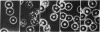Abstract
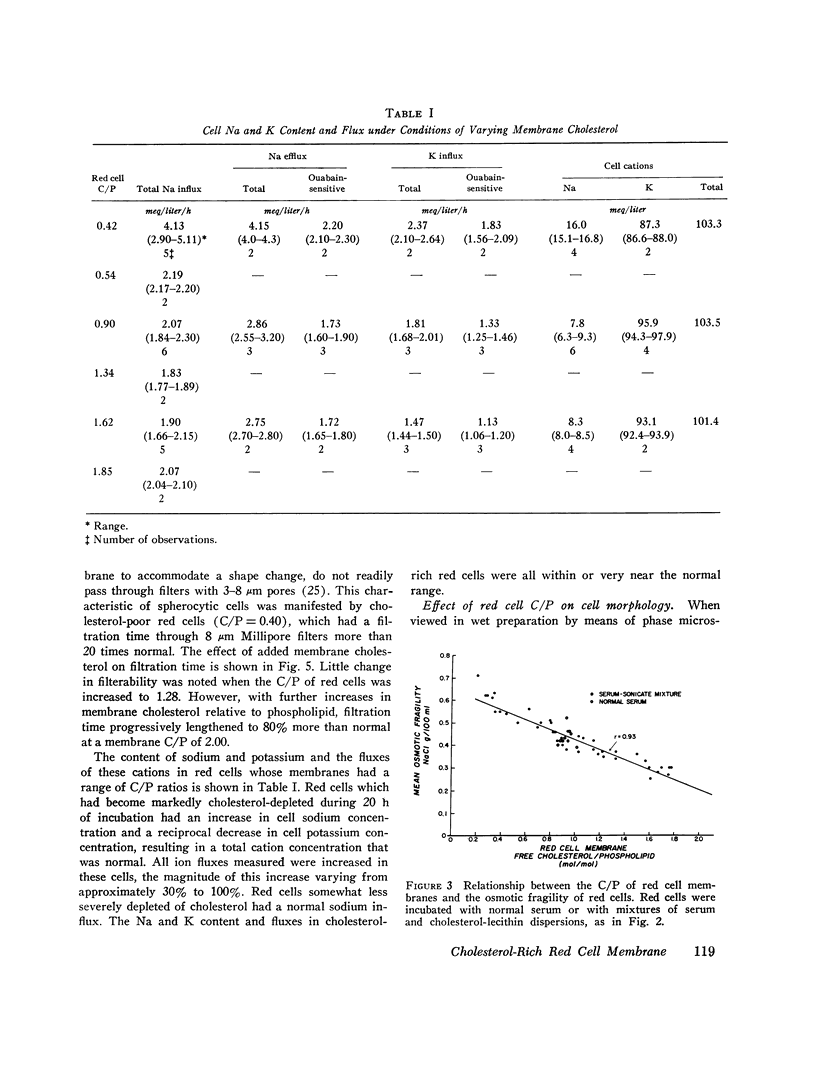
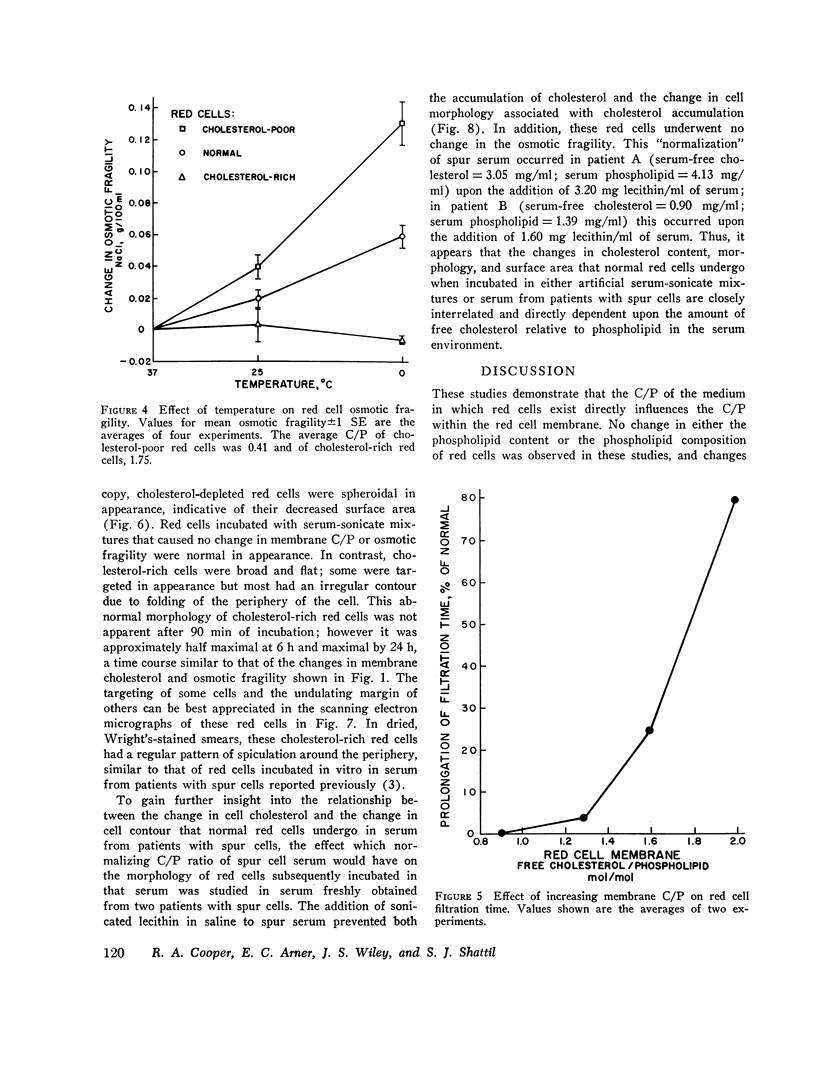
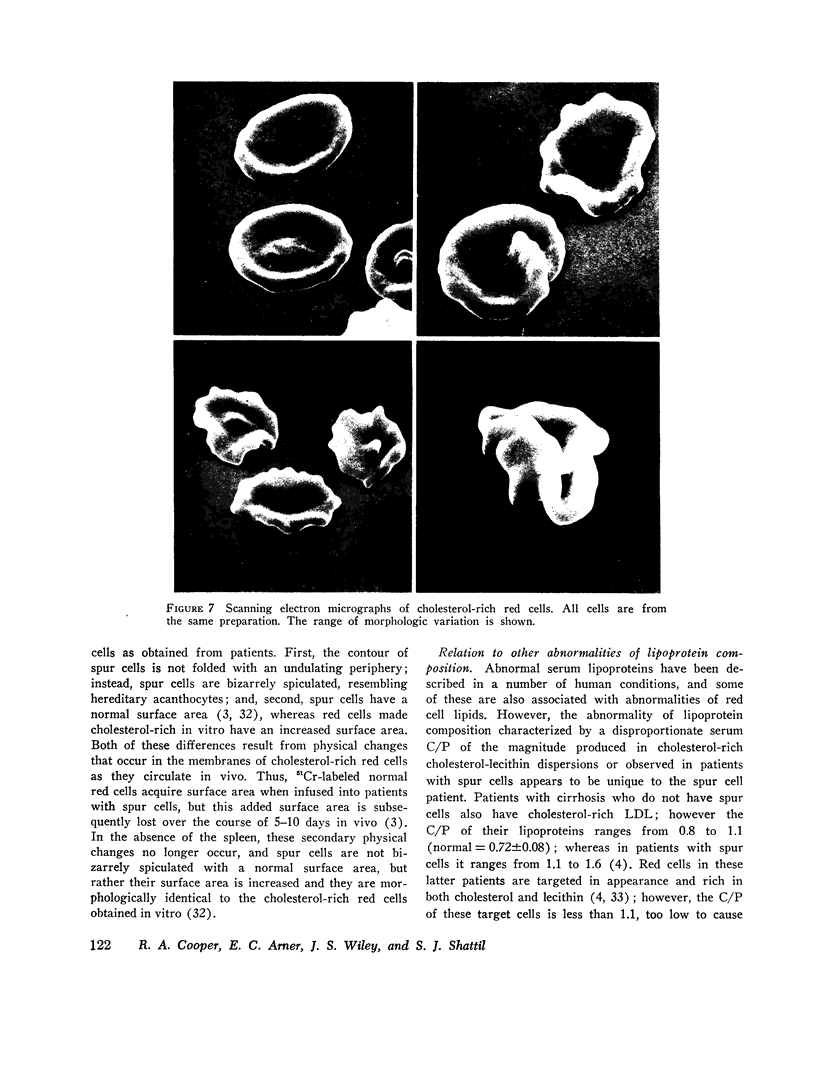
Cholesterol-rich membranes are the hallmark of "spur" red cells. Spur cells accumulate cholesterol from cholesterol-rich serum lipoproteins. Previous studies suggested that this added cholesterol is responsible for both the altered morphology and the destruction of spur cells. To examine this process in the absence of other serum factors, cholesterol-lecithin dispersions with varying amounts of unesterified cholesterol (C) relative to phospholipid (P) were prepared, and their influence on normal human red cells was studied. Cholesterol-rich lipid dispersions (C/P mole ration greater 1.0) transferred cholesterol to both red cell membranes and serum lipoproteins, and cholesterol-poor dispersions (C/P mole ration less 1.0) depleted red cells of