Abstract
The establishment of neuronal circuits relies on the stabilization of functionally appropriate connections and the elimination of inappropriate ones. Here we report that postsynaptic AMPA receptors play a critical role in regulating the stability of glutamatergic synapses. Removal of surface AMPA receptors leads to a decrease in the number and stability of excitatory presynaptic inputs, whereas overexpression increases synapse number and stability. Furthermore, overexpression of AMPA receptors along with Neuroligin-1 in 293T cells is sufficient to stabilize presynaptic inputs from cortical neurons onto heterologous cells. The stabilization of presynaptic inputs by AMPA receptors is not dependent on receptor-mediated current and instead relies on structural interactions mediated by the N-terminal domain of the glutamate receptor 2 (GluR2) subunit. These observations indicate that transsynaptic signaling mediated by the extracellular domain of GluR2 regulates the stability of presynaptic terminals.
Keywords: GluR2 N-terminal domain, Presynaptic stabilization, Presynaptic input dynamics
The development of neural circuits is characterized by exuberant synapse formation followed by elimination of inappropriate connections (1–4). Although there is considerable evidence that activity-dependent mechanisms are involved in synapse elimination, the mechanisms responsible for regulating and maintaining stable synapses are largely unknown.
Synapse formation is a dynamic process and involves the rapid recruitment of several synaptic proteins to sites of axo-dendritic contact. Transport packets containing essential components of the presynaptic active zone are highly motile before they reach synaptic sites (5). Likewise, synaptic vesicles traffic rapidly along axons before stabilizing at dendritic contact sites (6), suggesting that a dendrite-associated signal regulates presynaptic stability. At glutamatergic synapses, AMPA and NMDA receptors are recruited to the postsynaptic density in 1 h or less (7, 8), raising the possibility that they may play an important role in regulating synapse stability.
Electrophysiological and immunohistochemical experiments suggest that a large percentage of young synapses contain NMDA receptors but lack AMPA receptors (9, 10). AMPA receptors are recruited gradually to postsynaptic sites, resulting in an increase in the AMPA/NMDA ratio at these synapses (11–13). The increase in the AMPA/NMDA ratio correlates with the stabilization of dendritic spines.
Synapse formation requires the precise apposition of presynaptic and postsynaptic elements underlying the need for bidirectional signaling. Key postsynaptic proteins such as neuroligins, synaptic cell-adhesion molecules (SynCAMs), Ephrin type B (EphB) receptors, netrin G ligands, and leucine-rich repeat transmembrane (LRRTM) proteins signal transsynaptically to induce recruitment of presynaptic components (14–20). Postsynaptic adhesion molecules also are involved in the functional maturation of presynaptic inputs (21–23). Recent work demonstrated that inhibition of postsynaptic protein synthesis leads to axon withdrawal in the neuromuscular junction, suggesting that transsynaptic signaling also is important for stabilizing synapses (24). However, little is known about the molecules that regulate synapse stability.
In this study, we explore the hypothesis that reverse signaling by postsynaptic AMPA receptors regulates the stability of presynaptic inputs. Time-lapse imaging of synaptophysin-GFP (Syn-GFP) puncta in neuronal cultures revealed that only a fraction of presynaptic inputs were stable, and this stability was strongly correlated with the presence of postsynaptic AMPA receptors. Removal of AMPA receptors from the surface of postsynaptic cells led to a marked decrease in the number and stability of presynaptic inputs onto these cells. Conversely, overexpression of AMPA receptors increased the number and stability of inputs onto neurons. Furthermore, AMPA receptors expressed along with Neuroligin-1 (NLG-1) in 293T cells stabilized presynaptic inputs onto heterologous cells. The N-terminal 109 amino acids of glutamate receptor 2 (GluR2) were necessary for presynaptic stabilization, arguing strongly for a structural role of the extracellular N-terminal domain (NTD) of AMPA receptors in regulating presynaptic input stability. Together, these data suggest that a structural interaction between GluR2 and the presynaptic terminal mediates synapse stability.
Results
Dynamics of Presynaptic Inputs in Cortical Culture.
To determine whether synapse elimination occurs in cortical cultures, we established a time course for the number of presynaptic inputs onto dendrites across a window spanning synaptogenesis in these cultures (25). Neurons were stained with antibodies against the excitatory presynaptic markers vesicular glutamate transporter (VGLUT)-1 and -2 as well as the dendritic marker microtubule-associated protein 2 (MAP2), and the number of presynaptic inputs per dendrite length was quantified (SI Appendix, Fig. S1 A–E). We additionally stained for surface-expressed AMPA receptor subunits, glutamate receptor 1 (GluR1) and GluR2, along with VGLUT and quantified the number of colocalized puncta as a marker of excitatory synapses (SI Appendix, Fig. S2). Presynaptic inputs onto postsynaptic neurons were seen as early as 6 d in vitro (DIV), although the number of inputs was quite small (SI Appendix, Fig. S1 A and E). Inputs reached a peak at 14 DIV and decreased by 18 DIV (SI Appendix, Fig. S1 B and E). A similar peak in the number of colocalized VGLUT, GluR1, and GluR2 puncta was observed at 14 DIV (SI Appendix, Fig. S2). This result suggested that a subset of the inputs observed at 14 DIV either failed to stabilize or were eliminated by 18 DIV.
In a complementary set of experiments, we imaged presynaptic vesicle dynamics across synaptogenesis (SI Appendix, Fig. S1 F–J). Synaptic vesicles are a key component of presynaptic terminals, and GFP fusions of synaptic vesicle proteins such as Syn-GFP have been used widely to study the dynamics of presynaptic terminals. The stability of puncta containing presynaptic vesicle protein is a good indicator of functional presynaptic terminals (6, 26, 27). We therefore chose to image Syn-GFP dynamics in these experiments.
We imaged the dynamics of Syn-GFP puncta and quantified the fraction of puncta that were stable, newly added, or eliminated across 1 h (SI Appendix, Fig. S1H). At 11 DIV, the majority of puncta were either newly added (45%) or eliminated (41%). Only 14% of Syn-GFP puncta were stable across the hour. By 14 DIV, the fraction of stable puncta increased to 24%. By 17 DIV, the fraction of stable puncta increased further to 59%. This result suggested that stabilization of presynaptic inputs is developmentally regulated, with a significant increase in stability between 11 and 17 DIV.
