Abstract
Asthma is the result of chronic airway inflammation associated predominantly with CD4+ cells, eosinophils, mast cells, and basophils. Several T cells subsets, including NKT cells, play a critical role in orchestrating the inflammation in the airways predominantly, by secreting interleukin-4 and interleukin-13. Recently, Programmed death-1 (PD-1) with its ligands, programmed death ligand B7H1 (PD-L1) and B7DC (PD-L2), was shown to regulate T-cell activation and tolerance. PD-1 has been characterized as a negative regulator of conventional CD4+T cells. In addition, the relative roles of PD-L1 and PD-L2 in regulating the activation and function of T cells have recently been characterized. Recent studies have demonstrated that PD-L1 and PD-L2 have important but opposing roles in modulating and polarizing T cell functions in airway hyperreactivity. Whereas the severity of asthma is greatly enhanced in absence of PD-L2, PD-L1 deficiency resulted in reduced airway hyperresponsiveness (AHR) and only minimal inflammation. This observation is partially due to the polarization of NKT cells in PD-L1 and PD-L2 deficient mice. This review will discuss the recent literature regarding the role of PD-L1 and PD-L2 in allergic disease and asthma. Current understanding of the role of PD ligands in allergic asthma gives impetus to the development of novel therapeutic approaches.
Keywords: Asthma, PD-1, PD-L1, PD-L2
Introduction
Asthma prevalence has increased considerably in recent decades and asthma is now considered as one of the most common chronic disorders in the world (1). Asthma is a chronic disease of the airways characterized by bronchial hyperreactivity to nonspecific stimuli, chronic eosinophilic airway inflammation, goblet cells hyperplasia and airway structural changes in response to a chronic Th2 immune response to inhaled allergen, as well as Th2-dependent increased serum levels of allergen-specific IgE and IgG1(2, 3). The complex inflammation that occurs in asthma is associated with different types of inflammatory cells and more than hundred inflammatory mediators and gene products (4-6). In some forms of asthma, IFN-γ and neutrophils predominate in the airway (7), but in allergic asthma, the inflammatory process is associated predominantly with CD4+ cells, eosinophils, mast cells, and basophils (4). In patients with allergic asthma, CD4+ cells predominantly produce IL-4, IL-5, IL-9, and IL-13. These interleukins have essential roles in asthma, enhancing the growth, differentiation, and recruitment of eosinophils, basophils, mast cells, and IgE-producing B cells. Although allergen induced AHR is known to be dependent on CD4+ cells and is associated with an increase in Th2 cytokines in the lung, the mechanisms by which asthma is controlled are not fully understood. It has been reported that T reg cells secreting interleukin IL-10 can inhibit airway inflammation and AHR, but other inhibitory pathways also exist and play important roles in the development of asthma (8-10).
In the pathogenesis of asthma, the activation of T lymphocytes is dependent on two coordinate signals (11). The initial signal confers specificity to the immune response in recognizing major histocompatibility class-peptide complexes by T lymphocytes. However, this initial signal is not sufficient to completely activate T cells. A second, nonspecific costimulatory signal is required by T cells to become fully effective. These signals are often provided by surface costimulatory molecules to allow interactions between APCs and T cells. In recent studies, numerous new costimulatory molecules have been described leading to the recognition of many costimulation pathways. Costimulatory molecules are divided into 2 main families: i) molecules from the B7:CD28 family, such as CTLA-4 or programmed death (PD)-L1, and ii) the tumor necrosis factor receptor (TNFR) superfamily such as OX40 or CD27 (12). Costimulatory molecules have specific effects on T-cell activation, function and survival and are implicated in nearly all inflammatory diseases including asthma. PD-1 (also called CD279) and its ligands deliver inhibitory signals that regulate the balance between T- cell activation, tolerance and immunopathology (13). The interaction between PD-1 and its ligands results in a diverse range of pathogenetic effects in T-cell activation, T cell tolerance, and immune-mediated tissue damage (Table 1). The role of PD-1 and its ligands have already been observed in the regulation of autoimmune diseases (14-16) and in immunoregulatory functions in various microbial and infectious disease models (17-23). These ligands were also reported to play a major role in tumor immunity (24, 25) and tissue transplantation (26, 27). The relative contribution of the costimulatory ligands PD-L1 and PD-L2 to the development of allergic airway responses in bronchial asthma has recently been recognized (28). This review will highlight recent findings on the potential role of these costimulatory markers in the pathogenesis of asthma.
Table 1. Immunoregulatory functions of Programmed cell death ligands, PD-L1 and PD-L2.
| Disease | Role of Programmed cell death Ligand | Reference |
|---|---|---|
| Programmed cell death Ligand 1 (PD-L1) | ||
| Allergic asthma | • PD-L1-/- mice have reduced AHR. | Akbari et al.(28) 2010 |
| • Blocking antibodies against PD-L1 show increased Th1 cytokine response in mice. | Matsumoto et al.(58) 2004 | |
| Other allergic diseases | • Keratinocyte-associated PD-L1 downregulates the effector function of CD8 T cells at local inflammatory sites and plays a role in peripheral T cell tolerance against exogenous Antigens. | Ritprajak et al.(86) 2010 |
| Immunoregulation | • In NOD mice blockade of PD-L1 leads to insulitis and proinflammatory cytokine production by T cells. | Wang et al.(16) 2005 |
| • In experimental autoimmune encephalomyelitis (EAE), PD-L1 expressed by CNS myeloid APC negatively regulates T-cell activation. | Schreiner et al.(15) 2008 | |
| • PD-L1 can inhibit T cell responses by promoting both the induction and maintenance of induced regulatory T cells. | Francisco et al.(56) 2009 | |
| Microbial Pathogenesis and Infectious Disease | • PD-L1-/- mice clears adenovirus infection more rapidly but develops severe hepatocellular injury. | Iwai et al.(19) 2003 |
| • Blocking PD-1:PD-L1 interactions in-vitro reverses the exhaustion of HIV, HBV, HCV, and SIV specific CD8 and CD4 T-cells and restores proliferation and cytokine production. | Day et al.(18) 2006, Boni et al.(17) 2007, Urbani et al.(22) 2006, Velu et al. (23) 2007. | |
| • PD-L1-/- mice show reduced growth of cutaneous lesions and parasite burden upon infection with Leishmania mexicana. | Liang et al.(20) 2006 | |
| Transplantation | • Administration of PD-L1 blocking antibodies accelerates transplant rejection. | Hori et al.(26) 2006, Ito et al.(27) 2005. |
| Tumor Immunity | • PD-L1 expression on tumors inhibits T cell activation and lysis of tumor cells in mice. | Dong et al.(78) 2004. |
| Immunopathology | • Blockade of PD-L1 on vascular endothelial cells enhances IFN-γ production and cytolytic activity of CD8 T cells in-vitro. | Rodig et al.(42) 2003. |
| Programmed cell death Ligand 2 (PD-L2) | ||
| Allergic asthma | • PD-L2-/- mice developed significantly higher AHR. | Akbari et al.(28) 2010 |
| • Administration of PD-L2 blocking antibodies in mice model of asthma blocked the development of airway inflammation. | Radhakrishnan et al.(59) 2004 | |
| Other Allergic diseases | • Administration of PD-L2 blocking antibody enhances eosinophilic infiltration in experimental allergic conjunctivitis in mice. | Fukushima et al.(85) 2006 |
| Immunoregulation | • PD-L2-/- mice show increased CD68 (+) cells along with elevated circulating IgG in glomeruli in autoimmune kidney disease. | Menke et al.(14) 2007 |
| • PD-L2 negatively regulates T cell activation in-vitro and in-vivo and mediates oral tolerance. | Zhang et al.(62) 2006 | |
| Microbial Pathogenesis and Infectious Disease | • Administration of PD-L2 blocking antibodies inhibit T-cell proliferation on activated macrophages in Taenia crassiceps. | Terrazas et al.(21) 2005 |
| • PD-L2-/- mice developed exacerbated disease with increased parasite burden upon infection with Leishmania mexicana. | Liang et al.(20) 2006 | |
| Tumor Immunity | • PD-L2 has been identified as being highly expressed in Hodgkin lymphoma cells by microarray analysis. | Rosenwald et al.(25) 2003 |
PD-1 and its ligands PD-L1 and PD-L2
The PD-1 receptor was first observed in T cells undergoing cell death (29). Since then, several studies involving these receptors have demonstrated an important role in immune tolerance and in various regulatory functions. Important findings came with the observation that PD-1 deficient mice develop spontaneous autoimmune diseases, suggesting a crucial role in the establishment and/or maintenance of immunological self-tolerance (30). Furthermore, augmented PD-1 expression has been observed on synovial fluid T cells in rheumatoid arthritis (RA) and on salivary T cells in Sjogren's syndrome, suggesting that PD-1 may actually exert its regulatory functions in target organs (31-33).
