skeletal muscle and subcutaneous white fat are highly inducible to differentiate into brown-like adipocytes upon stimulation with bone morphogenetic protein 7 (BMP7). Consistent with these findings, human preadipocytes isolated from subcutaneous white fat also exhibit the greatest inducible capacity to become brown adipocytes compared with cells isolated from mesenteric or omental white fat. When muscle-resident ScaPCs are re-engrafted into skeletal muscle of syngeneic mice, BMP7-treated ScaPCs efficiently develop into adipose tissue with brown fat-specific characteristics. Importantly, ScaPCs from obesity-resistant mice exhibit markedly higher thermogenic capacity compared with cells isolated from obesity-prone mice. These data establish the molecular characteristics of tissue-resident adipose progenitors and demonstrate a dynamic interplay between these progenitors and inductive signals that act in concert to specify brown adipocyte development.
In mammals, thermogenesis is essential for maintaining physiological homeostasis. Brown adipose tissue (BAT) is specialized for thermogenic energy expenditure with unique expression of uncoupling protein 1 (UCP1). Given its specialized function to dissipate chemical energy, BAT provides a natural defense against cold and obesity (1–3). Although BAT was once considered nonexistent in adult humans, recent morphological and imaging studies demonstrate that brown fat is present in adults and its presence and activity inversely correlate with age-related adiposity and indices of metabolic syndrome (4–10).
Recent studies have suggested that brown fat and skeletal muscle may share a common developmental ancestry (11, 12). Myf5-expressing progenitors can give rise to both skeletal muscle and interscapular brown fat (13). In accordance with these observations, brown-fat progenitors have also been identified in human skeletal muscle (14). However, not all brown-fat cells are derived from myf5-expressing progenitors. For example, the brown-fat cells emerging in white fat in response to β3-adrenergica stimulation are not marked by myf5-driven fluorescent protein (13). Following stimulation with peroxisome proliferator-activated receptor-γ (PPARγ) agonists, they express molecular characteristics distinct from interscapular brown-fat cells (15). Interestingly, the brown-fat cells found in white fat and between muscle bundles appear to be more abundant in obesity-resistant strains of mice (16–18), suggesting a potential role for these systemic brown adipocytes in protection against obesity (19).
Cell-fate determination in stem/progenitor cells is controlled by the integration of cell-intrinsic factors with extrinsically inductive cues (20). Bone morphogenetic proteins (BMPs) have been implicated as developmental factors that provide instructive signals to guide differentiation of stem-cell populations during embryogenesis and organogenesis (21). Emerging evidence suggests that different members of the BMP superfamily play a significant role in adipocyte development and energy metabolism (22, 23). Whereas BMP2 and BMP4 enhance white adipogenesis (24, 25), we recently discovered that BMP7 specifically promotes brown-fat differentiation in multipotent mesenchymal cells (26). BMP7 knockout mice have a marked paucity of interscapular brown fat at birth, suggesting that BMP7 plays an essential role in embryonic brown-fat development.
In this study, we prospectively isolated a subpopulation of adipose progenitors from different fat depots and skeletal muscle of adult mice using combinations of cell-surface markers (Sca-1+, CD45−, Mac1−). These cells express unique patterns of developmental markers. Furthermore, these cells display different degrees of sensitivity to inducers of brown-fat differentiation. Importantly, Sca-1+ adipogenic cells isolated from tissues of obesity-resistant strains of mice display increased brown adipogenic capacity compared with cells isolated from obesity-prone mice. Taken together, these data define a population of tissue-resident, inducible brown-fat progenitors in adult mice. These cells may provide an important target for the development of new therapeutic approaches for the treatment of obesity and its many associated metabolic complications.
Results
Tissue Resident Sca-1+ Progenitors Possess Differential Degrees of Brown Adipogenic Capacities and Express Unique Molecular Signatures.
Recent studies have demonstrated the presence of white-fat progenitors residing in white adipose tissue (WAT) as well as in skeletal muscle in adult mice (27–30). These progenitor cells express the cell surface marker stem cell antigen-1 (Sca-1), among others, and can differentiate into white adipocytes both in vitro and following implantation into mice. We conducted a comprehensive study to characterize nonhematopoietic, Sca-1+ progenitor cells (ScaPCs, Sca-1+/CD45−/Mac1−) isolated from interscapular BAT (iBAT), subcutaneous white adipose tissue (sWAT), epididymal WAT (eWAT), and skeletal muscle (MUS) by FACS. Additional analysis of surface marker expression on freshly isolated and short-term cultured MUS-ScaPCs revealed that this population of cells homogeneously expresses surface markers commonly found on adipose progenitors (Fig. S1).
