Abstract
Primary biliary cirrhosis (PBC) is a chronic cholestatic liver disease in which an immune-mediated injury targets the small intrahepatic bile ducts. PBC is further characterized by highly specific serum antimitochondrial autoantibodies (AMA) and autoreactive T cells, a striking female predominance, a strong genetic susceptibility, and a plethora of candidate environmental factors to trigger the disease onset. For these reasons PBC appears ideal to represent the developments of the clonal selection theory over the past decades. First, a sufficiently potent autoimmunogenic stimulus in PBC would require the coexistence of numerous pre-existing conditions (mostly genetic, as recently illustrated by genome-wide association studies and animal models) to perpetuate the destruction of the biliary epithelium by the immune system via the persistence of forbidden clones. Second, the proposed modifications of mitochondrial autoantigens caused by infectious agents and/or xenobiotics well illustrate the possibility that peculiar changes in the antigen structure and flexibility may contribute to tolerance breakdown. Third, the unique apoptotic features demonstrated for cholangiocytes are the ideal setting for the development of mitochondrial autoantigen presentation to the immune system through macrophages and AMA thus turning the non traditional mitochondrial antigen into a traditional one. This article will review the current knowledge on PBC etiology and pathogenesis in light of the clonal selection theory developments.
Keywords: anti-mitochondrial antibodies, autoimmunity, environmental factors, thymic selection, tolerance
The clonal selection theory and the history of biliary autoimmunity
Two remarkable insights in 1948 and 1957 by Frank Macfarlane Burnet were to set the course for modern immunology 1, 2. In 1948 he deduced the nature of immunological inertness to self, named this as “tolerance” and proposed it to be a characteristic acquired in developmental life rather than innate as earlier believed 3. His intuition led to seminal consequences in our understanding of autoimmunity. First, in 1957 (while the hypothesis was elaborated in19594) he proposed that specificity of antigen recognition existed in cells of the immune system before any exposure to antigen for which the role was to select cells for clonal proliferation rather than acting in any “instructional” way to do so. Second, these concepts coincided closely in time with the renaissance of the notion of autoimmunity, in the late 1940s, so leading to the idea that an event such as a somatic mutation among cells of an antigen-stimulated clone could result in an aberrant proliferative response to a self antigen. This, together with a resistance to an ill-defined immunological homeostasis, could result in a ‘“forbidden clone” as the basis for an autoimmune disease 4. The ‘forbidden clone’ hypothesis was elaborated in our monograph of 1963 2, and still retained by Burnet in 1972, but developing knowledge in the 1960s including the B and T cell dichotomy and other advances left the concept increasingly harder to sustain. In particular, T lymphocytes do not undergo post-diversification mutations of their antigen receptor (TCR) whereas hypermutagenesis of genes for the B cell receptor (BCR) in germinal centers of lymph nodes is normally required for affinity maturation, and presumably gives rise constantly to self-reactive BCRs, but such cells for various reasons, including, lack of T-cell help, fail to thrive.
Also many advances were made in the 1960s on the expressions of autoimmunity among which was recognition of two separate albeit overlapping groups of diseases, “organ-specific” (prototypically lymphocytic thyroiditis) in which the identifiable autoantigens (named traditional) were constituents of the affected tissue, and “multisystemic” (prototypically systemic lupus erythematosus -SLE) in which the identifiable autoantigen(s) (non-traditional) were distributed in all cells throughout the body. This latter group includes the clinically, histologically and immunologically well-defined liver disease primary biliary cirrhosis (PBC) wherein there is consistent reactivity with a mitochondrially located autoantigen recognized over some 50 years as eliciting antimitochondrial antibody (AMA) reactivity, and researched from many angles over the past 25 years in our laboratory, as described in this encompassing review. In general terms, the 2010 products of the seminal evidence proposed by Frank Macfarlane Burnet of mutagenesis as the major feature of immune degeneration leading to autoimmunity is the objective of this Special Feature issue to celebrate the 50-year anniversary since the Nobel Prize was awarded for the enunciation of immune tolerance.