cholesterol. Changes in membrane cholesterol paralleled changes in membrane surface area, as calculated from osmotic fragility, with a 0.22 percent variation in surface area per 1.0 percent variation in cholesterol content. Cold-induced compression of membrane surface area was increased in cholesterol-poor red cells (C/P equals 0.4), whereas the surface area of cholesterol-rich membranes (C/P equals 1.80) underwent no compression. Although the Na and K permeability of red cells severely depleted of cholesterol was increased, lesser degrees of depletion had no effect, and the permeability of cholesterol-rich cells was normal. However, increasing membrane cholesterol caused a progressive decrease in red cell deformability, as measured by filtration. Cholesterol-poor red cells were spherocytic in appearance and cholesterol-rich cells were broad and flat, indicative of their surface areas. In addition, cholesterol-rich cells had an irregular contour due to folding of the periphery of the cell. This shape abnormality was identical to that of both spur cells after splenectomy and normal red cells incubated in spur serum. Normalization of the C/P of spur serum by added phospholipid prevented the increase in membrane cholesterol and surface area and the transformation of cell shape. These studies establish that the cholesterol content of red cells is dependent on the C/P of their milieu, either lipoproteins or cholesterol-lecithin dispersions. Moreover, the surface area, deformability, and contour of cholesterol-rich red cells are a direct function of their increased membrane C/P. Although cholesterol-rich spur cells are further modified in the circulation of patients with spleens, this abnormality of the membrane lipid bilayer, induced by cholesterol-rich cholesterol-lecithin dispersions, represents the primary spur cell defect.
