Abstract
Calcium-dependent activator protein for secretion 2 (CAPS2) is a dense-core vesicle-associated protein that is involved in the secretion of BDNF. BDNF has a pivotal role in neuronal survival and development, including the development of inhibitory neurons and their circuits. However, how CAPS2 affects BDNF secretion and its biological significance in inhibitory neurons are largely unknown. Here we reveal the role of CAPS2 in the regulated secretion of BDNF and show the effect of CAPS2 on the development of hippocampal GABAergic systems. We show that CAPS2 is colocalized with BDNF, both synaptically and extrasynaptically in axons of hippocampal neurons. Overexpression of exogenous CAPS2 in hippocampal neurons of CAPS2-KO mice enhanced depolarization-induced BDNF exocytosis events in terms of kinetics, frequency, and amplitude. We also show that in the CAPS2-KO hippocampus, BDNF secretion is reduced, and GABAergic systems are impaired, including a decreased number of GABAergic neurons and their synapses, a decreased number of synaptic vesicles in inhibitory synapses, and a reduced frequency and amplitude of miniature inhibitory postsynaptic currents. Conversely, excitatory neurons in the CAPS2-KO hippocampus were largely unaffected with respect to field excitatory postsynaptic potentials, miniature excitatory postsynaptic currents, and synapse number and morphology. Moreover, CAPS2-KO mice exhibited several GABA system-associated deficits, including reduced late-phase long-term potentiation at CA3–CA1 synapses, decreased hippocampal theta oscillation frequency, and increased anxiety-like behavior. Collectively, these results suggest that CAPS2 promotes activity-dependent BDNF secretion during the postnatal period that is critical for the development of hippocampal GABAergic networks.
Keywords: dense-core vesicles, brain-derived neurotrophic factor, GABAergic synapse
Calcium-dependent activator protein for secretion (CAPS) was identified initially as a cytosolic protein associated with dense-core vesicles (DCVs) in endocrine and neuroendocrine cells and was implicated in Ca2+-dependent DCV secretion (1–3). Neuronal CAPS is localized to DCVs where it is involved in Ca2+-activated DCV exocytosis (2). A recent KO mouse study suggested that CAPS proteins also play a role in priming glutamatergic synaptic vesicle (SV) exocytosis (4).
The CAPS protein family consists of two distinct members, CAPS1 and CAPS2 (5, 6). Our previous studies have shown that CAPS2 is involved in the secretion of BDNF in cerebellar granule cells and cerebral cortical neurons (7, 8). BDNF plays a critical role in neuronal survival and differentiation and in synaptic development and plasticity (9–12). It also exhibits a neurotrophic action on the development of GABAergic interneurons and their networks in the cerebral cortex (13–15) and hippocampus (16, 17). BDNF is secreted from DCV-like secretory vesicles from neuronal dendrites and axons (18). Together with CAPS2, synaptotagmin-IV also controls BDNF secretion, although these two proteins act in opposite ways: CAPS2 promotes secretion (7, 8), whereas synaptotagmin-IV inhibits secretion (19). Little is known, however, about how CAPS2 affects the dynamics of BDNF secretion, and the biological significance of CAPS2-induced BDNF secretion is unknown.
In the present study, we analyzed the role of CAPS2 in the regulation of BDNF secretion and in the development and function of hippocampal GABAergic neurons at cellular and microcircuitry levels. We discovered that expression of CAPS2 enhanced activity-dependent BDNF secretion kinetics, frequency, and amplitude in hippocampal neurons from CAPS2-KO mice. Moreover, we found that CAPS2-KO mice have significant deficits in hippocampal GABAergic systems at multiple levels, ranging from inhibitory synaptic architectures and synaptic function to related behaviors such as anxiety. Our results suggest an indispensable role of CAPS2 in enhancing BDNF secretion, which is important for the proper development of hippocampal GABAergic interneuron networks.
Results
Expression of Exogenous CAPS2 Enhances BDNF Secretion in CAPS2-KO Mouse Hippocampal Neurons.
To investigate the function of CAPS2, we first examined its subcellular localization in cultured hippocampal neurons by immunocytochemistry (Fig. 1A). We found that CAPS2-immunopositive puncta were largely localized in Tau+ and MAP2− axons (Fig. S1). Of CAPS2-immunopositive puncta, 26.7% colocalized with BDNF (Fig. 1A). However, only 17.7% of CAPS2 puncta that overlapped with BDNF were coincidently detected with bassoon puncta, indicating that the majority (82.3%) of CAPS2-associated BDNF vesicles was located at extrasynaptic sites rather than at presynaptic sites.
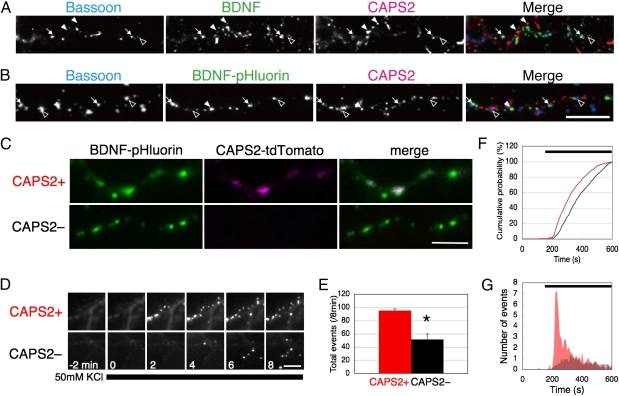
Fig. 1.