The first description of possible biliary cirrhosis, probably obstructive, was by the celebrated Italian pathologist Giovanni Battista Morgagni in 1761 and the earliest report of non-obstructive biliary cirrhosis is attributed to Addison and Gull in 1851 5. The disease was put on the map clinically by Ahrens et al in 1950 6. Serum “anti-tissue” autoantibodies were described in 1959 and PBC was nominated as an autoimmune disease in 1963 4. The anti-tissue autoantibodies were identified as antimitochondrial in 1965 7. After a considerable pause, a cDNA encoding the AMA autoantigen was cloned in 1986 and the antigen was identified as subunits (E2) of the pyruvate dehydrogenase complex (PDC) 8, 9 is nuclear encoded and expressed but is imported to become located on the inner mitochondrial membrane.
In the wider field of autoimmunity numerous self antigens became recognized as functional structures of the cell, particularly nuclear components, the autoepitopes were mapped, but adverse effector/inhibitory properties of autoantibodies in vivo remained incompletely defined 10. Among the characterized autoantigens, functional sites were found within cell nuclei as chromatin, nucleoli and ribonucleoproteins additional to the mitochondrial proteins. DNA molecules and the associated histones were among the most common of the reactive nuclear autoantigens, being recognized by almost all sera from patients with SLE giving reactivity for ANA. Other ANA included anti-Scl70 antibodies directed against topoisomerase I (Scl-70), a nuclear non-histone protein that uncoils condensed chromatin during mitosis 11, the anti anti-Sm 12 antibodies, and SS-B (or La) antibodies directed at eukaryotic RNA polymerase III in Sjögren’s syndrome and SLE. The genesis of all of these “non-traditional” autoantibodies seemed harder to explain than that of the “traditional” autoantibodies of organ-specific autoimmunity such as thyroid peroxidase (TPO) and thyrotropin receptor (TSHR) recognized by autoantibodies in autoimmune thyroid diseases. The discovery of PBC-specific antinuclear antibodies (ANA) came after AMA description and led to further possible implications in the pathogenesis of the disease, although our knowledge on the ANA onset and role in PBC remains largely incomplete. We will herein provide a conspectus of mitochondrial autoimmunity before returning to the question of how clonal selection theory might relate to the non-traditional, if not paradoxical, autoimmunity of PBC by invoking our recent discoveries on patterns and pathways of apoptosis in the target cell, the cholangiocyte.
Biochemical properties of the PBC autoantigens
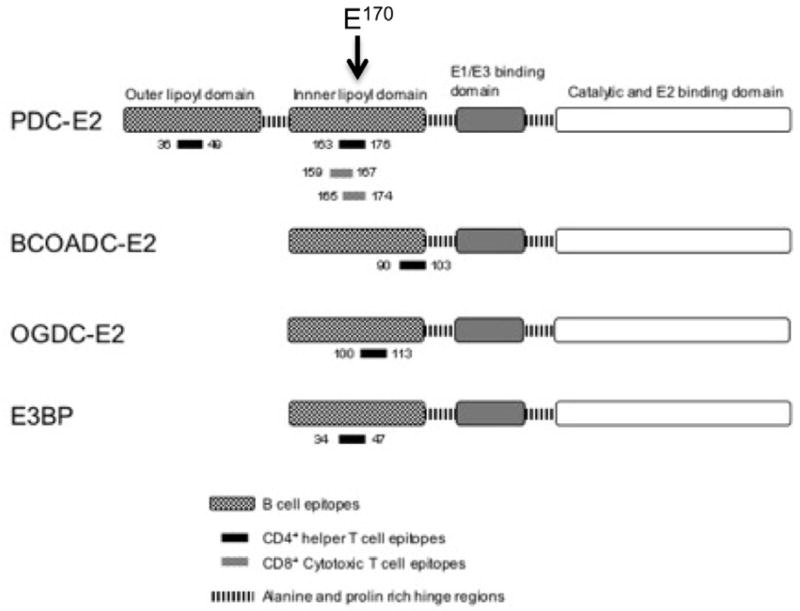
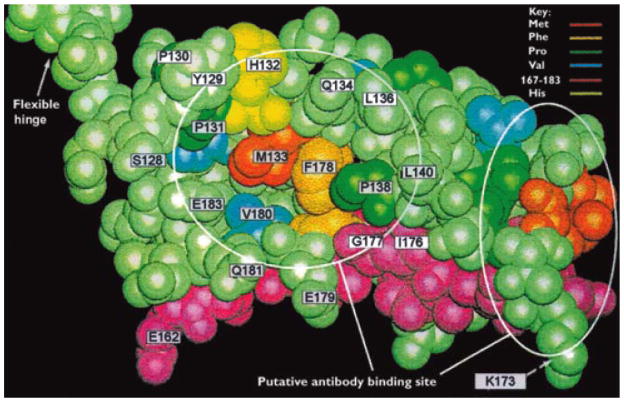
