Abstract
The intestinal epithelium is maintained by a population of rapidly cycling (Lgr5+) intestinal stem cells (ISCs). It has been postulated, however, that slowly cycling ISCs must also be present in the intestine to protect the genome from accumulating deleterious mutations and to allow for a response to tissue injury. Here, we identify a subpopulation of slowly cycling ISCs marked by mouse telomerase reverse transcriptase (mTert) expression that can give rise to Lgr5+ cells. mTert-expressing cells distribute in a pattern along the crypt–villus axis similar to long-term label-retaining cells (LRCs) and are resistant to tissue injury. Lineage-tracing studies demonstrate that mTert+ cells give rise to all differentiated intestinal cell types, persist long term, and contribute to the regenerative response following injury. Consistent with other highly regenerative tissues, our results demonstrate that a slowly cycling stem cell population exists within the intestine.
Every day the small intestinal epithelium of a normal mouse sheds ∼2 × 108 cells into the lumen of the gut, placing it among the most highly regenerative mammalian tissues (1). It is well established that the absorptive and secretory lineages of the continuously renewing intestinal epithelium are maintained by multipotent intestinal stem cells (ISCs) (2), although their identity remained elusive until recently due to a lack of definitive ISC markers (3). Using lineage-tracing techniques, Hans Clevers’ group identified Lgr5 (4) and Ascl2 (5) as markers for highly proliferative crypt base columnar (CBC) cells, which contribute to all intestinal lineages during extended chase. Their high rate of proliferation, however, was a surprising characteristic, given most mammalian stem cell populations are thought to be maintained in a slowly cycling (largely quiescent) state (6). Additional ISC markers have recently been identified, although their cell cycle status has yet to be established. For example, the Capecchi group defined Bmi-1, a member of the Polycomb group gene family, as an ISC marker that appears to mark cells that are largely distinct from Lgr5+ cells (7), although more recent evidence supports some overlap. Whereas the coexistence of quiescent and active stem cells has been demonstrated in other mammalian tissues, the presence of quiescent ISCs remains controversial (8).
Relative resistance to cellular senescence, despite multiple rounds of cell division, is a common characteristic of stem cells. Telomerase is a ribonucleoprotein complex that helps maintain the telomeric ends of chromosomes, normally shortened with each cell division. Because loss of telomeric DNA beyond a critical threshold induces senescence in most somatic cells, maintenance or induction of telomerase activity provides a means of preventing cellular senescence (9) that may be relevant for the self-renewal of tissue stem cells. Consistent with this hypothesis, loss of telomerase has been shown to result in intestinal villus atrophy, suggesting a functional requirement for telomerase activity and/or telomere maintenance in ISC function (10). In addition, several reports have recently implicated mouse telomerase reverse transcriptase (mTERT) in the direct regulation of stem cell proliferation and mobilization (11, 12).
At the whole tissue level, telomerase activity and expression have been identified within self-renewing tissues such as testis, bone marrow, and intestine (13, 14). However, with the exception of testis, telomerase is expressed at very low levels (15–19) and has been localized to single telomerase-expressing cells within the lower crypt (20). Previously, we generated a mTert–GFP transgenic mouse model system in which GFP expression recapitulates endogenous mTert expression and telomerase activity (14). Using this model, we have shown that mTert marks embryonic and adult stem cells as well as induced pluripotent stem (iPS) cells (14, 21). In the intestine, prior studies showed that mTert–GFP marks long-term label-retaining cells (LRCs) within the intestinal crypt, suggesting it may mark quiescent ISCs (14). Here, we report that mTert expression marks a slowly cycling ISC population distinct from Lgr5+ cells. mTert+ cells contribute to all differentiated intestinal cell types as well as the Lgr5+ cell population, persist long-term, are resistant to injury, and contribute to the regenerative response following tissue injury. Thus, a slowly cycling stem cell exists within the intestine alongside and perhaps upstream of the Lgr5+ population.
Results
mTert–GFP Expression Marks Single Cells in the Intestinal Crypt.
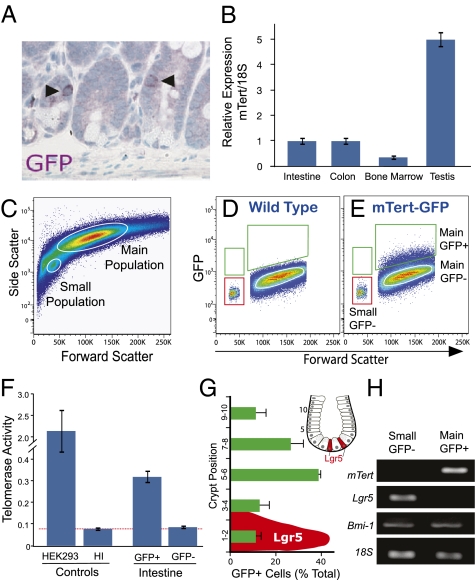
Previously we reported a rare population of single telomerase-positive cells present within intestinal crypts occurring at an average frequency of 1 GFP+ cell per 150 crypts (Fig. 1A and ref. 14) consistent with its relatively low expression level (Fig. 1B). To further study these cells, we used EDTA chelation to isolate intestinal epithelial cells for flow cytometry. GFP+ cells were present in the main population of cells at a frequency of 1.7 ± 0.3% (Fig. 1E) and were found in both proximal and distal intestine at a ratio of ∼2:1, respectively. For comparison, two GFP− populations were also studied, which included cells from the main population and from a population of smaller cells (Fig. 1E), which was enriched for Lgr5+ cells (Fig. 1H). Consistent with our prior findings (14), telomerase expression and activity was detected within GFP+ crypt cells but was absent from the smaller GFP− cells (Fig. 1 F and H). Whereas GFP− cells from the main population (Fig. 1E) were largely negative for telomerase activity, occasionally we detected low levels of activity, which may be attributed to inefficient sorting of the somewhat dim GFP+ cells or possibly to our reporter system marking a subpopulation of mTert-expressing cells. To rule out any effect of genomic integration on transgene expression, two independent lines of mTert–GFP mice were studied. Both lines exhibited an overlapping GFP expression profile, so for simplicity, results from one line are presented.