CAPS2 enhances BDNF secretion in hippocampal neurons, (A and B) Colocalization of CAPS2 and bassoon with (A) endogenous BDNF or (B) exogenously expressed BDNF-pHluorin in WT hippocampal cultures. Colocalization of BDNF (or BDNF-pHluorin) and CAPS2 in synapses (arrows) and in extrasynaptic zones (filled arrowheads) and of CAPS2 without BDNF (or BDNF-pHluorin) in synapses (open arrowheads) are indicated. (Scale bar, 10 μm.) (C) Hippocampal neurons from CAPS2-KO mice were transfected with BDNF-pHluorin, with (Upper Row) or without (Lower row) CAPS2-tdTomato. (Scale bar, 2 μm.) (D) BDNF-pHluorin exocytosis events were induced by stimulation with 50 mM KCl (indicated by thick bar at bottom). Images were taken every 2 min from 2 min before until 8 min after stimulation. (Scale bar, 2 μm.) (E) Number of total events during an 8-min recording. Error bars represent SEM. *P < 0.05 using Student's t test. (F) Cumulative distribution of BDNF-pHluorin fluorescence events in the presence (red) and absence (black) of CAPS2-tdTomato. The thick bar indicates the duration of 50-mM KCl stimulation. P < 0.05 using the Kolmogorov–Smirnov test. (G) Histogram of events in the presence (red) and absence (gray) of CAPS2-tdTomato. (E–G) n = 12 cells from three (CAPS2+) or four (CAPS2–) different cultures per transfection.
To monitor BDNF secretion from hippocampal neurons, we exogenously expressed BDNF-fused superecliptic pHluorin (a pH-sensitive GFP derivative) (20) whose fluorescence is quenched in the acidic lumen of vesicles but which becomes fluorescent upon vesicle exocytosis because of the neutral pH outside of vesicles. In fixed WT neurons, fluorescent puncta of exogenous BDNF-pHluorin colocalized with endogenous CAPS2 immunosignals (Fig. 1B), as did endogenous BDNF immunosignals (Fig. 1A). To determine whether CAPS2 deficiency altered BDNF secretion, we performed time-lapse live-cell fluorescence imaging of BDNF-pHluorin in CAPS2-KO neurons with and without cotransfection of CAPS2-fused red fluorescent protein tdTomato (referred to as “CAPS2+” and “CAPS2−” cells, respectively) (Fig. 1 C and D). Fluorescent puncta of BDNF-pHluorin and CAPS2-tdTomato colocalized in axons of cotransfected neurons (Fig. 1C), as did endogenous BDNF and CAPS2 immunosignals (Fig. 1A and Fig. S1). BDNF-pHluorin secretion was elicited by the application of 50 mM KCl in the presence of kynurenic acid and picrotoxin (PTX) (inhibitors of excitatory and inhibitory transmission, respectively) (Fig. 1D and Movie S1). Following cotransfection with CAPS2-tdTomato, the average number of BDNF-pHluorin puncta that appeared along neuronal axons during a period of 8 min after KCl stimulation was increased significantly (CAPS2+ 95.4 ± 2.5 puncta per area vs. CAPS2− 51.6 ± 8.3 puncta per area) (Fig. 1 D and E). By contrast, the number of BDNF-pHluorin puncta before KCl stimulation (Fig. 1C) and the size of BDNF-pHluorin fluorescent puncta (Fig. S2) were not different in CAPS2+ and CAPS2− cells. BDNF-pHluorin secretion was significantly faster from CAPS2+ neurons than from CAPS2− neurons (τ = 217.9 s and 298.1 s, respectively) (Fig. 1F). Moreover, time-to-peak and peak frequency of BDNF-pHluorin secretion were significantly faster and larger, respectively, as a result of CAPS2-tdTomato cotransfection (time-to-peak, 220 s in CAPS2+ vs. 320 s in CAPS2− neurons; peak frequency: 7.13 ± 1.22 events in CAPS2+ vs. 1.35 ± 0.38 events in CAPS2− neurons) (Fig. 1G).
After KCl stimulation, analysis of single exocytosis events revealed more rapid appearance of fluorescent puncta in CAPS2+ neurons than in CAPS2− neurons (Fig. 2). The fluorescence intensity of the majority of exocytosis events was significantly greater in CAPS2+ neurons than in CAPS2− neurons (Fig. 2B). The average fractional change in fluorescence (ΔF/F) 8 min after KCl application also was larger in CAPS2+ neurons (161.6 ± 15.8) than in CAPS2− neurons (117.7 ± 2.8) (Fig. 2C). Taken together, these results suggest that CAPS2 promotes activity-dependent BDNF secretion in hippocampal neurons by enhancing the frequency, kinetics, and quantal size of BDNF vesicle exocytosis events.
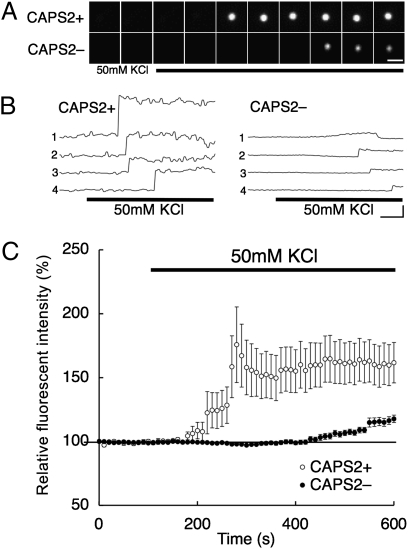
Fig. 2.