To determine if there was a relationship between the size of Syn-GFP puncta and synapse stability, we examined the relationship between dwell time (Methods) and Syn-GFP puncta size. Stable puncta were much larger than transient puncta, indicating that puncta size is a good measure of presynaptic stability (SI Appendix, Fig. S3A). A similar relationship between the size of presynaptic puncta and stability also was reported recently in Caenorhabditis elegans (28). Further, larger Syn-GFP puncta were much more likely to colocalize with bassoon, a component of the active zone (SI Appendix, Fig. S3B). The majority of larger Syn-GFP puncta colocalized with synaptotagmin uptake staining, a marker of active release sites (SI Appendix, Fig. S3C). A careful analysis of the distribution of Syn-GFP dwell time at 14 DIV showed that the transient and stable puncta could be distinguished clearly (SI Appendix, Fig. S1 I and J). Together, these results demonstrate that both stable and dynamic populations of presynaptic puncta are present during synaptogenesis and that the fraction of stable puncta increases significantly by the end of synaptogenesis.
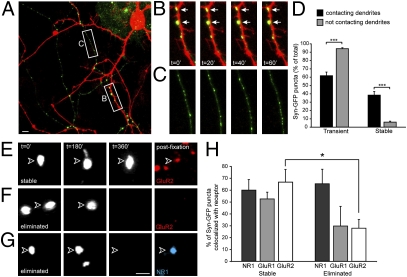
To determine if contact with postsynaptic dendrites affected the stability of Syn-GFP puncta, we transfected a subset of neurons with Syn-GFP and a separate subset with mCherry to visualize dendrites. We then imaged Syn-GFP punctum dynamics in regions containing mCherry-positive neurons (Fig. 1). We separated puncta into two groups: Syn-GFP puncta contacting dendrites and Syn-GFP puncta not contacting dendrites. To confirm that the Syn-GFP puncta not contacting mCherry-positive dendrites were in areas devoid of dendrites, we visualized all dendrites (transfected and untransfected) using differential interference contrast (DIC) imaging at the beginning and end of each experiment. We observed numerous examples of stable Syn-GFP puncta in contact with dendrites (white arrows in Fig. 1B). In fact, 61 of the 76 stable puncta observed in these experiments were in direct contact with dendrites, suggesting that a dendrite-dependent signal stabilizes presynaptic vesicles. In support of this notion, most puncta (94%) not in contact with dendrites were transient (Fig. 1 C and D). Surprisingly, 62% of Syn-GFP puncta that contacted dendrites also were transient (Fig. 1D). Thus, although contact with a dendrite may be a prerequisite for stabilization, this contact alone is not sufficient for stabilization. This finding argues for a dendrite-derived stabilizing factor that is found only at select sites along the dendrite.
Fig. 1.
Stable presynaptic puncta are associated with postsynaptic AMPA receptors. Time-lapse imaging revealed that Syn-GFP puncta preferentially stabilized on dendrites and are associated with postsynaptic AMPA receptors the majority of the time. (A) Example of a location in which Syn-GFP–expressing axons (green) contacted mCherry-expressing dendrites (red). The white boxes depict areas that are magnified in B and C. In each imaging experiment, we confirmed by DIC imaging that no untransfected dendrites were in the field of view. (Scale bar, 5 μm.) (B and C) Micrographs of locations identified in A at 0, 20, 40, and 60 min. (B) A dendrite segment receiving stable Syn-GFP inputs (white arrows). (C) Syn-GFP puncta in an area without dendrites. Note the lack of stable puncta in this field of view, suggesting that dendrite contact is necessary for presynaptic input stabilization. (D) Quantification of the dynamics of Syn-GFP puncta at and away from sites of dendritic contact. ***P < 0.001. (E–G) Stable and eliminated Syn-GFP puncta with retrospective staining for AMPA and NMDA receptors. Arrowheads indicate the position of Syn-GFP puncta at the time point indicated. (E) A stable Syn-GFP punctum. Live-labeling at the 6-h mark revealed the presence of a surface-expressed GluR2 punctum that colocalized with the Syn-GFP punctum. (F) A Syn-GFP punctum that was present at 0 and 3 h but was eliminated by 6 h. No staining for surface GluR2 was seen at the elimination site. (G) Another example of an eliminated Syn-GFP punctum. Note the presence of an NR1 punctum at the elimination site. (Scale bar, 1 μm.) (H) Quantification of the fraction of stable and eliminated Syn-GFP puncta that colocalized with total NR1 or surface-expressed GluR1 or GluR2. *P < 0.05.
Correlation Between Presynaptic Stability and Postsynaptic AMPA Receptors.
A central goal of this work was to identify molecules that regulate presynaptic input stability. Previous experiments suggested that this molecule is developmentally up-regulated (SI Appendix, Figs. S1 and S2) and present at a subset of postsynaptic sites (Fig. 1). We further reasoned that this signal should be easily trafficked into or out of individual synaptic sites to allow for stabilization or elimination of specific inputs. AMPA receptors were an intriguing candidate molecule because several nascent synapses contain NMDA receptors but not AMPA receptors (29), and AMPA receptor trafficking is tightly regulated (30, 31).
To determine whether presynaptic inputs that colocalized with AMPA receptors were more likely to be stabilized, we imaged the dynamics of Syn-GFP puncta across 6 h in 14 DIV cultures and then live-labeled surface GluR1 or GluR2 receptors at the end of the imaging period before fixing and staining neurons (Fig. 1 E–H). There was a strong correlation between the presence of postsynaptic AMPA receptors and the stability of presynaptic inputs. Although a majority of stable Syn-GFP puncta were associated with the presence of postsynaptic AMPA receptors (55 and 67% for GluR1 and GluR2, respectively), AMPA receptors were present less than 30% of the time at sites of eliminated presynaptic inputs (Fig. 1 F and G). In contrast, there was no correlation between the stability of presynaptic inputs and the presence of postsynaptic NMDA receptors (Fig. 1 G and H). These observations suggested that postsynaptic AMPA receptors may play a role in regulating synapse stability.