Fig. 1.
Analysis of mTert-expressing cells in the intestine. (A) Immunohistochemical analysis of GFP in the intestine. Arrowheads indicate single GFP+ crypt cells. Magnification, 40×. (B) Quantitative RT-PCR analysis of mTert expression in adult mouse small intestine, colon, bone marrow, and testis. To normalize the level of input RNA, 18S was used. A representative analysis is shown. Bars represent mean ± SEM, performed in duplicate. (C–E) FACS analysis of single intestinal cells harvested from mTert–GFP mice. (C) A representative forward and side-scatter plot depicts events already gated for live intestinal cells. The small and main cell populations are circled. (D and E) Representative wild type (D) and mTert–GFP (E) forward-scatter and GFP plots are shown. Events depicted have already been gated for live, CD45− small and main cell populations. GFP+ cells, green gate; GFP− cells from the small cell population, red gate; GFP− cells from the main cell population, white circle. (F) Telomerase activity for GFP+ or GFP− FACS-isolated intestinal cells from the main and small cell populations, respectively. HEK293 cell extracts, positive control; heat inactivated (HI) HEK293 cell extracts, negative control. Data shown are from two to four experiments, each performed in duplicate. (G) Distribution of GFP+ cells within the intestinal crypt relative to the crypt bottom. Bars represent mean ± SEM of two separate experiments with a total of 96 cells counted. Distribution of Lgr5+ cells (in red) as previously published (4). Inset shows a schematic illustration of a crypt with Lgr5+ cells in red and crypt cell numbering. (H) RT-PCR analysis for mTert, Lgr5, Bmi-1, and 18S on FACS-purified GFP− and GFP+ cells corresponding to the gates in E.
mTert–GFP Cells Are Distinct from Lgr5+ Cells.
Historically, the location of a cell along the crypt axis has been used to infer its function. For example, slowly cycling LRCs, presumed to be stem cells, are distributed throughout the lower crypt with a peak frequency between positions 4 and 9, commonly referred to as “position +4” (2). Using these previously established criteria, we scored the location of GFP+ cells along the crypt axis. The majority of cells were present between positions 5 and 8 (Fig. 1G), consistent with prior colocalization of mTert–GFP and LRCs (14). A similar distribution has been reported for Bmi-1+ intestinal stem cells (7). In addition, Bmi-1+ and GFP+ cells are occasionally present in positions 1 and 2, near the crypt bottom (Fig. 1G) (7), the region in which Lgr5-expressing CBC cells are primarily located (4).
Given the slight overlap in position between mTert–GFP+ and Lgr5+ cells (Fig. 1G) and the stronger overlap with Bmi-1+ cells (7), we next sought to determine whether GFP+ cells coexpressed either marker. Analysis of gene expression from FACS-isolated cells demonstrated that GFP+ cells do not express Lgr5 (Fig. 1H), which was detected only within GFP− small cells (Fig. 1H and Fig. S1). This finding was confirmed using Lgr5–GFP–IRES–CreER mice. Therefore, subsequent studies using a GFP− population used this Lgr5-enriched population. Intriguingly, Bmi-1 expression was detected in all populations (Fig. 1E and Fig. S1). In summary, mTert expression marks a population of crypt cells distinct from Lgr5+ CBC cells and also marks a subpopulation of Bmi-1+ cells.
mTert–GFP+ Cells Are Slowly Cycling.
Telomerase expression is generally associated with cellular proliferation (22). Given the highly regenerative nature of the intestinal epithelium, one would speculate that mTert-expressing cells are rapidly dividing, similar to Lgr5+ cells. However, the fact that mTert–GFP marks LRCs (14) would suggest these cells are slowly cycling. To address this discrepancy, we determined the proliferative rate of mTert-expressing cells. Confocal microscopic analysis of crypt sections revealed GFP+ cells were negative for Ki67 (Fig. 2A), in contrast to the adjacent transit-amplifying cells. To confirm and quantify this observation, we performed Ki67 staining in conjunction with flow cytometry and analyzed the fraction of Ki67+ cells in the GFP+ population as well as the GFP− Lgr5-enriched small cell population. A significant fraction of the GFP− population was Ki67+ (43.3 ± 3.7%), consistent with the rapidly cycling nature of Lgr5+ cells (4), whereas only a small fraction of the GFP+ population was Ki67+ (5.3 ± 0.9%) (Fig. 2 B and C). Similar results were obtained when Ki67 staining was performed on FACS-isolated GFP+ cells (GFP+Ki67+, 8.2 ± 1.6%) (Fig. S2). Taken together, these results establish that 90–95% of GFP+ crypt cells are in G0 in contrast to CBC cells (4).