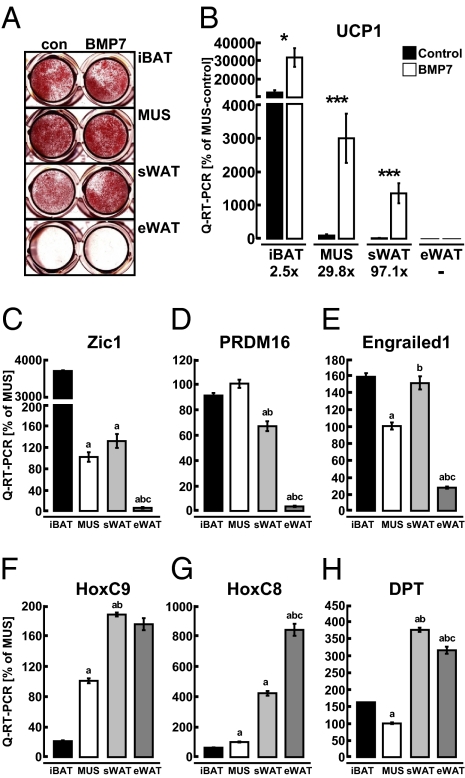
BMPs have been implicated as niche factors in body patterning (31) and stem cell differentiation (32). We recently discovered that BMP7 specifically promotes brown-fat differentiation in both committed and uncommitted adipose progenitors (26). To determine whether BMP7 could trigger commitment of ScaPCs into the brown adipocyte lineage, we treated ScaPCs isolated from all four tissues with BMP7 for 3 d, followed by adipogenic differentiation without further exposure to BMP7 (Fig. S2). ScaPCs isolated from iBAT, MUS, and sWAT efficiently differentiated into adipocytes, as indicated by lipid specific Oil Red O staining and expression of fatty acid synthase (Fig. 1A and Fig. S3A). However, ScaPCs isolated from eWAT displayed very poor differentiation capacities that were marginally enhanced by the BMP7 treatment. Regardless of the similar extent of lipid accumulation in differentiated ScaPCs derived from iBAT, MUS, and sWAT, they showed very drastically different levels of expression of the brown fat-defining marker, UCP1 (Fig. 1B). ScaPCs isolated from iBAT expressed the highest levels of UCP1, indicating that a strong commitment toward the brown adipogenic lineage was already present in these progenitors. The brown adipogenic morphogen, BMP7, induced expression of UCP1 in all three types of ScaPCs. Whereas this increase was 2.5-fold in iBAT-derived cells, the same treatment led to a profound induction of UCP1 in ScaPCs derived from MUS and sWAT, reaching levels of induction up to 30- and 97-fold, respectively. There was no detectable UCP1 expression in ScaPCs derived from eWAT, consistent with their lower adipogenic potential (Fig. 1 A and B). A comparable pattern of induction was also observed for another brown-fat marker, cell death-inducing DFFA-like effector A (CIDEA) (33) (Fig. S3B). These findings demonstrate that the ScaPCs residing in different fat depots and MUS possess distinct sensitivity to induction of a brown adipocyte phenotype. Hence, the default setting for these MUS and sWAT resident ScaPCs is to undergo white adipogenesis, but in the presence of appropriate inductive cues, such as BMP7, they become committed to a brown adipocyte lineage.
Fig. 1.
ScaPCs from muscle and sWAT, but not from eWAT, can be induced to undergo brown adipogenesis. Distinct expression profiles are associated with brown adipogenic capacity. (A) Oil Red O staining of lipid-accumulating cells in both control and BMP7 pretreated cells from iBAT, MUS, sWAT, and eWAT. (B) Quantitative RT-PCR of UCP1 in differentiated ScaPCs with and without BMP7 pretreatment. Fold-changes are given for each tissue type of BMP7-treatment over control. Black bars indicate control conditions; white bars indicate BMP7-pretreatment. Data are presented as mean ± SEM (n = 3). Asterisks denote significant differences between control and BMP7-pretreated cells. *P < 0.05; **P < 0.01; ***P < 0.001. (C–H) Quantitative RT-PCR analysis of undifferentiated Sca-1+ progenitor cells isolated from iBAT, MUS, sWAT, and eWAT: (C) Zic-1, (D) PRDM16, (E) Engrailed1 (Eng1), (F) HoxC9, (G) HoxC8, and (H) dermatopontin (DPT). Data are presented as mean ± SEM (n = 3); “a” depicts statistically significant differences (P < 0.05) between iBAT and the indicated tissue; “b” depicts statistically significant (P < 0.05) differences between MUS and indicated tissue; “c” depicts statistically significant (P < 0.05) differences between sWAT and eWAT.
To define further the molecular characteristics of ScaPCs isolated from all four tissues, we quantified the expression of several developmental markers in undifferentiated ScaPCs that have been associated with different adipogenic lineages. Zic-1, a transcription factor that is highly expressed in iBAT (12, 15), displayed a 30- to 40-fold higher level of expression in ScaPCs isolated from iBAT compared with cells from other tissues; however, its expression was still significantly higher in ScaPCs from muscle and sWAT compared with eWAT (Fig. 1C). PRD1-BF1-RIZ1 homologous containing protein-16 (PRDM16) has been previously identified as being highly expressed in mature brown fat compared with low expression in epididymal white fat (34). Consistent with these findings, we found that expression of PRDM16 was high not only in ScaPCs derived from iBAT, but also in cells isolated from muscle and sWAT, and it was expressed at a very low level in ScaPCs from eWAT (Fig. 1D). Engrailed1, a homeobox transcription factor that marks progenitors giving rise to iBAT, dorsal dermis, and epaxial muscle in in vivo fate mapping experiments (11) showed relatively high levels of expression in ScaPCs derived from iBAT, sWAT, and MUS (Fig. 1E). Moreover, expression of the white (pre)adipocyte markers HoxC9, HoxC8, and dermatopontin (12, 15) was elevated in ScaPCs from both white-fat depots (Fig. 1 F–H). HoxC8 and HoxC9 were expressed at intermediate levels in muscle-derived cells. Together, these data indicate that Sca-1+ progenitors derived from different fat depots and skeletal muscle express unique molecular signatures that can help establish functional similarities among these cells. Although the strongest distinction is observed between iBAT-ScaPCs and eWAT-ScaPCs, the ScaPCs isolated from muscle and sWAT express markers common to both brown and white fat, placing these cells in an intermediate position.
