Abstract
Stathmin/oncoprotein 18, a protein that regulates microtubule dynamics, is highly expressed in a number of tumors including leukemia, lymphoma, neuroblastoma, breast, ovarian, and prostate cancers. High stathmin levels have been associated with the development of resistance to the widely-used anticancer drug taxol (®Taxol, paclitaxel). The mechanisms of stathmin-mediated taxol resistance are not well-understood at the molecular level. To better understand the role of stathmin in taxol resistance, we stably overexpressed stathmin two-fold in BT549 human breast cancer cells and characterized several cell processes involved in the mechanism of action of taxol. After stable overexpression of stathmin, neither the cell doubling time nor the mitotic index was altered and the microtubule polymer mass was reduced only modestly (by 18%). Unexpectedly, microtubule dynamicity was reduced by 29% after stathmin overexpression, resulting primarily from reduction in the catastrophe frequency. Sensitivity to taxol was reduced significantly (by 44%) in a clonogenic assay, and stathmin appeared to protect the cells from the spindle-damaging effects of taxol. The results suggest that in the stably-stathmin overexpressing clones, compensatory gene expression occurred that resulted in normal rates of cell proliferation and prevented the increase in catastrophe frequency expected in response to stathmin. Stathmin overexpression protected the cells from taxol-induced abnormal mitoses, and thus induced taxol resistance. Using offgel IEF/PAGE difference gel electrophoresis, we identified a number of proteins whose expression is reduced in the taxol-resistant stathmin-overexpressing cell lines, including proteins involved in the cytoskeleton and cell structure, the stress response, protein folding, glycolysis, and catalysis.
Keywords: stathmin, taxol, microtubule dynamics, proteomics, mitosis, resistance
INTRODUCTION
The effectiveness of taxol in treating breast cancer is often limited due to inherent or developed taxol resistance[1]. Overexpression of stathmin (a.k.a. oncoprotein 18) in different cancers is associated with resistance to taxol treatment [1]. Stathmin regulates microtubule dynamics [2] by its tubulin sequestering and microtubule catastrophe-promoting activities [3–8]. At the mitotic phase of the cell cycle, stathmin is inactivated by phosphorylation, thus promoting mitotic spindle assembly and cell division. In carcinoma cells, overexpression of stathmin leads to decreased taxol sensitivity [9]. The mechanisms responsible for stathmin-mediated taxol resistance are not well-understood at the molecular level.
We stably overexpressed stathmin in BT549 breast cancer cells with the idea that stable rather than transient overexpression might better reveal the physiologically relevant protein alterations that accompany the increased stathmin expression associated with resistance to taxol. Here we have characterized taxol’s effects on several processes in these cells that are involved in its mechanism of action and examined associated gene expression by quantitative proteomic analysis. First, we characterized the effects of stathmin overexpression on cell proliferation, mitotic index, microtubule polymer mass and dynamic instability, and sensitivity to taxol, including the frequency of abnormal mitotic spindles. Second, we developed an enhanced approach for difference gel electrophoresis (DIGE) using Offgel isoelectric focusing of high starting protein amounts followed by one dimensional SDS PAGE to improve the depth of proteome coverage. Quantitative proteomic analysis has been successful in identifying proteins and their relative expression changes. Central to the success of the quantification in yielding mechanistically insightful biological changes is, (1) proper development of the proteomic analysis model to study system-wide changes associated with misexpression of a protein, and (2) extending proteome coverage to include low abundant proteins.
Two-dimensional difference gel electrophoresis (2D-DIGE, [10]) overcomes some of the drawbacks of traditional two dimensional gels such as run-to-run variability and provides improved dynamic range and sensitivity with the use of electrophilic versions of fluorescent cyanine dyes (Cy3 and Cy5) for quantitation. Both of these two dimensional techniques are, however, limited to protein amounts of ≤ 500 μg in order to achieve optimal separation of the proteins. Low abundance proteins are thus missed due to the dynamic range of protein abundances. Although protein enrichment or fractionation can allow study of low abundance proteins, these strategies do not permit a global, unbiased analysis of the proteome changes. We present a strategy that combines the benefits of DIGE with the use of offgel protein fractionation to study quantitative protein changes associated with stathmin-mediated taxol resistance.
MATERIALS AND METHODS
Vector construction, cell culture and reagents
To generate stathmin overexpressing cell lines, the full length human stathmin gene was subcloned into a pcDNA3.1-myc, his vector (Invitrogen) (pcDNA3.1-stat-myc, his). The empty vector was used as the stathmin overexpression control (pcDNA3.1-myc, his). BT549 human breast epithelial cancer cells (obtained from ATCC, American Type Culture Collection) were cultured in RPMI-1640 medium (Sigma-Aldrich, St. Louis, MO) supplemented with 10% fetal bovine serum (Hyclone, Logan, UT) and 0.1% penicillin/streptomycin at 37°C in a humidified 5% CO2 environment. The cells were transfected with one of the vectors, either pcDNA3.1-stat-myc, his or pcDNA3.1-myc, his, using Superfect transfection reagent (QIAGEN Inc., Valencia, CA) following the manufacturer’s instructions. For stable cell lines, colonies were selected with 0.2 mg/ml G418 (BioWhittaker, Walkersville, MD). Positive clones were selected by screening for stathmin expression by RT-PCR and Western blotting for stathmin protein. Cell lines were named as follows: stathmin overexpression cell lines are OE1 and OE2, and their empty vector control cell line is OEc.
Taxol (Drug Synthesis Branch, National Cancer Institute) was dissolved in DMSO (dimethylsulfoxide) at a concentration of 10 mM. This stock solution was stored in aliquots at −80°C until used.
For proteomic analysis, BT549 breast cancer cells that were stably-altered for stathmin levels and the controls were grown/maintained in RPMI-1640 (Invitrogen) containing 0.2mg/ml G418 (Invitrogen) supplemented with 10% fetal bovine serum (Gemini Bioproducts). Cells were treated with 20 nM taxol for 24 h. The taxol concentration was chosen because it induced a rapid and pronounced effect on the cells (18–28% mitotic arrest) in the absence of significant cell killing during a 24-h incubation. Cells were washed three times in cold Hanks buffer and stored at −80°C until use.
Unless specified otherwise, reagents were obtained from Sigma. All were of “reagent” quality or better.
RT-PCR
Expression levels of stathmin mRNA were examined by RT-PCR. First-strand cDNA was synthesized using random primers (Promega, Madison, WI) with 50 ng of total RNA, and cDNA was subsequently amplified with the following primers: stathmin forward, 5′-TCTCAGCCCTCGGTCAAAAG-3′; stathmin reverse, 5′-TCTCGTGCTCTCGTTTCTCAG-3′; GAPDH forward, 5′-TGGTATCGTGGAAGGACTCATGAC-3′; and GAPDH reverse, 5′-ATGCCAGTGAGCTTCCCGTTCAGC-3′ (N-055). GAPDH was used as internal control. PCR reactions are subjected to 20 (for stathmin) or 25 (for GAPDH) PCR cycles after denaturation (5 min at 96°C) as follows: 30 s at 96°C; annealing: 30 s at 55°C; and extension 60 s at 72°C.
Western blot analysis
For quantification of protein levels, whole cell extracts were obtained by sonication, 10 sec × 3 on ice, in cell lysis buffer (10 mM Tris, pH 7.4, containing 1 mM EDTA, 20 mM NaF, and 0.1 % NP-40 and protease inhibitor cocktail (complete Mini, EDTA-free, Roche)). After centrifugation at 13,000 rpm at 4°C for 20 min, cleared lysate samples were obtained. Samples were separated in 12.5 % acrylamide SDS-PAGE gels followed by transfer to PVDF membrane (Immobilon-Psq, Millipore, Billerica, MA). The following antibodies were used for immunoblotting: rabbit anti-stathmin polyclonal antibody (1:20,000, Calbiochem, San Diego, CA), or mouse anti-myc monoclonal antibody (1:1,000, Invitrogen, Carlsbad, CA). Exogenous stathmin was detected only by the anti-myc antibody because it was tagged with myc at the C-terminus. GAPDH detected by mouse anti-GAPDH monoclonal antibody (1:3,000, 6C5, Abcam, Cambridge, MA) was used as an internal control. The bound antibodies were visualized by chemiluminescence (ECL; Amersham Pharmacia Biotech, Piscataway, NJ). For determination of number of phosphorylated sites, cell lysates were separated by native-PAGE, and stathmin was detected by immunoblot with anti-stathmin antibody.