Fig. 2.
Telomerase-positive cells are slowly cycling and do not express activated stem cell markers. (A) Confocal analysis of GFP and Ki67 expression in intestinal crypts. Arrowhead indicates a GFP+Ki67− cell. Magnification, 63×. Gamma correction was applied to reduce background fluorescence. (B) Representative Ki67 and side-scatter plots depict events already gated for live, CD45−, main GFP+ (Upper) or small GFP− (Lower) cell populations. (C) Percentage of Ki67+ cells within the GFP+ and GFP− cell populations. Bars represent mean ± SEM of two independent experiments performed with five replicates. (D–F) Coimmunofluorescence for GFP and P-β-catS552 in intestinal crypts. DAPI (blue) counterstain. Magnification, 60×.
Coexpression of mTert–GFP with Putative ISC Markers.
To investigate the relationship of mTert–GFP+ cells to so-called “activated” stem cells present in position +4 we performed immunohistochemistry using antibodies for P-β-catS552 (23) and P-PTEN (24). Interestingly, neither P-β-catS552 nor P-PTEN colocalized with GFP (Fig. 2 D–F and Fig. S3A) despite the fact that P-PTEN+ cells shared a similar distribution profile to GFP+ cells within the crypt (compare Fig. 1G and Fig. S3B), thereby indicating that mTert–GFP marks ISCs in the nonactivated state. Intriguingly, whereas P-β-catS552 and GFP did not colocalize within the same cell, GFP+ cells were predominantly found adjacent to the more abundant P-β-catS552+ cells (Fig. 2F and Fig. S4). Analysis of other proposed ISC markers revealed the majority of GFP+ cells (79.7 ± 1.9%) coexpressed β1-integrin (25), whereas only a small percentage coexpressed BMP-R1a (24) (6.8 ± 1.7%), Sca-1 (26) (5.9 ± 1.9%), or DCAMKL-1 (27) (18%) (Fig. S5). Taken together, these results show that mTert–GFP expression partially overlaps with previously proposed ISC markers, indicating it may mark a specific ISC population or a specific differentiation state.
mTert–GFP+ Cells Are Resistant to Injury.
In response to injury, the intestine has a large regenerative capacity, which may result from the mobilization of a population of quiescent stem cells present in position +4 (2). Paradoxically, cells in this position have been shown to be highly sensitive to both low- and high-dose radiation (4, 28), although such studies have not used ISC-specific markers, leaving open the possibility that multiple cell types may be present in this region. In contrast, Lgr5+ stem cells have been shown to be resistant to low-dose (1 Gy) although sensitive to high-dose (10 Gy) radiation (4). To assess the sensitivity of mTert–GFP+ cells to intestinal injury, we administered whole body gamma irradiation (1 or 10 Gy) to mTert–GFP mice and scored for cell death using the dual criteria of rounded apoptotic body morphology and activated-caspase-3 staining (28). Despite a dose-dependent increase in the percentage of apoptotic crypt cells, analysis of ∼9,000 crypts and 44 GFP+ cells revealed no evidence for apoptotic induction within the GFP+ population (1 Gy, 0/19 cells; 10 Gy, 0/25 cells) (Fig. S6). These results indicate that mTert–GFP+ cells at position +4 are resistant to the effects of ionizing radiation, in contrast to the variable sensitivity of classical position +4 and CBC cells to low- (1 Gy) and high-dose (10 Gy) radiation, respectively (4), and suggest functional heterogeneity among cells in this position. Interestingly, these results are consistent with other regenerative and neoplastic systems where slowly cycling stem cells are generally radioresistant, whereas rapidly cycling cells are radiosensitive.
mTert–CreER Expression Marks a Subpopulation of Multipotent ISCs.
To functionally establish that mTert marks ISCs, we generated tamoxifen-inducible mTert–CreER::R26R mice (Fig. S7) and performed short- and long-term lineage-tracing studies. Shortly after induction, histological analysis demonstrated single LacZ-stained cells (Fig. 3 A–C) in a distribution similar to mTert–GFP+ cells. In some cases, staining progressed to small clusters of LacZ-marked cells (Fig. 3 D–I) and ultimately to complete stripes emanating from the crypt base to the villus tip (Fig. 3 J–L), still detected after 2 y of chase. Interestingly, as previously described for Bmi-1–induced Cre recombination (7), we also observed a gradient along the intestine with most recombination events restricted to the proximal half of the small bowel. Analysis of LacZ-stained villus-crypt units following long-term chase confirmed that mTert-expressing cells give rise to both intestinal lineages (absorptive and secretory), including all four differentiated cell types (Fig. 3 M–R). Furthermore, quantitative analysis of LacZ-stained clones and adjacent nonstained clones showed a similar frequency of each differentiated cell type, confirming mTert expression marks multipotent ISCs (Table S1). No LacZ staining was detected in placebo-treated control mice.
Fig. 3.