Down-Regulation of AMPA Receptors Leads to a Decrease in the Number and Stability of Presynaptic Inputs.
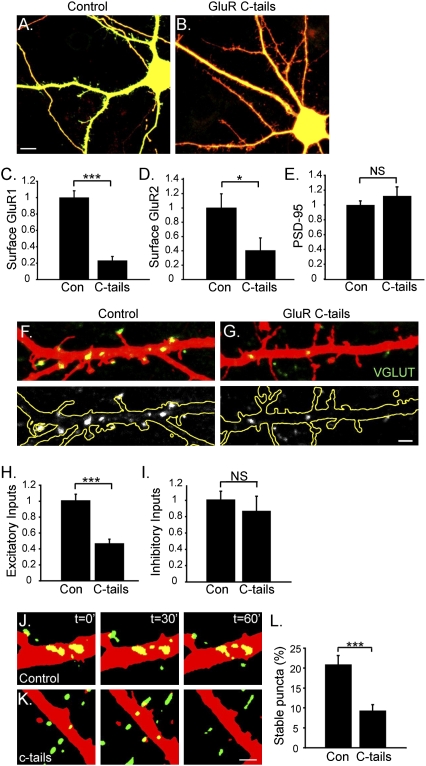
To explore further the potential requirement for AMPA receptors in presynaptic input stabilization, we actively down-regulated AMPA receptor expression on the postsynaptic surface. Cortical neurons were transfected with GluR1 and GluR2 C-terminal domains fused to CFP (CFP:GluR1 C-tail and CFP:GluR2 C-tail, henceforth referred to as “GluR C-tails”) or with CFP alone (control) at 7 DIV, and the effects on VGLUT (excitatory) and glutamic acid decarboxylase (GAD6) (inhibitory) inputs were assessed at 14 DIV (Fig. 2). The C-tail constructs have been shown previously to disrupt synaptic targeting of GluR1 and GluR2 (32, 33), probably by competing for endogenous proteins involved in the normal trafficking of these receptors.
Fig. 2.
AMPA receptor down-regulation decreases the number and stability of presynaptic inputs. (A and B) A control neuron cotransfected with mCherry (red) and CFP (green) (A) and a neuron cotransfected with mCherry and CFP:GluR1 and -2 C-tails (B). C-tails were expressed robustly and targeted to dendrites. (Scale bar, 5 μm.) (C) Quantification of the number of surface GluR1 puncta per length of dendrite (normalized to controls). ***P < 0.0001. (D) Quantification of the number of surface GluR2 puncta per length of dendrite (normalized to controls). *P = 0.03. (E) Quantification of the number of PSD-95 puncta per length of dendrite (normalized to controls). (F and G) Endogenous VGLUT-positive inputs onto control (F) or C-tail–expressing neurons (G) at 14 DIV. (Upper) VGLUT-positive inputs (green) onto mCherry-filled dendrites (red). (Lower) The same VGLUT inputs are shown in white with the dendrite outlined in yellow, allowing better visualization of inputs. (Scale bar, 2 μm.) (H) Quantification of the number of excitatory, VGLUT-positive inputs per length of dendrite (normalized to controls). ***P < 0.0001. (I) Quantification of the number of inhibitory, GAD6-positive inputs per length of dendrite (normalized to controls). NS, not significant. (J and K) Examples from imaging experiments in which Syn-GFP–expressing axons (green) contacted mCherry expressing dendrites (red) that also were expressing CFP or CFP:GluR1 and two C-tails. (J) Micrographs from an experiment in which Syn-GFP puncta contacted an mCherry-expressing dendrite that also was expressing CFP (control). The panels show time points at 0, 30, and 60 min. Several Syn-GFP puncta were stabilized on the control neuron for the duration of 1 h, whereas other puncta either appeared or disappeared. (K) Micrographs from the imaging experiment shown in J, but in this case Syn-GFP puncta contacted an mCherry-expressing dendrite that also was expressing GluR C-tails. Although several Syn-GFP puncta contacted the dendrite expressing C-tails, no puncta were stabilized across the hour. (Scale bar, 2 μm.) (L) Quantification of the fraction of stable Syn-GFP inputs contacting control dendrites or dendrites expressing C-tails. There was a significant decrease in stable inputs onto neurons expressing C-tails relative to controls. ***P = 0.0003.
Neurons transfected with GluR C-tails (Fig. 2B) were morphologically similar to control neurons transfected with CFP alone (Fig. 2A). GluR C-tail expression significantly decreased total surface levels of GluR1 by fourfold (P < 0.0001) and of GluR2 by 2.4-fold (P = 0.03) (Fig. 2 C and D). Levels of postsynaptic density protein 95 (PSD-95) were not altered, however, indicating that the C-tails did not globally disrupt the postsynaptic complex (P = 0.37) (Fig. 2E). Neurons expressing GluR C-tails had a 50% decrease in excitatory input number relative to controls (P < 0.0001) (Fig. 2 F–H), consistent with the idea that AMPA receptors are necessary for presynaptic terminal stabilization. The number of inhibitory inputs onto these neurons was not altered (P = 0.51) (Fig. 2I), indicating that loss of AMPA receptors selectively affects excitatory presynaptic terminals.
In the next set of experiments, we directly examined the role of postsynaptic AMPA receptors in presynaptic stability by time-lapse imaging (Fig. 2 J–L). A subset of neurons was transfected with Syn-GFP and another subset with mCherry (to visualize neurons) and with either CFP or GluR C-tails. At 12 DIV, we identified areas where axons expressing Syn-GFP contacted mCherry-positive dendrites and imaged the dynamics of those axo-dendritic contacts across 1 h (Fig. 2 J and K). Numerous Syn-GFP puncta contacted both control and C-tail–expressing dendrites during each imaging period (average of 42 puncta per neuron for control neurons, 66 puncta per neuron for C-tail–expressing neurons). Twenty-one per cent of Syn-GFP puncta in contact with control neurons were stable across 1 h of imaging (Fig. 2 J and L). Strikingly, only 9.3% of Syn-GFP puncta in contact with GluR C-tail–expressing cells were stable (P = 0.0003) (Fig. 2 K and L). This finding represents a 50% decrease in stable inputs onto C-tail–expressing neurons over the course of 1 h and strongly supports the hypothesis that AMPA receptors are necessary for presynaptic stabilization.