Activation of Full Brown Adipogenic Capacity in Muscle-Resident ScaPCs by BMP7.
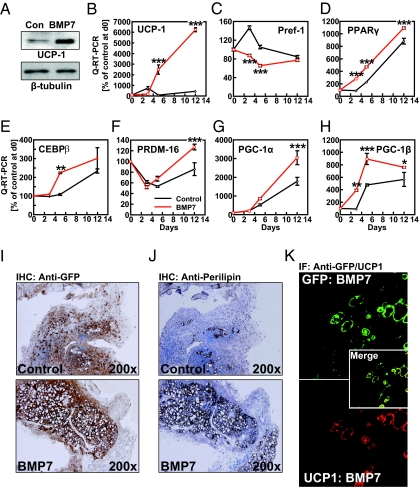
To further characterize the molecular mechanisms underlying BMP7-induced brown adipogenesis in ScaPCs, which we identified in MUS and sWAT, we performed a detailed analysis of molecular events throughout the entire time course of adipogenesis in ScaPCs isolated from MUS. Pretreatment of ScaPCs with BMP7 accelerated adipogenic differentiation, as shown by increases in lipid accumulation at both day 5 and 7 (Fig. S2). However, at day 12 more than 90% of ScaPCs had become lipid-containing cells, regardless of BMP7 treatment. Despite the comparable levels of lipid accumulation, the BMP7-primed cells had markedly increased expression of the brown-fat marker UCP1 (Fig. 2 A and B). Expression of the adipogenic inhibitor, preadipocyte factor-1 (35) was significantly reduced following 3 d of BMP7 treatment, providing a potential mechanism for BMP7 action (Fig. 2C). Conversely, expression of the proadipogenic factors PPARγ and CCAAT/enhancer-binding proteinß (C/EBPβ) (36) was elevated in the ScaPCs pretreated with BMP7 from day 3 to day 12 compared with cells treated with vehicle (Figs. 2 D and E). Importantly, BMP7 induced the expression of key transcriptional regulators that determine brown-fat fate, including proliferator-activated receptor-γ coactivator (PGC)-1α (37), PGC-1β (38), and PRDM16 (34) at day 3 or later time points (Figs. 2 F–H).
Fig. 2.
Pretreatment of muscle-resident ScaPCs with BMP7 results in formation of brown adipocytes in vitro and in vivo. (A) Protein levels of UCP1 following pretreatment with BMP-7 and 48 h of adipogenic induction. (B–H) Quantitative RT-PCR analysis of gene expression during differentiation of ScaPCs: 16 h after seeding cells (day 0), ScaPCs were pretreated with 3.3 nM BMP7 for 3 d (day 3), followed by adipogenic induction for 48 h (day 5) and 7 d of adipogenic maturation (day 12). Expression of (B) UCP1, (C) preadipocyte factor-1 (pref-1), (D) PPARγ, (E) C/EBPβ, (F) PRDM16, (G) PGC-1α, and (H) PGC-1β. Red lines depict expression levels for BMP-7 treated cells, and controls cells are indicated in black. Data are presented as mean ± SEM (n = 3). (I) GFP immunohistochemical staining in skeletal muscle of non-GFP isogenic recipient mice transplanted with ScaPCs treated with BMP7 or vehicle for 4 d (original magnification: 200×). (J) Perilipin immunohistochemical staining of a tissue section adjacent to the section stained for GFP, as shown in panel I (original magnifications 200×). (K) Immunofluorescence detection of colocalization of GFP expression with UCP1 expression. (Upper) Detection of GFP (green); (Lower) expression of UCP1 (red). Merge (Inset) demonstrates colocalization of GFP and UCP1 (yellow). Sections adjacent to those showing GFP-positive staining in J were used for IF (original magnification: 400×). *P < 0.05; **P < 0.01; ***P < 0.001.
Microarray analysis of ScaPCs treated with BMP7 or vehicle for 3 d identified a highly coordinated enrichment in >200 genes involved in mitochondrial biogenesis and energy metabolism (Fig. S4A and Table S1). Notably, this analysis also revealed a threefold decrease in expression of zinc-finger protein, Zfp423. The latter is an upstream regulator of PPARγ via interaction with Smad proteins (39), which in turn are known BMP targets. These observations provide an additional mechanism for BMP7’s adipogenic action. In conjunction with the induction of mitochondrial gene expression, we observed increased uncoupled respiration in MUS- and sWAT-derived ScaPCs treated with BMP7 (Fig. S4 B and C).
Consistent with previous observations (29, 40, 41), we found that muscle-derived ScaPCs are nonmyogenic and nonosteogenic, also when treated with 3.3 nM of BMP7 (Fig. S5). Additionally, we also demonstrated that ScaPCs do not derive from the myf5-positive lineage (Fig. S6) that gives rise to muscle and constitutive BATs (13). These data suggest that the muscle-derived ScaPCs are committed adipogenic progenitors and represent an inducible population of brown-fat precursors that are distinct from the myf5-expressing progenitor cells.
BMP7-Treated ScaPCs Reconstitute Brown-Fat Pads in MUS.
