Abstract
CO2 is both a critical regulator of animal physiology and an important sensory cue for many animals for host detection, food location, and mate finding. The free-living soil nematode Caenorhabditis elegans shows CO2 avoidance behavior, which requires a pair of ciliated sensory neurons, the BAG neurons. Using in vivo calcium imaging, we show that CO2 specifically activates the BAG neurons and that the CO2-sensing function of BAG neurons requires TAX-2/TAX-4 cyclic nucleotide-gated ion channels and the receptor-type guanylate cyclase GCY-9. Our results delineate a molecular pathway for CO2 sensing and suggest that activation of a receptor-type guanylate cyclase is an evolutionarily conserved mechanism by which animals detect environmental CO2.
Keywords: guanylyl cyclase, olfaction, transcriptional profiling, regulator of G protein signaling, chemosensation
The ability to detect and respond to changing concentrations of environmental CO2 is widespread among animals and plays a critical role in locating food, finding hosts and mates, and avoiding danger (1–4). CO2 exposure can also have profound physiological effects, including altered respiration, motility, fecundity, and emotional state (5–7). CO2 is sensed as an aversive cue by many free-living animals, including humans (3, 6, 8, 9). By contrast, many parasites and disease vectors are attracted to CO2, which serves as a sensory cue for host location (1, 10).
Nematodes constitute a large and highly diverse phylum that includes both free-living and parasitic species. Many parasitic nematodes, including some of the most devastating human- and plant-parasitic nematodes, are attracted to CO2. By contrast, adults of the free-living species Caenorhabditis elegans are repelled by CO2 (11–14). CO2 avoidance by C. elegans requires a pair of head neurons called the BAG neurons (13), which also mediate responses to decreases in ambient oxygen levels (15). Whether the BAG neurons directly sense CO2 is not known, and the signaling pathways that mediate CO2 detection are poorly understood.
We show here that environmental CO2 specifically activates the BAG neurons and not other neurons that drive avoidance behavior, suggesting that the BAG neurons are primary sensory neurons that detect CO2. Prolonged CO2 exposure causes desensitization of avoidance behavior and the BAG neurons themselves, indicating that behavioral adaptation to CO2 occurs at the level of the BAG neurons. In addition, we show that the CO2-evoked activity of the BAG neurons requires a cGMP signaling pathway consisting of the receptor guanylate cyclase GCY-9 and the cGMP-gated cation channel TAX-2/TAX-4. Insects detect CO2 using a pair of gustatory receptors (16, 17), whereas some mammals detect CO2 using the receptor-type guanylate cyclase, guanylate cyclase D (GC-D), and soluble adenylate cyclase (18–20). Our results show that the mechanism of CO2 detection in C. elegans more closely resembles that of mammals than insects and suggest an evolutionarily ancient role for receptor-type guanylate cyclases in mediating environmental CO2 detection by animals.
Results
BAG Neurons Are Activated by CO2.
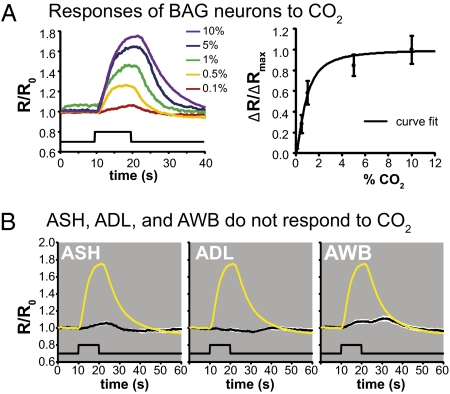
BAG neurons are located in the head, extend ciliated dendrites to the tip of the nose (21), and are required for avoidance of CO2 across concentrations (Figs. S1 and S2). To determine whether the BAG neurons respond to environmental CO2, we monitored the activity of BAG neurons using the genetically encoded ratiometric calcium indicator cameleon (22). The gcy-33 promoter was used to drive expression of cameleon specifically in the BAG neurons (23). We first confirmed that animals expressing gcy-33::cameleon show normal CO2 avoidance behavior (Fig. S3). We then imaged these animals and found that CO2 exposure evoked rapid and reversible calcium transients in the cell bodies of BAG neurons (Fig. 1A and Fig. S4). By contrast, other sensory neurons known to mediate C. elegans avoidance behavior, the amphid neurons ASH, ADL, and AWB, did not respond to a 10% CO2 stimulus, indicating that the CO2 response of BAG neurons is cell type-specific (Fig. 1B). This result suggests that the BAG neurons are the primary sensory neurons that detect CO2.
Fig. 1.
BAG neurons are activated by CO2. (A Left) BAG neuron cell bodies respond to CO2. R/R0 is the YFP to CFP ratio (R) divided by the average YFP to CFP ratio of the first 10 frames (R0). (A Right) Dose-response curve for CO2. ΔR/ΔRmax is the maximal ratio change caused by presentation of a given CO2 stimulus normalized to the maximal ratio change measured (evoked by 10% CO2). (B) ASH, ADL, and AWB do not respond to 10% CO2. The yellow trace is the average response of the BAG neurons to 10% CO2. The white area around each trace represents the SEM; the lower black traces indicate the stimulus onset and duration (n = 5–18 animals for each condition or genotype). For all graphs, error bars represent SEMs.