Dynamics of BDNF-pHluorin secretion events. (A) Representative time-lapse images of depolarization-induced single BDNF-pHluorin exocytosis events in axons of CAPS2-KO hippocampal neurons cotransfected with (CAPS2+, Upper Row) and without (CAPS2−, Lower Row) CAPS2-tdTomato. Images shown were taken every min from 2 min before to 8 min after the onset of 50-mM KCl stimulation at 0 min (third panel from the left). (Scale bar, 1 μm.) (B) Sample traces of BDNF-pHluorin fluorescence dynamics induced during 50-mM KCl stimulation (indicated by bar at bottom). (Scale bars: horizontal bar, 100 s; vertical bar, 100 AU) (C) Average traces of BDNF-pHluorin fluorescence dynamics in the presence and absence of CAPS2-tdTomato. Thick bar indicates 50-mM KCl stimulation. Average traces were normalized to maximum punctum fluorescence intensity before depolarization. Between 160 and 480 s after 50-mM KCl administration, CAPS2+ neurons (n = 28 from three different cultures) exhibited an average intensity of 169.5 ± 18.3%, whereas CAPS2- neurons (n = 36 from four different cultures) exhibited an average intensity of 105.2 ± 2.4% (P < 0.01, Student's t test). Error bars indicate SEM.
Morphological Abnormalities in GABAergic Interneuron Systems in the Hippocampus of CAPS2-KO Mice.
The promotion of BDNF secretion by CAPS2 prompted the hypothesis that CAPS2 deficiency affects hippocampal neurons and synapses, for instance by affecting their development or maturation. To address this issue, we first compared hippocampal interneuronal development in WT and CAPS2-KO mice by counting glutamate decarboxylase 67 (GAD67) promoter-driven GFP-tagged interneurons (SI Materials and Methods). We observed a reduction in the number of GFP-fluorescent interneurons in the CA1 and dentate gyrus of CAPS2-KO/GAD67-GFP mice compared with WT/GAD67-GFP mice in the adolescent (postnatal day 28, P28) stage but not in the adult stage (Fig. S3). Next we compared hippocampal excitatory and inhibitory synapses in WT and CAPS2-KO mice by immunostaining with anti-vesicular glutamate transporter 1 (vGluT1), an excitatory presynapse marker, and anti-vesicular GABA transporter (vGAT), an inhibitory presynapse marker (Fig. 3 A and B). The number of vGluT1-immunopositive puncta in the CA1 stratum radiatum region was not different in WT and KO mice at P28 (WT = 68.6 ± 2.0 vs. KO 70.2 ± 2.4) or at 8 wk (WT 66.4 ± 1.2 vs. KO 63.3 ± 1.7). Conversely, the number of vGAT-immunopositive puncta was significantly decreased in CAPS2-KO mice at both P28 (WT 35.9 ± 1.7 vs. KO 28.3 ± 1.9) and at 8 wk (WT 33.5 ± 1.1 vs. KO 29.3 ± 1.2) (Fig. 3B).
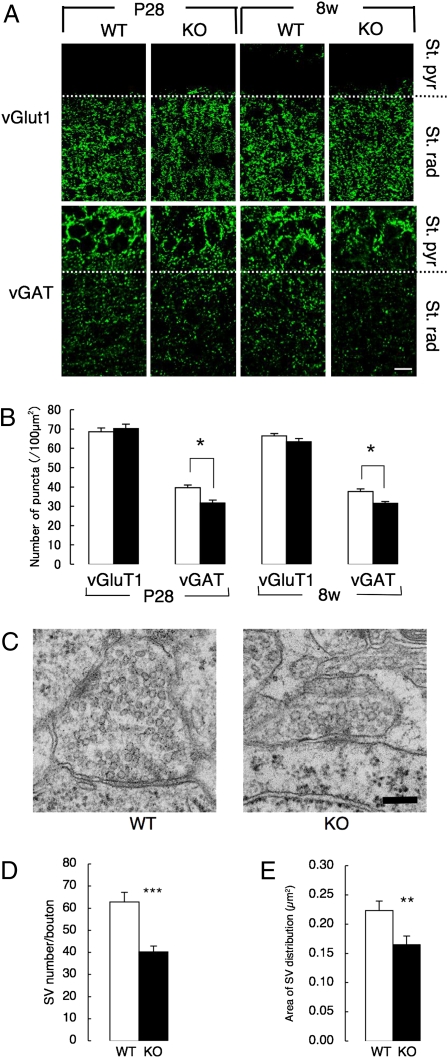
Fig. 3.
Morphological abnormalities of GABAergic interneurons in the CAPS2-KO mouse hippocampus. (A) Representative immunofluorescence images of vGluT1 and vGAT in the hippocampal CA1 region of WT and CAPS2-KO mice at P28 and 8 wk. St. pyr, stratum pyramidale; St. rad, stratum radiatum. (Scale bar, 10 μm.) (B) Number of vGluT1+ and vGAT+ puncta in the stratum radiatum. Error bars represent SEM. *P < 0.05, using Student's t test (n = 12, 16, 14, and 14 regions in P28 WT and CAPS2-KO mice and in 8-wk-old WT and CAPS2-KO mice, respectively, from at least six slices from three mice in each category). (C) Representative ultrastructure of GABAergic terminals. (Scale bar, 250 nm.) (D and E) Number of SVs per GABAergic terminal (D) and area of SV distribution (E). Error bars represent SEM. **P < 0.01, and ***P < 0.001 using Student's t test (n = 26 synapses in 8-wk-old WT mice and 29 synapses in 8-wk-old CAPS2-KO mice, from six pictures from three mice in each genotype).