Mitotic arrest and immunocytochemistry
Cells were fixed with 10% formalin in PBS for 30 min at room temperature. After incubation with blocking buffer (20 mM tris(hydroxymethyl)aminomethane containing 150 mM NaCl, 2% bovine serum albumin (BSA) and 0.1% TritonX-100) for 1 h at room temperature, cells were incubated with mouse anti-α-tubulin monoclonal antibody (1:1,000, DM1A, Sigma) at 4°C for overnight followed by Cy3 donkey anti-mouse IgG (H+L) (1:1,000, Jackson Immunolaboratories, Westgrove, PA) for 1 h at room temperature. Nuclei were stained with 4′,6-diamidino-2-phenylindole (DAPI). Mitotic cells were counted by fluorescence microscopic examination.
To determine the percentages of mitotic cells, 1,000–1,500 interphase and mitotic cells were counted. To determine the percentages of abnormal mitotic cells, at least 100 mitotic cells were counted. Abnormal mitotic cells included those with multipolar mitotic spindles, irregular uncongressed chromosome arrangements, and cells undergoing cytokinesis into three daughter cells.
Cell proliferation
Cell proliferation was determined in the absence and presence of taxol by two methods. In the short-term assay, cells were seeded into 96-well plates at the density of 3,000 cells/well. Twenty-four hours after seeding, cells were incubated with taxol for up to 72 h at concentrations of 0, 2 and 4 nM. The taxol concentration range included the concentrations that inhibited proliferation by 50% in all three cell lines (see Results). Cellular protein was determined using a sulforhodamine B (SRB) assay [11]. In brief, cells were fixed every 24 h for 72 h by addition of 50 μl of 50% cold trichloroacetic acid and incubated at 4°C for 1 h. After washing with water, cells were stained with SRB solution (0.4% (w/v) of SRB containing 1% acetic acid) at room temperature for 30 min, then washed with 1 % acetic acid and lysed with 100 μl of 10 mM unbuffered tris(hydroxymethyl)aminomethane, pH 10.5. Optical density (OD) was read at 490 nm using a 1420 Multilabel Counter, VICTOR3V (PerkinElmer, Shelton, CT). The cell growth was determined by plotting the OD versus duration of taxol incubation, and the growth inhibition by taxol was calculated as percent inhibition of growth in the absence of taxol.
To measure the long-term effects of taxol on cell growth after taxol removal, cells were seeded in 24-well plates at a density of 150 cells/well. One day later, taxol was added at a range of concentrations (0.50 – 4 nM). The next day, the medium was replaced with medium lacking taxol, and incubation was continued for 7–10 days. Cells were stained with crystal violet, lysed in 50% ethanol containing 1 % acetic acid, and then the absorbance of the cells at 595 nm was determined on the plate using a Victor3V multilabel plate reader.
Microtubule dynamic instability in cells
Microtubule dynamics experiments were performed as previously described with modifications [12]. Briefly, cells were seeded into 4-well plates (Nunc) at a density of 8–10 × 103 cells/well onto poly-L-lysine (Sigma)-treated glass cover slips. To visualize intracellular microtubules, the cells were microinjected with 0.1 μg/μl of enhanced green fluorescence protein (EGFP)-tagged tubulin expression vector (Clontech, Mountain View, CA) 24 h after the seeding. Twenty-four hours after microinjection, cells were incubated in culture medium containing a reduced concentration of fetal bovine serum (2%) to promote cell flattening with or without taxol (2 nM), for another 24 h. For imaging, the glass cover slips with attached cells were transferred to recording medium (RPMI-1640 medium without phenol red (Sigma-Aldrich) and without taxol, containing Oxyrase (1:33, Oxyrase, Inc., Mansfield, OH)). Microtubules were visualized and recorded by fluorescence microscopy for 30 min to 1 h at 37°C.
The positions of the plus ends of microtubules over time were tracked using MetaMorph 5.0 imaging software (Universal Imaging Corp., Media, PA), and analyzed using Real Time Measurement software (a kind gift of Neal Gliksman and E. D. Salmon, University of North Carolina, Chapel Hill, NC). Changes in the length of individual microtubules were graphed as a function of time. Individual growth and shortening rates were determined by linear regression. Changes of ≥ 0.5 μm were considered growth or shortening events. Changes in length of < 0.5 μm were considered to be periods of attenuated dynamics or pause. Only microtubules undergoing both catastrophe and rescue phenomena were used in calculating transition frequencies. Dynamicity is the length grown and shortened divided by the total life span of the microtubule. Results are shown as the mean and standard deviation (SD), and an unpaired t test was used to make comparisons.
DIGE - protein offgel isoelectric focusing/sodium dodecyl sulfate polyacrylamide gel electrophoresis (DIGE-Offgel IEF/SDS PAGE)
Cells were lysed by brief sonication in 40 mM Tris, pH 9.0, containing protease inhibitor cocktail (Invitrogen) and benzonase, followed by acetone-induced precipitation of the proteins. The proteins were solubilized in rehydration buffer (8M urea, 2M thiourea, 4% CHAPS 3-[(3-chloamidopropyl)dimethylamonio]-1-propanesulfonate, 20 mM dithiothreitol, and 0.2% ampholyte). DIGE labeling was carried out with 1 mg of protein per label using electrophilic fluorescent dyes, PrCy3-N-hydroxysuccinimide ester (Cy3) and MeCy5-N-hydroxysuccinimide ester (Cy5) as described previously [13]. Pair wise comparisons were carried out with OEc vs OEc taxol, OE1 vs OE1 taxol, OE2 vs OE2 taxol. Labeled samples were pooled and fractionated by Offgel isoelectric focusing using 24 cm, 3–10L IPG strips into 24 fractions (Agilent 3100 OffGel Fractionator, GE Healthcare). From each fraction, 50 μg of protein was loaded on 1D gel lanes for separation by SDS PAGE.
Densitometry
Gel images were acquired with a custom built CCD camera at excitation wavelengths 545 nm and 635 nm, respectively, for Cy3- and Cy5-labeled protein samples [13]. Images were viewed with Image J (NIH, MD, USA). Raw TIFF images were brought into ImageJ where the background within each pair was subtracted with equal rolling ball radii. All lanes were selected, plotted, and lines were drawn at the top of the peaks to set the background level. The area under the curve was measured to give the relative intensities of the desired bands.
Gel imaging/band picking/in-gel tryptic digests
The 1D SDS-PAGE gels were stained with BioRad Sypro Ruby Protein Gel Stain according to the manufacturer’s protocol. Gel imaging was done on a Digilab ProPic II. In order to maximize the amount of protein used in the in-gel digests, the spot picker on the ProPic was only used to mark the position of the gel bands chosen by the DIGE analysis. Each chosen gel band was then manually excised in its entirety with a razor blade and cut into 1-mm2 pieces and placed into an individual well of a 96-well plate. The Intavis Digest Pro robot was used for high-throughput in-gel trypsin digestion. After dehydrating the gel pieces, 40 μl of porcine trypsin (20 ng/ul in 25 mM ammonium bicarbonate) was added to each well in the Intavis Digest Pro robot. After rehydrating the gel pieces at room temperature for 1 h, the 96-well plate was incubated at 37 °C for 8 h. The supernatant of the digests and a 20 μl rinse with 25 mM ammonium bicarbonate were then transferred to another 96-well plate. The digests were concentrated to 10 μl on a speed-vac. A 0.5 μl aliquot of the concentrated supernatant of each sample was spotted onto an ABI Opti-TOF 384 MALDI plate insert. The samples were allowed to air dry. A 0.5 μl aliquot of CHCA matrix (10 mg/ml in 1:1 acetonitrile:0.1% aqueous formic acid) was subsequently spotted on top of the analyte droplet and also allowed to air dry.
MALDI-TOF-MS and -TOF/TOF-MS/MS analysis
Peptide mass fingerprinting analysis was done by MALDI-TOF-MS analysis on an ABI 4800 MALDI-TOF/TOF-MS/MS Analyzer (Applied Biosystems, Foster City, CA). Positive ion mass spectra were acquired in MS reflector mode with 1000 laser shots per spectrum. Mass calibration used the tryptic autolysis peptide signal at m/z 842 as an internal calibrant. Six precursor ions with the highest intensity and an m/z value greater than 1000 were selected for MALDI-TOF/TOF-MS/MS analyses. Tandem mass spectra were acquired using 2kV collision energy with 3000 laser shots per spectrum.
Data analysis for MALDI-TOF-MS and -TOF/TOF-MS/MS spectra
Analysis of the spectra from MALDI-TOF-MS and -TOF/TOF-MS/MS spectra was performed with Protein Pilot 3.0. Database searching used the Mascot program (version 2.1.0) using an IPI human database (version 3.48, 71401 sequences, 30194169 residues). The search parameters used trypsin as the proteolytic enzyme with one missed cleavage permitted, oxidation of methionines as a variable modification, and mass tolerance of 50 ppm and 0.4 Da for precursor ions and fragment ions respectively.