Prolonged CO2 Exposure Desensitizes BAG Neurons.
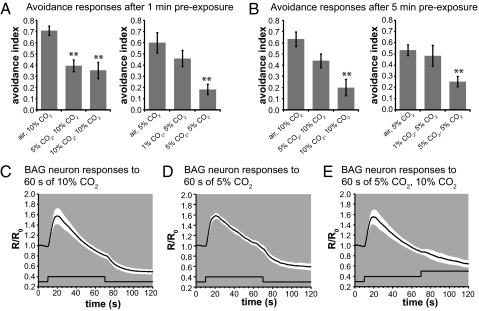
Prolonged exposure to many sensory stimuli results in behavioral adaptation, which can occur either in the primary sensory neuron or in circuitry downstream of the sensory neuron (24–28). We tested whether CO2 avoidance behavior adapts to prolonged CO2 exposure by exposing animals to 1%, 5%, or 10% CO2 for either 1 or 5 min and then testing their ability to respond to 5% or 10% CO2 in an acute avoidance assay. We found that prolonged exposure to either 5% or 10% CO2 greatly reduced subsequent behavioral responses to the same concentration of CO2, and responses to 10% CO2 were significantly decreased after a 1-min exposure to 5% CO2 (Fig. 2 A and B). Thus, C. elegans displays behavioral adaptation in response to prolonged CO2 exposure.
Fig. 2.
Prolonged CO2 exposure desensitizes BAG neurons. (A and B) Acute CO2 avoidance after either a (A) 1-min or (B) 5-min exposure to CO2. For each treatment condition, the stimulus used during the preexposure is indicated followed by the stimulus used for the acute avoidance assay (n = 11–15 trials for each treatment). (C and D) Prolonged pulses of either (C) 10% or (D) 5% CO2 results in BAG neuron desensitization (n = 7 animals for each condition). (E) Prior exposure to 5% CO2 blocks the response to 10% CO2 (n = 7 animals). The white area around each trace represents the SEM; the lower black traces indicate the stimulus onset and duration.
To test whether behavioral adaptation to 5% or 10% CO2 occurs at the level of the BAG neurons, we recorded the calcium response of BAG neurons evoked by prolonged exposure to CO2. We found that a 1-min exposure to either 5% or 10% CO2 caused an initial increase in BAG neuron calcium, which then dropped to below-baseline levels (Fig. 2 C and D). This drop in BAG neuron calcium was the result of desensitization; a 1-min exposure to 5% CO2 blocked the calcium response to a subsequent 10% CO2 stimulus (Fig. 2E). These results show that the BAG neurons desensitize during prolonged exposure to CO2 and suggest that behavioral adaptation to CO2 occurs at the level of the BAG sensory neuron.
cGMP-Gated Channel TAX-2/TAX-4 Is Required for BAG Neuron Responses to CO2.
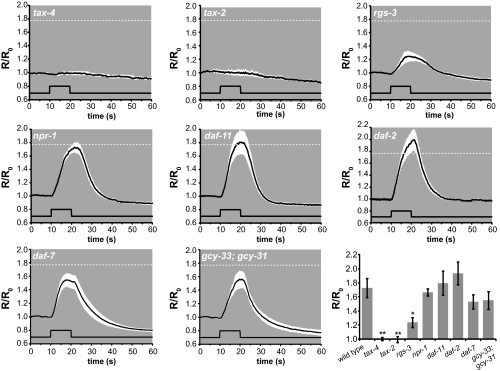
CO2 avoidance behavior by C. elegans requires multiple signaling pathways, including a cGMP, a G protein, an insulin, and a TGF-β pathway (13, 14). To test whether any of these pathways are required for the CO2-evoked calcium response of the BAG neurons, we measured CO2-evoked calcium transients of BAG neurons in animals that are mutant for these pathways and behaviorally defective in acute CO2 avoidance. We found that mutations in tax-2 and tax-4, which encode subunits of a cGMP-gated cation channel (29), eliminated the CO2 calcium response of BAG neurons, indicating a direct requirement for TAX-2/TAX-4 in the signal transduction cascade that leads to CO2 detection (Fig. 3).
Fig. 3.
A subset of genes required for acute CO2 avoidance is required for activation of BAG neurons by CO2. tax-2 and tax-4 mutations eliminate and an rgs-3 mutation reduces CO2-evoked calcium transients in the BAG neurons. npr-1, daf-11, daf-2, daf-7, and gcy-33; gcy-31 mutations do not affect BAG neuron responses to CO2 (n = 6–18 animals for each genotype). Dashed line indicates the mean maximum response of WT animals. The white area around each trace represents the SEM; the lower black traces indicate the stimulus onset and duration. (Lower Right) Summary of CO2 responses for each genotype. Mean responses were calculated as the mean response after CO2 exposure in the time interval 19.0–19.9 s, normalized to the mean response before CO2 exposure in the time interval 0.4–1.3 s. Error bars represent average SEMs after CO2 exposure (19.0–19.9 s).