The 2-OADC autoantigens are multi-enzyme complexes essential in energy metabolism 13.Since this enzyme family has been repeatedly reviewed in the context of PBC, the data are presented in summary form in Table 1 and Figure 2 for the constituent pyruvate dehydrogenase complex (PDC), the 2-oxo glutarate dehydrogenase complex (OGDC), and the branched chain 2-oxoacid dehydrogenase complex (BCOADC). Each of the three complexes consists of three subunits, i.e. E1, E2 and E3. The E2 components consist of several functional domains. There is the inner catalytic domain containing the active site, one or more lipoyl domains containing the lysine residue to which the essential cofactor lipoic acid is attached, and an E3-binding domain. PDC-E2 and E3BP are the major autoantigens for serum AMA. Both PDC-E2 and E3BP fold into distinct domains linked by flexible regions rich in alanine and proline residues; interestingly, such flexibility is important for the enzyme catalytic function 14. Moreover, both polypeptides have a central core region, responsible for binding to other polypeptides. The E2 core, moreover, contains residues essential for its catalytic activity and is linked to a binding domain, which accounts for the binding to E1 (and possibly E3). On the other hand, the corresponding E3BP region binds E3 only. Both polypeptides include at their amino terminals compact domains containing the covalently attached lipoic acid co-factor 13. PDC-E2 has two and E3BP a single lipoylated domain 15. These lipoyl domains are exposed on the surface of the E2 core, a necessity for the function of the molecules. In all three instances, the domain is composed by a single lipoic acid residue covalently attached to a lysine residue in a constant DKA sequence motif. There are no crystallographic structural data but there is available for the inner lipoyl domain of human PDC-E2 a three dimensional model derived by nuclear magnetic resonance (Figure 1)16.
Table 1.
Molecular weights and functions of the 2-oxo-acid dehydrogenase complexes
| Enzymes | MW (kDa) | Function |
|---|---|---|
| Pyruvate dehydrogenase complexes | (PDC) | |
| E1a decarboxylase | 41 | Decarboxylates pyruvate with thiamine pyrophosphate (TTP) as a co-factor |
| E1b decarboxylase | 36 | Decarboxylates pyruvate with TTP as a co-factor |
| E2 acetyltransferase | 74 | Transfers acetyl group from E1 to coenzyme A (CoA) |
| E3 lipoamide dehydrogenase | 55 | Regenerates disulphide of E2 by oxidation of lipoic acid |
| E3 binding protein (protein X) | 56 | Anchoring E2 to the E2 core of pyruvate dehydrogenase complex |
| 2-oxoglutarate dehydrogenase complex | (OGDC) | |
| E1 oxoglutarate dehydrogenase | 113 | Decarboxylates a-ketoglutarate with TTP as a co-factor |
| E2 succinyl transferase | 48 | Transfers succinyl group from E2 to CoA |
| E3 lipoamide dehydrogenase | 55 | Regenerates disulphide of E2 by oxidation of lipoic acid |
| Branched-chain 2-oxo-acid dehydrogenase complex | (BCOADC) | |
| E1a decarboxylase | 46 | Decarboxylates a-keto acids |
| E1b decarboxylase | 38 | Derived from leucine, isoleucine, and valine with TTP as a co-factor |
| E2 acyltransferase | 52 | Transfers acyl group from E1 to CoA |
| E3 lipoamide dehydrogenase | 55 | Regenerates disulphide of E2 by oxidation of lipoic acid |
Figure 2.
The mapping of AMA (B cell) and T cell (CD4, CD8) epitopes recognized in PBC. Glutamic acid (E) at position 170 is highlighted for clarity purposes.
Figure 1.