The ligands for PD-1 have given even more insight into their role in immune regulation. The PD-1 receptor ligands PD-L1 (B7-H1) and PD-L2 (B7-DC) belong to the B7:CD28 family of ligands (34). PD-L1 and PD-L2 are type I transmembrane glycoproteins composed of IgC- and IgV-type extracellular domains (35-38). The location of PD-L1 and PD-L2 genes are on mouse chromosome 19 at a distance of 22-kb between them. PD-L1 shares a 20% amino acid identity with B7.1, a ligand for CD28, whereas PD-L2 shares a 20% amino acid homology with B7.2, a well established ligand for CTLA-4. Whereas PD-L1 and PD-L2 share 70% amino acid homology in mice, the homology among the two ligands in humans is 40%. The expression profiles of human PD-L1 and PD-L2 are comparable to the murine system (39). Both these markers have short cytoplasmic tails with no known sequence motif for signal transduction, suggesting that these ligands do not transduce signals upon interaction with PD-1. Some earlier reports suggest that cross-linking of PD-L2 induces stimulatory signals in dendritic cells resulting in augmented antigen presentation (40, 41).
PD-L1 and PD-L2 are expressed in various tissues, with high levels of expression in lungs, heart and liver and with lower expressions in spleen, lymph nodes and thymus (35-38). Expression of PD-L1 is observed in both lymphoid and non-lymphoid tissues which indicate that the PD-1–PD-L1 pathway modulates immune responses in secondary lymphoid organs as well as in target organs. Expression of PD-L1 and PD-L2 on antigen-presenting cells has been well described (42). It was observed that PD-L1 is expressed on resting B cells, T cells, macrophages and DCs and their expression is further up-regulated by various types of stimulation in vitro: i) anti-IgM, LPS and anti-CD40 for B cells, ii) anti-CD3 for T cells, iii) anti-CD40, LPS, IFNγ and granulocyte macrophage colony stimulating factor (GM-CSF) for macrophages and iv) anti-CD40, IFNγ, IL-4, IL-12 and GM-CSF for DCs (43). In contrast to PD-L1, PD-L2 is rarely expressed on resting cells and can hardly be induced on B cells and on T cells. PD-L2 has been induced on macrophages by IL-4 and IFNγ and on DCs by anti-CD40, GM-CSF, IL-4, IFNγ and IL-12 (43). It is reported that IL-4 induces PD-L2 more strongly than IFNγ, while IFNγ induces PD-L1 more strongly than IL-4 on macrophages, suggesting that Th1 and Th2 responses mobilize PD-L1 and PD-L2 differentially (44).
It is now well established that PD-L1 is expressed on a wide variety of tumors, and high levels of PD-L1 expression strongly correlate with an unfavorable prognosis in several cancers. The pathogenetically crucial role of PD-L1 in different tumors has made PD-L1 a potential candidate for tumor immunotherapy (45-50). In addition, Dong and coworkers have described a significant role of tumor associated PD-L1 in T cell apoptosis and tumor invasion (24), and blockade of PD-L1 was reported to augment the effects of T- cell immunotherapy in certain models (51, 52). Several groups have subsequently reported that engagement of PD-1 by PD-L1 or PD-L2 results in inhibition of proliferation and polarized or altered cytokine production (16, 53, 54). Moreover, the inhibitory signals provided by engagement of PD-1 was demonstrated by the development of autoimmune diseases in PD-1-deficient mice (55). PD-L1 is also reported to protect tissues from autoreactive T effector cells by inhibiting their function and also by increasing the frequency and function of T reg cells (56). Taken together, PD-1 and the ligands, PD-L1 and PD-L2 appear to play a crucial role in the maintenance of T cell homeostasis, not only in cancer but also in allergic diseases.