We also found that G-protein signaling modulated BAG neuron responses to CO2: mutation of rgs-3, which encodes a regulator of G protein signaling (30), resulted in reduced BAG neuron responses to CO2 (Fig. 3). To test whether rgs-3 functions in BAG neurons to regulate CO2 responses, we expressed rgs-3 specifically in the BAG neurons of rgs-3 mutants and found that this expression restored normal CO2 avoidance behavior (Fig. S5A). Because regulator of G protein signaling (RGS) proteins negatively regulate G-protein signaling (31), our results indicate that a G-protein signaling pathway negatively regulates BAG neurons.
A number of genes that are required for behavioral avoidance of CO2 (13) were not required for CO2-evoked calcium responses of the BAG neurons. These genes include npr-1, which encodes a G protein-coupled receptor similar to the neuropeptide Y receptor, daf-11, which encodes a receptor guanylate cyclase, daf-2, which encodes an insulin receptor, and daf-7, which encodes a TGF-β receptor. The BAG neurons of these mutants displayed robust calcium responses to CO2, suggesting that these genes act either downstream of or in parallel to the calcium signal or that they act to regulate CO2 avoidance in cells other than BAG neurons (Fig. 3). daf-7 was reported to be expressed specifically in the amphid neurons ASI (32, 33), and expression of daf-7 specifically in ASI neurons rescued the defect in CO2 avoidance of daf-7 mutants (Fig. S5B). Thus, daf-7 acts in cells other than the BAG neurons to regulate acute CO2 avoidance. Ablation of the ASI neurons does not affect CO2 avoidance behavior (Fig. S5C), indicating that, unlike the BAG neurons, ASI neurons are not required for CO2 detection. We also tested whether two BAG neuron-specific guanylate cyclases, gcy-31 and gcy-33, are required for CO2-evoked BAG neuron activity. gcy-31 and gcy-33 encode soluble guanylate cyclases that are required for BAG neuron responses to acute hypoxia (15). We found that the BAG neurons of gcy-33; gcy-31 double mutants showed normal CO2-evoked activity and that gcy-33; gcy-31 mutants had normal behavioral responses to CO2. Therefore, the CO2- and O2-sensing functions of the BAG neurons require distinct signaling pathways (Fig. 3 and Fig. S6).
Transcriptional Profiling of Embryonic BAG Neurons Identifies Signaling Molecules That Might Function in CO2 Sensation.
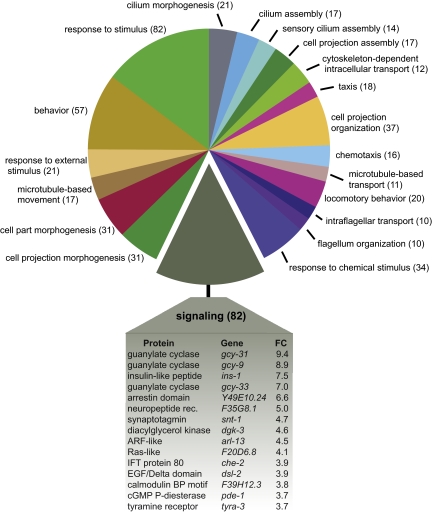
To identify signaling pathways that function in BAG neurons in CO2 sensing, we performed transcriptional profiling of embryonic BAG neurons using Affymetrix C. elegans tiling arrays (Fig. S7). We identified 850 mRNA transcripts that were significantly enriched in the BAG neurons relative to the aggregate of all other embryonic cells (Dataset S1). The BAG neuron transcriptional profile is consistent with the role of BAG as a sensory neuron: the most highly enriched gene ontology terms include chemotaxis, response to external stimuli, response to chemical stimulus, and signaling [false discovery rate (FDR) ≤ 1%] (Fig. 4). BAG-enriched transcripts include a number of genes reported to be specifically expressed by BAG neurons (gcy-31, gcy-33, and flp-17) and genes with known roles in CO2 sensing by BAG neurons (tax-4 and tax-2) (13, 15, 34).
Fig. 4.
Transcriptional profiling of embryonic BAG neurons. BAG neuron-enriched genes were organized according to gene ontology (GO) terms referring to biological process. The chart depicts the 15 most frequent GO terms, with the number of genes in each listed in parentheses. Shown below are the most abundant transcripts in the largest category (signaling), with fold change (FC) indicating relative enrichment in the BAG neuron profile.
Among the genes most highly enriched in the BAG neurons is gcy-9 (Fig. 4), which encodes a receptor-type guanylate cyclase (23). Nothing was previously known about the function of gcy-9, and reporter gene constructs for gcy-9 resulted in either no expression or variable nonneuronal expression (23, 35). We found that gcy-9 shows an approximately ninefold enrichment in BAG neurons relative to other embryonic cells, suggesting a role for gcy-9 in BAG neuron function.
gcy-9 Is Required for BAG Neurons to Respond to CO2.