The decrease in synapse input number and stability in response to AMPA receptor down-regulation could be caused either by disruption of structural interactions between AMPA receptors and interacting proteins or by loss of activity through AMPA receptors. To differentiate between these two scenarios, we pharmacologically blocked AMPA receptors with 6,7-dinitroquinoxaline-2,3-dione (DNQX) from 7 to 14 DIV (SI Appendix, Fig. S4). In contrast to the effect of physically removing AMPA receptors, we found that selective blockade of AMPA receptors led to a significant increase in VGLUT-positive inputs per dendrite length (a twofold increase; P < 0.0001) (SI Appendix, Fig. S4 A, B, and D). Thus, loss of AMPA receptor-mediated activity could not account for the decrease in excitatory input number we observed with receptor down-regulation.
The twofold increase in excitatory input number in response to DNQX treatment was particularly interesting because DNQX treatment also caused a twofold increase in the surface expression of GluR1 (P = 0.0002) and GluR2 (P < 0.0001) (SI Appendix, Fig. S4 E and F). Meanwhile, pharmacologically blocking NMDA receptors with 2-amino-5-phosphonovaleric acid (APV) from 7 to 14 DIV had no effect on either surface expression of GluR1/GluR2 or excitatory input number (SI Appendix, Fig. S4 C, D–F). These data suggest that structural interactions mediated by surface-expressed AMPA receptors were responsible for the increase in input number.
Overexpression of AMPA Receptors Increases the Number and Stability of Presynaptic Inputs.
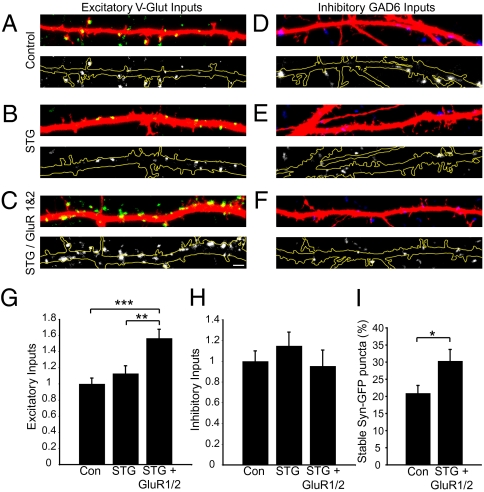
To determine if AMPA receptor overexpression could indeed increase the number of presynaptic inputs, we transfected neurons with GFP-tagged GluR1 and GluR2 subunits at 7 DIV and assessed excitatory and inhibitory input number at 14 DIV. Transfection of neurons with GFP-GluR1 and GFP-GluR2 led to highly variable interexperiment levels of surface expression; therefore, to facilitate trafficking of AMPA receptors to the synapse, we additionally cotransfected neurons with stargazin (STG), a protein integral to proper AMPA receptor trafficking (34). This cotransfection led to robust and reliable overexpression of AMPA receptors on the membrane surface. Neurons cotransfected with STG and GFP-GluR1 and -2 showed a significant increase in the number of VGLUT-positive inputs per length of dendrite when compared with vector (P < 0.001) or vector plus STG (P < 0.01) transfection controls (overall P = 0.0004) (Fig. 3 A–C and G). This increase was selective for excitatory inputs, because there was no significant difference in the number of GAD6-positive inputs among the three conditions (P = 0.24) (Fig. 3 D–F and H).
Fig. 3.
Overexpression of AMPA receptors increases presynaptic input number and stability. (A–C) Representative images of VGLUT-positive inputs (green) onto 14-DIV neurons transfected with vector (A), STG (B), or STG plus GFP-GluR1 and -2 (C), along with mCherry for visualization of dendrites (red). Lower panels show the same image but with the VGLUT-positive inputs in white and the dendrite outlined in yellow, allowing better visualization of inputs. (Scale bar, 1 μm.) (D–F) Representative images of GAD6-positive inputs (blue) onto 14-DIV neurons transfected with vector (D), STG (E), or STG plus GFP-GluR1 and -2 (F), along with mCherry for visualization of dendrites (red). Lower panels show the same image, but with the GAD6-positive inputs in white and the dendrite outlined in yellow. (G) Quantification of the number of excitatory (VGLUT-positive) inputs per length of dendrite, normalized to control values. Overall P = 0.0004. Intergroup comparisons: ***P < 0.001; **P < 0.01. (H) Quantification of the number of inhibitory (GAD6-positive) inputs per length of dendrite, normalized to controls. (I) Quantification of Syn-GFP stabilization onto control and AMPA receptor-expressing neurons. *P = 0.03.
To determine whether the increase in excitatory synapse number was caused by the increased stability of presynaptic inputs, we imaged the dynamics of Syn-GFP puncta in contact with neurons expressing mCherry plus STG versus neurons expressing mCherry plus STG plus GluR1 and -2 (Fig. 3I). We observed a 1.5-fold increase in stable inputs onto neurons overexpressing AMPA receptor across 1 h (P = 0.03). These observations indicate that increased surface delivery of AMPA receptors increases the number and stability of presynaptic inputs onto neurons.
Coexpression of AMPA Receptors and NLG-1 in 293T Cells Is Sufficient to Stabilize Syn-GFP Inputs onto Heterologous Cells.