PD-L1/PD-L2 in Airway hyperreactivity
Studies are just beginning to elucidate the function of PD-L1 and PD-L2 in allergy and asthma. Our group has recently found a novel mechanism by which PD-L2 distinctly regulates airways inflammation and AHR (28). We demonstrated that a lack of PD-L2 expression results in increased AHR and increased lung inflammation which indicates that PD-L2 expression in the lung protects against the initiation and progression of airway inflammation (28). In a similar study, Matsumoto and colleagues demonstrated that PD-L2 is highly expressed on pulmonary DCs and macrophages of sensitized mice, and the authors showed that administration of blocking antibodies against PD-L2, but not against PD-1 or PD-L1, during allergen challenge enhances the airway hyperresponsiveness and production of Th2 cytokines (57, 58). It was further observed that this effect is mediated by IFN-γ as no enhancement was observed in IFN-γ deficient mice after treatment with anti-PD-L2 antibody (57). In relation to asthma it is also reported that administration of the sHIgM12 antibody (an antibody inducing reverse signaling through PD-L2) in a mouse model of allergic asthma blocks the development of AHR (59). Administration of PD-L2-Fc in a mouse model of allergic asthma resulted in elevated levels of serum IgE and in increased eosinophilic and lymphocytic infiltration (60). In this line, we were able to observe that PD-L2 deficiency substantially increased the development of AHR in mice (Figure 1). To further differentiate the role of PD-L1 and PD-L2 in AHR, it was reported that upon challenge with α-GalCer, PD-L1 deficient mice showed a reduced level of AHR and minimal airway inflammation, while PD-L2 deficient mice developed increased AHR when compared with wild type mice. These findings suggest that PD-L2 preferentially modulates the effector phase of the asthmatic response. The inhibitory role of PD-L2 has already been reported in earlier studies (37, 61). Previously, Zhang et al. showed that PD-L2 negatively regulates T cell activation in a murine model, and PD-L2 appeared to be crucial in the development of oral tolerance (62). Data from our group indicate that PD-L1 expression inhibits IFN-γ production. Thus, lack of PD-L1 lead to reduced levels of AHR, minimal inflammation and mucous secretion in lungs, thus defining its opposing role with that of PD-L2 in asthma pathogenesis. It is also observed that the combined deficiency of PD-L1 and PD-L2 expression in PD-L1/PD-L2 double knockout mice neutralizes the effects of the single knockout mice, as PD-L1/PD-L2 double knockout mice developed airway hyperreactivity comparable to wild type mice (28). These data indicate that PD-L1 and PD-L2 might have opposing roles in the pathogenesis of allergic diseases and asthma; while PD-L1 might be crucial for the development of AHR in mice, activation of the PD-1/PD-L2 pathway might lead to protective immune responses.
Figure 1. Role of programmed cell death ligands, PD-L1 and PD-L2 in allergic asthma.
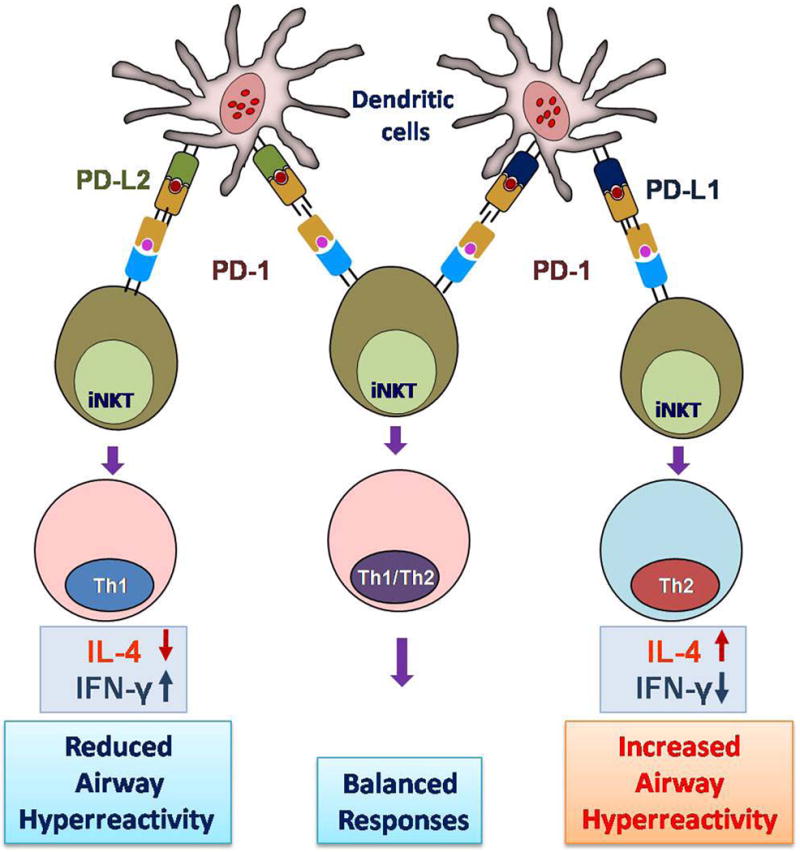
Upon recognition and activation, pulmonary dendritic cells express PD-L1 and PD-L2. PD-1/PD-L1 interaction produces a Th2 response with more IL-4 production which leads to increased AHR (right). However, PD-1/PD-L2 interaction initiates a Th1 type response with increased expression of IFN-γ and subsequently reduces the AHR (left). Simultaneous expression of PD-L1 and PD-L2 neutralizes the single effects and does not lead to immediate polarization of T cells (middle).
PD-L1/PD-L2 and Dendritic cells
Dendritic cells (DCs) play functional roles in regulating immune responses. While DCs generally present antigens, they are a heterogeneous group of cells that differ in origin, location, cell-surface phenotype, and function (63). After interaction with T-cells, DCs determine the type of resulting immune response from inducing tolerance to the establishment of effector T cell responses that protect the host against potentially harmful antigens. (64). This differentiation is dependent on a number of factors, such as the nature of the antigen, the cytokine milieu in the surrounding tissue, and the type of DC subset, expressing specific costimulatory molecules (65). It is well established, that PD-L1 is constitutively expressed on mouse DCs, macrophages, B cells and T cells and that it is further up-regulated upon activation (13, 66). It has also been shown that PD-1 is up-regulated on T cells upon activation, and its ligands have distinct expression patterns, with PD-L1 being expressed much more abundantly than PD-L2. PD-L2 expression in contrast was reported to be elevated on DCs from the lung and from draining pulmonary lymph nodes after antigen challenge (58) and seems to be upregulated on splenic DCs after in vivo activation of iNKT cells by the administration of α-GalCer (67). Similar to previous studies, we recently observed that lung DCs showed increased expression of PD-L2 upon OVA challenge and also observed that expression of PD-L2 was upregulated on lung DCs from naive mice upon culture with IL-4. However, treatment with IFN-γ plus LPS inhibits PD-L2 expression. These finding suggests that the local cytokine microenvironment in the lungs plays an important role in defining the expression pattern of PD-L2 (28). As PD-L2 inhibits IL-4 production and iNKT-cell-mediated AHR, our studies add to the notion that PD-L2 constitutes a feedback loop in the lung during the course of acute inflammation by being upregulated by IL-4, and subsequently reducing IL-4 production, thereby modulating the severity of asthma. In similar experimental settings, it was reported that DCs from mice cultured with IL-4 plus lipopolysaccharide showed higher expression of PD-L2. In contrast, culture with IFN-γ resulted in an enhanced PD-L1 expression (28). Interestingly, the enhanced PD-L1 expression observed on lung DCs, macrophages, and B cells after OVA challenge inhibits IFN-γ production, which could mitigate AHR. Similarly, loss of PD-L1 results in increased IFN-γ and reduced AHR. These findings add to the idea that both markers might have opposing roles in acute inflammatory processes. Benedict et al. have studied the relative contribution of the PD-L1/PD-1 pathway in MCMV-infection and have reported that PD-L1/PD-1 interactions directly encounter infected DC both in culture and in vivo, whereas there is a negligible contribution for the PD-L1/PD-1 pathway when murine cytomegalovirus (mCMV) antigen is cross-presented by uninfected APC. Their findings suggested that maintaining the PD-L1/PD-1 interaction is a critical component of the overall program used by mCMV to suppress the antiviral T cell response of the host (68). In another study, Agarwal and colleagues observed higher expression levels of PD-L1 and PD-L2 of CD11chigh,CD11blow DCs in Flt3 ligand–treated OVA-sensitized mice. These authors observed a positive correlation of Flt3 ligand treatment and increased PDL2 expression which can be the inhibitory effect of PD-L2/PD-1 interaction on T-cell proliferation (69). The role of PD-L1 was also reported in immunoregulation by plasmacytoid dendritic cells (pDCs) in airway inflammation. Recently, it was reported that pDCs induce the formation of regulatory T cells via the expression of PD-L1. It was reported that pDCs exert their suppressive function through expression of the costimulatory molecule PD-L1, and also PD-L1 deficient pDCs did not down-regulate eosinophilic inflammation (70). These findings give impetus on the protective role of PD-1/PD-L1 interaction in dendritic cells. The relatively greater role of IFN-γ in stimulating PD-L1 expression and IL-4 in stimulating PD-L2 expression suggests once more that PD-L1 and PD-L2 have opposing functions in regulating Th1 and Th2 responses.