NanoLC-ESI-MS/MS analysis
All digests from gel bands that did not give high confidence protein identifications were analyzed by nanoLC-ESI-MS/MS on a LCQ Deca XP Plus (Thermo) quadrupolar ion trap mass spectrometer. The LC system (Surveyor, Thermo) consisted of a sample trap followed by a C18 column (BioBasic C18 PicoFrit column, 10 cm × 75μm, New Objective, Inc., Woburn, MA). The elution gradient was formed with solvent A (water with 0.1% formic acid) and solvent B (acetonitrile with 0.1% formic acid). The elution was performed with a linear increase from 5% to 50% solvent B in 25 min, further linear increase from 50% to 90% solvent B for 5 min, linear reduction to 5% solvent B in 5 min, then isocratic at 5% B for 10 min. Flow rate of the system was 160 nl/min. A full MS scan was done (m/z 300–2000) followed by three MS/MS scans on the three most intense peaks with dynamic exclusion. Data was analyzed with Bioworks 3.2 Browser with the Sequest search engine. The search used IPI human database, indexed for a trypsin digest, two missed cleavages, and two modifications (oxidation of methionines, carbamidomethylation). The search results that were accepted contained cross-correlation scores (XCorr) for singly charged peptides > 1.5, doubly charged peptides >2.0 and triply charged peptides >3.0.
RESULTS
Characterization of stathmin-overexpressing BT549 clones
Three clones of BT549 cells stably overexpressing either myc-tagged stathmin (overexpression clones 1 and 2, termed OE1 and OE2) or the parental BT549 empty vector control (termed OEc) were constructed, and viable clones overexpressing stathmin were selected and expanded. Stathmin mRNA levels were increased more than two-fold in OE1 and OE2 cell lines as compared with the control OEc cell line as shown by RT-PCR in Fig. 1A. Increased stathmin protein levels in OE1 and OE2 were visualized by anti-myc antibody staining since the myc-tag of the overexpressed stathmin obscured the stathmin epitope (Fig. 1B). The growth rates of the overexpressing cell lines were very similar to those of the control cell line (doubling times were OEc, 23.4 ± 0.2 h; OE1, 21.9 ± 1.1 h; and OE2, 26.5 ± 0.5 h)
Fig. 1. Stathmin mRNA (A), and protein (B) levels, in the stable BT549 transfectants.
A) mRNA levels determined by RT-PCR indicate that stathmin mRNA levels were increased more than 2-fold in OE1 and OE2 as compared with the control OEc. B) Stathmin protein expression levels as determined by western blotting using stathmin antibody to determine the levels of non-myc-tagged stathmin and myc antibody to determine the levels of transfected myc-tagged stathmin (Materials and Methods). The myc antibody signal represents the additional stathmin expression, which was not capable of being visualized by staining with stathmin antibody.
Microtubule polymer levels were measured by lysing cells into a taxol-containing microtubule stabilizing buffer, centrifuging the lysate, and analyzing the tubulin content of the pellets and supernatants by SDS-PAGE and immunoblotting. Polymerized tubulin comprised 43% of total tubulin in the control and was only modestly reduced (by 18%, to 34% of total tubulin, Fig. 2) after stathmin overexpression. All three cell lines were relatively similar in appearance; interphase cells were well spread and sometimes assumed a somewhat elongated shape (data not shown).
Fig. 2. The percentage of tubulin in the form of polymerized microtubules was decreased by 18% after stable stathmin overexpression.
Cells were lysed into taxol containing lysis buffer and incubated at 37 °C for 5 min (Materials and Methods). Polymerized tubulin was sedimented by centrifugation. Supernatants containing soluble tubulin (S) and pellets containing polymerized tubulin (P) were separated and analyzed by SDS-PAGE followed by immunoblotting with anti-α-tubulin antibody (A).
Stable stathmin overexpression suppressed microtubule dynamics in living BT549 cells
To determine the effects of stable stathmin overexpression on microtubule dynamics, OEc and OE1 cells were microinjected with fluorescent, EGFP-tagged tubulin and imaged using time-lapse low light level fluorescence microscopy (Materials and Methods). As in other cell types, microtubules alternated between phases of growing, shortening, and pause (a state of attenuated dynamic instability). Life history plots of the changes in length of individual microtubules in the thin lamellar edge of cells during interphase were recorded (data not shown) and analyzed to determine the parameters of dynamic instability (Table 1).
Table 1.
Effects of stable stathmin overexpression on microtubule dynamic instability in BT549 cells.
| Parameter | OEc (control) | OE1 (stathmin overexpression) |
|---|---|---|
| Growth Rate (μm/min) | 18.1 ± 7.6 | 17.3 ± 10.0 |
| Shortening Rate (μm/min) | 43.0 ± 14.9 | 39.4 ± 16.6 |
| Catastrophe frequency (/cell)(/min) | 1.82 ± 0.45 | 1.01 ± 0.50** |
| “ (/μm) | 0.24 ± 0.10 | 0.11 ± 0.07* |
| Rescue frequency (/cell)(/min) | 3.40 ± 1.56 | 3.04 ± 1.58 |
| “ (/μm) | 0.07 ± 0.04 | 0.08 ± 0.06 |
| Percentage of time in growth | 34.0 % | 33.9 % |
| Percentage of time in shortening | 20.9 % | 15.1 % |
| Percentage of time in pause | 45.0 % | 51.0 % |
| Dynamicity (μm/min) | 17.5 ± 3.6 | 12.5 ± 3.9* |
Values shown are mean ± S.D. OEc, control cell line, OE1, stathmin overexpression cell line. Statistical significance, unpaired t-test.
p < 0.05,
p < 0.01.
Between 28 and 31 microtubules from 7–10 cells were measured for each cell type.
Plus ends of microtubules in control BT549 OEc cells shortened at 43.0 ± 14.9 μm/min, faster than their mean growing rate of 18.1 ± 7.6 μm/min (Table 1). In the OE1 stathmin overexpressing cells, the rates of shortening and growth were not significantly different from these values. Similarly the frequency of rescue from a shortening event (the rescue frequency) did not differ significantly between OEc and OE1. Interestingly, the frequency of catastrophe was 45% lower after stable stathmin overexpression (OEc, 1.82 ± 0.45/min compared with OE1, 1.01 ± 0.50/min). When measured on the basis of length grown prior to a catastrophe, the catastrophe frequency was 54% lower after stathmin overexpression (OEc, 0.24 ± 0.10/μm, and OE1, 0.11 ± 0.07/μm). The reduction in catastrophe frequency combined with a slight increase in the percentage of time attenuated (13%), resulted in reduction in the overall microtubule dynamicity of 29% in the stathmin overexpressing cells.
Stable stathmin overexpression induced taxol resistance
We assessed sensitivity to taxol by two methods, a 72 h SRB proliferation assay and a long-term taxol-washout clonogenic assay. In the first assay, cells were cultured in the continuous presence of 0–4 nM taxol for 72 h, and proliferation was measured by SRB assay after 24, 48 and 72 h (Fig. 3 top). Taxol inhibited cell proliferation in all three cell lines in a concentration-dependent manner. Cell growth was inhibited by 30% by 2 nM taxol (72 h) in both stathmin overexpressing cell lines as compared with 37% inhibition in the parental control OEc (IC50’s: OEc, 2.6 nM compared with OE1, 2.9 nM; OE2, 3.0 nM). Thus, stable stathmin overexpression induced only a modest taxol resistance, if any (13% change), when tested in the short-term 72 h assay.
Fig. 3. The effects of taxol on the proliferation of BT549 cells stably overexpressing stathmin (OE1 and OE2) and their control (OEc).

(Upper panels) Cells were grown in the absence or presence (2 nM and 4 nM) of taxol, collected and stained by SRB at one, two, or three days after adding taxol. The percent inhibition after 3 days incubation with 2 nM taxol was OEc, 37%; OE1, 30%; and OE2, 30%. At 4 nM the percent inhibition was OEc, 70%; OE1, 67%; and OE2, 63%. Results are mean and standard deviation. (Lower panel) Cell survival was determined by long-term clonogenic assay (Materials and Methods). Open bars, control; filled bars, OE1; hatched bars, OE2. Results are mean and standard deviation, * p< 0.05, ** p < 0.01, ***, p< 0.001. Unpaired t-test.