Moreover, electron microscopic analyses of inhibitory synaptic architectures in the CA1 region at age 8 wk revealed a decreased number and smaller distribution area of SV in CAPS2-KO mice than in their WT littermates (Fig. 3 C–E). However, there was no significant difference in WT and CAPS2-KO in the number of excitatory SVs or in their distribution (Fig. S4). These results suggest impaired development and/or survival of GABAergic interneurons during early postnatal stages in the CAPS2-KO mouse hippocampus, probably leading to or associated with deficits in inhibitory synapse architectures in adulthood.
Functional Impairments in GABAergic Interneuron Systems in the Hippocampus of CAPS2-KO Mice That Likely Affect GABAergic Network Activity and Behavior.
To determine whether developmentally impaired GABAergic interneurons affect inhibitory synaptic function in the CAPS2-KO hippocampus, miniature inhibitory postsynaptic currents (mIPSCs) were recorded in CA1 pyramidal cells of acute hippocampal slices (Fig. 4 A–C). Juvenile CAPS2-KO mice (age 2–3 wk) exhibited significantly reduced mIPSC frequency compared with WT littermates [frequency per 3 min: number of peaks analyzed, WT = 8,350 (n = 9) and CAPS2-KO = 11,146 (n = 12); amplitude: WT = 24.79 ± 2.26 pA and CAPS2-KO = 22.45 ± 1.79 pA; frequency: WT = 1.73 ± 0.11 Hz and CAPS2-KO = 1.06 ± 0.15 Hz] (Fig. 4B). In young adult CAPS2-KO mice (age 6–8 wk), mIPSC frequency and amplitude were significantly reduced [frequency per 3 min: number of peaks analyzed, WT = 15,843 (n = 9) and CAPS2-KO = 10,000 (n = 9); amplitude: WT = 24.54 ± 2.43 pA and CAPS2-KO = 19.80 ± 1.41 pA; frequency: WT = 1.95 ± 0.25 Hz and CAPS2-KO = 0.69 ± 0.10 Hz] (Fig. 4C). In contrast, stimulus–response curves for field excitatory postsynaptic potentials (fEPSPs) in the CA1 stratum radiatum were not significantly different in CAPS2-KO and WT mice (Fig. S5A). In addition, the amplitude and frequency of miniature excitatory postsynaptic currents (mEPSCs) were unchanged in CAPS2-KO mice (Fig. S5B), suggesting that excitatory neurons are not affected by CAPS2 KO.
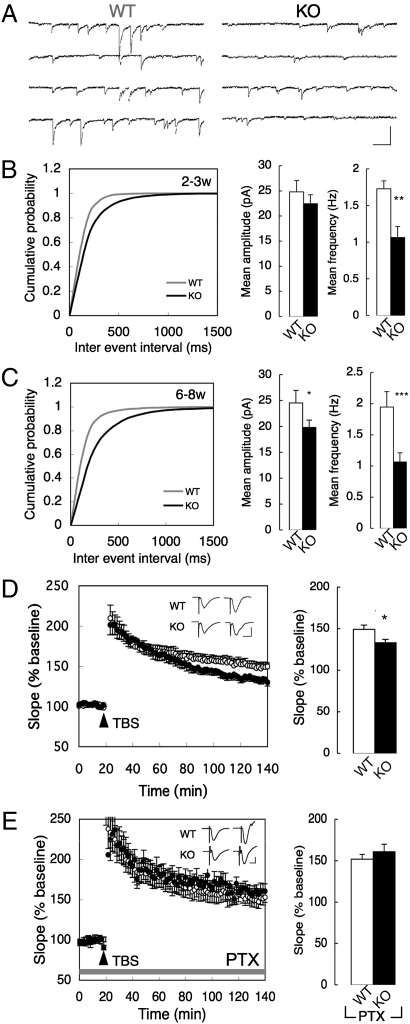
Fig. 4.
Functional impairments of GABAergic interneurons in the CAPS2-KO mouse hippocampus. (A) Examples of mIPSCs in 8-wk-old WT and CAPS2-KO mice. (Scale bars: horizontal, 100 ms; vertical, 100 pA.) (B and C) Cumulative plot of mIPSC frequency (Left), mean amplitude (Center), and frequency (Right) in 2- to 3-wk-old (B) and 6- to 8-wk-old (C) WT and CAPS2-KO mice. P < 0.05 using the Kolmogorov–Smirnov test for cumulative plot. *P < 0.05, **P < 0.01, and ***P < 0.001 using Student's t test for mean amplitude and frequency. Error bars represent SEM. (n = 9 from four 2- to 3-wk-old WT mice, 12 from five 2- to 3-wk-old CAPS2-KO mice, 9 from four 6- to 8-wk-old WT mice, and 9 from five 6- to 8-wk-old CAPS2-KO mice). (D) (Left) Time course of TBS-induced LTP at CA3-CA1 synapses. (Inset) Representative traces immediately before (Left) and 120 min after (Right) TBS for each genotype. (Scale bars: horizontal bar, 10 ms; vertical bar, 1 mV.) (Right) Results 120 min after TBS (n = 10 each for five WT mice and seven CAPS2-KO mice). Error bars represent SEM. *P < 0.05 using Student's t test. (E) (Left) Time course of TBS-LTP in the presence of PTX. (Inset) Representative traces immediately before (Left) and 120 min after (Right) TBS for each genotype. (Scale bars: horizontal bar, 10 ms; vertical bar, 200 μV.) (Right) Results 120 min after TBS (n = 9 slices for 6- to 8-wk-old WT and CAPS2-KO mice, four or five mice for each genotype). Error bars represent SEM.