Role of PD-L1/PD-L2 on T cells and NKT cells
T-regulatory cells (Tregs) are known to be potent immunomodulators in allergic asthma. It is well established that there is a reduction of Tregs cells and an increase of Th2 cells and this imbalance initiates the development of AHR and airway inflammation in allergic asthma (71, 72). Tregs are known to secrete immunosuppressive cytokines which modulate immune responses. An earlier report has already suggested that suppression by Tregs cells may occur through the expression of the cell surface marker programmed death PD-1 (73). Recently, PD-L1 was found to be directly responsible for the generation of regulatory T cells (74). Earlier, McGee and Agarwal reported that PD-1 has a role in the function of Tregs and their ability to suppress AHR and airway inflammation. In a mouse model, these authors observed that PD-1 expressing inducible Tregs (iTregs) from spleens and lungs completely reversed lung inflammation and AHR to methacholine. It was suggested that PD-1 is critically involved in the mechanism of action of these iTreg cells (75). Recent studies from our own group confirmed this, as we were able to show that the severity of asthma is greatly enhanced in PD-L2 deficient mice, and this was due to a higher production of IL-4 by iNKT cells, indicating that PD-L2 engagement inhibited IL-4 production by iNKT cells (28). These observations may help explain the inhibitory role of PD-L2 in asthma. Studies with PD-L1 deficient mice showed reduced levels of AHR, minimal inflammation, and mucous secretion, as well as enhanced production of IFN-γ by iNKT cells. In addition, other groups have reported that the PD-1/PD-L inhibitory pathway can potently inhibit the proliferation and IL-2 production of murine T cells (76). Some previous studies have also shown that the PD-1/PD-L1 pathway enhances the α-galactosylceramide (α-GalCer)-mediated induction of NKT cell anergy (44, 67). Earlier, Kinter et al. had observed that the cytokines IL-2, IL-7, IL-15, and IL-21 directly induce the expression of PD-1 and PD-L1 on purified T cells as well as PD-1 ligands on APCs. Their findings suggest that the expression of PD-1/PD-L ligand does not appear to negatively impact the ability of T cells to expand, function, or survive in response to further γc cytokine exposure, but does render them susceptible to PD-1 ligand-mediated suppression of TCR-triggered function (77).
Although PD-L1 and PD-L2 are the most important ligands for PD-1, some additional receptors for these B7 family members have been reported (78-80). Some earlier studies showed that PD-L2 co-stimulates CD4 + T-cell proliferation and cytokine production and this co-stimulation was found to be independent of PD-1 (78-80). Further, Freeman et al. reported that PD-L1 interacts not only with PD-1 but also with a second receptor, B7-1, on activated T cells, and that this interaction negatively regulates T-cell expansion (81). Recently, we reported that the addition of a mAb that blocks the PD-L1 interaction with both PD-1 and B7-1 (9G2) resulted in greatly enhanced levels of IFN-γ production by splenic iNKT cells stimulated with α-GalCer, but did not affect IL-4 production. However, culture in the presence of PD-L1 mAb that selectively blocks the PD-L1 / B7-1 interaction (2H11) had no effect on the production of IFN-γ or IL-4 (28). These findings suggest that PD-1/PD-L1 interaction is primarily responsible for inhibiting the production of IFN-γ by iNKT cells. Previous studies have demonstrated that PD-1 is up-regulated on T cells following TCR-mediated activation (37, 82-84).
PD-L1 and PD-L2 in other allergic diseases
The role of PD-1/PD-L1/PD-L2 interaction has also been reported in other allergic diseases. Fukushima et al. reported that PD-1 and its ligands are involved in the development of experimental allergic conjunctivitis in mice. They showed that treatment with anti-PD-L2 mAb enhanced the local infiltration of eosinophils during the effector phase but not during the induction phase. This indicates that the interaction between PD-L2 and its putative receptor other than PD-1 is important for the recruitment of eosinophils into the conjunctiva (85). Recently, It was observed that Keratinocyte-associated PD-L1 plays an important role in down regulation of effector function of CD8 T cells at local inflammatory sites and plays a crucial role in peripheral T cell tolerance against exogenous antigens(86).
PD-L1 and PD-L2 as a therapeutic targets
Since the ligands for PD-1 were identified, PD-1:PD-L interactions have been shown to exert a vital and diverse range of immunoregulatory roles in T cell activation, tolerance, and immune-mediated diseases. Regarding therapeutic approaches, PD-1 ligands have been targets in different disease models such as viral infections and tumors. Inhibition of the PD-1/PD-L1 pathway initiates functional recovery of exhausted T cells in chronic viral infections. In HIV infection, PD-1/PD-L1 interactions are operative during a persistent viral infection in humans, and cause a reversible defect in HIV-specific T-cell function (18). Also, PD-L1 expression serves as a potent mechanism for potentially immunogenic tumors to escape from host immune responses, and blockade of its interaction with PD-1 provide a promising strategy for specific tumor immunotherapy (87). Because of the critical role of PD-1 and interactions with its ligands in animal models of asthma, there has been a growing interest in analyzing these ligands and to use them as new therapeutic targets in human lung diseases and asthma. Neutralizing and blocking antibodies to the human PD-1, PDL-1 and PD-L2 are available. Their cross-reactivity with the equivalent molecules encoded by the genomes of non-human primates will provide an opportunity to test the importance of their regulatory pathway in the pathogenesis of allergic diseases and asthma. Particularly, PD-L1 blockade or the activation of PD-L2 pathways by fusion proteins or antibodies might provide an effective way in reducing asthma pathogenesis. However, because of the complexity of the crosstalk between immunoregulatory pathways, these approaches preferentially need to be managed locally and subjects needs to be carefully monitored.