Lineage-tracing in the small intestine and colon. Histological or whole mount analysis of intestinal LacZ staining following pulse (A–F) or greater than 1-mo chase (G–L). (M–R) Histological analysis of LacZ staining in the various intestinal epithelial cell lineages after 1-mo chase. Colabeling of LacZ and periodic acid-Schiff (PAS) positive cells corresponding to goblet (M and N) and Paneth (O and P) cells. (Q and R) Colocalization of the enteroendocrine marker, chromogranin-A (Q) and LacZ staining (R). Arrow indicates an enteroendocrine cell. (S–U) Analysis of long-term labeling of colonic crypts by whole mount analysis following 1-mo (U) or 6-mo (S and T) chase. Arrows demarcate LacZ+ colonic crypts at low power (S) and crypt openings at higher power (T and U). A crypt duplication event is shown by arrowhead and hatched box in S and at higher magnification (T, white arrow). (V and W) Colabeling of LacZ+ and PAS+ colonic goblet cells after 6-mo chase. Representative pictures are shown for all results. Magnification for histological images is 60× and 2–10× for whole mount images.
To determine whether telomerase marks stem cells in related organs, we next examined the colon and cecum of bigenic mice following long-term chase. Both tissues demonstrated LacZ-stained crypts extending from the basal to the luminal surface, which included both colonocytes as well as goblet cells, confirming permanent marking of long-lived, multipotent colonic stem cells (Fig. 3 S–W).
Majority of Cells Marked by mTert–CreER Expression Are Slowly Cycling.
Given only a small fraction of mTert–GFP cells are cycling at any given time (Fig. 2 A–C), we speculated that the majority of mTert–CreER-expressing cells might not be actively contributing to villus stripes under basal conditions. To investigate this possibility, we developed a whole mount LacZ crypt assay, which allows for the microscopic analysis of individual crypts at the single cell level. Consistent with our hypothesis, the majority of LacZ-marked crypts (93%) contained only single or few (2–4) LacZ+ cells, whereas only 7% gave rise to full lineage stripes following 4 d or 1 mo of chase (Fig. 4A). These results indicate that the majority of cells marked by mTert–CreER are quiescent or slowly cycling consistent with the mTert–GFP data (Fig. 2 B and C and Fig. S2). The similarity between the percentage of marked crypts giving rise to full lineage stripes and the proportion of mTert–GFP+ cells that are cycling (Fig. 2C) signifies that the cycling mTert+ cells give rise to the differentiated cell lineages. To investigate whether the quiescent cell population progressively contributes to the active stem cell pool over time, we compared the proportion of LacZ marked crypts containing single cells (no stripes) vs. multiple cells (full lineage stripes) at baseline (<1 mo) and after an extended period of chase (>1 y). Interestingly, with time, there was a dramatic shift toward marked crypts giving rise to full lineage stripes (7 vs. 65%) (Fig. S8). Taken together these results indicated that, whereas mTert-expressing cells contribute minimally to normal intestinal homeostasis, over the life of the organism they progressively contribute to the crypt/villus unit presumably through the active stem cell pool.
Fig. 4.
Frequency of LacZ-marked crypts under basal and regenerative conditions. Analysis of intestinal LacZ-marked crypts using the whole mount LacZ crypt assay. (A) Analysis of crypts marked with single, few (two to four) or multiple (five or more) LacZ+ cells under basal conditions. Crypts marked with five or more cells correspond to fully marked crypt/villus units (stripe). Inset is a representative image (arrow) and schematic illustration of a crypt containing a single LacZ+ cell. The percentage for each group is shown. (B) Analysis of total LacZ-marked crypts following varying doses of radiation. (C) Fold increase in the fraction of crypts from B marked by single cells or multiple cells. Bars represent mean ± SEM obtained from two to three animals per group, ANOVA P < 0.001, (*) Bonferroni posttest.
mTert-Expressing Cells Contribute to the Regenerative Response Following Injury.
The intestine is capable of regeneration following irradiation-induced injury (29). To investigate whether mTert+ ISCs play a role in this process, mTert–CreER mice were exposed to varying doses of ionizing radiation (0, 1, 10, and 15 Gy) followed by tamoxifen administration. Analysis of crypts at the peak of the regenerative response (4 d) revealed mTert-expressing cells contribute to intestinal regeneration in a dose-dependent manner, as demonstrated by an 8- to 10-fold increase in the frequency of total LacZ-marked intestinal crypts (Fig. 4B). Whether this increase is due to preferential survival of mTert-expressing cells or to induction of mTert expression remains to be determined. Furthermore, the fraction of crypts containing multiple LacZ-marked cells, compared with single LacZ-marked cells, increased 12- to 15-fold with high-dose radiation (Fig. 4C). These results establish a role for mTert-expressing cells in the regenerative response and provide a potential mechanism by which radiation-sensitive ISCs might be restored following intestinal injury.
mTert-Expressing Cells Can Give Rise to Lgr5-Expressing Cells.