Three-dimensional structure of the PDC-E2 inner lipoylated domain based on published NMR structure 16
Antigen-specific adaptive response in PBC
AMA specifically recognizes lipoylated domains of the 2-oxoacid dehydrogenase (2-OADC) family of enzymes (Table 1) of the mitochondrial respiratory chain. All immunodominant epitopes contain a ExDKA (glutamic acid –E-, x, aspartic acid, -D-, lysine –K-, and alanine –A-) motif, with lipoic acid attached to K at position 173, which is necessary and/or sufficient for antigen recognition 17. Amongst the 2-OADC constituents, the major autoantigen is the E2 subunit of pyruvate dehydrogenase complex (PDC-E2). Less frequent autoantigenic are the E2 components of 2-oxo glutarate dehydrogenase (OADC-E2) and branched-chain 2-oxo acid dehydrogenase (BCOADC-E2) complexes and the E3 binding protein (E3BP) 8, 18. In addition, nucleotide sequence analysis of genes encoding specific human monoclonal antibodies to PDC-E2 and combinatorial Fabs strongly suggest that these autoantibodies have been selected from a restricted set of somatically mutated immunoglobulin germline genes.
T helper (CD4+) TCR αβ+ and CD8+ T cells are present in portal tracts, around damaged bile ducts, strongly suggesting the participation of cellular immune mechanisms in the biliary damage 19–26. Autoreactive CD4 T cells that specifically target PDC-E2-self-antigen are present in peripheral blood and liver. There is a specific 100–150 fold increase in number of PDC-E2-specific CD4 T cells in the hilar lymph nodes and liver versus peripheral blood in patients with PBC 21, while it is of interest that their presence is independent of the serum AMA status 22. Autoreactive CD8 T cells likewise have been characterized in PBC, and are considered the major effectors of tissue injury in PBC. The HLA class I restricted epitope for CD8 T cells, namely the 159–167 aa sequence, maps in close vicinity to the epitopes recognized by CD4 T cells as well as by AMA (Figure 2). Notably the autoepitope for T cells, both CD4 and CD8 T cells, overlaps with the B cell (AMA) counterpart and includes the lipoylated amino acid K173 of the inner lipoyl domain. Similar to CD4 autoreactive T cells, there is a 10-fold higher frequency of PDC-E2 159–167 specific CD8 T cells within the liver versus peripheral blood Moreover, the precursor frequency of PDC-E2-specific autoreactive CD8 T cells is significantly higher in early rather than late stage of the disease. The autoreactive CD8 T cells in PBC have specific cytotoxicity against PDC-E2 antigen and, as well, produce IFN-g rather than IL-4/IL-10 cytokines 27.
The role of lipoic acid in PDC-E2 recognition
The three-dimensional model of PDC-E2, and its unique oxidative mechanisms, raise the idea that foreign compounds that mimic or alter lipoic acid could bind AMA when the lipoic acid molecule becomes conjugated to any carrier molecule, such that immunization of rabbits with a bovine serum albumin (BSA) conjugate of a lipoic acid mimic (6-bromohexanoic acid) would induce AMA28, and it was later demonstrated that lipoic acid can indeed be mounted on a protein background 29. Thus PBC could be due to chemical exposure, and lipoic acid or a lipoic acid mimic could be important in failure of tolerance to mitochondrial antigens 28, 30, 31. Several lipoic acid (IA) mimotopes have been identified with the use of mimotope conjugated carrier molecules and affinity purified anti-PDC-E2 antibodies; specifically, 79/97 (81%) of AMA-positive PBC sera reacted to lipoylated human albumin (HSA-LA), and a high reactivity to HSA-LA correlated with the level of reactivity to PDC-E2. Also, PDC-E2 affinity purified sera reacted with HSA-LA, suggesting that some of the antibodies to HSA-LA are a subset of anti-PDC-E2 specificities. The antibody reactivity to lipoylated PDC-E2 and PDC-E2 is predominantly IgG and IgM whereas that to HSA-LA is predominantly IgM. Bruggraber and colleagues 17 have demonstrated the presence in sera of patients with PBC of (a) antibodies with specificity against PDC-E2 that are capable of recognizing both PDC-E2 and lipoic acid and (b) antibodies to lipoic acid that recognize a conjugated form of lipoic acid but not the PDC-E2 backbone. Notably, antibodies to PDC-E2 LA and antibodies to lipoylated peptide conjugates are not merely cross-reactive antibodies: they differ in their epitope specificity and also in their Ig subclass and affinity for antigens. Previous studies had failed to detect anti-lipoic acid antibodies when free lipoic acid was used as an antigen 32, yet our findings otherwise on the presence in PBC of autoantibodies to lipoic acid 17 are of particular interest in resembling the immune response to iodine in autoimmune thyroiditis observed in chickens, rats and NOD mice 33–37.