Full text
PDF






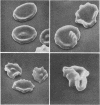
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHWORTH L. A., GREEN C. THE TRANSFER OF LIPIDS BETWEEN HUMAN ALPH-LIPOPROTEIN AND ERYTHROCYTES. Biochim Biophys Acta. 1964 Apr 20;84:182–187. doi: 10.1016/0926-6542(64)90075-7. [DOI] [PubMed] [Google Scholar]
- BARTLETT G. R. Phosphorus assay in column chromatography. J Biol Chem. 1959 Mar;234(3):466–468. [PubMed] [Google Scholar]
- BASFORD J. M., GLOVER J., GREEN C. EXCHANGE OF CHOLESTEROL BETWEEN HUMAN BETA-LIPOPROTEINS AND ERYTHROCYTES. Biochim Biophys Acta. 1964 Dec 2;84:764–766. doi: 10.1016/0926-6542(64)90039-3. [DOI] [PubMed] [Google Scholar]
- Bar R. S., Deamer D. W., Cornwell D. G. Surface area of human erythrocyte lipids: reinvestigation of experiments on plasma membrane. Science. 1966 Aug 26;153(3739):1010–1012. doi: 10.1126/science.153.3739.1010. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Demel R. A., De Gier J., van Deenen L. L. The effect of partial replacements of membrane cholesterol by other steroids on the osmotic fragility and glycerol permeability of erythrocytes. Biochim Biophys Acta. 1969 Jul 15;183(2):334–345. doi: 10.1016/0005-2736(69)90089-3. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Edwards P. A., Green C. Properties of aqueous dispersions of phospholipid and cholesterol. Eur J Biochem. 1968 May;4(4):506–511. doi: 10.1111/j.1432-1033.1968.tb00241.x. [DOI] [PubMed] [Google Scholar]
- Bruckdorfer K. R., Graham J. M., Green C. The incorporation of steroid molecules into lecithin sols, beta-lipoproteins and cellular membranes. Eur J Biochem. 1968 May;4(4):512–518. doi: 10.1111/j.1432-1033.1968.tb00242.x. [DOI] [PubMed] [Google Scholar]
- Cooper R. A. Anemia with spur cells: a red cell defect acquired in serum and modified in the circulation. J Clin Invest. 1969 Oct;48(10):1820–1831. doi: 10.1172/JCI106148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Diloy Puray M., Lando P., Greenverg M. S. An analysis of lipoproteins, bile acids, and red cell membranes associated with target cells and spur cells in patients with liver disease. J Clin Invest. 1972 Dec;51(12):3182–3192. doi: 10.1172/JCI107145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Garcia F. A., Trey C. X. The effect of lithocholic acid on red cell membranes in vivo. J Lab Clin Med. 1972 Jan;79(1):7–18. [PubMed] [Google Scholar]
- Cooper R. A., Jandl J. H. Bile salts and cholesterol in the pathogenesis of target cells in obstructive jaundice. J Clin Invest. 1968 Apr;47(4):809–822. doi: 10.1172/JCI105775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Jandl J. H. Red cell cholesterol content: a manifestation of the serum affinity for free cholesterol. Trans Assoc Am Physicians. 1969;82:324–330. [PubMed] [Google Scholar]
- Cooper R. A., Jandl J. H. The selective and conjoint loss of red cell lipids. J Clin Invest. 1969 May;48(5):906–914. doi: 10.1172/JCI106049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper R. A., Kimball D. B., Durocher J. R. Role of the spleen in membrane conditioning and hemolysis of spur cells in liver disease. N Engl J Med. 1974 Jun 6;290(23):1279–1284. doi: 10.1056/NEJM197406062902303. [DOI] [PubMed] [Google Scholar]
- DODGE J. T., MITCHELL C., HANAHAN D. J. The preparation and chemical characteristics of hemoglobin-free ghosts of human erythrocytes. Arch Biochem Biophys. 1963 Jan;100:119–130. doi: 10.1016/0003-9861(63)90042-0. [DOI] [PubMed] [Google Scholar]