Conclusion
It has become clear that PD-1 plays critical roles in the regulation of autoimmunity, tumor immunity, infectious immunity, transplantation immunity and in the definition of immune privilege. A novel function of PD-1 and its ligands in asthma pathogenesis was recently studied and revealed a crucial costimulatory function of the PD-1 ligands PD-L1 and PD-L2 in asthma pathogenesis. There is strong evidence that PD-L1 is involved in the maintenance of peripheral tolerance and that PD-L1 contributes to the induction of asthma. In addition, there is evidence from animal models of allergy and asthma that PD-L1 and PD-L2 have opposing roles in modulating and polarizing T cell functions in AHR and airway inflammation. With an increased understanding of the role of PD ligands for controlling airway hyperresponsiveness and asthma, new pathways leading to therapeutic strategies in the treatment of allergic diseases are expected.
Acknowledgments
Supported by: NIH R01 AI066020 (O.A.)
References
- 1.Anandan C, Nurmatov U, van Schayck OC, Sheikh A. Is the prevalence of asthma declining? Systematic review of epidemiological studies. Allergy. 65(2):152–67. doi: 10.1111/j.1398-9995.2009.02244.x. [DOI] [PubMed] [Google Scholar]
- 2.Galli SJ, Tsai M, Piliponsky AM. The development of allergic inflammation. Nature. 2008;454(7203):445–54. doi: 10.1038/nature07204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.von Garnier C, Wikstrom ME, Zosky G, Turner DJ, Sly PD, Smith M, et al. Allergic airways disease develops after an increase in allergen capture and processing in the airway mucosa. J Immunol. 2007;179(9):5748–59. doi: 10.4049/jimmunol.179.9.5748. [DOI] [PubMed] [Google Scholar]
- 4.Busse WW, Lemanske RF., Jr Asthma. N Engl J Med. 2001;344(5):350–62. doi: 10.1056/NEJM200102013440507. [DOI] [PubMed] [Google Scholar]
- 5.Kiley J, Smith R, Noel P. Asthma phenotypes. Curr Opin Pulm Med. 2007;13(1):19–23. doi: 10.1097/MCP.0b013e328011b84b. [DOI] [PubMed] [Google Scholar]
- 6.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3(8):715–20. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 7.Brusasco V, Crimi E, Pellegrino R. Airway hyperresponsiveness in asthma: not just a matter of airway inflammation. Thorax. 1998;53(11):992–8. doi: 10.1136/thx.53.11.992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Akbari O, Stock P, Meyer E, Kronenberg M, Sidobre S, Nakayama T, et al. Essential role of NKT cells producing IL-4 and IL-13 in the development of allergen-induced airway hyperreactivity. Nat Med. 2003;9(5):582–8. doi: 10.1038/nm851. [DOI] [PubMed] [Google Scholar]
- 9.Lloyd CM, Hawrylowicz CM. Regulatory T cells in asthma. Immunity. 2009;31(3):438–49. doi: 10.1016/j.immuni.2009.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stock P, Akbari O, Berry G, Freeman GJ, Dekruyff RH, Umetsu DT. Induction of T helper type 1-like regulatory cells that express Foxp3 and protect against airway hyper-reactivity. Nat Immunol. 2004;5(11):1149–56. doi: 10.1038/ni1122. [DOI] [PubMed] [Google Scholar]
- 11.Lafferty KJ, Cooley MA, Woolnough J, Walker KZ. Thyroid allograft immunogenicity is reduced after a period in organ culture. Science. 1975;188(4185):259–61. doi: 10.1126/science.1118726. [DOI] [PubMed] [Google Scholar]
- 12.Croft M. Co-stimulatory members of the TNFR family: keys to effective T-cell immunity? Nat Rev Immunol. 2003;3(8):609–20. doi: 10.1038/nri1148. [DOI] [PubMed] [Google Scholar]
- 13.Keir ME, Butte MJ, Freeman GJ, Sharpe AH. PD-1 and its ligands in tolerance and immunity. Annu Rev Immunol. 2008;26:677–704. doi: 10.1146/annurev.immunol.26.021607.090331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Menke J, Lucas JA, Zeller GC, Keir ME, Huang XR, Tsuboi N, et al. Programmed death 1 ligand (PD-L) 1 and PD-L2 limit autoimmune kidney disease: distinct roles. J Immunol. 2007;179(11):7466–77. doi: 10.4049/jimmunol.179.11.7466. [DOI] [PubMed] [Google Scholar]
- 15.Schreiner B, Bailey SL, Shin T, Chen L, Miller SD. PD-1 ligands expressed on myeloid-derived APC in the CNS regulate T-cell responses in EAE. Eur J Immunol. 2008;38(10):2706–17. doi: 10.1002/eji.200838137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang J, Yoshida T, Nakaki F, Hiai H, Okazaki T, Honjo T. Establishment of NOD-Pdcd1-/- mice as an efficient animal model of type I diabetes. Proc Natl Acad Sci U S A. 2005;102(33):11823–8. doi: 10.1073/pnas.0505497102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Boni C, Fisicaro P, Valdatta C, Amadei B, Di Vincenzo P, Giuberti T, et al. Characterization of hepatitis B virus (HBV)-specific T-cell dysfunction in chronic HBV infection. J Virol. 2007;81(8):4215–25. doi: 10.1128/JVI.02844-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Day CL, Kaufmann DE, Kiepiela P, Brown JA, Moodley ES, Reddy S, et al. PD-1 expression on HIV-specific T cells is associated with T-cell exhaustion and disease progression. Nature. 2006;443(7109):350–4. doi: 10.1038/nature05115. [DOI] [PubMed] [Google Scholar]
- 19.Iwai Y, Terawaki S, Ikegawa M, Okazaki T, Honjo T. PD-1 inhibits antiviral immunity at the effector phase in the liver. J Exp Med. 2003;198(1):39–50. doi: 10.1084/jem.20022235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liang SC, Greenwald RJ, Latchman YE, Rosas L, Satoskar A, Freeman GJ, et al. PD-L1 and PD-L2 have distinct roles in regulating host immunity to cutaneous leishmaniasis. Eur J Immunol. 2006;36(1):58–64. doi: 10.1002/eji.200535458. [DOI] [PubMed] [Google Scholar]
- 21.Terrazas LI, Montero D, Terrazas CA, Reyes JL, Rodriguez-Sosa M. Role of the programmed Death-1 pathway in the suppressive activity of alternatively activated macrophages in experimental cysticercosis. Int J Parasitol. 2005;35(13):1349–58. doi: 10.1016/j.ijpara.2005.06.003. [DOI] [PubMed] [Google Scholar]