We next examined whether the impaired synaptic transmission in inhibitory synapses of CAPS2-KO mice affected synaptic plasticity at CA3–CA1 synapses. High-frequency stimulation-induced long-term potentiation (LTP) revealed no obvious differences between CAPS2-KO and WT mice (Fig. S5C). However, theta-burst stimulation (TBS)-induced late-phase LTP (TBS-L-LTP) was impaired in CAPS2-KO mice (percent of baseline at 140 min: WT = 149.3 ± 5.2%, CAPS2-KO = 133.0 ± 4.2%) (Fig. 4D). BDNF is known to promote TBS-L-LTP at CA3–CA1 synapses (21); therefore, the reduced TBS-L-LTP might be caused by reduced BDNF secretion during TBS in CAPS2-KO mice. However, bath application of recombinant BDNF did not completely ameliorate the reduced TBS-L-LTP in the CAPS2-KO mice (percent of baseline at 140 min: 141.6 ± 10.6%) (Fig. S5D). On the other hand, PTX, a GABAA receptor antagonist, abolished changes in TBS-L-LTP in CAPS2-KO mice (percent of baseline at 140 min: WT/PTX = 151.7 ± 6.0%; CAPS2-KO/PTX = 160.8 ± 9.2%) (Fig. 4E), suggesting a correlation between reduced TBS-L-LTP and impaired GABAergic neurotransmission in the CAPS2-KO mouse hippocampus.
Finally, we investigated the biological significance of enhanced BDNF secretion by CAPS2. In the hippocampus, GABAergic interneurons are involved in network activities, such as hippocampal oscillation (for reviews, see ref. 22). Notably, hippocampal EEGs during the awake phase revealed significantly decreased theta oscillation frequency in the CA1 region of CAPS2-KO mice than in WT littermates (Fig. S6). Impaired hippocampal GABA neurotransmission and decreased BDNF levels are thought to affect hippocampus-related learning and anxiety-like behavior (23–25). Interestingly, CAPS2-KO mice displayed increased anxiety-like behavior (Table S1) but showed little impairment in learning and memory tests. Together, these results suggest that reduced BDNF secretion in the CAPS2-KO hippocampus leads to significant deficits in the development and function of hippocampal GABAergic interneurons and synapses, thereby resulting in impaired hippocampal interneuronal network activity and behavior.
Discussion
The present study determined a regulatory role for CAPS2 in BDNF secretion kinetics and characterized the biological significance of CAPS2 in hippocampal GABAergic system development and function. These results revealed that CAPS2 enhances the kinetics, frequency, and amplitude of regulated BDNF secretion from hippocampal neurons. In addition, the reduced BDNF secretion in CAPS2-KO mice resulted in impaired development and function of hippocampal GABAergic interneurons, synapses, networks, and related behavior.
Time-lapse live-cell imaging of BDNF-pHluorin in the present study demonstrated that CAPS2 protein positively controls BDNF release in terms of frequency, amplitude, and kinetics. CAPS2-KO neurons retained some degree of regulated BDNF secretion activity, despite decreased secretion and slow kinetics. Therefore, our results suggest that CAPS2 is critical for the enhancement of regulated BDNF secretion but that CAPS2 is not essential for regulated secretion. Recent studies have shown that CAPS promotes trans-soluble N-ethylmaleimide–sensitive factor attachment protein receptor (SNARE) complex formation in DCV exocytosis (26), and an alternative pathway or proteins, such as mammalian homologue of C. elegans unc13-1 (Munc13-1) (27) and CAPS1 (4), could substitute for CAPS2 in CAPS2-KO neurons. In the latter study, CAPS2 was proposed to promote SV exocytosis. However, given our finding in hippocampal cell cultures that CAPS2 largely colocalizes with BDNF at bassoon-immunonegative extrasynaptic sites of axons, it is possible that the defects in synaptic transmission observed in that study could be attributed to an indirect effect of CAPS2 on presynaptic function, such as SV recycling via regulation of BDNF release (28).
BDNF promotes GABAergic inhibitory interneuronal development (13–17). Previous results have demonstrated that knocked-down BDNF expression in cultured cortical neurons decreases the number of GABAergic synapses, resulting in reduced mIPSC frequency (29). Overexpression of the BDNF gene, as well as chronic treatment with BDNF, promotes maturation of GABAergic innervations in the hippocampus (17). In the present study, several abnormalities were observed in hippocampal GABAergic interneurons of CAPS2-KO mice, in addition to the defective BDNF secretion kinetics. The number of vGAT+ GABAergic synapses, the number and distribution of SVs in inhibitory presynapses, and mIPSC frequency and amplitude in the CA1 region were all reduced in CAPS2-KO mice. In contrast, CAPS2-KO mice did not exhibit changes in architecture or transmission properties of excitatory synapses compared with WT mice. Collectively, these results suggest a correlation between impaired BDNF secretion and defective GABAergic inhibitory neurons in the CAPS2-KO mouse hippocampus.