The long-term, clonogenic assay indicated a more significant taxol resistance (Fig. 3, bottom panel). Cell survival was measured after 24 h of incubation with a range of taxol concentrations (0 – 4 nM) and subsequent removal of medium and continued culture in drug-free medium for 7–10 days. Taxol (0.5 nM) inhibited survival of the parental cells by 45% (open bars), whereas survival of OE1 and OE2 was inhibited by only 25% and 7% (filled bars and hatched bars, respectively), indicating taxol resistance at this concentration. The two stathmin-overexpressing cells lines differed in their resistance to taxol. At higher taxol concentrations (3 and 4 nM), OE1 did not exhibit resistance to taxol, For example, at 3 nM taxol, OEc exhibited 94% inhibition of survival compared with 92% inhibition of OE1, whereas OE2 was resistant and 3 nM taxol inhibited survival by only 74%). The IC50’s were higher with stathmin overexpression (IC50’s: OEc, 0.9 nM taxol, compared with OE1, 1.1 nM taxol, and OE2, 1.5 nM taxol), representing an average increase of 44% in taxol IC50 after stable stathmin overexpression. Thus, stable stathmin overexpression induced significant taxol resistance that varied with the cell line.
Effects of altered stathmin levels on mitotic index and taxol-induced mitotic abnormalities
In OEc, OE1 and OE2, the mitotic indices in the absence of taxol were similar, ranging from 2.9% to 3.8% (Fig. 4A). Taxol (2 nM) increased mitotic accumulation to 7.3% in the control cells whereas no additional mitotic accumulation occurred in OE1 (3.1%) and accumulation was similar to parental controls in OE2 (7.8%). However, after incubation with 20 nM taxol, mitotic accumulation was higher in all three cell lines, but was significantly higher in control than in the overexpressing cell lines (OEc, 28%; OE, 13%; OE2, 17%).
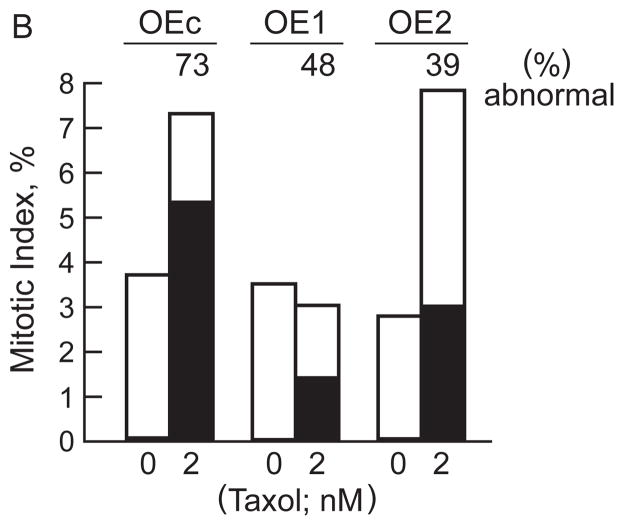
Fig. 4. Overexpression of stathmin reduced both mitotic arrest and spindle abnormalities induced by taxol.
A) The percent of all cells that were mitotic after 24 h incubation with or without taxol is shown (0 nM, clear bars; 2 nM, black bars; and 20 nM, hatched bars). Results represent the mean and S.D. of three independent experiments in which 1000–1500 cells were counted from 2–3 samples. B) The height of the bars represents the percentage of total cells that were mitotic (including prometaphase through cytokinesis) with and without incubation with 2 nM taxol, and the filled portion of the bars represents the fraction of those mitotic cells that were abnormal in that they had multipolar spindles, irregular chromosome arrangement (lagging chromosomes), or divided into three daughter cells. The number above the bars indicates the percentages of mitotic cells that were abnormal.
In many cell types, taxol induces formation of abnormal spindles in association with mitotic arrest and multinucleate interphase cells [14]. Even in the absence of taxol, nearly all spindles in all three cell lines were normal and bipolar with a fully congressed metaphase plate of chromosomes. Taxol did, however, induce two kinds of abnormal spindles in both the control and stathmin-overexpressing cell lines; these consisted primarily of abnormal tripolar spindles that resulted in tripartite division of cells during cytokinesis and also of spindles that were bipolar but contained uncongressed chromosomes located at the spindle poles. Interestingly, both stathmin-overexpressing cell lines showed a significantly reduced incidence of spindle abnormalities compared with the controls. In OEc, 73% of spindles were abnormal after incubation with 2 nM taxol whereas in OE1 and OE2 only 48% and 39% of spindles were abnormal, respectively, as shown by the filled portions of the bars (Fig. 4B). These results suggest that stathmin overexpression results in protection against taxol-induced abnormalities. Thus, stathmin may increase taxol resistance, at least in part, by reducing both mitotic abnormalities and mitotic arrest.
Differential expression of proteins with taxol treatment
We used DIGE Offgel IEF/SDS PAGE to study protein level changes associated with taxol treatment of BT549 cell lines with stable overexpression of stathmin. PrCy3OSu was used to label the proteins extracted from BT549 cells, while MeCy5OSu was used to label proteins from taxol-treated cells (Table 2). In total, 2 mg of dye-labeled, pooled protein samples were separated by isoelectric focusing using an Agilent 3000 Offgel Fractionator to produce 24 fractions. Each of these fractions was separated by one dimensional SDS-PAGE. Image analysis gave band volume ratios corresponding to changes in protein abundance levels. These bands corresponded to large differences in intensity between Cy3 and Cy5 (>1000 units) or missing bands, where a band may be present in the Cy3 filter but not under Cy5, or vice versa (Table 2). In OE comparisons, sixty-two bands respectively showed a change in intensity due to taxol treatment. Robotic spot excision and digestion with trypsin allowed high throughput peptide mass fingerprinting analysis and was performed on differentially expressed bands from OE comparisons (combinations D, E and F) for the present study. If a confident protein identification was not established by MALDI-TOF-MS and -TOF/TOF-MS/MS, an aliquot was analyzed by nanoLC-ESI-MS/MS. The identified proteins in Table 2 represent proteins that decreased in expression from taxol treatment. We estimated thirty unique proteins to be differentially regulated by accounting for presence of a particular protein in more than one band due to protein isoform redundancy, post-translational modifications and proteins shared between the three pairs of OE comparisons. They were categorized as shown in the Venn diagram (Fig. 5).
Table 2. List of proteins reduced in levels after taxol (T) treatment in stathmin-overexpressing BT549 cell lines, OE1 and OE2, vs. their vector control cells, OEc.
The table contains proteins identified with high confidence scores using Mascot and/or Sequest database searches. “Best” denotes the protein to which the best statistical match was made. Numerical scores listed are from Mascot, while ‘S’ is used to denote Sequest scores that met with criteria for high confidence identifications as described in methods. More than one protein was identified from some of the gel bands (denoted by an asterisk), and this is noted in the protein description column; thus, the detected quantitative changes could be due to altered levels in one or more proteins present.