It has recently been proposed that Lgr5+ cells in skin arise from a more “primitive” stem cell (30). Whether a similar hierarchy exists in the intestine remains to be determined. Interestingly, our lineage-tracing analysis revealed LacZ+ cells at the base of the crypt (Fig. 3 H and I) where Lgr5+ CBC cells reside, suggesting they may be derived from mTert+ cells. To investigate this possibility we performed short-term pulse-chase experiments with mTert–CreER::R26R mice in combination with flow cytometry. This approach was chosen because adequate LGR5-specific antibodies are not currently available. On the basis of our lineage-tracing data (Fig. 3), we predicted that immediately following induction, a small number of LacZ+ cells would be present in the main population but not in the Lgr5-enriched small population (Fig. 5A). If Lgr5-expressing cells arise from mTert-expressing cells, then following a period of chase, LacZ+ Lgr5+ cells should appear in the small population (Fig. 5A, model 1). Alternatively, if mTert-expressing cells do not contribute, then LacZ+ cells should be absent from the Lgr5+ small cell population (Fig. 5A, model 2). Following induction, LacZ+ cells were detected only in the main population (Fig. 5 C and D). No LacZ+ cells were detected in the small population. Consistent with model 1, following a 5-d chase, LacZ+ cells were now present in both cell populations (Fig. 5 F and G).
Fig. 5.
mTert+ cells contribute to Lgr5+ cells. (A) Schematic illustration of flow cytometry plots showing the small cell (Lgr5+) and main cell (mTert+) populations at pulse and chase. Open circles, unstained cells; blue circles, LacZ+ cells. LacZ+ cells in the main population give rise to both small and main cell populations (model 1) or only to the main cell population (model 2). (B–G) Flow cytometric analysis of LacZ+ cells at pulse (B–D) or chase (E–G). Representative fluorescein and forward-scatter plots depict events already gated for live, CD45− intestinal cells and indicate LacZ+ cells within the small (dashed gate) and main (red gate) cell populations. Control cells from wild-type or placebo-treated bigenic mice used to set the fluorescent gates. (D and G) Quantitation of the percentage of LacZ+ cells from the small or main cell populations following pulse (D) or chase (G). A representative experiment is shown; bars represent mean ± SEM. Nine mice were assayed in duplicate or triplicate. (H and I) RT-PCR analysis of Lgr5 and 18S expression in FACS-isolated small and main cell populations following pulse (H) or chase (I). Positive (+) control corresponds to whole intestine cDNA.
To confirm that Lgr5+ cells were present within the LacZ+ small population but not within the main population, we collected LacZ+ cells from both populations by FACS and performed RT-PCR analysis. Cells isolated from the main population (Fig. 5 C and F, red gates) during either the pulse or chase period proved to be Lgr5− (Fig. 5 H and I), whereas LacZ+ cells collected from the small population following chase (Fig. 5F, dashed gate) were Lgr5+ (Fig. 5I). These results provide important proof-of-principle evidence that Lgr5+ CBC cells can arise from mTert-expressing ISCs, suggesting a possible mechanism by which the active stem cell population may be renewed throughout the life of the organism.
Discussion
The traditional view that mammalian stem cells are slowly cycling has recently been challenged by the discovery of rapidly cycling (Lgr5+) stem cells in intestine (4). Here we report the identification of a slowly cycling ISC marked by mTert expression. This ISC population is long lived, multipotent, and distinct from rapidly cycling Lgr5+ ISCs. In addition, whereas Lgr5+ cells and putative ISCs present at position +4 are sensitive to the effects of ionizing radiation, mTert-expressing cells are resistant to high-dose radiation. Furthermore, our data indicate that mTert-expressing cells contribute to the regenerative response following injury and can give rise to Lgr5+ cells, demonstrating a relationship between these populations (Fig. S9). Finally, our results demonstrate that both rapidly and slowly cycling stem cells can coexist in the intestine, similar to prior findings in skin (31), suggesting this may be a general feature of many organ systems (8).
The coexistence of separate populations of stem cells within mammalian tissues has raised questions about the mechanisms of tissue maintenance and repair under both homeostatic and regenerative conditions. For example, rapidly cycling stem cells are postulated to play a major role in tissue homeostasis, whereas slowly cycling cells may function more in tissue regeneration. Consistent with this hypothesis, “dormant” stem cells have recently been shown to function following tissue injury, while only minimally contributing to tissue homeostasis. Whether mTert-expressing cells represent such a population in the intestine remains to be determined; however, their ability to give rise (albeit at low levels) to rapidly cycling Lgr5+ cells during normal intestinal homeostasis is proof of principle that they may play a role in restoring the Lgr5+ ISC population. In fact, given the relative sensitivity of Lgr5-expressing cells to ionizing radiation, compared with mTert-expressing cells, it is plausible this is a mechanism by which rapidly cycling Lgr5+ cells might be reestablished following injury. Alternatively, it is theoretically possible that Lgr5-expressing cells could periodically exit the cell cycle to protect themselves from injury. It will also be of interest to determine whether Lgr5-expressing cells contribute to the mTert-expressing population.
Despite the recent identification of additional molecular markers for ISCs, evidence that a slowly cycling ISC population exists has remained elusive. Whereas Bmi-1 has been proposed to mark slowly cycling cells, our studies would indicate that it is expressed in both rapidly and slowly cycling stem cell populations, consistent with previous findings (32). In addition, whereas DCAMKL-1 marks slowly cycling cells within the intestine (27), definitive lineage-tracing data have yet to confirm their role as stem cells. The identification of a population of mTert-expressing ISCs provides important functional evidence for the existence of a slowly cycling ISC population within the intestinal crypt. The variable coexpression of mTert–GFP and the proposed ISC markers BMP-R1a, Sca-1, and β1-integrin, as well as Ki67, suggest these cells represent a heterogenous population. It will be of interest to determine at the single cell level whether or not multiple markers are coexpressed on these cells and their correlation with proliferation status. Curiously, we noted that a similar percentage of mTert–GFP+ cells were positive for the cell surface marker BMP-R1a as were positive for Ki67, raising the possibility that BMP-R1a may be marking cycling GFP+ cells. BMP signaling is active in slowly cycling cells present in position +4 and it has been suggested to play a role in stem cell self-renewal and the response to tissue injury (8).