Cholangiocyte apoptosis and “traditional” mitochondrial autoantigens
The mitochondrial antigens recognized by both B and T cell autoimmune responses in PBC are ubiquitously expressed in all nucleated cells, and are highly conserved in phylogenesis 38. Mitochondrial 2-OADC antigens are not “cryptic” to the immune system, and normally there is tolerance to these, even if there are responses to bacterial homologs which are phylogenetically distant from human proteins. During spontaneous or induced apoptosis, numerous---perhaps all--- cell types express mitochondrial antigens on the intact plasma membrane and within apoptotic blebs 39, 40 which then acquire the capability to initiate an autoimmune response by presentation of 2-OADC-derived autoantigens 41. Notably this latter process is specific to cholangiocytes, explaining in part why PBC recurs after liver transplantation 42, 43. Indeed, AMA may react, though weakly, against biliary epithelial cells of normal subjects 44 and specifically reactive T cells45, 46, and B cells, and serum AMA 47, have been found (at low levels) in the serum of non-PBC subjects. Nevertheless, the immune system starts a progressive autoimmune attack against cholangiocytes only in patients destined to develop PBC, and this occurs irrespective of whether the biliary epithelium is derived from a patient with PBC or a control subject 48. Accordingly, liver infiltrating autoreactive T cells to 2-OADC were found only in patients with PBC49, irrespective of their serum AMA status 22. The mitochondrial autoantigens undergo a particular cell-specific processing that may well contribute to, if not entirely explain, the organ specificity of PBC 50, 51. The lack of putative post-translational modifications alters protein degradation leading to the accumulation and exposure of large amounts of autoantigens, as postulated for the “traditional” autoantigens of the organ-specific autoimmune diseases 52. In most cell types, lysine-lipoylated sequences when released from mitochondria during apoptosis 50 are oxidized by glutathiones; the oxidated forms are not immunogenic and are not recognized by serum AMA because glutathionylation masks the autoantibody recognition site 50 53. On the other hand, cholangiocytes and cells from certain other epithelia fail to covalently link glutathione to lysine-lipoyl groups during apoptosis 50. In cholangiocytes cleavage of the immunodominant PDC-E2 epitope has not been detected in vivo during either apoptosis 50, 54 or phagocytosis 55. Moreover, the enhanced expression of 2-OADC proteins, with a particular luminal concentration, is seen early in cholangiocytes in PBC versus other chronic inflammatory biliary disease e.g. primary sclerosing cholangitis 44. This abnormal expression of PDC-E2 may depend on self-antigens being presented by cholangiocytes after binding to HLA molecules, although HLA class II on cholangiocytes have more an intrahepatic basolateral than luminal surface expression 56–60 and anyway, are expressed only weakly and in the early stages of disease 60. This observation could be also secondary to immune complexes deposition rather than membrane protein expression. There are different opinions on exposure of self-antigens on the cell membrane during cholangiocyte apoptosis 39, 50, or during the rearrangement of lipid rafts as seen after TLR activation by microbial infection 61 or after ingestion of apoptotic cholangiocytes by other cholangiocytes 55. Whilst cholangiocyte phagocytosis of neighboring apoptotic cholangiocytes is not specific to PBC, this effect could be involved in the presentation process of mitochondrial antigen observed in PBC indicating that mitochondrial autoantigens are similar to the ‘traditional’ group.
The intact PDC-E2 in apoptotic fragments could be uptaken by local antigen presenting cells and transferred to regional lymph nodes for priming of cognate T cells thus initiating PBC. This is indeed an attractive possibility, however solid data of such antigen presentation are awaited and it could not be excluded that the reported mechanisms are not PBC specific. A major contribution came from Lleo and colleagues who first demonstrated that PDC-E2 is found in the blebs of human intrahepatic bile duct cells undergoing apoptosis 40 and subsequently that macrophages are capable to uptake the autoantigen found in apoptotic blebs (coined apotopes) 41. The addition of serum AMA to the coculture of macrophages and apotopes led to a significant increase in proinflammatory cytokine secretion. These phenomena were not observed in other epithelial cell lines and appears to confirm the importance of apoptosis in the perpetuation of the autoimmune injury 62 as well as the view that PBC bile duct cells are not unique 48.