- Darke A., Finer E. G., Flook A. G., Phillips M. C. Complex and cluster formation in mixed lecithincholesterol bilayers. Cooperativity of motion in lipid systems. FEBS Lett. 1971 Nov 1;18(2):326–330. doi: 10.1016/0014-5793(71)80478-7. [DOI] [PubMed] [Google Scholar]
- Gjone E., Torsvik H., Norum K. R. Familial plasma cholesterol ester deficiency. A study of the erythrocytes. Scand J Clin Lab Invest. 1968;21(4):327–332. doi: 10.3109/00365516809077001. [DOI] [PubMed] [Google Scholar]
- Glomset J. A., Norum K. R., King W. Plasma lipoproteins in familial lecithin: cholesterol acyltransferase deficiency: lipid composition and reactivity in vitro. J Clin Invest. 1970 Oct;49(10):1827–1837. doi: 10.1172/JCI106400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gottfried E. L., Robertson N. A. Glycerol lysis time as a screening test for erythrocyte disorders. J Lab Clin Med. 1974 Feb;83(2):323–333. [PubMed] [Google Scholar]
- HAGERMAN J. S., GOULD R. G. The in vitro interchange of cholesterol between plasma and red cells. Proc Soc Exp Biol Med. 1951 Oct;78(1):329–332. doi: 10.3181/00379727-78-19064. [DOI] [PubMed] [Google Scholar]
- HAVEL R. J., EDER H. A., BRAGDON J. H. The distribution and chemical composition of ultracentrifugally separated lipoproteins in human serum. J Clin Invest. 1955 Sep;34(9):1345–1353. doi: 10.1172/JCI103182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamilton R. L., Havel R. J., Kane J. P., Blaurock A. E., Sata T. Cholestasis: lamellar structure of the abnormal human serum lipoprotein. Science. 1971 Apr 30;172(3982):475–478. doi: 10.1126/science.172.3982.475. [DOI] [PubMed] [Google Scholar]
- Hubbell W. L., McConnell H. M. Molecular motion in spin-labeled phospholipids and membranes. J Am Chem Soc. 1971 Jan 27;93(2):314–326. doi: 10.1021/ja00731a005. [DOI] [PubMed] [Google Scholar]
- JANDL J. H., SIMMONS R. L., CASTLE W. B. Red cell filtration and the pathogenesis of certain hemolytic anemias. Blood. 1961 Aug;18:133–148. [PubMed] [Google Scholar]
- Kroes J., Ostwald R. Erythrocyte membranes--effect of increased cholesterol content on permeability. Biochim Biophys Acta. 1971 Dec 3;249(2):647–650. doi: 10.1016/0005-2736(71)90147-7. [DOI] [PubMed] [Google Scholar]
- Kroes J., Ostwald R., Keith A. Erythrocyte membranes--compression of lipid phases by increased cholesterol content. Biochim Biophys Acta. 1972 Jul 3;274(1):71–74. doi: 10.1016/0005-2736(72)90281-7. [DOI] [PubMed] [Google Scholar]
- Ladbrooke B. D., Williams R. M., Chapman D. Studies on lecithin-cholesterol-water interactions by differential scanning calorimetry and X-ray diffraction. Biochim Biophys Acta. 1968 Apr 29;150(3):333–340. doi: 10.1016/0005-2736(68)90132-6. [DOI] [PubMed] [Google Scholar]
- Levine Y. K., Wilkins M. H. Structure of oriented lipid bilayers. Nat New Biol. 1971 Mar 17;230(11):69–72. doi: 10.1038/newbio230069a0. [DOI] [PubMed] [Google Scholar]
- Lippert J. L., Peticolas W. L. Laser Raman investigation of the effect of cholesterol on conformational changes in dipalmitoyl lecithin multilayers. Proc Natl Acad Sci U S A. 1971 Jul;68(7):1572–1576. doi: 10.1073/pnas.68.7.1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy J. R. The influence of pH and temperature on some physical properties of normal erythrocytes and erythrocytes from patients with hereditary spherocytosis. J Lab Clin Med. 1967 May;69(5):758–775. [PubMed] [Google Scholar]
- Neerhout R. C. Abnormalities of erythrocyte stromal lipids in hepatic disease. J Lab Clin Med. 1968 Mar;71(3):438–447. [PubMed] [Google Scholar]