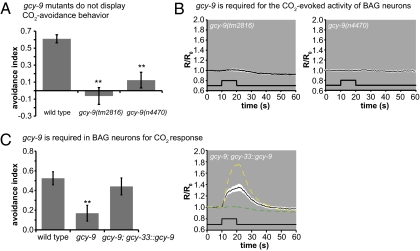
We examined the behavioral response of gcy-9 mutants to CO2 and found that gcy-9 mutants did not respond to CO2 (Fig. 5A and Fig. S8 A and B). To test whether gcy-9 is required for CO2-evoked activity of BAG neurons, we imaged the BAG neurons of gcy-9 mutants. We found that the BAG neurons of gcy-9 mutants did not show CO2-evoked calcium transients, showing that gcy-9 is necessary for BAG neuron responses to CO2 (Fig. 5B). Expression of WT gcy-9 specifically in the BAG neurons of gcy-9 mutants rescued the defect in CO2 avoidance behavior of gcy-9 mutants and partially rescued the defect in CO2-evoked neural activity of the BAG neuron (Fig. 5C), showing that gcy-9 functions cell autonomously in CO2 detection by the BAG neurons. Mutations in other guanylate cyclase genes did not affect acute CO2 avoidance (Fig. S8C) (13), with the exception of daf-11, which affects behavioral avoidance (13) but does not affect BAG neuron responses to CO2 (Fig. 3). Thus, gcy-9 is specifically required for CO2-evoked BAG neuron activity. Taken together, our results suggest that GCY-9 acts upstream of TAX-2/TAX-4 in BAG neurons to mediate CO2 detection.
Fig. 5.
gcy-9 is required for CO2 sensing by BAG neurons. (A) gcy-9 mutants do not respond to CO2 (n = 10–32 trials for each genotype). (B) gcy-9 mutants do not show CO2-evoked activity in the BAG neurons (n = 9 animals). (C) Expression of gcy-9 specifically in the BAG neurons completely rescues CO2 avoidance behavior (Left) and partially rescues CO2-evoked calcium transients of BAG neurons (Right). Left has n = 22–39 trials for each genotype, and Right has n = 8 animals. The yellow dashed line shows the mean response of WT animals, and the green dashed line shows the mean response of gcy-9(tm2816) animals. The white area around each trace represents the SEM; the lower black traces indicated the stimulus onset and duration. For this experiment, CO2 avoidance behavioral assays were performed on all three genotypes in parallel.
Discussion
Our data indicate that the BAG neurons are activated by CO2 and are likely the primary sensory neurons that detect CO2. BAG neurons are sensitive detectors of CO2, responding to CO2 concentrations as little as twofold above the ambient level of ∼0.04%. Behavioral adaptation to environmental CO2 is also mediated by the BAG neurons, which desensitize during prolonged CO2 stimuli.
The CO2 avoidance circuit is modulated by input from other sensory neurons. Our data show that daf-7 is required in ASI neurons for CO2 avoidance behavior, suggesting that sensory cues detected by ASI regulate CO2 avoidance. Because the BAG neurons of daf-7 mutants respond normally to CO2, it is possible that ASI acts on the neural circuit that mediates CO2 avoidance downstream of the BAG neurons. This circuit remains to be defined. The primary synaptic output of the BAG neurons is onto five interneurons: the ventral cord interneurons AVA and AVE and the ring interneurons RIA, RIB, and RIG (21). In preliminary studies, we did not observe CO2-evoked responses in the cell bodies of these interneurons. However, we cannot exclude the possibility that CO2-evoked responses might be restricted to the processes of interneurons, which has been reported for interneurons in thermotaxis and chemotaxis circuits of C. elegans (36, 37).
It is also possible that the CO2 avoidance circuit involves extrasynaptic signaling (for example, through neuropeptide secretion from BAG). Our analysis of BAG neuron transcripts identified more than a dozen neuropeptides that show enriched expression in the BAG neurons (Dataset S1). A role for neuropeptide signaling in the regulation of egg laying by the BAG neurons has been shown: the FMRF-amide like neuropeptide FLP-17 is secreted by the BAG neurons and acts on the G protein-coupled receptor EGL-6 on the HSN hermaphrodite-specific motor neurons to inhibit egg laying (34). FLP-17 peptides are not required for acute CO2 avoidance (Fig. S9), indicating that BAG neurons regulate egg laying and avoidance behavior through distinct signaling pathways and circuits.
How do BAG neurons detect CO2? CO2 detection by insects requires the gustatory receptors GR21a and GR63a, which seem to act through a G-protein signaling pathway (16, 17, 38). By contrast, mammalian olfactory receptor neurons that respond to CO2 express the receptor-type guanylate cyclase GC-D (8), which is activated by the CO2 metabolite bicarbonate (20, 39). Because CO2 sensing by the BAG neurons of C. elegans requires the receptor-type guanylate cyclase GCY-9, we propose that GCY-9 might function as a receptor for CO2 or a metabolite of CO2. GCY-9 is one of a large number of C. elegans guanylate cyclases (Fig. S10), many of which are expressed in small subsets of sensory neurons and are required for specific chemosensory behaviors (23, 29). Although some GCY proteins seem to function as chemoreceptors, others have been proposed to function downstream of G protein-coupled receptors (23, 40–42). It is, therefore, also possible that GCY-9 acts downstream of a yet to be identified receptor for CO2.