- 22.Urbani S, Amadei B, Tola D, Massari M, Schivazappa S, Missale G, et al. PD-1 expression in acute hepatitis C virus (HCV) infection is associated with HCV-specific CD8 exhaustion. J Virol. 2006;80(22):11398–403. doi: 10.1128/JVI.01177-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Velu V, Kannanganat S, Ibegbu C, Chennareddi L, Villinger F, Freeman GJ, et al. Elevated expression levels of inhibitory receptor programmed death 1 on simian immunodeficiency virus-specific CD8 T cells during chronic infection but not after vaccination. J Virol. 2007;81(11):5819–28. doi: 10.1128/JVI.00024-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dong H, Strome SE, Salomao DR, Tamura H, Hirano F, Flies DB, et al. Tumor-associated B7-H1 promotes T-cell apoptosis: a potential mechanism of immune evasion. Nat Med. 2002;8(8):793–800. doi: 10.1038/nm730. [DOI] [PubMed] [Google Scholar]
- 25.Rosenwald A, Wright G, Leroy K, Yu X, Gaulard P, Gascoyne RD, et al. Molecular diagnosis of primary mediastinal B cell lymphoma identifies a clinically favorable subgroup of diffuse large B cell lymphoma related to Hodgkin lymphoma. J Exp Med. 2003;198(6):851–62. doi: 10.1084/jem.20031074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Hori J, Wang M, Miyashita M, Tanemoto K, Takahashi H, Takemori T, et al. B7-H1-induced apoptosis as a mechanism of immune privilege of corneal allografts. J Immunol. 2006;177(9):5928–35. doi: 10.4049/jimmunol.177.9.5928. [DOI] [PubMed] [Google Scholar]
- 27.Ito T, Ueno T, Clarkson MR, Yuan X, Jurewicz MM, Yagita H, et al. Analysis of the role of negative T cell costimulatory pathways in CD4 and CD8 T cell-mediated alloimmune responses in vivo. J Immunol. 2005;174(11):6648–56. doi: 10.4049/jimmunol.174.11.6648. [DOI] [PubMed] [Google Scholar]
- 28.Akbari O, Stock P, Singh AK, Lombardi V, Lee WL, Freeman GJ, et al. PD-L1 and PD-L2 modulate airway inflammation and iNKT-cell-dependent airway hyperreactivity in opposing directions. Mucosal Immunol. 3(1):81–91. doi: 10.1038/mi.2009.112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ishida Y, Agata Y, Shibahara K, Honjo T. Induced expression of PD-1, a novel member of the immunoglobulin gene superfamily, upon programmed cell death. EMBO J. 1992;11(11):3887–95. doi: 10.1002/j.1460-2075.1992.tb05481.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishimura H, Nose M, Hiai H, Minato N, Honjo T. Development of lupus-like autoimmune diseases by disruption of the PD-1 gene encoding an ITIM motif-carrying immunoreceptor. Immunity. 1999;11(2):141–51. doi: 10.1016/s1074-7613(00)80089-8. [DOI] [PubMed] [Google Scholar]
- 31.Bolstad AI, Eiken HG, Rosenlund B, Alarcon-Riquelme ME, Jonsson R. Increased salivary gland tissue expression of Fas, Fas ligand, cytotoxic T lymphocyte-associated antigen 4, and programmed cell death 1 in primary Sjogren's syndrome. Arthritis Rheum. 2003;48(1):174–85. doi: 10.1002/art.10734. [DOI] [PubMed] [Google Scholar]
- 32.Hatachi S, Iwai Y, Kawano S, Morinobu S, Kobayashi M, Koshiba M, et al. CD4+ PD-1+ T cells accumulate as unique anergic cells in rheumatoid arthritis synovial fluid. J Rheumatol. 2003;30(7):1410–9. [PubMed] [Google Scholar]
- 33.Kobayashi M, Kawano S, Hatachi S, Kurimoto C, Okazaki T, Iwai Y, et al. Enhanced expression of programmed death-1 (PD-1)/PD-L1 in salivary glands of patients with Sjogren's syndrome. J Rheumatol. 2005;32(11):2156–63. [PubMed] [Google Scholar]
- 34.Okazaki T, Honjo T. PD-1 and PD-1 ligands: from discovery to clinical application. Int Immunol. 2007;19(7):813–24. doi: 10.1093/intimm/dxm057. [DOI] [PubMed] [Google Scholar]
- 35.Dong H, Zhu G, Tamada K, Chen L. B7-H1, a third member of the B7 family, co-stimulates T-cell proliferation and interleukin-10 secretion. Nat Med. 1999;5(12):1365–9. doi: 10.1038/70932. [DOI] [PubMed] [Google Scholar]
- 36.Freeman GJ, Long AJ, Iwai Y, Bourque K, Chernova T, Nishimura H, et al. Engagement of the PD-1 immunoinhibitory receptor by a novel B7 family member leads to negative regulation of lymphocyte activation. J Exp Med. 2000;192(7):1027–34. doi: 10.1084/jem.192.7.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Latchman Y, Wood CR, Chernova T, Chaudhary D, Borde M, Chernova I, et al. PD-L2 is a second ligand for PD-1 and inhibits T cell activation. Nat Immunol. 2001;2(3):261–8. doi: 10.1038/85330. [DOI] [PubMed] [Google Scholar]
- 38.Tseng SY, Otsuji M, Gorski K, Huang X, Slansky JE, Pai SI, et al. B7-DC, a new dendritic cell molecule with potent costimulatory properties for T cells. J Exp Med. 2001;193(7):839–46. doi: 10.1084/jem.193.7.839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Brown JA, Dorfman DM, Ma FR, Sullivan EL, Munoz O, Wood CR, et al. Blockade of programmed death-1 ligands on dendritic cells enhances T cell activation and cytokine production. J Immunol. 2003;170(3):1257–66. doi: 10.4049/jimmunol.170.3.1257. [DOI] [PubMed] [Google Scholar]
- 40.Nguyen LT, Radhakrishnan S, Ciric B, Tamada K, Shin T, Pardoll DM, et al. Cross-linking the B7 family molecule B7-DC directly activates immune functions of dendritic cells. J Exp Med. 2002;196(10):1393–8. doi: 10.1084/jem.20021466. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 41.Radhakrishnan S, Nguyen LT, Ciric B, Ure DR, Zhou B, Tamada K, et al. Naturally occurring human IgM antibody that binds B7-DC and potentiates T cell stimulation by dendritic cells. J Immunol. 2003;170(4):1830–8. doi: 10.4049/jimmunol.170.4.1830. [DOI] [PubMed] [Google Scholar]
- 42.Rodig N, Ryan T, Allen JA, Pang H, Grabie N, Chernova T, et al. Endothelial expression of PD-L1 and PD-L2 down-regulates CD8+ T cell activation and cytolysis. Eur J Immunol. 2003;33(11):3117–26. doi: 10.1002/eji.200324270. [DOI] [PubMed] [Google Scholar]