BDNF and GABA play a role in the modulation of synaptic plasticity. LTP enhancement and maintenance are associated with the activity-dependent BDNF signaling pathway (30). In addition, GABAergic neurotransmission influences LTP maintenance (31). The present study showed that TBS-L-LTP at CA3–CA1 synapses was reduced significantly in CAPS2-KO mice. Interestingly, acute BDNF application rescued reduced TBS-L-LTP only partially, whereas administration of the GABAA receptor antagonist PTX completely abolished differences in TBS-L-LTP between CAPS2-KO and WT mice. Previous studies have demonstrated that the induction of LTP requires the inhibition of GABAergic transmission by GABAB autoreceptor activation (32) and/or GABAB receptor-mediated GABAA receptor disinhibition (33). In the hippocampus of CAPS2-KO mice, impaired development and physiology of GABAergic neurons, which are not acutely ameliorated by BDNF, might compromise these GABAergic actions required for TBS-L-LTP.
GABAergic interneuronal activity is thought to influence neuronal network activities, such as theta oscillations (34), that are critical for temporal coding of neuronal ensembles and modification of synaptic efficacy (22). Moreover, theta oscillation (35) and the hippocampal GABAergic system play a prominent role in certain forms of emotional processing, with special emphasis on anxiety-like behavior (23–25). The present study showed that CAPS2-KO mice exhibited not only decreased frequency of hippocampal theta oscillations but also increased anxiety-like behavior. Thus, we suggest that CAPS2-mediated secretion may contribute to GABA- or BDNF-related network activities and may play a role in psychiatric behavior.
In conclusion, the present study demonstrates that CAPS2 promotes regulated BDNF secretion by affecting BDNF release kinetics. Our results also show that enhanced BDNF secretion by CAPS2 is indispensable for proper development and function of hippocampal GABAergic neurons and synapses. This finding suggests that the loss of enhanced BDNF secretion by a deficit of CAPS2 causes impairments in hippocampal GABAergic interneuronal networks, resulting in impaired CA3-CA1 L-LTP and hippocampal theta oscillations and increased anxiety-like behavior. Further studies are needed to elucidate the detailed mechanisms of CAPS2-mediated promotion of BDNF secretion kinetics and to gain a deeper understanding of the biological function of CAPS2.
Materials and Methods
Detailed experimental procedures are described in SI Materials and Methods
Animals.
All experimental protocols were approved by the RIKEN Institutional Animal Care and Use Committee.
Hippocampal Primary Cell Culture and Transfection.
Primary hippocampal neurons from CAPS2-KO mice were prepared and cultured according to a previously described method (36), with slight modifications. Briefly, on days 6–8 in vitro, neurons were transfected with BDNF-4× pHluorin, with or without CAPS2-tdTomato, using Lipofectamine 2000 (Invitrogen). Time-lapse live-cell imaging was performed 24–48 h after transfection.
Time-Lapse Imaging of BDNF Secretion.
For time-lapse experiments, glass-bottomed culture dishes were transferred to a warm chamber (Tokai Hit), and Hepes buffered saline solution (119 mM NaCl, 2.5 mM KCl, 2 mM CaCl2, 2 mM MgCl2, 30 mM glucose, 25 mM Hepes, 1 mM kynurenic acid, and 100 μM PTX) was perfused into the recording chamber at room temperature via a thermo controller (Warner Instruments) and then was removed via a circulation controller (K.T. Labs). Images of BDNF-pHluorin and CAPS2-tdTomato expression were acquired sequentially at 5-s intervals using a Nikon ECLIPSE TE2000-E inverted microscope with a CoolSNAP HQ2-cooled CCD camera (Photometrics). Images were acquired by MetaMorph software. The number of fluorescent puncta in each area (24 μm × 24 μm) was quantified every 5 s, and the values were plotted as a cumulative curve over a period of 10 min.
Hippocampal Slice Preparation and Electrophysiology.
Acute hippocampal slice preparations were prepared according to previously described methods (37), with slight modifications. mIPSCs were recorded in the presence of 1 mM kynurenic acid (TCI) and 1 μM tetrodotoxin (Tocris). mEPSCs were recorded in the presence of 100 μm PTX and 1 μM tetrodotoxin.
Immunocytochemistry and Immunohistochemistry.
Immunochemical staining of hippocampal cultures and slices was performed as previously described (7). The following antibodies were used: guinea pig polyclonal anti-CAPS2 (8) (1:10,000); rabbit polyclonal anti-BDNF (38) (1:200); mouse monoclonal anti-Bassoon (1:500; Stressgen); guinea pig anti-vGluT1 (Millipore) (1:2,000); and mouse monoclonal anti-vGAT (1:500; Synaptic Systems) The number of interneuron terminals in CA1 stratum radiatum regions of interest (10 μm × 10 μm) was quantified.
Electron Microscopy.