GCY-9 orthologs are present in multiple nematode species, including the plant-parasitic nematodes Heterodera glycines and Meloidogyne incognita (43) and the human-parasitic nematode Brugia malayi (44). The requirement for GCY-9 in mediating a behavioral response to CO2 and the importance of CO2 as a host-seeking cue for many parasitic nematodes raise the possibility that compounds that block GCY-9 activity might be useful in the development of strategies for nematode control.
Materials and Methods
Standard techniques are listed in SI Materials and Methods.
Behavioral Assay for Acute CO2 Avoidance.
CO2 assays were performed as previously described (13). Briefly, for each assay, about 10–20 L4 hermaphrodites were placed on 5-cm assay plates overnight and tested as young adults. Plates consisted of nematode growth medium (NGM) agar seeded with a thin lawn of Escherichia coli OP50 bacteria. Gases were medical grade certified mixes (Air Liquide) consisting of the indicated CO2 concentration, 10% O2, and the remaining percentage of N2. A concentration of 10% CO2 was used unless otherwise indicated. Two 50-mL syringes were filled with gas, one with CO2 and one without CO2. The mouths of the syringes were connected to tubes attached to Pasteur pipettes, and gases were pumped through the Pasteur pipettes using a syringe pump at a rate of 1.5 mL/min. Worms were exposed to gases by placing the tip of the Pasteur pipette near the head of a forward-moving worm, and a response was scored if the worm reversed within 4 s. An avoidance index was then calculated by subtracting the fraction of animals that reversed in response to the air control from the fraction of animals that reversed in response to the CO2 (Fig. S2A).
Behavioral Assay for Adaptation to CO2.
For each assay, about five L4 hermaphrodites were placed on 9-cm assay plates overnight and tested as young adults. Plates consisted of NGM agar seeded with a thin lawn of OP50 in the center of the plate. Gas was pumped into the plate through a hole on one side of the lid at a rate of 1 L/min; a hole on the other side allowed gas to escape. Worms were preexposed to gas for either 1 or 5 min and then tested immediately in an acute avoidance assay.
Imaging BAG Neurons in Restrained Animals.
Young adults were immobilized with cyanoacrylate veterinary glue (Surgi-Lock; Meridian Animal Health) on a cover glass coated with a 2% agarose pad made with 10 mM Hepes (pH 7.4). The cover glass was affixed to a custom-made air chamber. The specimen was illuminated with 435-nm excitation light and imaged using a 40× Nikon long-working distance objective (0.75 numerical aperture). The emission image was passed through a DV2 image splitter (Photometrics), and the CFP and YFP emission images were projected onto two halves of a cooled CCD camera (Andor). Images were acquired at 10 Hz, with exposure times between 10 and 50 ms. Gas perfusion was controlled by three-way valves (Numatics) driven by a custom-made valve controller unit. Excitation light, image acquisition, and hardware control were performed by the Live Acquisition software package (Till Photonics). Custom certified gas mixes used for imaging were obtained from Airgas.
Image Analysis.
The mean pixel value of a background region of interest was subtracted from the mean pixel value of a region of interest that circumscribed the specimen. A correction factor, which we measured in images of samples that express only CFP, was applied to the YFP channel to compensate for bleed through of CFP emissions into the YFP channel (YFPadjusted = YFP − 0.86 × CFP). YFP to CFP ratios were normalized to the average value of the first 10 frames (1 s), and a boxcar filter of 5 frames (0.5 s) was applied to the time series.
Generating a Gene Expression Profile of Embryonic BAG Neurons.
A synchronized population of nIs242[gcy-33::GFP] hermaphrodites was treated with hypochlorite to release embryos. Embryos were dissociated with chitinase as previously described (45, 46). GFP-labeled BAG neurons were isolated from the freshly dissociated suspension of embryonic cells by FACS; sorted cells were confirmed to be >80% BAG neurons by direct inspection in a fluorescent microscope. A reference population comprised of all viable embryonic cells was also collected by FACS. Dead cells were marked with propidium-iodide and excluded from these preparations. Sorting was performed with a Becton-Dickinson FACS-Aria (75-μm nozzle; ∼15,000 events/s). Approximately 30,000 cells were obtained per sort. Cells were sorted directly into TRIzol LS for extraction with phenol chloroform. RNA was precipitated with isopropanol and purified using a ZYMO DNA-free RNA kit. RNA integrity and concentration were evaluated using an Agilent Bioanalyzer. RNA (1–2 ng) was amplified with the WT-Ovation Pico RNA Amplification System (NuGEN). Double-stranded (ds) cDNA was generated with a WT-Ovation Exon module (NuGEN), and it was fragmented and labeled with an Encore Biotin module (NuGEN) for application to the C. elegans Affymetrix 1.0R whole-genome tiling array; ds cDNA targets were used for hybridization, because all probes on the Affymetrix 1.0R array match a single DNA strand, whereas transcripts are derived from both strands. Tiling array results were obtained from three independent replicates (interse Pearson correlations ≥ 0.89). Methods for tiling array analysis are briefly summarized here. A more detailed description will be reported elsewhere. Unique PM (Perfect Match) probes from exonic regions of gene models were selected to generate a probe set for each gene listed in WormBase (WS199). Intensity values were quantile-normalized, and probe-specific effects were reduced by Robust Multichip Analysis (RMA) (47). An empirical null model of background expression was derived from intergenic probes, and genes with intensity values exceeding this threshold at ≤5% FDR were scored as significantly expressed genes (EGs). Transcripts that were significantly elevated in BAG neurons were identified by comparison with a reference dataset obtained from all viable embryonic cells. Differentially expressed genes were estimated using a linear model and Bayes-moderated t statistic (48, 49); 850 transcripts with FDR ≤ 10% and 1.5-fold elevated vs. the reference dataset were scored as significantly enriched in BAG neurons (Dataset S1). Gene ontology analysis was performed with the gene ontology (GO) enrichment analysis widget on the modENCODE intermine website (http://intermine.modencode.org). The 850 enriched BAG transcripts were uploaded to the modMINE website on July, 5, 2010; 464 transcripts were annotated with GO terms and compared with all genes for enrichment using the hypergeometric test with FDR ≤ 1%. All transcripts annotated with enriched GO terms are listed in Dataset S2.