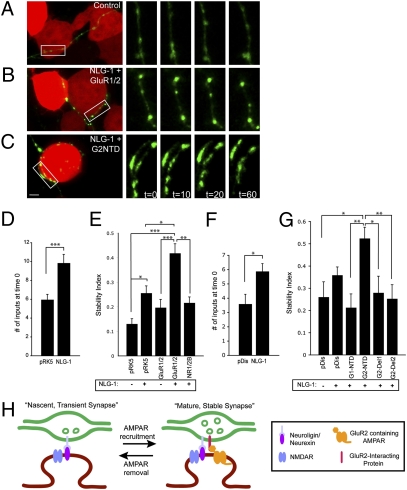
Next, we asked whether AMPA receptors could induce presynaptic input stability in a reduced heterologous culture system. Scheiffele et al. (17) previously demonstrated that coculturing neurons with NLG-1–expressing 293T cells promotes the induction of presynaptic inputs onto those cells. We used a modified version of this assay to test whether 293T cells expressing AMPA receptors are capable of stabilizing Syn-GFP puncta in axons from cocultured neurons. We cotransfected 293T cells with mCherry (for visualization) along with one of the following combinations of plasmids: (i) vector, (ii) NLG-1, (iii) GluR1 and -2, (iv) NLG-1 plus GluR1 and -2, or (iv) NLG-1 plus NMDA receptor subunit NR1 (NR1) and NMDA receptor subunit 2B (NR2B) (see SI Appendix, Fig. S5 for confirmation of expression). We then overlaid these 293T cells onto neurons previously transfected with Syn-GFP and imaged interactions between 293T cells and Syn-GFP puncta 18–24 h later (Fig. 4).
Fig. 4.
The NTD of GluR2 in concert with NLG-1 is sufficient to induce presynaptic input stability. (A–C) Live-imaged 293T cells (red) expressing vector (A), NLG-1 plus GluR1 and -2 (B), or NLG-1 plus GluR2 NTD (G2NTD) (C) contacted by Syn-GFP–expressing axons (green) from cocultured neurons. The white rectangles in the left panels of A–C demarcate an area of a Syn-GFP–expressing axon that is magnified in the panels on the right. The panels on the right correspond to various time points during the imaging period (0, 10, 20, and 60 min). A large fraction of Syn-GFP puncta contacting control 293T cells were trafficking (present in only one imaging window) (A). In contrast, a large fraction of Syn-GFP puncta contacting NLG-1 plus GluR2 NTD-expressing 293T cells were stable across 60 min (C). (Scale bar, 5 μm.) (D) Confirmation that NLG-1 induces presynaptic inputs in our imaging paradigm. The 293T cells expressing NLG-1 received a significantly greater number of Syn-GFP inputs than control cells expressing pRK5 vector at the beginning of the imaging period. ***P = 0.001. (E) A stability index, defined as the ratio of stable puncta to total (stable plus trafficking) puncta, for axons contacting 293T cells expressing the indicated constructs. Overall P < 0.0001. Intergroup comparisons: ***P < 0.001; **P < 0.01; *P < 0.05. n = 24–43 cells for each condition. (F The 293T cells expressing NLG-1 received a significantly greater number of Syn-GFP inputs than control (pDisplay-expressing) cells at the beginning of the imaging period. *P = 0.013. (G) Quantification of stability indexes for 293T cells expressing indicated constructs shows that the first 109 amino acids of the NTD of GluR2 are necessary for presynaptic input stabilization. **P < 0.01; *P < 0.05. (H) Model of the proposed role of the NTD of GluR2 in regulating presynaptic stability. AMPAR, AMPA receptor; NMDAR, NMDA receptor.
First, we confirmed that NLG-1 increased Syn-GFP input number onto 293T cells relative to controls (P = 0.0005) (Fig. 4D). Next, we imaged the dynamics of Syn-GFP puncta at 10-min intervals across 1 h and determined a stability index for each 293T cell, defined as the fraction of stable relative to total (trafficking plus stable) Syn-GFP inputs contacting that cell (Fig. 4 A–C and E). This index is a measure of the ability of the 293T cell to attract and stabilize trafficking Syn-GFP puncta effectively. Vector-transfected control 293T cells were not very efficient at stabilizing presynaptic inputs (stability index 0.13 ± 0.02) (Fig. 4 A and E). NLG-1–expressing 293T cells had an increased stability index (P < 0.05), suggesting that postsynaptic NLG-1 has presynaptic input-stabilizing properties. The 293T cells expressing only AMPA receptors were statistically no better than control cells at stabilizing presynaptic inputs (Fig. 4E). However, 293T cells expressing NLG-1 in combination with AMPA receptors had a greater than threefold increase in their stability index relative to controls (stability index: 0.42 ± 0.04; P < 0.001) (Fig. 4 B and E). Replacement of AMPA receptors by NMDA receptors (NR1 plus NR2B) abolished the increase in stability over control values, suggesting that presynaptic input stability is dependent specifically on AMPA receptors.
The N-Terminal Domain of GluR2 Is Sufficient for AMPA Receptor-Dependent Presynaptic Stabilization.
AMPA receptor subunits contain a large NTD that is located in the synaptic cleft. We speculated that structural interactions between this NTD and presynaptic membrane proteins could lead to the stabilization of presynaptic inputs. To test this assumption, we subcloned GluR1 NTD and GluR2 NTD into the Invitrogen pDisplay vector (Methods). When expressed in cells, GluR NTDs were inserted into the membrane with the NTD located in the extracellular space (SI Appendix, Fig. S5).
We transfected 293T cells with pDisplay vector alone or with NLG-1 plus (i) pDisplay, (ii) GluR1 NTD-pDisplay, or (iii) GluR2 NTD-pDisplay and calculated a stability index for these cells (Fig. 4 C and G). NLG-1 increased presynaptic input number relative to the pDisplay vector control and led to a modest increase in input stability, although this increase was not significant in this data set, probably because of increased background stability from pDisplay (Fig. 4G). The stability index of cells expressing GluR1 NTD was not significantly different from controls (Fig. 4G). In contrast, the stability index of cells expressing GluR2 NTD was twofold higher than controls (P < 0.05) (Fig. 4 C and G). This result demonstrates that the GluR2 NTD in combination with NLG-1 is sufficient to induce stabilization of presynaptic inputs onto nonneuronal cells and argues strongly that structural interactions mediated specifically by the extracellular domain of AMPA receptors are responsible for presynaptic input stabilization. We also transfected neurons with GluR2 NTD-deletion constructs lacking either the first 109 amino acids (GluR2 NTD-Del1) or the first 173 amino acids (GluR2 NTD-Del2) of the NTD (Fig. 4G). Expression of either of these constructs abolished the stability effect. These observations suggest the N-terminal 109 amino acids of GluR2 are required for the stabilization of presynaptic inputs in the presence of NLG-1 and further support the hypothesis of a transsynaptic interaction that mediates stability.