| Cell Line Comparison | Best Protein Accession | Best Protein Mass | Mascot Protein Score | Best Protein Description | Fold Intensity Change (Underexpression in Taxol-Treated Cells) |
|---|---|---|---|---|---|
| OEc vs. OEc+T | IPI00216298 | 11730 | 63 | TXN (Thioredoxin) | * |
| IPI00003362.2 | 72422 | S | HSPA5 (HSPA5 protein) | 2.5 | |
| IPI00418471 | 53619 | 620 | VIM (Vimentin) | 1.5 | |
| IPI00003865 | 70854 | 298 | HSPA8 (isoform 1 of Heat Shock Cognate 71 kDa protein) | 2.3 | |
| IPI00007765 | 73635 | 78 | HSPA9 (Stress-70 protein, mitochondrial) | 2.6 | |
| IPI00307162.2 | 123799 | 86 | VCL (isoform 2 of Vinculin) | * | |
| IPI00290770.3 | 60463 | S | CCT3 (Chaperonin Containing TCP1, subunit 3 isoform b) | * | |
| IPI00218918 | 38690 | 94 | ANXA1 (Annexin A1) | * | |
| IPI00465248 | 47139 | 254 | ENO1 (isoform 1 of Enolase, aka α-Enolase) | 1.9 | |
| IPI00419585.9 | 18012 | S | PPIA (Peptidyl-Prolyl cis-trans Isomerase A); also, Histone H2B | * | |
| IPI00418169.3 | 40411 | S | ANXA2 (Annexin A2 isoform 1) | * | |
| IPI00012011.6 | 18502 | S | CFL1 (Cofilin-1) | * | |
| IPI00877002 | 17960 | 102 | PPIA (Peptidyl-Prolyl cis-trans Isomerase A) | * | |
| IPI00216691.5 | 15054 | S | PFN1 (Profilin-1) | * | |
| IPI00012011.6 | 18502 | S | CFL1 (Cofilin-1) | * | |
| IPI00419585.9 | 18012 | S | PPIA (Peptidyl-Prolyl cis-trans Isomerase A) | * | |
| IPI00220362.5 | 10932 | S | HSPE1 (10 kDa Heat Shock Protein, mitochondrial) | * | |
| IPI00465439 | 39395 | 201 | ALDOA (Fructose-Bisphosphate Aldolase A) | 2 | |
| IPI00797221 | 28649 | 205 | GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) | 2.1 | |
| Q53HN8_HUMAN | 38564 | 66 | Annexin A2 (isoform 2 variant, fragment); also, LDHA (L-Lactate Dehydrogenase A chain); also, VDAC1 (Voltage Dependent Anion-selective Channel protein 1) | * | |
| IPI00640741 | 18964 | 107 | PRDX1 (Peroxiredoxin 1) | * | |
| IPI00012011.6 | 18502 | S | CFL1 (Cofilin-1) | * | |
| IPI00216691.5 | 15054 | S | PFN1 (Profilin-1); also, PRDX5 (Peroxiredoxin 5) | * | |
| IPI00220362.5 | 10932 | S | HSPE1 (10 kDa Heat Shock Protein, mitochondrial) | * | |
| IPI00217966.8 | 39837 | S | LDHA (cDNA FLJ55792, highly similar to L-Lactate Dehydrogenase A chain) | * | |
| IPI00465439 | 39395 | 301 | ALDOA (Fructose-Bisphosphate Aldolase A) | 2.1 | |
| IPI00219018 | 36030 | 187 | GAPDH (Glyceraldehyde-3-Phosphate Dehydrogenase) | 1.9 | |
| IPI00414696 | 35984 | 109 | HNRNPA2B1 (isoform A2 of Heterogeneous Nuclear Ribonucleoproteins A2/B1) | * | |
| IPI00479306.1; IPI00550363.3 |
28480; 22391 |
S | PSMB5 (Proteasome Subunit β type-5); also, TAGLN2 (Transgelin-2) | * | |
| IPI00012011.6 | 18502 | S | CFL1 (Cofilin-1) | * | |
| IPI00216691.5 | 15054 | S | PFN1 (Profilin-1) | * | |
| IPI00218918.5 | 38714 | S | ANXA1 (Annexin A1); also, HSPE1 (10 kDa Heat Shock Protein, mitochondrial) | * | |
| IPI00220362.5 | 10932 | S | HSPE1 (10 kDa Heat Shock Protein, mitochondrial) | * | |
| IPI00646304 | 23728 | 132 | PPIB (Peptidyl-Prolyl Isomerase B precursor) | * | |
| IPI00216691 | 15045 | 102 | PFN1 (Profilin-1) | 5.7 | |
| OE1 vs. OE1+T | IPI00872379 | 35783 | 64 | ANXA5 (Annexin A5 fragment) | * |
| IPI00000811.2 | 25358 | S | PSMB6 (Proteasome Subunit β type-6) | * | |
| IPI00376005.2 | 20170 | S | EIF5A (isoform 2 of Eukaryotic Translation Initiation Factor 5A-1) | * | |
| IPI00025512 | 22768 | 356 | HSPB1 (Heat Shock Protein β-1) | * | |
| IPI00008530 | 34252 | 329 | RPLP0 (60S acidic Ribosomal Protein P0) | * | |
| IPI00025512 | 22768 | 383 | HSPB1 (Heat Shock Protein β-1) | * | |
| IPI00025512 | 22768 | 465 | HSPB1 (Heat Shock Protein β-1) | * | |
| IPI00026964.2 | 29668 | S | UQCRFS1 (Cytochrome b-c1 complex subunit Rieske, mitochondrial) | * | |
| IPI00465028 | 30772 | 119 | TPI1 (isoform 1 of Triosephosphate Isomerase | * | |
| IPI00455315 | 38580 | 607 | ANXA2 (Annexin A2) | * | |
| IPI00455315 | 38580 | 512 | ANXA2 (Annexin A2) | * | |
| IPI00220301 | 25019 | 120 | PRDX6 (Peroxiredoxin-6) | * | |
| IPI00647915 | 24438 | 260 | TAGLN2 (Transgelin-2) | * | |
| IPI00006935.3 | 16793 | S | EIF5A2 (Eukaryotic Translation Initiation Factor 5A-2) | * | |
| IPI00000874.1, IPI00640741.1, IPI00550363.3, IPI00025019.3 | 22110 | S | PRDX1 (Peroxiredoxin-1); also, TAGLN2 (Transgelin-2); also, PEBP1 (Phosphatidylethanolamine-Binding Protein 1); also, PSMB1 (Proteasome subunit β type-1) | * | |
| IPI00418471 | 53619 | 296 | VIM (Vimentin) | * | |
| OE2 vs OE2+T | IPI00179953 | 85186 | 75 | NASP (isoform 1 of Nuclear Autoantigenic Sperm Protein) | * |
| IPI00784295 | 84607 | 125 | HSP90AA1 (isoform 1 of Heat Shock Protein 90-α | ||
| IPI00465028 | 30772 | 270 | TPI1 (isoform 1 of Triosephosphate Isomerase) | * | |
| IPI00000874.1 | 22110 | S | PRDX1 (Peroxiredoxin-1) | * | |
| IPI00465028 | 30772 | 73 | TPI1 (isoform 1 of Triosephosphate isomerase) | * | |
| IPI00012011 | 18491 | 230 | CFL1 (Cofilin-1) | * | |
| IPI00419585 | 18001 | 220 | PPIA (Peptidyl-Prolyl cis-trans Isomerase A) | * | |
| IPI00216691 | 15045 | 256 | PFN1 (Profilin-1) | * | |
| IPI00012011 | 18491 | 174 | CFL1 (Cofilin-1) | * | |
| IPI00419585 | 18001 | 188 | PPIA (Peptidyl-Prolyl cis-trans Isomerase A) | * | |
| IPI00747319.1 | 23412 | S | MXRA7 (Matrix-Remodelling Associated protein 7); also, ITGA5 (Integrin α-5) | * | |
| IPI00012011 | 18491 | 184 | CFL1 (Cofilin-1) | * |
Fig. 5.

Venn diagram showing the Gene Ontology (GO) terms associated with the proteins found to have altered levels after taxol (T) treatment in stathmin-overexpressing BT549 cell lines, OE1 and OE2, vs. their vector control cells, OEc.
The entire list of proteins identified to be down-regulated with taxol treatment was imported into ProteinCenter (version 3.0, Proxeon) for annotation with Gene Ontology (GO) terms and statistical analysis. Comparison statistics (<5% false discovery rate) for the functional terms in the GO categories for cellular components, molecular functions and biological processes was obtained by comparison of the stathmin dataset with the reference International Protein Index (IPI) human database. This showed that the GO molecular function terms protein binding (34 proteins in the stathmin dataset), antioxidant activity (5), phospholipase inhibitor activity (3) and catalytic activity (28) were overrepresented. The term cytoplasm was overrepresented in the GO cellular component category. In GO biological processes, glycolysis (7), response to chemical stimulus (15), and protein folding (7) were some of the overrepresented terms. The overrepresentation of certain GO terms provides an overview of the functional categories affected in the taxol-treated cells.
It was not unexpected that some of the LC-MS analyses showed more than one high-confidence protein identification within a gel band. As with other discovery based proteomics techniques, the protein identifications from the Offgel DIGE approach require validation for quantitative differences in expression using additional methods.
DISCUSSION
To examine the effects of tumor stathmin levels on sensitivity or resistance to the antitumor drug taxol, we developed viable clones of BT549 cells that stably express increased levels of the microtubule-regulatory protein stathmin. We characterized the resulting alterations in cell proliferation, microtubule behavior, mitosis, and taxol resistance, as well as the accompanying changes in gene expression. Stably increasing stathmin levels by more than two-fold induced no significant changes in the rate of cell proliferation and no significant mitotic arrest. In contrast, in a study of the effects of transient rather than stable stathmin overexpression, Marklund et al. [15] found that the growth rate of K562 cells was markedly reduced (by approximately 80%) but, similar to our findings, these cells also showed almost no alteration in cell cycle distribution. The results suggest that in the stable stathmin-overexpressing BT549 clones, compensatory gene expression or protein modifications occurred that promoted normal rates of cell proliferation. Thus, despite the observation that highly proliferative or malignant tumors frequently overexpress stathmin [17,18], our results suggest that increased stathmin expression may not induce increased cell proliferation. In other words, the increased stathmin expression in tumor cells may be related to other tumor properties, and not directly to increased proliferative rates.
Stable stathmin overexpression reduced microtubule polymer mass only slightly
Stable stathmin overexpression in the OE1 and OE2 cell lines induced only an 18% decrease in the percentage of tubulin in microtubule polymer form. Consistent with the modest reduction in microtubule polymer mass, we observed no detectable change in the mass or organization of microtubules by immunofluorescence microscopy. These results are in stark contrast to the effects of stathmin in vitro with purified microtubules. Stathmin has a marked ability to destabilize microtubules in vitro, both by sequestering tubulin and by increasing the catastrophe frequency of microtubule dynamic instability at both microtubule plus and minus ends [3–5, 8]. Previous results in cells after transient upregulation of stathmin expression are consistent with these in vitro observations. Specifically, increasing the level of stathmin by transient overexpression or microinjection markedly decreases microtubule polymer mass, sometimes by as much 80–90% or more [6, 18–22]. Our data with stably-overexpressed stathmin suggest that, over time, cells regulate their microtubule polymer levels within a relatively narrow range in order to survive and proliferate; thus, over the long term, cells may adjust to the increased stathmin levels by altering expression of other proteins, such as tau and MAP4, that can compensate for the short-term destabilizing effects.