To determine whether ScaPCs can develop into brown-fat cells in vivo, ScaPCs purified from muscle of GFP-transgenic mice were treated with BMP7 and then transplanted into the gastrocnemius muscle of GFP− recipient mice. We chose this approach over simple subcutaneous implantations because ScaPCs injected directly under the skin did not form any tissue. Engraftment of GFP+ cells and differentiation into mature adipocytes was evident in recipient mice that received BMP7-treated ScaPCs as early as 10 d after injection, as determined by direct epifluorescence and subsequent immunohistochemical staining for GFP (Fig. 2I). Further immunohistochemical analysis revealed that the BMP7-treated ScaPCs differentiated into distinct fat pads in vivo, as shown by positive staining for the lipid-droplet–associated protein, perilipin (Fig. 2J). Notably, without BMP7 treatment, ScaPCs differentiated poorly following implantation and only a few GFP+ adipocytes could be found after sectioning the entire GFP+ area of the recipient muscle. Immunohistochemical analysis revealed that BMP7-treated MUS-ScaPCs could develop into brown fat-like multilocular cells mixed with some unilocular adipocytes within muscle (Fig. 2J), but with low levels of UCP1 protein. It has been noted that full activation of BAT-mediated thermogenesis in vivo requires sympathetic stimulation (1, 19). We therefore treated recipient mice with CL 316,243, a selective β3-adrenergic receptor agonist, for 8 d following implantation of ScaPCs into muscle. This process resulted in a significant induction of UCP1 protein in all GFP+ adipocytes derived from engrafted BMP7-treated ScaPCs (Fig. 2K). Although BMP7 alone was able to induce a brown-fat phenotype in vitro, the reconstituted fat pad may need additional stimuli, such as CL316,243, to activate its full thermogenic capacity.
To determine whether engraftment and differentiation of ScaPCs is influenced by the microenvironment, we injected the muscle-derived ScaPCs into the epididymal and subcutaneous white-fat depots. Although BMP7-treated cells injected into sWAT differentiated comparably to those engrafts found in MUS, cells injected into eWAT displayed relatively lower levels of adipocyte formation (Fig. S7 A and B). Similarly, injection of BMP7-treated ScaPCs derived from sWAT into MUS resulted in adipocyte formation (Fig. S7C). Thus, although the ScaPCs isolated from various depots could differentiate into lipid-containing cells in vitro with or without BMP7 pretreatment (Fig. 1A and Fig. S2), the ability of these cells to successfully engraft and differentiate into fat tissues in vivo is largely influenced by BMP7 treatment and to a lesser extent by the microenvironment.
Genetic Background Determines the Brown Adipogenic Capacity of ScaPCs in MUS and sWAT.
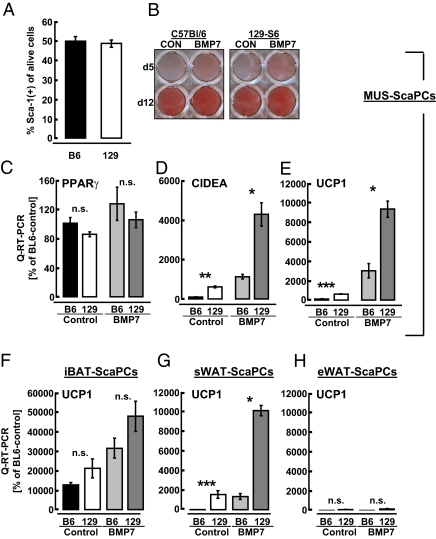
UCP1-expressing brown adipocytes in MUS or white fat have been proposed to contribute to genetic resistance toward obesity (16, 18). To determine whether ScaPCs are a potential source of these inducible brown adipocytes, we first compared the frequency and differentiation of muscle-derived ScaPCs isolated from obesity-prone C57BL/6 and obesity-resistant 129-S1 mice. Both strains showed comparable abundance of Sca-1+ cells (Fig. 3A). ScaPCs isolated from the MUS of these two strains of mice also exhibited the same degree of adipogenic ability, as assessed by Oil Red O staining (Fig. 3B) and gene expression of PPARγ (Fig. 3C). However, already under basal conditions, expression of brown-fat markers CIDEA and UCP1 in differentiated ScaPCs was 6.2- and 6.1-fold higher, respectively, in cells isolated from 129-S1 compared with cells from C57BL/6 mice (Fig. 3 D and E). Expression of these markers was further increased in BMP7-treated cells, and strain-dependent differences remained. Similar findings were observed in ScaPCs isolated from another obesity-resistant strain of mice, the A/J mice, compared with cells from C57BL/6 mice (Fig. S8 A–C).
Fig. 3.
Genetic background determines the propensity of ScaPCs isolated from muscle and sWAT, but not from iBAT, to undergo brown adipogenesis. (A–E) Analyses of ScaPCs derived from skeletal muscle. (A) Frequency of ScaPCs isolated from obesity-prone (C57BL/6) or obesity-resistant (129-S1) mice. (B) Oil Red O staining of ScaPCs derived from both strains of mice at days 5 and 12 of adipogenic differentiation. (C–E) Quantitative RT-PCR analysis of (C) PPARγ, (D) CIDEA, and (E) UCP1 expression in muscle-derived ScaPCs, comparing the C57BL/6 mouse strain to the 129-S1 strain. Bars indicate untreated cells from C57BL/6 (black), 129-S1 (white), BMP7-pretreated from C57BL/6 (light gray), and BMP7-pretreated from 129-S1 mice (dark gray). (F–H) Quantitative RT-PCR analysis of UCP1 expression in ScaPCs derived from (F) iBAT, (G) sWAT, and (H) eWAT under basal (black/white bars) and BMP7-stimulated conditions (light/dark gray bars). All data are presented as mean ± SEM (n = 3). *P < 0.05; **P < 0.01; ***P < 0.001.