Discussion
The establishment of neural circuits requires the stabilization of correct inputs and the elimination of incorrect ones. However, very little is known about the role of specific proteins in regulating synapse stability. Here, we show that AMPA receptors play a critical role in this process and provide evidence that AMPA receptors function as a transsynaptic signal to stabilize presynaptic inputs.
Our investigation of the role of AMPA receptors in regulating synapse stability was motivated by the observation that there was a strong correlation between the presence of postsynaptic AMPA receptors and synapse stability, suggesting that AMPA receptors may play an instructive role in this process. Consistent with such a possibility, we found that overexpression of AMPA receptors led to a 1.5-fold increase in the number of excitatory inputs onto cells as well as a 1.5-fold increase in the stabilization of Syn-GFP puncta onto those cells. Conversely, removal of AMPA receptors led to a twofold reduction in the number of excitatory inputs onto dendrites and a 50% decrease in the fraction of stable Syn-GFP inputs. Thus postsynaptic AMPA receptors appear to be an important determinant of presynaptic stability.
How might AMPA receptors influence presynaptic stability? AMPA receptors potentially could influence stability through structural interactions with proteins on the presynaptic membrane. Alternatively, AMPA receptor-mediated activity could trigger downstream signaling that results in the recruitment of proteins that provide a retrograde signal to the presynaptic terminal. Our observations suggest that AMPA receptors regulate presynaptic stability via a structural interaction. Functional blockade of AMPA receptors does not phenocopy the effect of loss of AMPA receptors, arguing against stability being mediated via neurotransmission. In support of a structural role, we found that coexpression of the GluR2 receptor along with NLG-1 was extremely efficient at stabilizing presynaptic inputs and that this effect requires the NTD of GluR2. In complementary experiments, we found that the NTD of GluR2 in concert with NLG-1 was sufficient to induce presynaptic stability. It therefore appears that the NTD of AMPA receptors signal transsynaptically to regulate presynaptic stability.
Although we demonstrate a role for the NTD of GluR2 in regulating presynaptic stability, previous work has implicated the GluR2 NTD in regulating spine size and density (35, 36). We should note that the role of GluR2 in spine morphogenesis is not fully resolved, because Lu et al. (37) found that genetic deletion of GluR2 does not affect spine density significantly. However, spine size and stability were not measured in that study, so it is possible that those aspects of spine development require GluR2. The observation that the GluR2 NTD can regulate both postsynaptic spine morphogenesis and presynaptic stability raises an intriguing question regarding the interdependence of these two phenomena. Namely, is the induction of spines necessary for presynaptic stabilization? Our experiments suggest that the answer is no, because the majority of synapses, including stable synapses, are shaft synapses in our cultures during the time that these experiments were carried out, and Harris and colleagues have reported that the majority of early synapses in the hippocampus in vivo are present on dendritic shafts (38). Although there are also stable inputs onto spines, spines do not appear to be a prerequisite for input stabilization. Strengthening this finding is the fact that presynaptic inputs were stabilized onto 293T cells that lack spines. Thus, AMPA receptors appear to regulate independently two critical aspects of synapse maturation, namely, stabilization of presynaptic inputs and postsynaptic spine morphogenesis.
Although synapses are quite dynamic early in development, they later maintain stability for weeks, months, or perhaps even years (39, 40). The importance of this stability is highlighted by reports that the disassembly of synapses late in life may be linked to Alzheimer's disease (41). In fact, recent work suggests that β-amyloid–induced loss of AMPA receptors may lead to this late-stage destabilization of synapses (42). Although we have focused on the possibility that proper trafficking of AMPA receptors to synapses mediates cortical wiring during development, it will be equally important to determine whether their prolonged presence at synapses is essential for stabilizing synaptic inputs in adult life and old age.
Methods
Primary Cell Culture and Transfections.
Cortical neurons were cultured from embryonic day 18 Long–Evans rats (Charles River Lab) and plated at a density of 85,000/cm2 on chamber slides (Nalgene Nunc International) or glass coverslips coated with poly-d-lysine (0.03 mg/mL final) (Millipore) and laminin (0.003 mg/mL final) (BD Biosciences). Neurons were cultured in Neurobasal Medium (Invitrogen) supplemented with 1,000 U/mL penicillin G and streptomycin sulfate (Invitrogen), 1× GlutaMAX (Invitrogen), 2% FBS (Invitrogen), and 2× B27 (Invitrogen). Media were refreshed every 3 d. Neurons were transfected using the calcium phosphate method (43). Neurons were transfected with Syn-GFP at 4 DIV to allow sufficient time for proper targeting to axons. All other transfections were carried out at 7 DIV. For drug-blockade experiments, neurons were grown in the presence of 20 μM DNQX (Tocris), 50 μM d-AP5 (Tocris), or vehicle (DMSO) from 7 to 14 DIV. Drugs were refreshed daily.
Plasmids.
Synaptophysin-GFP was provided by Hollis Cline (Cold Spring Harbor Laboratory, Cold Spring Harbor, NY). mCherry was provided by Roger Tsien (University of California, San Diego, La Jolla, CA). STG was provided by Susumu Tomita (Yale School of Medicine, New Haven, CT) and Roger Nicoll (University of California, San Francisco, CA). Untagged and GFP-tagged GluR1 and GluR2 were provided by Richard Huganir (Johns Hopkins University, Baltimore, MD). Flag-NLG-1 was provided by Palmer Taylor (University of California, San Diego, La Jolla, CA). The GFP control constructs pEGFP-N1 and pECFP-C1 are commercially available (BD Biosciences). CFP:GluR1 C-tail and CFP:GluR2 C-tail constructs were made following the method described by Shi et al. (33). GluR1 and GluR2 NTD constructs were made by subcloning the first 400 amino acids of GluR1 and GluR2 into the pDisplay vector (Invitrogen).
Immunostaining.