- Oldfield E., Chapman D. Dynamics of lipids in membranes: Heterogeneity and the role of cholesterol. FEBS Lett. 1972 Jul 1;23(3):285–297. doi: 10.1016/0014-5793(72)80300-4. [DOI] [PubMed] [Google Scholar]
- Poznansky M., Kirkwood D., Solomon A. K. Modulation of red cell K+ transport by membrane lipids. Biochim Biophys Acta. 1973 Dec 22;330(3):351–355. doi: 10.1016/0005-2736(73)90245-9. [DOI] [PubMed] [Google Scholar]
- ROSE H. G., OKLANDER M. IMPROVED PROCEDURE FOR THE EXTRACTION OF LIPIDS FROM HUMAN ERYTHROCYTES. J Lipid Res. 1965 Jul;6:428–431. [PubMed] [Google Scholar]
- Robins S. J., Miller A. Red cell cholesterol depletion and the formation of spiculated cells in vivo. J Lab Clin Med. 1974 Mar;83(3):436–443. [PubMed] [Google Scholar]
- SMITH J. A., LONERGAN E. T., STERLING K. SPUR-CELL ANEMIA: HEMOLYTIC ANEMIA WITH RED CELLS RESEMBLING ACANTHOCYTES IN ALCOHOLIC CIRRHOSIS. N Engl J Med. 1964 Aug 20;271:396–398. doi: 10.1056/NEJM196408202710804. [DOI] [PubMed] [Google Scholar]
- Sardet C., Hansma H., Ostwald R. Characterization of guinea pig plasma lipoproteins: the appearance of new lipoproteins in response to dietary cholesterol. J Lipid Res. 1972 Sep;13(5):624–639. [PubMed] [Google Scholar]
- Sardet C., Hansma H., Ostwald R. Effects of plasma lipoproteins from control and cholesterol-fed guinea pigs on red cell morphology and cholesterol content: an in vitro study. J Lipid Res. 1972 Nov;13(6):705–715. [PubMed] [Google Scholar]
- Sessa G., Weissmann G. Phospholipid spherules (liposomes) as a model for biological membranes. J Lipid Res. 1968 May;9(3):310–318. [PubMed] [Google Scholar]
- Shinitzky M., Inbar M. Difference in microviscosity induced by different cholesterol levels in the surface membrane lipid layer of normal lymphocytes and malignant lymphoma cells. J Mol Biol. 1974 Jan 5;85(4):603–615. doi: 10.1016/0022-2836(74)90318-0. [DOI] [PubMed] [Google Scholar]
- Silber R., Amorosi E., Lhowe J., Kayden H. J. Spur-shaped erythrocytes in Laennec's cirrhosis. N Engl J Med. 1966 Sep 22;275(12):639–643. doi: 10.1056/NEJM196609222751204. [DOI] [PubMed] [Google Scholar]
- Singer S. J., Nicolson G. L. The fluid mosaic model of the structure of cell membranes. Science. 1972 Feb 18;175(4023):720–731. doi: 10.1126/science.175.4023.720. [DOI] [PubMed] [Google Scholar]
- Skipski V. P., Peterson R. F., Barclay M. Quantitative analysis of phospholipids by thin-layer chromatography. Biochem J. 1964 Feb;90(2):374–378. doi: 10.1042/bj0900374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanderkooi J., Fischkoff S., Chance B., Cooper R. A. Fluorescent probe analysis of the lipid architecture of natural and experimental cholesterol-rich membranes. Biochemistry. 1974 Apr 9;13(8):1589–1595. doi: 10.1021/bi00705a006. [DOI] [PubMed] [Google Scholar]
- Verma S. P., Wallach D. F. Effects of cholesterol on the infrared dichroism of phosphatide multibilayers. Biochim Biophys Acta. 1973 Dec 13;330(2):122–131. doi: 10.1016/0005-2736(73)90216-2. [DOI] [PubMed] [Google Scholar]
- Whittam R., Wiley J. S. Some aspects of adenosine triphosphate synthesis from adenine and adenosine in human red blood cells. J Physiol. 1968 Dec;199(2):485–494. doi: 10.1113/jphysiol.1968.sp008664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley J. S., Cooper R. A. A furosemide-sensitive cotransport of sodium plus potassium in the human red cell. J Clin Invest. 1974 Mar;53(3):745–755. doi: 10.1172/JCI107613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ZLATKIS A., ZAK B., BOYLE A. J. A new method for the direct determination of serum cholesterol. J Lab Clin Med. 1953 Mar;41(3):486–492. [PubMed] [Google Scholar]