Emergence of brown adipocytes following stimuli, such as cold exposure or β3-adrenergic stimulation, has mostly been found in white-fat depots, but the mass of constitutive brown fat found in the interscapular region remains unaffected. It has also been noted that genetic factors are important for induction of brown adipogenesis (16, 17, 42). Consistent with these findings, no significant difference was observed between iBAT-ScaPCs derived from C57BL/6 versus 129-S1 mice (Fig. 3F). In contrast, UCP1 was expressed at significantly higher levels in ScaPCs derived from sWAT of 129-S1 mice under both basal and BMP7 pretreatment conditions (Fig. 3G). UCP1 expression trended to be increased even in eWAT from the obesity-resistant mouse strain, but this difference did not reach statistical significance (Fig. 3H).
BMP7 Acts Synergistically with Other Brown Adipogenic Agents to Promote Brown-Fat Formation in Vitro and in Vivo.
To determine whether ScaPCs can respond to other brown adipogenic stimuli and differentiate into brown adipocytes, ScaPCs isolated form sWAT and iBAT were pretreated with rosiglitazone, BMP7, or both, before exposure to adipogenic induction. Previously, Petrovic et al. have demonstrated that thiazolidinediones can induce brown adipogenesis in adipose precursors isolated from white fat (15). Although rosiglitazone alone slightly induced UCP1 expression in sWAT-derived ScaPCs, the combination of both synergistically induced UCP1 expression to a greater extent (Fig. S9A). Cells derived from iBAT did not respond to rosiglitazone treatment, but a combination of BMP7 and rosiglitazone resulted in an almost fivefold induction of UCP1 (Fig. S9B). These data suggest that BMP7 can act in concert with other brown adipogenic agents to induce brown-fat formation.
To further evaluate the potentially synergistic effect of BMP7 and other brown adipogenic stimuli on the formation of systemic brown-fat depots in vivo, we injected BMP7 and CL316,243 into mice maintained on a high-fat diet. Interestingly, a combination of BMP7 and CL316,243 resulted in significant increases in the expression of brown-fat marker genes UCP1 and CIDEA in sWAT, eWAT, and iBAT compared with tissues from mice receiving CL316,243 injection alone (Figs. S9 C–H). Somewhat unexpectedly, the synergistic effect of BMP7 and CL316,243 was not observed in iBAT and also MUS. Although the exact mechanisms involved in these effects remain to be determined, these data highlight the possibility that BMP7 may act in concert with other brown adipogenic agents to promote the formation of energy-dissipating brown adipocytes from tissue-resident brown-fat cells.
BMP7-Induced Brown Adipogenesis in Human Preadipocytes.
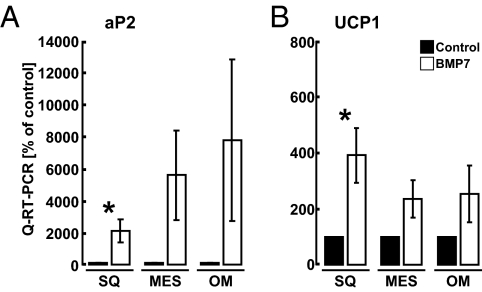
To test whether BMP7 was able to promote brown adipogenesis in human adipogenic cells, we treated preadipocytes isolated from different white-fat depots with BMP7. These adipose progenitors are known to possess depot-specific patterns of gene expression and differentiation capacities (43–45). Unlike murine primary ScaPCs, human primary preadipocytes required continuous exposure to BMP7 to differentiate into lipid-containing cells, as demonstrated by the expression of adipocyte marker aP2 (FABP4) (Fig. 4A). There were variable degrees of brown adipogenic ability in cells isolated from different individuals, possibly reflecting the heterogeneous genetic backgrounds of the subjects. However, we observed significant induction of UCP1 expression in cells derived from sWAT (Fig. 4B). This finding is consistent with our observations in mice, in which sWAT showed marked inducible brown adipogenic capacity.
Fig. 4.
BMP7 induces brown adipogenesis in primary human preadipocytes. Quantitative RT-PCR analysis of (A) fatty acid binding protein 4 (FABP4, aP2) and (B) UCP1 in human primary preadipocytes derived from subcutaneous (SQ), mesenteric (Mes), and omental (OM) white fat. Cells were seeded and cultured in the presence of either vehicle (black bars), or BMP7 (3.3 nM; white bars) throughout differentiation. All data are presented as mean ± SEM (primary cells from five to seven individual subjects were used). *P < 0.05.
Discussion
Given the ability of brown fat to dissipate stored energy, identification of brown-fat progenitors holds therapeutic promise for the treatment of obesity and its related complications (19). Recently, brown-fat progenitors have been identified in skeletal muscle and white fat of humans (14, 46), providing the potential for increasing the oxidative capacity of these tissues by targeting these endogenous precursor cells to differentiate in vivo into energy-dissipating brown adipocytes. However, a more complete understanding of the physiological characteristics of these progenitors, as well as identifying factors that regulate their differentiation, is urgently needed to make feasible any such clinical interventions. In this study, we undertook a comprehensive analysis of Sca-1+ progenitors derived from skeletal muscle and different fat depots in mice, and thereby defined three types of brown-fat progenitors (Fig. S10). Whereas the ScaPCs from interscapular BAT serve as the constitutively committed brown-fat progenitors, the Sca-1+ populations of myf5−/Sca-1+ progenitor cells from MUS and sWAT are highly inducible to become mature brown adipocytes. Importantly, these progenitors represent a population of cells that can give rise to white adipocytes or inducible brown-fat cells following stimulation with appropriate signals, such as BMP7.