Statistical Analysis.
Statistical tests were performed using GraphPad Instat. Statistical comparisons were made using a one-way ANOVA with Dunnett's posttest, except that Fig. 1A used a paired t test and Fig. 5A used an unpaired t test. For Fig. 1A, the dose-response curve shows the least squares fit of a Hill equation to the data points, with a Kd of 0.9% CO2 and a Hill coefficient of 1.6 (for all graphs, ***P < 0.001, **P < 0.01, and *P < 0.05). The GCY dendrogram was generated using ClustalW. The gcy-9 intron–exon structure was generated using Exon–Intron Graphic Maker by Nikhil Bhatla (http://www.wormweb.org/exonintron).
Supplementary Material
Acknowledgments
We thank Aravi Samuel, Chris Gabel, and Harrison Gabel (Harvard University, Cambridge, MA); Cori Bargmann (Rockefeller University, New York); Leon Avery (University of Texas Southwestern Medical Center, Dallas); Ikue Mori and Atsushi Kuhara (Nagoya University, Nagoya, Japan); Denise Ferkey (SUNY Buffalo, Buffalo, NY); Larry Salkoff (Washington University School of Medicine, St. Louis); Shohei Mitani (Tokyo Women's Medical University School of Medicine, Tokyo); Anne Hart (Brown University, Providence, RI); and the Caenorhabditis Genetics Center for strains and reagents. We thank the Vanderbilt Flow Cytometry Core and Vanderbilt Functional Genomics Shared Resource (VFGSR) for help with microarray experiments. We thank Julia Brandt and Sonya Aziz-Zaman for assistance in cloning the gcy-9 cDNA. H.R.H. and P.W.S. are Investigators of the Howard Hughes Medical Institute. This work was supported by a Helen Hay Whitney postdoctoral fellowship (to E.A.H.), a National Institutes of Health Pathway to Independence award (to E.A.H.), National Institutes of Health Grants U01 HG004263 (to D.M.M.), R01 NS26115 (to D.M.M.), and R01 GM24663 (to H.R.H.), the Howard Hughes Medical Institute (P.W.S.), a Whitehead Fellowship for Junior Faculty in Biomedical and Biological Sciences (to N.R.), and funds from the Helen L. and Martin S. Kimmel Center for Biology and Medicine (to N.R.).
Footnotes
The authors declare no conflict of interest.
Data deposition: The sequence reported in this paper has been deposited in the GenBank database (accession no. HQ636455). The gene expression data have been deposited in the Gene Expression Omnibus database (accession no. GSE23769).
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1017354108/-/DCSupplemental.
References
- 1.Haas W. Parasitic worms: Strategies of host finding, recognition and invasion. Zoology (Jena) 2003;106:349–364. doi: 10.1078/0944-2006-00125. [DOI] [PubMed] [Google Scholar]
- 2.Guerenstein PG, Hildebrand JG. Roles and effects of environmental carbon dioxide in insect life. Annu Rev Entomol. 2008;53:161–178. doi: 10.1146/annurev.ento.53.103106.093402. [DOI] [PubMed] [Google Scholar]
- 3.Suh GS, et al. A single population of olfactory sensory neurons mediates an innate avoidance behaviour in Drosophila. Nature. 2004;431:854–859. doi: 10.1038/nature02980. [DOI] [PubMed] [Google Scholar]
- 4.Faucher C, Forstreuter M, Hilker M, de Bruyne M. Behavioral responses of Drosophila to biogenic levels of carbon dioxide depend on life-stage, sex and olfactory context. J Exp Biol. 2006;209:2739–2748. doi: 10.1242/jeb.02297. [DOI] [PubMed] [Google Scholar]
- 5.Sharabi K, et al. Elevated CO2 levels affect development, motility, and fertility and extend life span in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2009;106:4024–4029. doi: 10.1073/pnas.0900309106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ziemann AE, et al. The amygdala is a chemosensor that detects carbon dioxide and acidosis to elicit fear behavior. Cell. 2009;139:1012–1021. doi: 10.1016/j.cell.2009.10.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lahiri S, Forster RE., 2nd CO2/H(+) sensing: Peripheral and central chemoreception. Int J Biochem Cell Biol. 2003;35:1413–1435. doi: 10.1016/s1357-2725(03)00050-5. [DOI] [PubMed] [Google Scholar]
- 8.Hu J, et al. Detection of near-atmospheric concentrations of CO2 by an olfactory subsystem in the mouse. Science. 2007;317:953–957. doi: 10.1126/science.1144233. [DOI] [PubMed] [Google Scholar]
- 9.Bensafi M, Frasnelli J, Reden J, Hummel T. The neural representation of odor is modulated by the presence of a trigeminal stimulus during odor encoding. Clin Neurophysiol. 2007;118:696–701. doi: 10.1016/j.clinph.2006.10.022. [DOI] [PubMed] [Google Scholar]