- 43.Yamazaki T, Akiba H, Iwai H, Matsuda H, Aoki M, Tanno Y, et al. Expression of programmed death 1 ligands by murine T cells and APC. J Immunol. 2002;169(10):5538–45. doi: 10.4049/jimmunol.169.10.5538. [DOI] [PubMed] [Google Scholar]
- 44.Loke P, Allison JP. PD-L1 and PD-L2 are differentially regulated by Th1 and Th2 cells. Proc Natl Acad Sci U S A. 2003;100(9):5336–41. doi: 10.1073/pnas.0931259100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Inman BA, Sebo TJ, Frigola X, Dong H, Bergstralh EJ, Frank I, et al. PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer. 2007;109(8):1499–505. doi: 10.1002/cncr.22588. [DOI] [PubMed] [Google Scholar]
- 46.Nakanishi J, Wada Y, Matsumoto K, Azuma M, Kikuchi K, Ueda S. Overexpression of B7-H1 (PD-L1) significantly associates with tumor grade and postoperative prognosis in human urothelial cancers. Cancer Immunol Immunother. 2007;56(8):1173–82. doi: 10.1007/s00262-006-0266-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Nomi T, Sho M, Akahori T, Hamada K, Kubo A, Kanehiro H, et al. Clinical significance and therapeutic potential of the programmed death-1 ligand/programmed death-1 pathway in human pancreatic cancer. Clin Cancer Res. 2007;13(7):2151–7. doi: 10.1158/1078-0432.CCR-06-2746. [DOI] [PubMed] [Google Scholar]
- 48.Zhang P, Su DM, Liang M, Fu J. Chemopreventive agents induce programmed death-1-ligand 1 (PD-L1) surface expression in breast cancer cells and promote PD-L1-mediated T cell apoptosis. Mol Immunol. 2008;45(5):1470–6. doi: 10.1016/j.molimm.2007.08.013. [DOI] [PubMed] [Google Scholar]
- 49.Konishi J, Yamazaki K, Azuma M, Kinoshita I, Dosaka-Akita H, Nishimura M. B7-H1 expression on non-small cell lung cancer cells and its relationship with tumor-infiltrating lymphocytes and their PD-1 expression. Clin Cancer Res. 2004;10(15):5094–100. doi: 10.1158/1078-0432.CCR-04-0428. [DOI] [PubMed] [Google Scholar]
- 50.Thompson RH, Gillett MD, Cheville JC, Lohse CM, Dong H, Webster WS, et al. Costimulatory B7-H1 in renal cell carcinoma patients: Indicator of tumor aggressiveness and potential therapeutic target. Proc Natl Acad Sci U S A. 2004;101(49):17174–9. doi: 10.1073/pnas.0406351101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Iwai Y, Ishida M, Tanaka Y, Okazaki T, Honjo T, Minato N. Involvement of PD-L1 on tumor cells in the escape from host immune system and tumor immunotherapy by PD-L1 blockade. Proc Natl Acad Sci U S A. 2002;99(19):12293–7. doi: 10.1073/pnas.192461099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Strome SE, Dong H, Tamura H, Voss SG, Flies DB, Tamada K, et al. B7-H1 blockade augments adoptive T-cell immunotherapy for squamous cell carcinoma. Cancer Res. 2003;63(19):6501–5. [PubMed] [Google Scholar]
- 53.Ansari MJ, Salama AD, Chitnis T, Smith RN, Yagita H, Akiba H, et al. The programmed death-1 (PD-1) pathway regulates autoimmune diabetes in nonobese diabetic (NOD) mice. J Exp Med. 2003;198(1):63–9. doi: 10.1084/jem.20022125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Maier LM, Wicker LS. Genetic susceptibility to type 1 diabetes. Curr Opin Immunol. 2005;17(6):601–8. doi: 10.1016/j.coi.2005.09.013. [DOI] [PubMed] [Google Scholar]
- 55.Anderson MS, Bluestone JA. The NOD mouse: a model of immune dysregulation. Annu Rev Immunol. 2005;23:447–85. doi: 10.1146/annurev.immunol.23.021704.115643. [DOI] [PubMed] [Google Scholar]
- 56.Francisco LM, Salinas VH, Brown KE, Vanguri VK, Freeman GJ, Kuchroo VK, et al. PD-L1 regulates the development, maintenance, and function of induced regulatory T cells. J Exp Med. 2009;206(13):3015–29. doi: 10.1084/jem.20090847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Matsumoto K, Fukuyama S, Eguchi-Tsuda M, Nakano T, Matsumoto T, Matsumura M, et al. B7-DC induced by IL-13 works as a feedback regulator in the effector phase of allergic asthma. Biochem Biophys Res Commun. 2008;365(1):170–5. doi: 10.1016/j.bbrc.2007.10.156. [DOI] [PubMed] [Google Scholar]
- 58.Matsumoto K, Inoue H, Nakano T, Tsuda M, Yoshiura Y, Fukuyama S, et al. B7-DC regulates asthmatic response by an IFN-gamma-dependent mechanism. J Immunol. 2004;172(4):2530–41. doi: 10.4049/jimmunol.172.4.2530. [DOI] [PubMed] [Google Scholar]
- 59.Radhakrishnan S, Iijima K, Kobayashi T, Rodriguez M, Kita H, Pease LR. Blockade of allergic airway inflammation following systemic treatment with a B7-dendritic cell (PD-L2) cross-linking human antibody. J Immunol. 2004;173(2):1360–5. doi: 10.4049/jimmunol.173.2.1360. [DOI] [PubMed] [Google Scholar]
- 60.Oflazoglu E, Swart DA, Anders-Bartholo P, Jessup HK, Norment AM, Lawrence WA, et al. Paradoxical role of programmed death-1 ligand 2 in Th2 immune responses in vitro and in a mouse asthma model in vivo. Eur J Immunol. 2004;34(12):3326–36. doi: 10.1002/eji.200425197. [DOI] [PubMed] [Google Scholar]
- 61.Cai G, Karni A, Oliveira EM, Weiner HL, Hafler DA, Freeman GJ. PD-1 ligands, negative regulators for activation of naive, memory, and recently activated human CD4+ T cells. Cell Immunol. 2004;230(2):89–98. doi: 10.1016/j.cellimm.2004.09.004. [DOI] [PubMed] [Google Scholar]
- 62.Zhang Y, Chung Y, Bishop C, Daugherty B, Chute H, Holst P, et al. Regulation of T cell activation and tolerance by PDL2. Proc Natl Acad Sci U S A. 2006;103(31):11695–700. doi: 10.1073/pnas.0601347103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Shortman K, Liu YJ. Mouse and human dendritic cell subtypes. Nat Rev Immunol. 2002;2(3):151–61. doi: 10.1038/nri746. [DOI] [PubMed] [Google Scholar]
- 64.Moser M, Murphy KM. Dendritic cell regulation of TH1-TH2 development. Nat Immunol. 2000;1(3):199–205. doi: 10.1038/79734. [DOI] [PubMed] [Google Scholar]
- 65.Bharadwaj AS, Bewtra AK, Agrawal DK. Dendritic cells in allergic airway inflammation. Can J Physiol Pharmacol. 2007;85(7):686–99. doi: 10.1139/Y07-062. [DOI] [PubMed] [Google Scholar]