In addition, although all three populations of progenitors have high adipogenic capacity in common, their expression patterns of developmental marker genes are clearly distinct (Fig. S10). The expression of marker genes in the functionally related Sca-1+ inducible progenitors isolated from MUS and sWAT suggests physiological similarity. Furthermore, the data presented here provide evidence that these reservoirs of inducible brown-fat progenitors may contribute to enrichment of brown adipocytes in obesity-resistant strains of mice. In adult humans, it remains to be determined which population of progenitors gives rise to brown fat. However, human brown adipocytes are often found mixed with white adipocytes and appear to be very sensitive to activation by cold exposure and are influenced by genetic background, suggesting they may functionally resemble inducible brown adipocytes (19). Indeed, we have shown in this study that preadipocytes derived from human subcutaneous white fat respond to BMP7 by differentiating into UCP1-expressing brownfat cells. The molecular signatures for different adipose progenitors established in this study may assist future studies in the isolation and characterization of human brown-fat precursors.
These findings suggest a new cell-based approach to generate energy-expending cells as a potential antiobesity therapy, which holds great potential for further exploration. The feasibility of such an approach has been demonstrated by engineering brown fat through genetic manipulation of murine embryonic fibroblasts to overexpress the transcription factors PRDM16 and C/EBPβ (47). Our study suggests that by isolating progenitor cells from an obese individual, followed by ex vivo stimulation of brown adipogenesis with BMP7 and subsequent retransplantation, the generation of functional brown fat could be enhanced. Although we did not observe significant metabolic changes following engraftment of the relatively limited number of cells used in our study, the use of more controllable microenvironments, such as silk-based scaffolding biomaterials, may help to overcome such caveats (48).
Alternatively, it may be possible, through directed pharmacological intervention, to activate resident progenitor cells in situ, thereby stimulating endogenous brown adipogenesis. The ability to directly and prospectively isolate brown-fat progenitors for study will greatly facilitate therapeutic screening approaches to identify such small molecules that ultimately may yield novel brown adipogenic “drugs” for obesity treatment.
In current and previous studies, we have demonstrated that BMP7 functions as a brown-fat inducer in both committed and noncommitted adipose progenitors. Recently, Vegiopoulos et al. showed that Sca-1+ cells resident in white fat can respond to prostaglandin stimulation by differentiating into mature brown adipocytes (49). In addition, a subset of adipose progenitors resident in white fat acquires a brown-fat phenotype in response to rosiglitazone treatment (15). In our study, we demonstrate that BMP7 can act synergistically with rosiglitazone to induce brown adipogenesis in the tissue resident Sca-1+ cells. In addition, in vivo studies show that a combination of BMP7 and the β3-adrenergic agonist, CL316,243, can further enhance UCP1 expression in white adipose tissue compared with treatment with CL316,243 alone. Thus, our data suggest that one may design a potent remedy by combining different brown adipogenic agents to target endogenous progenitors to enhance the production of energy-dissipating cells in vivo for the treatment of obesity and its many associated metabolic complications.
Experimental Procedures
Detailed materials and methods are provided as online SI Experimental Procedures and Table S2. All animal procedures have been approved by the Institutional Animal Care and Use Committee (IACUC). Mice were maintained on a 12 h/12 h light/dark cycle and ad libitum access to food and water.
Supplementary Material
Acknowledgments
The authors thank J. LaVecchio, G. Buruzula, and J. Schroeder for expert technical assistance, and E. Kokkotou for helpful advice and critical reading of the manuscript. T.J.S. is supported by a research fellowship from the German Research Foundation. K.L.T. is supported by National Institutes of Health (NIH) training Grant T32 DK007260-32. This work was supported in part by NIH Grants R01 DK077097 and UL1 RR025758-01 (Harvard Catalyst/The Harvard Clinical and Translational Science Center), the Eli Lilly Research Foundation and Harvard Stem Cell Institute (to Y.-H.T.), NIH 1U01 HL100402-01, the Burroughs-Wellcome Fund, the Harvard Stem Cell Institute and W.M. Keck Foundation (to A.J.W.), NIH Grant R01 AG13925, the Noaber Foundation, and the Ted Nash Foundation (to J.L.K.), and by Flow Cytometry and Genomics Core facilities supported by The Joslin Diabetes Center's Diabetes and Endocrinology Research Center (NIH Grant P30DK036836 from the National Institute of Diabetes and Digestive and Kidney Diseases) and the Muscle Progenitor Core facility supported by the Boston Area Claude D. Pepper Center OAIC (P30 AG031679-02, from the National Institute on Aging).
Footnotes
Conflict of interest statement: D.S. and D.F. are employees of Stryker Biotech, which is actively developing BMP7 in several therapeutic areas. A.J.W. serves on the Scientific Advisory Board of iPierian, Inc.
The other authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1010929108/-/DCSupplemental.