- 10.Bowen MF. The sensory physiology of host-seeking behavior in mosquitoes. Annu Rev Entomol. 1991;36:139–158. doi: 10.1146/annurev.en.36.010191.001035. [DOI] [PubMed] [Google Scholar]
- 11.Haas W, et al. Behavioural strategies used by the hookworms Necator americanus and Ancylostoma duodenale to find, recognize and invade the human host. Parasitol Res. 2005;95:30–39. doi: 10.1007/s00436-004-1257-7. [DOI] [PubMed] [Google Scholar]
- 12.Prot JC. Migration of plant-parasitic nematodes towards plant roots. Rev Nematol. 1980;3:305–318. [Google Scholar]
- 13.Hallem EA, Sternberg PW. Acute carbon dioxide avoidance in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8038–8043. doi: 10.1073/pnas.0707469105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bretscher AJ, Busch KE, de Bono M. A carbon dioxide avoidance behavior is integrated with responses to ambient oxygen and food in Caenorhabditis elegans. Proc Natl Acad Sci USA. 2008;105:8044–8049. doi: 10.1073/pnas.0707607105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zimmer M, et al. Neurons detect increases and decreases in oxygen levels using distinct guanylate cyclases. Neuron. 2009;61:865–879. doi: 10.1016/j.neuron.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kwon JY, Dahanukar A, Weiss LA, Carlson JR. The molecular basis of CO2 reception in Drosophila. Proc Natl Acad Sci USA. 2007;104:3574–3578. doi: 10.1073/pnas.0700079104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jones WD, Cayirlioglu P, Kadow IG, Vosshall LB. Two chemosensory receptors together mediate carbon dioxide detection in Drosophila. Nature. 2007;445:86–90. doi: 10.1038/nature05466. [DOI] [PubMed] [Google Scholar]
- 18.Chen Y, et al. Soluble adenylyl cyclase as an evolutionarily conserved bicarbonate sensor. Science. 2000;289:625–628. doi: 10.1126/science.289.5479.625. [DOI] [PubMed] [Google Scholar]
- 19.Luo M, Sun L, Hu J. Neural detection of gases—carbon dioxide, oxygen—in vertebrates and invertebrates. Curr Opin Neurobiol. 2009;19:354–361. doi: 10.1016/j.conb.2009.06.010. [DOI] [PubMed] [Google Scholar]
- 20.Guo D, Zhang JJ, Huang XY. Stimulation of guanylyl cyclase-D by bicarbonate. Biochemistry. 2009;48:4417–4422. doi: 10.1021/bi900441v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.White JG, Southgate E, Thomson JN, Brenner S. The structure of the nervous system of the nematode Caenorhabditis elegans. Philos Trans R Soc Lond B Biol Sci. 1986;314:1–340. doi: 10.1098/rstb.1986.0056. [DOI] [PubMed] [Google Scholar]
- 22.Nagai T, Yamada S, Tominaga T, Ichikawa M, Miyawaki A. Expanded dynamic range of fluorescent indicators for Ca(2+) by circularly permuted yellow fluorescent proteins. Proc Natl Acad Sci USA. 2004;101:10554–10559. doi: 10.1073/pnas.0400417101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yu S, Avery L, Baude E, Garbers DL. Guanylyl cyclase expression in specific sensory neurons: A new family of chemosensory receptors. Proc Natl Acad Sci USA. 1997;94:3384–3387. doi: 10.1073/pnas.94.7.3384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Colbert HA, Smith TL, Bargmann CI. OSM-9, a novel protein with structural similarity to channels, is required for olfaction, mechanosensation, and olfactory adaptation in Caenorhabditis elegans. J Neurosci. 1997;17:8259–8269. doi: 10.1523/JNEUROSCI.17-21-08259.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hilliard MA, et al. In vivo imaging of C. elegans ASH neurons: Cellular response and adaptation to chemical repellents. EMBO J. 2005;24:63–72. doi: 10.1038/sj.emboj.7600493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.L'Etoile ND, et al. The cyclic GMP-dependent protein kinase EGL-4 regulates olfactory adaptation in C. elegans. Neuron. 2002;36:1079–1089. doi: 10.1016/s0896-6273(02)01066-8. [DOI] [PubMed] [Google Scholar]
- 27.Chalasani SH, et al. Neuropeptide feedback modifies odor-evoked dynamics in Caenorhabditis elegans olfactory neurons. Nat Neurosci. 2010;13:615–621. doi: 10.1038/nn.2526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hirotsu T, Iino Y. Neural circuit-dependent odor adaptation in C. elegans is regulated by the Ras-MAPK pathway. Genes Cells. 2005;10:517–530. doi: 10.1111/j.1365-2443.2005.00856.x. [DOI] [PubMed] [Google Scholar]