Asymmetric cellular division of a quiescent stem cell results in a self-renewing daughter cell and a transit-amplifying progenitor cell (33, 34). Whether this hierarchical model applies to the intestine has recently been studied using lineage-tracing models targeting the proliferative compartment (35, 36). These studies provide compelling evidence that rapidly cycling ISCs, which are essential for daily homeostasis, undergo symmetrical divisions following a pattern of neutral drift dynamics. As pointed out, however, these studies cannot rule out the existence of asymmetric division resulting from a slowly cycling ISC population (36). It may now be possible to determine whether asymmetric division, resulting from quiescent ISCs, occurs within the intestinal epithelium.
Our observation that mTert-expression marks a slowly cycling stem cell population stands in direct contrast to the commonly held view that it marks dividing cells, although the available data for this claim are mixed (22). The Artandi lab has demonstrated that mTERT induction is sufficient to activate quiescent epidermal stem cells (12) and, in the intestine, functions as a transcriptional modulator of the Wnt/β-catenin signaling pathway (37). While their data are highly intriguing, it is not readily apparent that direct comparisons can be made between their model and ours. For example, whereas overexpression of mTERT within quiescent stem cells (that might not normally express telomerase) may cause them to be activated, it is not clear that endogenous mTERT+ cells are similarly activated. It is also possible that sustained vs. transient mTERT expression could have differential effects on cellular proliferation. Alternatively, Wnt signaling may be suppressed in some endogenous mTERT+ cells depending on their BMP signaling status, as previously suggested (23), and derepression may trigger them to be activated and emerge from quiescence. In addition, mTERT may activate only Wnt-dependent cells. Finally, given the lack of an overt phenotype in early generation mTert (−/−) knockout mice, it has been suggested that mTERT may not be essential for stem cell proliferation under basal conditions (38).
In summary, we have identified a slowly cycling ISC that coexists with rapidly cycling stem cells. These cells are primarily located above the Paneth cell zone, coincident with the location of traditional LRCs. A detailed understanding of their role in the regenerative response to injury as well as the role of targeted mutations in neoplastic transformation may ultimately translate into improved therapeutic treatment options for individuals with intestinal disease and cancer.
Materials and Methods
Mice and Labeling Experiments.
mTert–GFP, Lgr5–GFP–IRES–CreER (Jax 008875), and Gt(ROSA)26Sortm1Sor (R26R) (Jax strain 003474) were described previously (4, 14). mTert–CreER mice were generated as described in SI Materials and Methods. Mice studied were 1–6 mo of age unless indicated. Procedures were approved by our institutional animal care and use committee. mTert–CreER::R26R mice were treated with 10–20 mg of tamoxifen (T) (Sigma), 4-hydroxytamoxifen (4-OHT) (Sigma), or corn oil (per os, s.c., or i.p.) as a single dose or over the course of 3–5 d. LacZ staining was analyzed after 1 d to 2 y using whole mount or histological analysis as described in SI Materials and Methods. Irradiation was administered using a Gammacell 40 irradiator at 80 cGy/min.
Flow Cytometry.
Intestinal crypt cells were isolated using a chelation method (39) and confirmed to contain >99% epithelial cells. Cells were routinely stained with anti–CD45-PE or -APC (1:100; BD Pharmingen or eBioscience) to exclude all hematopoietic cells. Propidium iodide (1–2 μg/mL) was used to exclude dead cells. In some experiments, cells were stained with rat anti-β1 integrin-PE (CD29; 1:400; Biolegend), rat anti–Sca-1-PE (1:100; BD Pharmigen), rabbit anti–BMP-R1a (1:20; Santa Cruz) with goat anti–rabbit-PE (1:200; Jackson Immunoresearch), rabbit anti-Ki67 (NeoMarker) with goat anti–rabbit-PB (1:100; Invitrogen). The FluoReporter lacZ Flow Cytometry kit (Molecular Probes) was used to detect β-galactosidase activity as described in SI Materials and Methods. BD FACSAria or MoFlo flow cytometers were used; data were analyzed using FlowJo (Tree Star).
Immunohistochemistry.
Staining was performed as described in SI Materials and Methods using the following antibodies: rabbit anti-GFP (1:8,000; Abcam), chicken anti-GFP (1:500; Aves Labs or 1:2,000; Abcam), anti–Phospho-PTEN (1:25; Cell Signaling), anti–P-β-catS552 (1:750), anti–DCAMKL-1 (1:200), anti-Ki67 (NeoMarker), and antiactivated caspase-3 (1:100; Cell Signaling). Sections from wild-type mice were used to confirm the specificity of GFP staining. Images were obtained using a Nikon Eclipse E800 microscope and Spot software, unless specified.
TRAP Assay.
Telomerase activity was assessed using cell extracts from 1,000 FACS-isolated cells and the TeloTAGGG Telomerase PCR ELISAPlus kit (Roche) in accordance with the manufacturer's recommendations. Telomerase-expressing HEK293 cells served as a (+) control and heat inactivated (HI) HEK293 cell extracts served as a (−) control.