Stable stathmin overexpression was associated with a reduction in the microtubule catastrophe frequency
Increases in stathmin levels in Xenopus egg extacts [23] result in an increased catastrophe frequency whereas, transient overexpression in porcine kidney epithelial LLCPK cells results in no significant change in catastrophe frequency [22]. In contrast, we found that stable stathmin overexpression in OE1 resulted in a 45–54% reduction in catastrophe frequency (Table 1). The results again suggest that compensatory expression of other proteins or the increased phosphorylation of stathmin that we observed in our stably transfected system may negate the inherent ability of stathmin to induce catastrophe. Interestingly, overall dynamicity was reduced by 29% in OE1 cells (Table 1), a comparable reduction to that found after transient stathmin overexpression in LLCPK cells by Ringhoff and Cassimeris [22] (32%). In contrast, in the LLCPK cells, the reduction in dynamicity resulted primarily from significant reduction in microtubule growth and shortening rates rather than from a reduction in catastrophe frequency.
Stable stathmin overexpression induced taxol resistance
Using two assays of the effects of taxol on cell proliferation, a 72 h proliferation assay and a long-term (7–10 days) clonogenic assay of viability after a brief one-day taxol exposure, we found that stathmin overexpression induced resistance to taxol. Although the change was minimal in the 72 h assay (a 13% increase in IC50), in the clonogenic assay, stathmin overexpression increased resistance by 44%. These results are consistent with previous results indicating that high levels of stathmin increase taxol resistance [9].
We note that resistance was significant and detectable in OE1 cells only at a very low taxol concentration (0.1 nM) whereas resistance was significant and clearly detectable at all taxol concentrations tested (0.5–4 nM) in OE2 cells. The reasons for this are not known but may reflect that cells can adapt and alter protein expression in different ways in response to a stressful primary alteration such as stathmin overexpression.
Stable stathmin overexpression reduced the incidence of taxol-induced spindle abnormalities
A major antitumor mechanism of taxol is suppression of microtubule dynamics leading to mitotic arrest and ultimately to cell death. Taxol-arrested mitotic spindles typically exhibit frequent abnormalities with uncongressed chromosomes and multipolar spindles [24]. Since stathmin regulates microtubule assembly and induces microtubule catastrophe, we were interested in the actions of taxol in mitotic cells with altered stathmin levels. We found that at taxol concentrations that inhibited proliferation, significant numbers of spindle abnormalities including uncongressed chromosomes and tripolar spindles were induced in all three cell lines. As shown in Fig. 4B, stathmin overexpression decreased the number of taxol-induced abnormal spindles (from 73% of all spindles in controls to 48% and 39% in OE1 and OE2, respectively). In contrast, we have also observed that stathmin knockdown increases the percentage of cells with taxol-induced abnormal spindles (C. Nakao, K. Kamath, L. Wilson, and M. A. Jordan, unpublished data). Stathmin appears to protect cells from the spindle-damaging effects of taxol, and thus play a role in the ability of stathmin to induce taxol resistance.
Stable stathmin overexpression induced significant changes in taxol-induced protein expression
Treatment of BT549 cell lines overexpressing stathmin with taxol resulted in decreased levels of many proteins (Table 2). This included the control OEc, since the BT549 cell line already expresses high levels of stathmin, even before cloning the myc-tagged stathmin. The proteins whose levels were altered by taxol are involved in the cytoskeleton and in cell structure, the stress response, protein folding, glycolysis pathways, and they possess some type of catalytic activity. From a technical point of view, several of these protein level alterations were reproducible as they occurred in two of the three OE comparisons. Peroxiredoxin 1 (PRDX1) and triosephosphate isomerase 1 (TPI1) levels were decreased after exposure to taxol in both the stathmin stably-overexpressing cell lines (OE1, OE2), while annexin A2 (ANXA2), eukaryotic translation initiation factor 5A (EIF5A), the proteasome subunit, beta type, 5 and 6 (PSMB5, PSMB6), vimentin (VIM), cofilin 1 (non-muscle) CFL1, profilin 1 (PFN1) and peptidylprolyl isomerase A (cyclophilin A) (PPIA) were decreased after taxol treatment in the scrambled vector control, OEc, and either of OE1 or OE2 cell lines. Several members of the heat shock protein (HSP) family along with other proteins functioning in stress response pathways (Table 2) were also decreased after taxol treatment.
In another chemoresistance proteomic study using MCF-7 breast cancer cells, twenty alterations were detected using DIGE analysis [25]. Eight proteins were identified to be altered in taxol resistant cell lines and seventeen in adriamycin-resistant cell lines. We detected alterations in twelve of these proteins after taxol treatment in OE cell lines, although only two, HSP27 and GAPDH, showed similar reduced levels as seen in chemoresistant MCF-7 cells. Reduced expression of HSP70 isoforms, PRDX6, ENO1, TPI1, HNRNPA2B1, ALDOA and other proteins were, however, reported in the taxol-resistant epithelial ovarian cancer cell line A2780 versus its taxol-sensitive counterpart A2780TC [26]. This DIGE analysis reported over 100 spot variations as significant changes. In a similar study, some of the protein changes we identified, ALDOA, PRDX6, ENO1, CFL1, were found decreased. These proteins were differentially expressed between taxol-sensitive A2780 and the taxol-resistant A2780TC1, as well as the inherently resistant OVCAR3 cell line [27]. The field of pharmacoproteomics is in its infancy, so proteomic investigations of chemotherapy response to anti-cancer agents are few [28]. Although findings in these studies both corroborate and contradict increase or decrease of a particular protein upon chemotherapy treatment, the lists of proteins identified in the literature as differentially expressed are similar to those in our study [29–34].
Dysregulation in cancer and the “dynamic range issue”
Notably, proteomic investigations to identify cancer biomarkers also show differential levels of several of the proteins identified in our study [35–38]. In the last decade, many of these investigations applied two dimensional electrophesis (2DE) for quantitative analysis. The ‘dynamic range’ challenge in proteome coverage, and known limitations of 2DE, leads one to ask the question of whether differential alterations are merely an artifact of the technique. Petrak et al. [39] performed a meta-analysis of 186 individual 2DE analyses that appeared in the journal Proteomics to compile a list of the top 15 proteins/protein families differentially expressed. In spite of our efforts to increase the depth of our proteome coverage, it is encouraging that many of the proteins we identified with altered levels are shared with the top 15 [39]. These authors performed a meta-analysis of transcriptomic data that corroborated the protein level changes in ENO1 and HSP27 observed in most proteomic studies related with cancer. Furthermore, other techniques, such as immunochemistry or transcriptomic analysis, also reveal altered expression of the proteins frequently reported in proteomic studies of cancer and response to chemotherapy, thus validating that these protein level changes are not an artifact of the proteomic technique employed. The ”dynamic range” issue, which biases towards detection of the most abundant proteins in the sample, cannot be wholly disregarded, as biomarkers detected in body fluids of cancer patients also consist of several of the proteins in the top 15. These are major proteins represented in most cells as shown by studies cataloguing the breast cancer proteome [40]. But the fact that HSP90 and other proteins are therapeutic targets attest to their biological role in cancer. A role for them in tumor biology is undeniable. It follows that they would be affected by chemotherapy. Likewise, though many of the proteins identified in this study fall into the top 15 proteins/protein families, it is still possible that these proteins are involved in tumor biology and also are affected by chemotherapy.
Mechanism of development of taxol resistance
The two principal effects of stathmin as elaborated in vitro in purified microtubule systems are its abilities to sequester tubulin, thereby reducing the fraction of tubulin in polymerized microtubule form, and to induce catastrophes at both the microtubule plus and minus ends, thereby leading to enhanced microtubule dynamicity. Taxol, in contrast, has somewhat opposite effects; it reduces microtubule dynamicity and enhances microtubule polymer mass, primarily by reducing the shortening rates of dynamic microtubules [41]. In the simplest interpretation, overexpression of stathmin might be expected to lead to taxol resistance by the opposing effects of stathmin and taxol. We found, however, that our cells stably overexpressing stathmin by more than two-fold underwent myriad changes in gene expression and posttranslational modification that complicate this interpretation. First, stathmin became more highly phosphorylated, thus most likely leading to inactivation of some portion of the total cellular stathmin as a tubulin-binding partner. In addition, since dynamic and functioning microtubules are essential to many cellular processes including signaling, transport, cell shape, and mitosis, it is likely that a cell would adjust to reduced microtubule mass by increasing expression of microtubule-stabilizing molecules including, for example, MAP4 and tau. The subtle changes in amounts of these molecules necessary to rescue microtubules would not be expected to become evident by proteomics at our current levels of resolution. In this respect, we note that in mouse embryo fibroblasts isolated from stathmin-null mice [22], found that the microtubule assembly-promoting proteins including MAP 2, CLASP1, and STOP were downregulated although, curiously, others including MAP4 were upregulated. At this point, we do not know how the postulated expression of additional microtubule stabilizing proteins after stathmin upregulation might affect the interaction of motor proteins and other microtubule interacting proteins (heat shock proteins) with the microtubules, thus most likely leading to extensive attempts by the cell to adjust to an abnormal balance of its components.