Electron microscopy was performed using a previously described method (39), with slight modifications. Under deep Nembutal anesthesia, WT and CAPS2-KO mice were transcardially perfused with 4% paraformaldehyde/2% glutaraldehyde/0.1 M phosphate buffer (pH 7.4). Serial ultrathin sections (70 nm thick) were stained with uranyl acetate/lead citrate. Interneuron terminals were distinguished by previously reported criteria (40).
Supplementary Material
Acknowledgments
We thank R. Y. Tsien (Department of Pharmacology, Department of Chemistry and Biochemistry, University of California at San Diego, La Jolla, CA) for kindly providing tdTomato and J. E. Rothman (Department of Cell Biology, Yale University School of Medicine, New Haven, CT) for pHluorin. This study was supported by research grants from the Ministry of Education, Culture, Sports, Science and Technology, the Japan Science and Technology Agency, and the Japan Society for the Promotion of Science.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1012220108/-/DCSupplemental.
References
- 1.Walent JH, Porter BW, Martin TF. A novel 145 kD brain cytosolic protein reconstitutes Ca(2+)-regulated secretion in permeable neuroendocrine cells. Cell. 1992;70:765–775. doi: 10.1016/0092-8674(92)90310-9. [DOI] [PubMed] [Google Scholar]
- 2.Berwin B, Floor E, Martin TF. CAPS (mammalian UNC-31) protein localizes to membranes involved in dense-core vesicle exocytosis. Neuron. 1998;21:137–145. doi: 10.1016/s0896-6273(00)80521-8. [DOI] [PubMed] [Google Scholar]
- 3.Tandon A, et al. Differential regulation of exocytosis by calcium and CAPS in semi-intact synaptosomes. Neuron. 1998;21:147–154. doi: 10.1016/s0896-6273(00)80522-x. [DOI] [PubMed] [Google Scholar]
- 4.Jockusch WJ, et al. CAPS-1 and CAPS-2 are essential synaptic vesicle priming proteins. Cell. 2007;131:796–808. doi: 10.1016/j.cell.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 5.Cisternas FA, Vincent JB, Scherer SW, Ray PN. Cloning and characterization of human CADPS and CADPS2, new members of the Ca2+-dependent activator for secretion protein family. Genomics. 2003;81:279–291. doi: 10.1016/s0888-7543(02)00040-x. [DOI] [PubMed] [Google Scholar]
- 6.Speidel D, et al. A family of Ca2+-dependent activator proteins for secretion: Comparative analysis of structure, expression, localization, and function. J Biol Chem. 2003;278:52802–52809. doi: 10.1074/jbc.M304727200. [DOI] [PubMed] [Google Scholar]
- 7.Sadakata T, et al. Autistic-like phenotypes in Cadps2-knockout mice and aberrant CADPS2 splicing in autistic patients. J Clin Invest. 2007;117:931–943. doi: 10.1172/JCI29031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sadakata T, et al. The secretory granule-associated protein CAPS2 regulates neurotrophin release and cell survival. J Neurosci. 2004;24:43–52. doi: 10.1523/JNEUROSCI.2528-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Greenberg ME, Xu B, Lu B, Hempstead BL. New insights in the biology of BDNF synthesis and release: Implications in CNS function. J Neurosci. 2009;29:12764–12767. doi: 10.1523/JNEUROSCI.3566-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Huang EJ, Reichardt LF. Neurotrophins: Roles in neuronal development and function. Annu Rev Neurosci. 2001;24:677–736. doi: 10.1146/annurev.neuro.24.1.677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lu B, Pang PT, Woo NH. The yin and yang of neurotrophin action. Nat Rev Neurosci. 2005;6:603–614. doi: 10.1038/nrn1726. [DOI] [PubMed] [Google Scholar]
- 12.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu Rev Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 13.Rutherford LC, DeWan A, Lauer HM, Turrigiano GG. Brain-derived neurotrophic factor mediates the activity-dependent regulation of inhibition in neocortical cultures. J Neurosci. 1997;17:4527–4535. doi: 10.1523/JNEUROSCI.17-12-04527.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kohara K, et al. Inhibitory but not excitatory cortical neurons require presynaptic brain-derived neurotrophic factor for dendritic development, as revealed by chimera cell culture. J Neurosci. 2003;23:6123–6131. doi: 10.1523/JNEUROSCI.23-14-06123.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hong EJ, McCord AE, Greenberg ME. A biological function for the neuronal activity-dependent component of Bdnf transcription in the development of cortical inhibition. Neuron. 2008;60:610–624. doi: 10.1016/j.neuron.2008.09.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Marty S, et al. Brain-derived neurotrophic factor promotes the differentiation of various hippocampal nonpyramidal neurons, including Cajal-Retzius cells, in organotypic slice cultures. J Neurosci. 1996;16:675–687. doi: 10.1523/JNEUROSCI.16-02-00675.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yamada MK, et al. Brain-derived neurotrophic factor promotes the maturation of GABAergic mechanisms in cultured hippocampal neurons. J Neurosci. 2002;22:7580–7585. doi: 10.1523/JNEUROSCI.22-17-07580.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsuda N, et al. Differential activity-dependent secretion of brain-derived neurotrophic factor from axon and dendrite. J Neurosci. 2009;29:14185–14198. doi: 10.1523/JNEUROSCI.1863-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Dean C, et al. Synaptotagmin-IV modulates synaptic function and long-term potentiation by regulating BDNF release. Nat Neurosci. 2009;12:767–776. doi: 10.1038/nn.2315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Miesenböck G, De Angelis DA, Rothman JE. Visualizing secretion and synaptic transmission with pH-sensitive green fluorescent proteins. Nature. 1998;394:192–195. doi: 10.1038/28190. [DOI] [PubMed] [Google Scholar]