RT-PCR and Quantitative RT-PCR Analysis.
For RT-PCR, 500–8,000 cells were sorted directly into TRIzol reagent (Sigma). For LacZ+ cells, equivalent numbers of fluorescein-positive cells were analyzed. RNA isolated from whole intestine was used as a positive control. Following RNA extraction, first strand cDNA synthesis was performed with the iScript Select cDNA Synthesis kit (Bio-Rad Laboratories) and one-tenth the volume was used to perform each RT-PCR analysis. For qRT-PCR analysis, total RNA was isolated from adult tissues using TRIzol reagent and cDNA was synthesized as described above. Relative quantification was performed using iQ SYBR green PCR master mix (Bio-Rad) and iCycler (iQ5) real-time PCR detection system (Bio-Rad).
Supplementary Material
Acknowledgments
We thank Drs. Joseph Majzoub, Amy Wagers, Susan Bonner-Weir, Wayne Lencer, Richard Grant, and Ramesh Shivdasani for critical review of the manuscript. In addition, we thank Dr. Margaret Thompson, Joyce Levecchio, Giri Buruzula, Suzanne White, and Luke Deary for expert technical assistance. This work was supported by Grants R01 DK 084056 (to D.T.B.), K08 DK 066305 (to D.T.B.), R21 DK 078198 (to D.T.B.), and R37 DK 32658 (to Richard Grand and R.K.M.); the Timothy Murphy Fund, Marco Polo Fonds, and Stichting Groninger Universiteitsfonds, Groningen, The Netherlands (to S.A.); Juvenile Diabetes Research Foundation International (to D.T.B.); Intellectual and Developmental Diseases Research Center Grant P30 HD 18655 (to D.T.B.); Harvard Stem Cell Institute (to D.T.B.); and Harvard Digestive Disease Center Grant P30 DK 34854 (to D.T.B. and R.K.M.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1013004108/-/DCSupplemental.
References
- 1.Potten CS, Loeffler M. Stem cells: Attributes, cycles, spirals, pitfalls and uncertainties. Lessons for and from the crypt. Development. 1990;110:1001–1020. doi: 10.1242/dev.110.4.1001. [DOI] [PubMed] [Google Scholar]
- 2.Scoville DH, Sato T, He XC, Li L. Current view: Intestinal stem cells and signaling. Gastroenterology. 2008;134:849–864. doi: 10.1053/j.gastro.2008.01.079. [DOI] [PubMed] [Google Scholar]
- 3.Montgomery RK, Breault DT. Small intestinal stem cell markers. J Anat. 2008;213:52–58. doi: 10.1111/j.1469-7580.2008.00925.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449:1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
- 5.van der Flier LG, et al. Transcription factor achaete scute-like 2 controls intestinal stem cell fate. Cell. 2009;136:903–912. doi: 10.1016/j.cell.2009.01.031. [DOI] [PubMed] [Google Scholar]
- 6.Fuchs E. The tortoise and the hair: Slow-cycling cells in the stem cell race. Cell. 2009;137:811–819. doi: 10.1016/j.cell.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sangiorgi E, Capecchi MR. Bmi1 is expressed in vivo in intestinal stem cells. Nat Genet. 2008;40:915–920. doi: 10.1038/ng.165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li L, Clevers H. Coexistence of quiescent and active adult stem cells in mammals. Science. 2010;327:542–545. doi: 10.1126/science.1180794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Blackburn EH. Structure and function of telomeres. Nature. 1991;350:569–573. doi: 10.1038/350569a0. [DOI] [PubMed] [Google Scholar]
- 10.Rudolph KL, et al. Longevity, stress response, and cancer in aging telomerase-deficient mice. Cell. 1999;96:701–712. doi: 10.1016/s0092-8674(00)80580-2. [DOI] [PubMed] [Google Scholar]
- 11.Flores I, Cayuela ML, Blasco MA. Effects of telomerase and telomere length on epidermal stem cell behavior. Science. 2005;309:1253–1256. doi: 10.1126/science.1115025. [DOI] [PubMed] [Google Scholar]
- 12.Sarin KY, et al. Conditional telomerase induction causes proliferation of hair follicle stem cells. Nature. 2005;436:1048–1052. doi: 10.1038/nature03836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Flores I, et al. The longest telomeres: A general signature of adult stem cell compartments. Genes Dev. 2008;22:654–667. doi: 10.1101/gad.451008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Breault DT, et al. Generation of mTert-GFP mice as a model to identify and study tissue progenitor cells. Proc Natl Acad Sci USA. 2008;105:10420–10425. doi: 10.1073/pnas.0804800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bekaert S, Derradji H, Baatout S. Telomere biology in mammalian germ cells and during development. Dev Biol. 2004;274:15–30. doi: 10.1016/j.ydbio.2004.06.023. [DOI] [PubMed] [Google Scholar]
- 16.Kolquist KA, et al. Expression of TERT in early premalignant lesions and a subset of cells in normal tissues. Nat Genet. 1998;19:182–186. doi: 10.1038/554. [DOI] [PubMed] [Google Scholar]
- 17.Ritz JM, et al. A novel transgenic mouse model reveals humanlike regulation of an 8-kbp human TERT gene promoter fragment in normal and tumor tissues. Cancer Res. 2005;65:1187–1196. doi: 10.1158/0008-5472.CAN-04-3046. [DOI] [PubMed] [Google Scholar]