We found that treatment of the stathmin-overexpressing BT549 cells with taxol resulted in decreased expression of the actin-binding proteins cofilin 1 and profilin 1, proteins that are involved in actin polymerization. In contrast, Po uha et al. [42] found that the levels of phospho-cofilin greatly increased (a phenomenon that appears important in spindle formation) in neuroblastoma cells that were selected for resistance to the microtubule-destabilizing agents vincristine and colchicine. At the concentration of taxol we used in the proteomic characterization (20 nM), the percentage of BT549 cells in mitosis was increased from 3–4% in controls to 13–28% in the presence of taxol, whereas the neuroblastoma cells were examined under a condition that reduced the mitotic index. Thus, it is conceivable that downregulation of the actin binding proteins we observed was a result of increased mitotic accumulation.
It is clear that microtubule dynamics influence cellular localization and function of proteins. For example, in malignant hematopoietic cells, peptidyl-prolyl isomerases such as cyclophilin A and pin1 were found to localize to the plasma membrane in response to taxol-induced microtubule stabilization [43]. It is curious that cell stress response proteins including several heat shock proteins were down-regulated after taxol incubation, a stress-inducing treatment, whereas their expression was increased in adriamycin and vincristine resistance [34,44]. The stress protein downregulation we observed may result from the microtubule-stabilizing effects of taxol on protein localization and function.
Interpretation of the results reported here should take into consideration that taxol binds not only to microtubules but also to other proteins in the cell such as Bcl2 [45] and perhaps to as yet unknown specific or non-specific binding targets. Understanding of the complex cellular changes involved in the development of drug-resistance will clearly require much additional experimentation.
Acknowledgments
Supported by NIH grants CA 057291 and RR 024153.
Abbreviations
- OD
optical density
- DAPI
4′,6-diamidino-2-phenylindole
- DIGE-Offgel IEF/SDS PAGE DIGE
difference gel electrophoresis-protein offgel isoelectric focusing/sodium dodecyl sulfate polyacrylamide gel electrophoresis
- DMSO
dimethyl sulfoxide
- EGFP
enhanced green fluorescence protein
- OE1 and OE2
stathmin-overexpressing cell lines
- OEc
empty vector control cell line
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Orr GA, Verdier-Pinard P, McDaid H, Horwitz SB. Mechanisms of Taxol resistance related to microtubules. Oncogene. 2003;22:7280–7295. doi: 10.1038/sj.onc.1206934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Holmfeldt P, Sellin ME, Gullberg M. Predominant regulators of tubulin monomer-polymer partitioning and their implication for cell polarization. Cell Mol Life Sci. 2009;66:3263–3276. doi: 10.1007/s00018-009-0084-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Belmont LD, Mitchison TJ. Identification of a protein that interacts with tubulin dimers and increases the catastrophe rate of microtubules. Cell. 1996;84:623–631. doi: 10.1016/s0092-8674(00)81037-5. [DOI] [PubMed] [Google Scholar]
- 4.Howell B, Deacon H, Cassimeris L. Decreasing oncoprotein 18/stathmin levels reduces microtubule catastrophes and increases microtubule polymer in vivo. J Cell Sci. 1999;112:3713–3722. doi: 10.1242/jcs.112.21.3713. [DOI] [PubMed] [Google Scholar]
- 5.Howell B, Larsson N, Gullberg M, Cassimeris L. Dissociation of the tubulin-sequestering and microtubule catastrophe-promoting activities of oncoprotein 18/stathmin. Mol Biol Cell. 1999;10:105–118. doi: 10.1091/mbc.10.1.105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Larsson N, Segerman B, Howell B, Fridell K, Cassimeris L, Gullberg M. Op18/stathmin mediates multiple region-specific tubulin and microtubule-regulating activities. J Cell Biol. 1999;146:1289–1302. doi: 10.1083/jcb.146.6.1289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Steinmetz MO, Kammerer RA, Jahnke W, Goldie KN, Lustig AJ, van Oostrum J. Op18/stathmin caps a kinked protofilament-like tubulin tetramer. EMBO J. 2000;19:572–580. doi: 10.1093/emboj/19.4.572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Manna T, Thrower D, Miller HP, Curmi P, Wilson L. Stathmin strongly increases the minus end catastrophe frequency and induces rapid treadmilling of bovine brain microtubules at steady state in vitro. J Biol Chem. 2006;281:2071–2078. doi: 10.1074/jbc.M510661200. [DOI] [PubMed] [Google Scholar]
- 9.Alli E, Bash-Babula J, Yang JM, Hait WN. Effect of stathmin on the sensitivity to antimicrotubule drugs in human breast cancer. Cancer Res. 2002;62:6864–6869. [PubMed] [Google Scholar]
- 10.Unlu M, Morgan ME, Minden JS. Difference gel electrophoresis: a single gel method for detecting changes in protein extracts. Electrophoresis. 1997;18:2071–2077. doi: 10.1002/elps.1150181133. [DOI] [PubMed] [Google Scholar]
- 11.Skehan P, Storeng R, Scudiero D, Monks A, McMahon J, Vistica D, Warren JT, Bokesch H, Kenney S, Boyd MR. New colorimetric cytotoxicity assay for anticancer-drug screening. J Natl Cancer Inst. 1990;82:1107–1112. doi: 10.1093/jnci/82.13.1107. [DOI] [PubMed] [Google Scholar]
- 12.Kamath K, Jordan MA. Suppression of microtubule dynamics by epothilone B in living MCF7 cells. Cancer Res. 2003;63:6026–6031. [PubMed] [Google Scholar]
- 13.Beckner ME, Chen X, An J, Day BW, Pollack IF. Proteomic characterization of harvested pseudopodia with differential gel electrophoresis and specific antibodies. Lab Invest. 2005;85:316–327. doi: 10.1038/labinvest.3700239. [DOI] [PubMed] [Google Scholar]
- 14.Jordan MA, Toso RJ, Thrower D, Wilson L. Mechanism of mitotic block and inhibition of cell proliferation by taxol at low concentrations. Proc Natl Acad Sci USA. 1993;90:9552–9556. doi: 10.1073/pnas.90.20.9552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Marklund U, et al. The phenotype of a “Cdc2 kinase target site-deficient mutant” of oncoprotein 18 reveals a role of this protein in cell cycle control. J Biol Chem. 1994;269:30626–30635. [PubMed] [Google Scholar]
- 16.Curmi PA, Noguès C, Lachkar S, Carelle N, Gonthier MP, Sobel A, Lidereau R, Bièche I. Overexpression of stathmin in breast carcinomas points out to highly proliferative tumours. Br J Cancer. 2000;82:142–150. doi: 10.1054/bjoc.1999.0891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Price DK, Ball JR, Bahrani-Mostafavi Z, Vachris JC, Kaufman JS, Naumann RW, Higgins RV, Hall JB. The phosphoprotein Op18/stathmin is differentially expressed in ovarian cancer. Cancer Invest. 2000;18:722–730. doi: 10.3109/07357900009012204. [DOI] [PubMed] [Google Scholar]
- 18.Horwitz S, Shen HJ, He L, Dittmar P, Neef R, Chen J, Schubart UK. The microtubule-destabilizing activity of metablastin (p19) is controlled by phosphorylation. J Biol Chem. 1997;272:8129–8132. doi: 10.1074/jbc.272.13.8129. [DOI] [PubMed] [Google Scholar]
- 19.Gavet O, Ozon S, Manceau V, Lawler S, Curmi P, Sobel A. The stathmin phosphoprotein family: intracellular localization and effects on the microtubule network. J Cell Sci. 1998;111:3333–3346. doi: 10.1242/jcs.111.22.3333. [DOI] [PubMed] [Google Scholar]