References
- 1.Cannon B, Nedergaard J. Brown adipose tissue: Function and physiological significance. Physiol Rev. 2004;84:277–359. doi: 10.1152/physrev.00015.2003. [DOI] [PubMed] [Google Scholar]
- 2.Ravussin E, Kozak LP. Have we entered the brown adipose tissue renaissance? Obes Rev. 2009;10:265–268. doi: 10.1111/j.1467-789X.2008.00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Frontini A, Cinti S. Distribution and development of brown adipocytes in the murine and human adipose organ. Cell Metab. 2010;11:253–256. doi: 10.1016/j.cmet.2010.03.004. [DOI] [PubMed] [Google Scholar]
- 4.Nedergaard J, Bengtsson T, Cannon B. Unexpected evidence for active brown adipose tissue in adult humans. Am J Physiol Endocrinol Metab. 2007;293:E444–E452. doi: 10.1152/ajpendo.00691.2006. [DOI] [PubMed] [Google Scholar]
- 5.Cypess AM, et al. Identification and importance of brown adipose tissue in adult humans. N Engl J Med. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N Engl J Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 7.Saito M, et al. High incidence of metabolically active brown adipose tissue in healthy adult humans: Effects of cold exposure and adiposity. Diabetes. 2009;58:1526–1531. doi: 10.2337/db09-0530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Virtanen KA, et al. Functional brown adipose tissue in healthy adults. N Engl J Med. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- 9.Zingaretti MC, et al. The presence of UCP1 demonstrates that metabolically active adipose tissue in the neck of adult humans truly represents brown adipose tissue. FASEB J. 2009;23:3113–3120. doi: 10.1096/fj.09-133546. [DOI] [PubMed] [Google Scholar]
- 10.Celi FS. Brown adipose tissue—When it pays to be inefficient. N Engl J Med. 2009;360:1553–1556. doi: 10.1056/NEJMe0900466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Atit R, et al. Beta-catenin activation is necessary and sufficient to specify the dorsal dermal fate in the mouse. Dev Biol. 2006;296:164–176. doi: 10.1016/j.ydbio.2006.04.449. [DOI] [PubMed] [Google Scholar]
- 12.Timmons JA, et al. Myogenic gene expression signature establishes that brown and white adipocytes originate from distinct cell lineages. Proc Natl Acad Sci USA. 2007;104:4401–4406. doi: 10.1073/pnas.0610615104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Seale P, et al. PRDM16 controls a brown fat/skeletal muscle switch. Nature. 2008;454:961–967. doi: 10.1038/nature07182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Crisan M, et al. A reservoir of brown adipocyte progenitors in human skeletal muscle. Stem Cells. 2008;26:2425–2433. doi: 10.1634/stemcells.2008-0325. [DOI] [PubMed] [Google Scholar]
- 15.Petrovic N, et al. Chronic PPAR{gamma} activation of epididymally derived white adipocyte cultures reveals a population of thermogenically competent, UCP1-containing adipocytes molecularly distinct from classical brown adipocytes. J Biol Chem. 2009;285:7153–7164. doi: 10.1074/jbc.M109.053942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guerra C, Koza RA, Yamashita H, Walsh K, Kozak LP. Emergence of brown adipocytes in white fat in mice is under genetic control. Effects on body weight and adiposity. J Clin Invest. 1998;102:412–420. doi: 10.1172/JCI3155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xue B, et al. Genetic variability affects the development of brown adipocytes in white fat but not in interscapular brown fat. J Lipid Res. 2007;48:41–51. doi: 10.1194/jlr.M600287-JLR200. [DOI] [PubMed] [Google Scholar]
- 18.Almind K, Manieri M, Sivitz WI, Cinti S, Kahn CR. Ectopic brown adipose tissue in muscle provides a mechanism for differences in risk of metabolic syndrome in mice. Proc Natl Acad Sci USA. 2007;104:2366–2371. doi: 10.1073/pnas.0610416104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tseng YH, Cypess AM, Kahn CR. Cellular bioenergetics as a target for obesity therapy. Nat Rev Drug Discov. 2010;9:465–482. doi: 10.1038/nrd3138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Jones DL, Wagers AJ. No place like home: Anatomy and function of the stem cell niche. Nat Rev Mol Cell Biol. 2008;9:11–21. doi: 10.1038/nrm2319. [DOI] [PubMed] [Google Scholar]
- 21.Chen D, Zhao M, Mundy GR. Bone morphogenetic proteins. Growth Factors. 2004;22:233–241. doi: 10.1080/08977190412331279890. [DOI] [PubMed] [Google Scholar]
- 22.Tobin JF, Celeste AJ. Bone morphogenetic proteins and growth differentiation factors as drug targets in cardiovascular and metabolic disease. Drug Discov Today. 2006;11:405–411. doi: 10.1016/j.drudis.2006.03.016. [DOI] [PubMed] [Google Scholar]
- 23.Schulz TJ, Tseng YH. Emerging role of bone morphogenetic proteins in adipogenesis and energy metabolism. Cytokine Growth Factor Rev. 2009;20:523–531. doi: 10.1016/j.cytogfr.2009.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tang QQ, Otto TC, Lane MD. Commitment of C3H10T1/2 pluripotent stem cells to the adipocyte lineage. Proc Natl Acad Sci USA. 2004;101:9607–9611. doi: 10.1073/pnas.0403100101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wang EA, Israel DI, Kelly S, Luxenberg DP. Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Factors. 1993;9:57–71. doi: 10.3109/08977199308991582. [DOI] [PubMed] [Google Scholar]