- 18.Niiyama H, et al. Quantitative analysis of hTERT mRNA expression in colorectal cancer. Am J Gastroenterol. 2001;96:1895–1900. doi: 10.1111/j.1572-0241.2001.03890.x. [DOI] [PubMed] [Google Scholar]
- 19.Martín-Rivera L, Herrera E, Albar JP, Blasco MA. Expression of mouse telomerase catalytic subunit in embryos and adult tissues. Proc Natl Acad Sci USA. 1998;95:10471–10476. doi: 10.1073/pnas.95.18.10471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Booth C, Potten CS. Gut instincts: Thoughts on intestinal epithelial stem cells. J Clin Invest. 2000;105:1493–1499. doi: 10.1172/JCI10229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Stadtfeld M, Maherali N, Breault DT, Hochedlinger K. Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell. 2008;2:230–240. doi: 10.1016/j.stem.2008.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Greider CW. Telomerase activity, cell proliferation, and cancer. Proc Natl Acad Sci USA. 1998;95:90–92. doi: 10.1073/pnas.95.1.90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.He XC, et al. PTEN-deficient intestinal stem cells initiate intestinal polyposis. Nat Genet. 2007;39:189–198. doi: 10.1038/ng1928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.He XC, et al. BMP signaling inhibits intestinal stem cell self-renewal through suppression of Wnt-beta-catenin signaling. Nat Genet. 2004;36:1117–1121. doi: 10.1038/ng1430. [DOI] [PubMed] [Google Scholar]
- 25.Fujimoto K, Beauchamp RD, Whitehead RH. Identification and isolation of candidate human colonic clonogenic cells based on cell surface integrin expression. Gastroenterology. 2002;123:1941–1948. doi: 10.1053/gast.2002.37065. [DOI] [PubMed] [Google Scholar]
- 26.Dekaney CM, Rodriguez JM, Graul MC, Henning SJ. Isolation and characterization of a putative intestinal stem cell fraction from mouse jejunum. Gastroenterology. 2005;129:1567–1580. doi: 10.1053/j.gastro.2005.08.011. [DOI] [PubMed] [Google Scholar]
- 27.May R, et al. Doublecortin and CaM kinase-like-1 and leucine-rich-repeat-containing G-protein-coupled receptor mark quiescent and cycling intestinal stem cells, respectively. Stem Cells. 2009;27:2571–2579. doi: 10.1002/stem.193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Marshman E, Ottewell PD, Potten CS, Watson AJ. Caspase activation during spontaneous and radiation-induced apoptosis in the murine intestine. J Pathol. 2001;195:285–292. doi: 10.1002/path.967. [DOI] [PubMed] [Google Scholar]
- 29.Potten CS. Radiation, the ideal cytotoxic agent for studying the cell biology of tissues such as the small intestine. Radiat Res. 2004;161:123–136. doi: 10.1667/rr3104. [DOI] [PubMed] [Google Scholar]
- 30.Snippert HJ, et al. Lgr6 marks stem cells in the hair follicle that generate all cell lineages of the skin. Science. 2010;327:1385–1389. doi: 10.1126/science.1184733. [DOI] [PubMed] [Google Scholar]
- 31.Tumbar T, et al. Defining the epithelial stem cell niche in skin. Science. 2004;303:359–363. doi: 10.1126/science.1092436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Snippert HJ, et al. Prominin-1/CD133 marks stem cells and early progenitors in mouse small intestine. Gastroenterology. 2009;136:2187–2194. e1. doi: 10.1053/j.gastro.2009.03.002. [DOI] [PubMed] [Google Scholar]
- 33.Cowan CR, Hyman AA. Asymmetric cell division in C. elegans: Cortical polarity and spindle positioning. Annu Rev Cell Dev Biol. 2004;20:427–453. doi: 10.1146/annurev.cellbio.19.111301.113823. [DOI] [PubMed] [Google Scholar]
- 34.Lechler T, Fuchs E. Asymmetric cell divisions promote stratification and differentiation of mammalian skin. Nature. 2005;437:275–280. doi: 10.1038/nature03922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lopez-Garcia C, Klein AM, Simons BD, Winton DJ. Intestinal stem cell replacement follows a pattern of neutral drift. Science. 2010;330:822–825. doi: 10.1126/science.1196236. [DOI] [PubMed] [Google Scholar]
- 36.Snippert HJ, et al. Intestinal crypt homeostasis results from neutral competition between symmetrically dividing Lgr5 stem cells. Cell. 2010;143:134–144. doi: 10.1016/j.cell.2010.09.016. [DOI] [PubMed] [Google Scholar]
- 37.Park JI, et al. Telomerase modulates Wnt signalling by association with target gene chromatin. Nature. 2009;460:66–72. doi: 10.1038/nature08137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Millar SE. Cell biology: The not-so-odd couple. Nature. 2009;460:44–45. doi: 10.1038/460044a. [DOI] [PubMed] [Google Scholar]
- 39.Mariadason JM, et al. Gene expression profiling of intestinal epithelial cell maturation along the crypt-villus axis. Gastroenterology. 2005;128:1081–1088. doi: 10.1053/j.gastro.2005.01.054. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.