- 29.Bargmann CI. Chemosensation in C. elegans. The C. elegans Research Community. WormBook. 2006 doi: 10.1895/wormbook.1.123.1. Available at www.WormBook.org. Accessed December 7, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ferkey DM, et al. C. elegans G protein regulator RGS-3 controls sensitivity to sensory stimuli. Neuron. 2007;53:39–52. doi: 10.1016/j.neuron.2006.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ross EM, Wilkie TM. GTPase-activating proteins for heterotrimeric G proteins: Regulators of G protein signaling (RGS) and RGS-like proteins. Annu Rev Biochem. 2000;69:795–827. doi: 10.1146/annurev.biochem.69.1.795. [DOI] [PubMed] [Google Scholar]
- 32.Ren P, et al. Control of C. elegans larval development by neuronal expression of a TGF-β homolog. Science. 1996;274:1389–1391. doi: 10.1126/science.274.5291.1389. [DOI] [PubMed] [Google Scholar]
- 33.Schackwitz WS, Inoue T, Thomas JH. Chemosensory neurons function in parallel to mediate a pheromone response in C. elegans. Neuron. 1996;17:719–728. doi: 10.1016/s0896-6273(00)80203-2. [DOI] [PubMed] [Google Scholar]
- 34.Ringstad N, Horvitz HR. FMRFamide neuropeptides and acetylcholine synergistically inhibit egg-laying by C. elegans. Nat Neurosci. 2008;11:1168–1176. doi: 10.1038/nn.2186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ortiz CO, et al. Searching for neuronal left/right asymmetry: Genomewide analysis of nematode receptor-type guanylyl cyclases. Genetics. 2006;173:131–149. doi: 10.1534/genetics.106.055749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Clark DA, Biron D, Sengupta P, Samuel AD. The AFD sensory neurons encode multiple functions underlying thermotactic behavior in Caenorhabditis elegans. J Neurosci. 2006;26:7444–7451. doi: 10.1523/JNEUROSCI.1137-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chalasani SH, et al. Dissecting a circuit for olfactory behaviour in Caenorhabditis elegans. Nature. 2007;450:63–70. doi: 10.1038/nature06292. [DOI] [PubMed] [Google Scholar]
- 38.Yao CA, Carlson JR. Role of G-proteins in odor-sensing and CO2-sensing neurons in Drosophila. J Neurosci. 2010;30:4562–4572. doi: 10.1523/JNEUROSCI.6357-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Sun L, et al. Guanylyl cyclase-D in the olfactory CO2 neurons is activated by bicarbonate. Proc Natl Acad Sci USA. 2009;106:2041–2046. doi: 10.1073/pnas.0812220106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ortiz CO, et al. Lateralized gustatory behavior of C. elegans is controlled by specific receptor-type guanylyl cyclases. Curr Biol. 2009;19:996–1004. doi: 10.1016/j.cub.2009.05.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gray JM, et al. Oxygen sensation and social feeding mediated by a C. elegans guanylate cyclase homologue. Nature. 2004;430:317–322. doi: 10.1038/nature02714. [DOI] [PubMed] [Google Scholar]
- 42.Kim K, et al. Two chemoreceptors mediate developmental effects of dauer pheromone in C. elegans. Science. 2009;326:994–998. doi: 10.1126/science.1176331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fitzpatrick DA, O'Halloran DM, Burnell AM. Multiple lineage specific expansions within the guanylyl cyclase gene family. BMC Evol Biol. 2006 doi: 10.1186/1471-2148-6-26. 10.1186/1471-2148-6-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ghedin E, et al. Draft genome of the filarial nematode parasite Brugia malayi. Science. 2007;317:1756–1760. doi: 10.1126/science.1145406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Christensen M, et al. A primary culture system for functional analysis of C. elegans neurons and muscle cells. Neuron. 2002;33:503–514. doi: 10.1016/s0896-6273(02)00591-3. [DOI] [PubMed] [Google Scholar]
- 46.Fox RM, et al. A gene expression fingerprint of C. elegans embryonic motor neurons. BMC Genomics. doi: 10.1186/1471-2164-6-42. 10.1186/1471-2164-6-42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Irizarry RA, et al. Exploration, normalization, and summaries of high density oligonucleotide array probe level data. Biostatistics. 2003;4:249–264. doi: 10.1093/biostatistics/4.2.249. [DOI] [PubMed] [Google Scholar]
- 48.Smyth GK. Linear models and empirical Bayes methods for assessing differential expression in microarray experiments. Stat Appl Genet Mol Biol. 2004 doi: 10.2202/1544-6115.1027. 10.2202/1544-6115.1027. [DOI] [PubMed] [Google Scholar]
- 49.Smyth GK. Limma: Linear models for microarray data. In: Gentleman R, Carey VJ, Huber W, Irizarry RA, Dudoit S, editors. Bioinformatics and Computational Biology Solutions Using R and Bioconductor. New York: Springer; 2005. pp. 397–420. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.