The following primary antibodies were used for this study: chicken anti-MAP2 (1:5,000; Abcam); guinea pig anti-VGLUT 1 (1:5,000; Millipore); guinea pig anti-VGLUT 2 (1:1,000; Millipore); mouse anti-GAD6 (1:50; Developmental Studies Hybridoma Bank,); goat anti-GFP (1:3,000; Abcam); mouse anti-Bassoon (1:1,000; Abcam); mouse anti-NR1 (1:500; BD Pharmingen); mouse anti-PSD-95 (1:250; Affinity Bioreagents); and rabbit anti-DsRed to visualize mCherry (1:1,000; Clontech). Appropriate secondary antibodies raised in donkey were used (1:1,000; Jackson Immunoresearch). The following primary antibodies were used for live-labeling experiments: rabbit anti-GluR1 (1:10; EMD Biosciences); mouse anti-GluR2 (1:100; Millipore); rabbit anti-GFP (Invitrogen; 1:1,000); mouse anti-Flag (1:1,000; Sigma); and mouse anti-myc (1:1,000; Covance).
Statistics.
Student's t test was used when comparing two conditions. For all comparisons of three conditions or more, the Kruskal–Wallis Test was used (nonparametric ANOVA), followed by Dunn's multiple comparisons test to determine statistical significance between individual conditions. All data presented in the text are mean ± SEM.
Additional experimental details, including methods for imaging experiments, are provided in SI Appendix, SI Methods.
Supplementary Material
Acknowledgments
We thank Yishi Jin and members of the A.G. laboratory for critical reading of the manuscript. We additionally thank Hollis Cline (The Scripps Research Institute, La Jolla, CA), Roger Tsien (University of California at San Diego, La Jolla, CA), Susumu Tomita (Yale University, New Haven, CT), Roger Nicoll (University of California, San Francisco), Richard Huganir (The Johns Hopkins University, Baltimore), and Palmer Taylor (University of California at San Diego, La Jolla, CA) for cDNA constructs. This work was supported by National Institutes of Health Grants NS052772 and NS064124 (to A.G.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1015163108/-/DCSupplemental.
References
- 1.Hashimoto K, Kano M. Postnatal development and synapse elimination of climbing fiber to Purkinje cell projection in the cerebellum. Neurosci Res. 2005;53:221–228. doi: 10.1016/j.neures.2005.07.007. [DOI] [PubMed] [Google Scholar]
- 2.Huberman AD. Mechanisms of eye-specific visual circuit development. Curr Opin Neurobiol. 2007;17:73–80. doi: 10.1016/j.conb.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 3.Rakic P, Bourgeois JP, Goldman-Rakic PS. Synaptic development of the cerebral cortex: Implications for learning, memory, and mental illness. Prog Brain Res. 1994;102:227–243. doi: 10.1016/S0079-6123(08)60543-9. [DOI] [PubMed] [Google Scholar]
- 4.Sanes JR, Lichtman JW. Development of the vertebrate neuromuscular junction. Annu Rev Neurosci. 1999;22:389–442. doi: 10.1146/annurev.neuro.22.1.389. [DOI] [PubMed] [Google Scholar]
- 5.Shapira M, et al. Unitary assembly of presynaptic active zones from Piccolo-Bassoon transport vesicles. Neuron. 2003;38:237–252. doi: 10.1016/s0896-6273(03)00207-1. [DOI] [PubMed] [Google Scholar]
- 6.Ahmari SE, Buchanan J, Smith SJ. Assembly of presynaptic active zones from cytoplasmic transport packets. Nat Neurosci. 2000;3:445–451. doi: 10.1038/74814. [DOI] [PubMed] [Google Scholar]
- 7.Friedman HV, Bresler T, Garner CC, Ziv NE. Assembly of new individual excitatory synapses: Time course and temporal order of synaptic molecule recruitment. Neuron. 2000;27:57–69. doi: 10.1016/s0896-6273(00)00009-x. [DOI] [PubMed] [Google Scholar]
- 8.Washbourne P, Bennett JE, McAllister AK. Rapid recruitment of NMDA receptor transport packets to nascent synapses. Nat Neurosci. 2002;5:751–759. doi: 10.1038/nn883. [DOI] [PubMed] [Google Scholar]
- 9.Isaac JT, Nicoll RA, Malenka RC. Evidence for silent synapses: Implications for the expression of LTP. Neuron. 1995;15:427–434. doi: 10.1016/0896-6273(95)90046-2. [DOI] [PubMed] [Google Scholar]
- 10.Liao D, Hessler NA, Malinow R. Activation of postsynaptically silent synapses during pairing-induced LTP in CA1 region of hippocampal slice. Nature. 1995;375:400–404. doi: 10.1038/375400a0. [DOI] [PubMed] [Google Scholar]
- 11.Crair MC, Malenka RC. A critical period for long-term potentiation at thalamocortical synapses. Nature. 1995;375:325–328. doi: 10.1038/375325a0. [DOI] [PubMed] [Google Scholar]
- 12.Isaac JT, Crair MC, Nicoll RA, Malenka RC. Silent synapses during development of thalamocortical inputs. Neuron. 1997;18:269–280. doi: 10.1016/s0896-6273(00)80267-6. [DOI] [PubMed] [Google Scholar]
- 13.Shepherd JD, Huganir RL. The cell biology of synaptic plasticity: AMPA receptor trafficking. Annu Rev Cell Dev Biol. 2007;23:613–643. doi: 10.1146/annurev.cellbio.23.090506.123516. [DOI] [PubMed] [Google Scholar]
- 14.Biederer T, et al. SynCAM, a synaptic adhesion molecule that drives synapse assembly. Science. 2002;297:1525–1531. doi: 10.1126/science.1072356. [DOI] [PubMed] [Google Scholar]
- 15.Kayser MS, McClelland AC, Hughes EG, Dalva MB. Intracellular and trans-synaptic regulation of glutamatergic synaptogenesis by EphB receptors. J Neurosci. 2006;26:12152–12164. doi: 10.1523/JNEUROSCI.3072-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kim S, et al. NGL family PSD-95-interacting adhesion molecules regulate excitatory synapse formation. Nat Neurosci. 2006;9:1294–1301. doi: 10.1038/nn1763. [DOI] [PubMed] [Google Scholar]
- 17.Scheiffele P, Fan J, Choih J, Fetter R, Serafini T. Neuroligin expressed in nonneuronal cells triggers presynaptic development in contacting axons. Cell. 2000;101:657–669. doi: 10.1016/s0092-8674(00)80877-6. [DOI] [PubMed] [Google Scholar]