- 20.Marklund U, Larsson N, Gradin HM, Brattsand G, Gullberg M. Oncoprotein 18 is a phosphorylation-responsive regulator of microtubule dynamics. EMBO J. 1996;15:5290–5298. [PMC free article] [PubMed] [Google Scholar]
- 21.Melander Gradin H, Marklund U, Larsson N, Chatila TA, Gullberg M. Regulation of microtubule dynamics by Ca2+/calmodulin-dependent kinase IV/Gr-dependent phosphorylation of oncoprotein 18. Mol Cell Biol. 1997;17:3459–3467. doi: 10.1128/mcb.17.6.3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ringhoff DN, Cassimeris L. Stathmin regulates centrosomal nucleation of microtubules and tubulin dimer/polymer partitioning. Mol Biol Cell. 2009;20:3451–3458. doi: 10.1091/mbc.E09-02-0140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Arnal I, Karsenti E, Hyman A. Structural transitions at microtubule ends correlate with their dynamic properties in Xenopus egg extracts. J Cell Biol. 2000;149:767–774. doi: 10.1083/jcb.149.4.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Jordan M, Kamath K. How do microtubule-targeted drugs work? An overview. Curr Cancer Drug Targets. 2007;7:730–742. doi: 10.2174/156800907783220417. [DOI] [PubMed] [Google Scholar]
- 25.Chuthapisith S, Layfield R, Kerr ID, Hughes C, Eremin O. Proteomic profiling of MCF-7 breast cancer cells with chemoresistance to different types of anti-cancer drugs. Int J Oncol. 2007;30:1545–1551. [PubMed] [Google Scholar]
- 26.Cicchillitti L, Di Michele M, Urbani A, Ferlini C, Donat MB, Scambia G, Rotilio D. Comparative proteomic analysis of paclitaxel sensitive A2780 epithelial ovarian cancer cell line and its resistant counterpart A2780TC1 by 2D-DIGE: the role of ERp57. J Proteome Res. 2009;8:1902–1912. doi: 10.1021/pr800856b. [DOI] [PubMed] [Google Scholar]
- 27.Di Michele M, Della Corte A, Cicchillitti L, Del Boccio P, Urbani A, Ferlini C, Scambia G, Donati MB, Rotilio D. A proteomic approach to paclitaxel chemoresistance in ovarian cancer cell lines. Biochim Biophys Acta. 2009;1794:225–236. doi: 10.1016/j.bbapap.2008.09.017. [DOI] [PubMed] [Google Scholar]
- 28.Martinkova J, Gadher SJ, Hajduch M, Kovarova H. Challenges in cancer research and multifaceted approaches for cancer biomarker quest. FEBS Lett. 2009;583:1772–1784. doi: 10.1016/j.febslet.2009.03.042. [DOI] [PubMed] [Google Scholar]
- 29.Chen ST, Pan TL, Tsai YC, Huang CM. Proteomics reveals protein profile changes in doxorubicin--treated MCF-7 human breast cancer cells. Cancer Lett. 2002;181:95–107. doi: 10.1016/s0304-3835(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 30.Poland J, Schadendorf D, Lage H, Schnölzer M, Celis JE, Sinha P. Study of therapy resistance in cancer cells with functional proteome analysis. Clin Chem Lab Med. 2002;40:221–234. doi: 10.1515/CCLM.2002.037. [DOI] [PubMed] [Google Scholar]
- 31.Somiari RI, Sullivan A, Russell S, Somiari S, Hu H, Jordan R, George A, Katenhusen R, Buchowiecka A, Arciero C, Brzeski H, Hooke J, Shriver C. High-throughput proteomic analysis of human infiltrating ductal carcinoma of the breast. Proteomics. 2003;3:1863–1873. doi: 10.1002/pmic.200300560. [DOI] [PubMed] [Google Scholar]
- 32.Urbani A, Poland J, Bernardini S, Bellincampi L, Biroccio A, Schnölzer M, Sinha P, Federici G. A proteomic investigation into etoposide chemo-resistance of neuroblastoma cell lines. Proteomics. 2005;5:796–804. doi: 10.1002/pmic.200401147. [DOI] [PubMed] [Google Scholar]
- 33.Yan XD, Pan LY, Yuan Y, Lang JH, Mao N. Identification of platinum-resistance associated proteins through proteomic analysis of human ovarian cancer cells and their platinum-resistant sublines. J Proteome Res. 2007;6:772–780. doi: 10.1021/pr060402r. [DOI] [PubMed] [Google Scholar]
- 34.Keenan J, Murphy L, Henry M, Meleady P, Clynes M. Proteomic analysis of multidrug-resistance mechanisms in adriamycin-resistant variants of DLKP, a squamous lung cancer cell line. Proteomics. 2009;9:1556–1566. doi: 10.1002/pmic.200800633. [DOI] [PubMed] [Google Scholar]
- 35.Dowling P, Meleady P, Dowd A, Henry M, Glynn S, Clynes M. Proteomic analysis of isolated membrane fractions from superinvasive cancer cells. Biochim Biophys Acta. 2007;1774:93–101. doi: 10.1016/j.bbapap.2006.09.014. [DOI] [PubMed] [Google Scholar]
- 36.Selicharová I, Smutná K, Sanda M, Ubik K, Matousková E, Bursíková E, Brozová M, Vydra J, Jirácek J. 2-DE analysis of a new human cell line EM-G3 derived from breast cancer progenitor cells and comparison with normal mammary epithelial cells. Proteomics. 2007;7:1549–1559. doi: 10.1002/pmic.200600907. [DOI] [PubMed] [Google Scholar]
- 37.Liang X, Huuskonen JK, Hajivandi M, Manzanedo R, Predki P, Amshey JR, Pope RM. Identification and quantification of proteins differentially secreted by a pair of normal and malignant breast-cancer cell lines. Proteomics. 2009;9:182–193. doi: 10.1002/pmic.200700957. [DOI] [PubMed] [Google Scholar]
- 38.Schulz DM, Böllner C, Thomas G, Atkinson M, Esposito I, Höfler H, Aubele M. Identification of differentially expressed proteins in triple-negative breast carcinomas using DIGE and mass spectrometry. J Proteome Res. 2009;8:3430–3438. doi: 10.1021/pr900071h. [DOI] [PubMed] [Google Scholar]
- 39.Petrak J, Ivanek R, Toman O, Cmejla R, Cmejlova J, Vyoral D, Zivny J, Vulpe CD. Déjà vu in proteomics. A hit parade of repeatedly identified differentially expressed proteins. Proteomics. 2008;8:1744–1749. doi: 10.1002/pmic.200700919. [DOI] [PubMed] [Google Scholar]
- 40.Pucci-Minafra I, Cancemi P, Fontana S, Minafra L, Feo S, Becch M, Freyria AM, Minafra S. Expanding the protein catalogue in the proteome reference map of human breast cancer cells. Proteomics. 2006;6:2609–2625. doi: 10.1002/pmic.200500627. [DOI] [PubMed] [Google Scholar]
- 41.Derry WB, Wilson L, Jordan MA. Substoichiometric binding of taxol suppresses microtubule dynamics. Biochemistry. 1995;34:2203–2211. doi: 10.1021/bi00007a014. [DOI] [PubMed] [Google Scholar]
- 42.Po’uha S, Shum M, Goebel A, Bernard O, Kavallaris M. LIM-kinase 2, a regulator of actin dynamics, is involved in mitotic spindle integrity and sensitivity to microtubule-destabilizing drugs. Oncogene. 2010;29:597–607. doi: 10.1038/onc.2009.367. [DOI] [PubMed] [Google Scholar]
- 43.Bane FT, Bannon JH, Pennington SR, Campaini G, Williams DC, Zisterer DM, McGee MM. The microtubule-targeting agents, PBOX-6 [pyrrolobenzoxazepine 7-[(dimethylcarbamoyl)oxy]-6-(2-naphthyl)pyrrolo-[2,1-d](1,5)-benzoxazepine] and paclitaxel, induce nucleocytoplasmic redistribution of the peptidyl-prolyl isomerases, cyclophilin A and pin1, in malignant hematopoietic cells. J Pharmacol Exp Ther. 2009;329:38–47. doi: 10.1124/jpet.108.148130. [DOI] [PubMed] [Google Scholar]
- 44.Yang YX, Sun XF, Cheng AL, Zhang GY, Yi H, Sun Y, Hu HD, Hu P, Ye F, Chen ZC, Xiao ZQ. Increased expression of HSP27 linked to vincristine resistance in human gastric cancer cell line. J Cancer Res Clin Oncol. 2009;135:181–189. doi: 10.1007/s00432-008-0460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ferlini C, Cicchillitti L, Raspaglio G, Bartollino S, Cimitan S, Bertucci C, Mozzetti S, Gallo D, Persico M, Fattorusso C, Campiani G, Scambia G. Paclitaxel directly binds to Bcl-2 and functionally mimics activity of Nur77. Cancer Res. 2009;69:6906–6914. doi: 10.1158/0008-5472.CAN-09-0540. [DOI] [PubMed] [Google Scholar]