- 66.Krupnick AS, Gelman AE, Barchet W, Richardson S, Kreisel FH, Turka LA, et al. Murine vascular endothelium activates and induces the generation of allogeneic CD4+25+Foxp3+ regulatory T cells. J Immunol. 2005;175(10):6265–70. doi: 10.4049/jimmunol.175.10.6265. [DOI] [PubMed] [Google Scholar]
- 67.Parekh VV, Lalani S, Kim S, Halder R, Azuma M, Yagita H, et al. PD-1/PD-L blockade prevents anergy induction and enhances the anti-tumor activities of glycolipid-activated invariant NKT cells. J Immunol. 2009;182(5):2816–26. doi: 10.4049/jimmunol.0803648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Benedict CA, Loewendorf A, Garcia Z, Blazar BR, Janssen EM. Dendritic cell programming by cytomegalovirus stunts naive T cell responses via the PD-L1/PD-1 pathway. J Immunol. 2008;180(7):4836–47. doi: 10.4049/jimmunol.180.7.4836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Shao Z, Bharadwaj AS, McGee HS, Makinde TO, Agrawal DK. Fms-like tyrosine kinase 3 ligand increases a lung DC subset with regulatory properties in allergic airway inflammation. J Allergy Clin Immunol. 2009;123(4):917–924 e2. doi: 10.1016/j.jaci.2009.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Kool M, van Nimwegen M, Willart MA, Muskens F, Boon L, Smit JJ, et al. An anti-inflammatory role for plasmacytoid dendritic cells in allergic airway inflammation. J Immunol. 2009;183(2):1074–82. doi: 10.4049/jimmunol.0900471. [DOI] [PubMed] [Google Scholar]
- 71.Bochner BS, Busse WW. Allergy and asthma. J Allergy Clin Immunol. 2005;115(5):953–9. doi: 10.1016/j.jaci.2005.02.032. [DOI] [PubMed] [Google Scholar]
- 72.Robinson DS, Larche M, Durham SR. Tregs and allergic disease. J Clin Invest. 2004;114(10):1389–97. doi: 10.1172/JCI23595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Akdis M, Blaser K, Akdis CA. T regulatory cells in allergy: novel concepts in the pathogenesis, prevention, and treatment of allergic diseases. J Allergy Clin Immunol. 2005;116(5):961–8. doi: 10.1016/j.jaci.2005.09.004. quiz 969. [DOI] [PubMed] [Google Scholar]
- 74.Wang L, Pino-Lagos K, de Vries VC, Guleria I, Sayegh MH, Noelle RJ. Programmed death 1 ligand signaling regulates the generation of adaptive Foxp3+CD4+ regulatory T cells. Proc Natl Acad Sci U S A. 2008;105(27):9331–6. doi: 10.1073/pnas.0710441105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.McGee HS, Yagita H, Shao Z, Agrawal DK. PD-1 Antibody Blocks Therapeutic Effects of T-regulatory Cells in Cockroach Antigen-induced Allergic Asthma. Am J Respir Cell Mol Biol. 2009 doi: 10.1165/rcmb.2009-0258OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Carter L, Fouser LA, Jussif J, Fitz L, Deng B, Wood CR, et al. PD-1:PD-L inhibitory pathway affects both CD4(+) and CD8(+) T cells and is overcome by IL-2. Eur J Immunol. 2002;32(3):634–43. doi: 10.1002/1521-4141(200203)32:3<634::AID-IMMU634>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- 77.Kinter AL, Godbout EJ, McNally JP, Sereti I, Roby GA, O'Shea MA, et al. The common gamma-chain cytokines IL-2, IL-7, IL-15, and IL-21 induce the expression of programmed death-1 and its ligands. J Immunol. 2008;181(10):6738–46. doi: 10.4049/jimmunol.181.10.6738. [DOI] [PubMed] [Google Scholar]
- 78.Dong H, Zhu G, Tamada K, Flies DB, van Deursen JM, Chen L. B7-H1 determines accumulation and deletion of intrahepatic CD8(+) T lymphocytes. Immunity. 2004;20(3):327–36. doi: 10.1016/s1074-7613(04)00050-0. [DOI] [PubMed] [Google Scholar]
- 79.Liu X, Gao JX, Wen J, Yin L, Li O, Zuo T, et al. B7DC/PDL2 promotes tumor immunity by a PD-1-independent mechanism. J Exp Med. 2003;197(12):1721–30. doi: 10.1084/jem.20022089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Shin T, Kennedy G, Gorski K, Tsuchiya H, Koseki H, Azuma M, et al. Cooperative B7-1/2 (CD80/CD86) and B7-DC costimulation of CD4+ T cells independent of the PD-1 receptor. J Exp Med. 2003;198(1):31–8. doi: 10.1084/jem.20030242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Butte MJ, Keir ME, Phamduy TB, Sharpe AH, Freeman GJ. Programmed death-1 ligand 1 interacts specifically with the B7-1 costimulatory molecule to inhibit T cell responses. Immunity. 2007;27(1):111–22. doi: 10.1016/j.immuni.2007.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Chemnitz JM, Parry RV, Nichols KE, June CH, Riley JL. SHP-1 and SHP-2 associate with immunoreceptor tyrosine-based switch motif of programmed death 1 upon primary human T cell stimulation, but only receptor ligation prevents T cell activation. J Immunol. 2004;173(2):945–54. doi: 10.4049/jimmunol.173.2.945. [DOI] [PubMed] [Google Scholar]
- 83.Freeman GJ, Wherry EJ, Ahmed R, Sharpe AH. Reinvigorating exhausted HIV-specific T cells via PD-1-PD-1 ligand blockade. J Exp Med. 2006;203(10):2223–7. doi: 10.1084/jem.20061800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Grakoui A, John Wherry E, Hanson HL, Walker C, Ahmed R. Turning on the off switch: regulation of anti-viral T cell responses in the liver by the PD-1/PD-L1 pathway. J Hepatol. 2006;45(4):468–72. doi: 10.1016/j.jhep.2006.07.009. [DOI] [PubMed] [Google Scholar]
- 85.Fukushima A, Yamaguchi T, Azuma M, Yagita H, Ueno H. Involvement of programmed death-ligand 2 (PD-L2) in the development of experimental allergic conjunctivitis in mice. Br J Ophthalmol. 2006;90(8):1040–5. doi: 10.1136/bjo.2006.091314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ritprajak P, Hashiguchi M, Tsushima F, Chalermsarp N, Azuma M. Keratinocyte-associated B7-H1 directly regulates cutaneous effector CD8(+) T cell responses. J Immunol. 184(9):4918–25. doi: 10.4049/jimmunol.0902478. [DOI] [PubMed] [Google Scholar]
- 87.Thompson RH, Dong H, Lohse CM, Leibovich BC, Blute ML, Cheville JC, et al. PD-1 is expressed by tumor-infiltrating immune cells and is associated with poor outcome for patients with renal cell carcinoma. Clin Cancer Res. 2007;13(6):1757–61. doi: 10.1158/1078-0432.CCR-06-2599. [DOI] [PubMed] [Google Scholar]