- 18.Linhoff MW, et al. An unbiased expression screen for synaptogenic proteins identifies the LRRTM protein family as synaptic organizers. Neuron. 2009;61:734–749. doi: 10.1016/j.neuron.2009.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Wit J, et al. LRRTM2 interacts with Neurexin1 and regulates excitatory synapse formation. Neuron. 2009;64:799–806. doi: 10.1016/j.neuron.2009.12.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ko J, Fuccillo MV, Malenka RC, Südhof TC. LRRTM2 functions as a neurexin ligand in promoting excitatory synapse formation. Neuron. 2009;64:791–798. doi: 10.1016/j.neuron.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Futai K, et al. Retrograde modulation of presynaptic release probability through signaling mediated by PSD-95-neuroligin. Nat Neurosci. 2007;10:186–195. doi: 10.1038/nn1837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Jüngling K, et al. N-cadherin transsynaptically regulates short-term plasticity at glutamatergic synapses in embryonic stem cell-derived neurons. J Neurosci. 2006;26:6968–6978. doi: 10.1523/JNEUROSCI.1013-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Varoqueaux F, et al. Neuroligins determine synapse maturation and function. Neuron. 2006;51:741–754. doi: 10.1016/j.neuron.2006.09.003. [DOI] [PubMed] [Google Scholar]
- 24.McCann CM, Nguyen QT, Santo Neto H, Lichtman JW. Rapid synapse elimination after postsynaptic protein synthesis inhibition in vivo. J Neurosci. 2007;27:6064–6067. doi: 10.1523/JNEUROSCI.0627-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hall BJ, Ripley B, Ghosh A. NR2B signaling regulates the development of synaptic AMPA receptor current. J Neurosci. 2007;27:13446–13456. doi: 10.1523/JNEUROSCI.3793-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Sabo SL, Gomes RA, McAllister AK. Formation of presynaptic terminals at predefined sites along axons. J Neurosci. 2006;26:10813–10825. doi: 10.1523/JNEUROSCI.2052-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhai RG, et al. Assembling the presynaptic active zone: A characterization of an active one precursor vesicle. Neuron. 2001;29:131–143. doi: 10.1016/s0896-6273(01)00185-4. [DOI] [PubMed] [Google Scholar]
- 28.Klassen MP, et al. An Arf-like small G protein, ARL-8, promotes the axonal transport of presynaptic cargoes by suppressing vesicle aggregation. Neuron. 2010;66:710–723. doi: 10.1016/j.neuron.2010.04.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Isaac JT. Postsynaptic silent synapses: Evidence and mechanisms. Neuropharmacology. 2003;45:450–460. doi: 10.1016/s0028-3908(03)00229-6. [DOI] [PubMed] [Google Scholar]
- 30.Bolton MM, Blanpied TA, Ehlers MD. Localization and stabilization of ionotropic glutamate receptors at synapses. Cell Mol Life Sci. 2000;57:1517–1525. doi: 10.1007/PL00000636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ehlers MD, Heine M, Groc L, Lee MC, Choquet D. Diffusional trapping of GluR1 AMPA receptors by input-specific synaptic activity. Neuron. 2007;54:447–460. doi: 10.1016/j.neuron.2007.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Haas K, Li J, Cline HT. AMPA receptors regulate experience-dependent dendritic arbor growth in vivo. Proc Natl Acad Sci USA. 2006;103:12127–12131. doi: 10.1073/pnas.0602670103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Shi S, Hayashi Y, Esteban JA, Malinow R. Subunit-specific rules governing AMPA receptor trafficking to synapses in hippocampal pyramidal neurons. Cell. 2001;105:331–343. doi: 10.1016/s0092-8674(01)00321-x. [DOI] [PubMed] [Google Scholar]
- 34.Ziff EB. TARPs and the AMPA receptor trafficking paradox. Neuron. 2007;53:627–633. doi: 10.1016/j.neuron.2007.02.006. [DOI] [PubMed] [Google Scholar]
- 35.Passafaro M, Nakagawa T, Sala C, Sheng M. Induction of dendritic spines by an extracellular domain of AMPA receptor subunit GluR2. Nature. 2003;424:677–681. doi: 10.1038/nature01781. [DOI] [PubMed] [Google Scholar]
- 36.Saglietti L, et al. Extracellular interactions between GluR2 and N-cadherin in spine regulation. Neuron. 2007;54:461–477. doi: 10.1016/j.neuron.2007.04.012. [DOI] [PubMed] [Google Scholar]
- 37.Lu W, et al. Subunit composition of synaptic AMPA receptors revealed by a single-cell genetic approach. Neuron. 2009;62:254–268. doi: 10.1016/j.neuron.2009.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fiala JC, Feinberg M, Popov V, Harris KM. Synaptogenesis via dendritic filopodia in developing hippocampal area CA1. J Neurosci. 1998;18:8900–8911. doi: 10.1523/JNEUROSCI.18-21-08900.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Grutzendler J, Kasthuri N, Gan WB. Long-term dendritic spine stability in the adult cortex. Nature. 2002;420:812–816. doi: 10.1038/nature01276. [DOI] [PubMed] [Google Scholar]
- 40.Trachtenberg JT, et al. Long-term in vivo imaging of experience-dependent synaptic plasticity in adult cortex. Nature. 2002;420:788–794. doi: 10.1038/nature01273. [DOI] [PubMed] [Google Scholar]
- 41.Selkoe DJ. Alzheimer's disease is a synaptic failure. Science. 2002;298:789–791. doi: 10.1126/science.1074069. [DOI] [PubMed] [Google Scholar]
- 42.Hsieh H, et al. AMPAR removal underlies Abeta-induced synaptic depression and dendritic spine loss. Neuron. 2006;52:831–843. doi: 10.1016/j.neuron.2006.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Threadgill R, Bobb K, Ghosh A. Regulation of dendritic growth and remodeling by Rho, Rac, and Cdc42. Neuron. 1997;19:625–634. doi: 10.1016/s0896-6273(00)80376-1. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.