- 26.Tseng YH, et al. New role of bone morphogenetic protein 7 in brown adipogenesis and energy expenditure. Nature. 2008;454:1000–1004. doi: 10.1038/nature07221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rodeheffer MS, Birsoy K, Friedman JM. Identification of white adipocyte progenitor cells in vivo. Cell. 2008;135:240–249. doi: 10.1016/j.cell.2008.09.036. [DOI] [PubMed] [Google Scholar]
- 28.Tang W, et al. White fat progenitor cells reside in the adipose vasculature. Science. 2008;322:583–586. doi: 10.1126/science.1156232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Joe AW, et al. Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat Cell Biol. 2010;12:153–163. doi: 10.1038/ncb2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Uezumi A, Fukada S, Yamamoto N, Takeda S, Tsuchida K. Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat Cell Biol. 2010;12:143–152. doi: 10.1038/ncb2014. [DOI] [PubMed] [Google Scholar]
- 31.Graff JM. Embryonic patterning: To BMP or not to BMP, that is the question. Cell. 1997;89:171–174. doi: 10.1016/s0092-8674(00)80196-8. [DOI] [PubMed] [Google Scholar]
- 32.Vukicevic S, Grgurevic L. BMP-6 and mesenchymal stem cell differentiation. Cytokine Growth Factor Rev. 2009;20:441–448. doi: 10.1016/j.cytogfr.2009.10.020. [DOI] [PubMed] [Google Scholar]
- 33.Zhou Z, et al. CIDEA-deficient mice have lean phenotype and are resistant to obesity. Nat Genet. 2003;35:49–56. doi: 10.1038/ng1225. [DOI] [PubMed] [Google Scholar]
- 34.Seale P, et al. Transcriptional control of brown fat determination by PRDM16. Cell Metab. 2007;6:38–54. doi: 10.1016/j.cmet.2007.06.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Smas CM, Sul HS. Pref-1, a protein containing EGF-like repeats, inhibits adipocyte differentiation. Cell. 1993;73:725–734. doi: 10.1016/0092-8674(93)90252-l. [DOI] [PubMed] [Google Scholar]
- 36.Farmer SR. Transcriptional control of adipocyte formation. Cell Metab. 2006;4:263–273. doi: 10.1016/j.cmet.2006.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Puigserver P, Spiegelman BM. Peroxisome proliferator-activated receptor-gamma coactivator 1 alpha (PGC-1 alpha): Transcriptional coactivator and metabolic regulator. Endocr Rev. 2003;24:78–90. doi: 10.1210/er.2002-0012. [DOI] [PubMed] [Google Scholar]
- 38.Lin J, Puigserver P, Donovan J, Tarr P, Spiegelman BM. Peroxisome proliferator-activated receptor gamma coactivator 1beta (PGC-1beta), a novel PGC-1-related transcription coactivator associated with host cell factor. J Biol Chem. 2002;277:1645–1648. doi: 10.1074/jbc.C100631200. [DOI] [PubMed] [Google Scholar]
- 39.Gupta RK, et al. Transcriptional control of preadipocyte determination by Zfp423. Nature. 2010;464:619–623. doi: 10.1038/nature08816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cerletti M, et al. Highly efficient, functional engraftment of skeletal muscle stem cells in dystrophic muscles. Cell. 2008;134:37–47. doi: 10.1016/j.cell.2008.05.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sherwood RI, et al. Isolation of adult mouse myogenic progenitors: Functional heterogeneity of cells within and engrafting skeletal muscle. Cell. 2004;119:543–554. doi: 10.1016/j.cell.2004.10.021. [DOI] [PubMed] [Google Scholar]
- 42.Collins S, Daniel KW, Petro AE, Surwit RS. Strain-specific response to beta 3-adrenergic receptor agonist treatment of diet-induced obesity in mice. Endocrinology. 1997;138:405–413. doi: 10.1210/endo.138.1.4829. [DOI] [PubMed] [Google Scholar]
- 43.Tchkonia T, et al. Fat depot-specific characteristics are retained in strains derived from single human preadipocytes. Diabetes. 2006;55:2571–2578. doi: 10.2337/db06-0540. [DOI] [PubMed] [Google Scholar]
- 44.Tchkonia T, et al. Fat depot origin affects adipogenesis in primary cultured and cloned human preadipocytes. Am J Physiol Regul Integr Comp Physiol. 2002;282:R1286–R1296. doi: 10.1152/ajpregu.00653.2001. [DOI] [PubMed] [Google Scholar]
- 45.Tchkonia T, et al. Identification of depot-specific human fat cell progenitors through distinct expression profiles and developmental gene patterns. Am J Physiol Endocrinol Metab. 2007;292:E298–E307. doi: 10.1152/ajpendo.00202.2006. [DOI] [PubMed] [Google Scholar]
- 46.Elabd C, et al. Human multipotent adipose-derived stem cells differentiate into functional brown adipocytes. Stem Cells. 2009;27:2753–2760. doi: 10.1002/stem.200. [DOI] [PubMed] [Google Scholar]
- 47.Kajimura S, et al. Initiation of myoblast to brown fat switch by a PRDM16-C/EBP-beta transcriptional complex. Nature. 2009;460:1154–1158. doi: 10.1038/nature08262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Zhang X, Reagan MR, Kaplan DL. Electrospun silk biomaterial scaffolds for regenerative medicine. Adv Drug Deliv Rev. 2009;61:988–1006. doi: 10.1016/j.addr.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vegiopoulos A, et al. Cyclooxygenase-2 controls energy homeostasis in mice by de novo recruitment of brown adipocytes. Science. 2010;328:1158–1161. doi: 10.1126/science.1186034. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.