- 21.Schinder AF, Poo M. The neurotrophin hypothesis for synaptic plasticity. Trends Neurosci. 2000;23:639–645. doi: 10.1016/s0166-2236(00)01672-6. [DOI] [PubMed] [Google Scholar]
- 22.Buzsáki G. Theta oscillations in the hippocampus. Neuron. 2002;33:325–340. doi: 10.1016/s0896-6273(02)00586-x. [DOI] [PubMed] [Google Scholar]
- 23.Zheng F, et al. Activin tunes GABAergic neurotransmission and modulates anxiety-like behavior. Mol Psychiatry. 2009;14:332–346. doi: 10.1038/sj.mp.4002131. [DOI] [PubMed] [Google Scholar]
- 24.Papadopoulos T, et al. Impaired GABAergic transmission and altered hippocampal synaptic plasticity in collybistin-deficient mice. EMBO J. 2007;26:3888–3899. doi: 10.1038/sj.emboj.7601819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Earnheart JC, et al. GABAergic control of adult hippocampal neurogenesis in relation to behavior indicative of trait anxiety and depression states. J Neurosci. 2007;27:3845–3854. doi: 10.1523/JNEUROSCI.3609-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.James DJ, Kowalchyk J, Daily N, Petrie M, Martin TF. CAPS drives trans-SNARE complex formation and membrane fusion through syntaxin interactions. Proc Natl Acad Sci USA. 2009;106:17308–17313. doi: 10.1073/pnas.0900755106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.García-Junco-Clemente P, Linares-Clemente P, Fernández-Chacón R. Active zones for presynaptic plasticity in the brain. Mol Psychiatry. 2005;10:185–200. 131. doi: 10.1038/sj.mp.4001628. [DOI] [PubMed] [Google Scholar]
- 28.Lessmann V. Neurotrophin-dependent modulation of glutamatergic synaptic transmission in the mammalian CNS. Gen Pharmacol. 1998;31:667–674. doi: 10.1016/s0306-3623(98)00190-6. [DOI] [PubMed] [Google Scholar]
- 29.Kohara K, et al. A local reduction in cortical GABAergic synapses after a loss of endogenous brain-derived neurotrophic factor, as revealed by single-cell gene knock-out method. J Neurosci. 2007;27:7234–7244. doi: 10.1523/JNEUROSCI.1943-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bramham CR, Messaoudi E. BDNF function in adult synaptic plasticity: The synaptic consolidation hypothesis. Prog Neurobiol. 2005;76:99–125. doi: 10.1016/j.pneurobio.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 31.Meredith RM, Floyer-Lea AM, Paulsen O. Maturation of long-term potentiation induction rules in rodent hippocampus: Role of GABAergic inhibition. J Neurosci. 2003;23:11142–11146. doi: 10.1523/JNEUROSCI.23-35-11142.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Davies CH, Starkey SJ, Pozza MF, Collingridge GL. GABA autoreceptors regulate the induction of LTP. Nature. 1991;349:609–611. doi: 10.1038/349609a0. [DOI] [PubMed] [Google Scholar]
- 33.Mott DD, Lewis DV. Facilitation of the induction of long-term potentiation by GABAB receptors. Science. 1991;252:1718–1720. doi: 10.1126/science.1675489. [DOI] [PubMed] [Google Scholar]
- 34.Paulsen O, Moser EI. A model of hippocampal memory encoding and retrieval: GABAergic control of synaptic plasticity. Trends Neurosci. 1998;21:273–278. doi: 10.1016/s0166-2236(97)01205-8. [DOI] [PubMed] [Google Scholar]
- 35.Siok CJ, Taylor CP, Hajós M. Anxiolytic profile of pregabalin on elicited hippocampal theta oscillation. Neuropharmacology. 2009;56:379–385. doi: 10.1016/j.neuropharm.2008.09.013. [DOI] [PubMed] [Google Scholar]
- 36.Shiraishi Y, Mizutani A, Yuasa S, Mikoshiba K, Furuichi T. Differential expression of Homer family proteins in the developing mouse brain. J Comp Neurol. 2004;473:582–599. doi: 10.1002/cne.20116. [DOI] [PubMed] [Google Scholar]
- 37.Liu L, et al. Role of NMDA receptor subtypes in governing the direction of hippocampal synaptic plasticity. Science. 2004;304:1021–1024. doi: 10.1126/science.1096615. [DOI] [PubMed] [Google Scholar]
- 38.Katoh-Semba R, Takeuchi IK, Semba R, Kato K. Distribution of brain-derived neurotrophic factor in rats and its changes with development in the brain. J Neurochem. 1997;69:34–42. doi: 10.1046/j.1471-4159.1997.69010034.x. [DOI] [PubMed] [Google Scholar]
- 39.Sadakata T, et al. Impaired cerebellar development and function in mice lacking CAPS2, a protein involved in neurotrophin release. J Neurosci. 2007;27:2472–2482. doi: 10.1523/JNEUROSCI.2279-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Harris KM, Landis DM. Membrane structure at synaptic junctions in area CA1 of the rat hippocampus. Neuroscience. 1986;19:857–872. doi: 10.1016/0306-4522(86)90304-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.