Antinuclear antibodies (ANA) in PBC
Serum ANA are detected in nearly 50% of patients with PBC with some reports suggesting that the prevalence may be higher in AMA-negative sera 63 PBC-specific indirect immunofluorescence patterns include ‘nuclear rim’, based on the recognition by the autoantibodies of gp210 and nucleoporin 62 (within the nuclear pore complex which also includes LBR) and ‘multiple nuclear dots’, based on the reactivity with Sp100, PML, and most recently, Sp140 (nuclear body proteins) 64, 65. In addition, the cross-reactivity with small ubiquitin-like modifiers (SUMO) bound to both Sp100 and PML have been suggested as independent antigens also specific for PBC 66. The prevalence of ‘nuclear rim’ ANA in PBC is similar with the different techniques utilized for the test, particularly when recombinant or isolated antigens are included. Gp210 consists of three main domains: a large glycosylated lumenal domain, a single hydrophobic transmembrane segment and a short cytoplasmic tail. The antigenic epitopes recognized by anti-gp210 ANA are located within the glycosylated lumenal domain (a 64 kD fragment) and the cytoplasmic tail (15 amino acids) 67. In general terms, anti-gp210 are detected in 26% of cases using the gp210-C terminal peptide a.a.1863–1887 68 and 27% when using isolated nuclear pore complexes 69. The major nuclear body protein is Sp100 which consists of at least three non-overlapping major autoantigenic domains in sp100 recognized by Sp100 positive PBC sera and two stretches of 16–20 amino acids are the predominant autoepitopes 67. One domain, which contains the sequence similarity with HIV nef proteins, was recognized by all anti-sp100 sera. The prevalence of anti-Sp100 ANA in PBC is estimated to range between 9% 68 and 30% 70 when different methods are used. It was first supposed that ANA-positive patients with PBC are more frequently AMA-negative, possibly because of the lack of a masking effect of these latter antibodies in such sera, yet this remains to be determined and current data do not support this view.
There is a third type of ANA associated with PBC and are directed against centromeric proteins (ACA) that occur in PBC mostly together with the usual “clinical partner” of ACA, limited CREST-type scleroderma, at a prevalence formerly cited as 10% 71 but now seemingly higher 68, although with specificity limited by the rheumatological comorbidity.
No studies have been able to discern any link between PBC-specific ANA or the antigens they recognize and the immunopathology of PBC, nor it is clear how or why these ANA are generated in individuals with PBC. Whether ANA-positivity should be regarded as the result of the unique cholangiocyte apoptotic features (discussed below) or of the T regulatory defect 72 is a fascinating hypothesis that awaits experimental confirmation and provides additional evidence that ‘nontraditional’ autoantigens in PBC could in fact be ‘traditional’. Nevertheless, significant associations between the presence of ANA and a worse prognosis have been independently reported 73, 74, different from AMA 75.
Innate immunity in PBC
Innate immunity as an activator of autoimmune responses is receiving much attention 76. The liver is a major organ of innate immunity in containing the largest resident population of innate immune system cells, including NK and NKT cells. As with other autoimmune diseases innate immune mechanisms likely contribute to the initiation and progression of liver damage 77, and in PBC in particular as judged by features such as epithelioid granulomas, elevated levels of polyclonal IgM, hyper-responsiveness of the immune system to CpG, increased levels in blood and liver NK cells 78 and an indicative cytokines response 77. There is a consistent elevation of serum polyclonal IgM in PBC, regardless of the AMA or ANA status 79, and reduction is usually observed during treatment 80. The hyper-IgM appears secondary to a chronic innate immune response of memory B cells to specific bacterial molecular motifs such as unmethylated CpG motifs 81. Furthermore patient peripheral B cells exposed to CpG motifs express increased amounts of TRL9 and CD86 so enhancing their production of AMA. This evidence strongly suggests a profound disease-specific dysregulation of B cells and supports the proposed link between bacterial infection and PBC i.e. B cells become hyper-responsive to innate stimuli, such as microbial CpG motifs, favoring perpetuation of the autoimmune process 82. Other innate immune cells such as monocytes have also been implicated in the pathogenesis of PBC since their pro-inflammatory activity is greatly enhanced in PBC. Functionally, monocytes become activated by pathogen-associated patterns through TLR to release pro-inflammatory cytokines, including IL-1, IL-6, IL-18, IL-12, and TNF-α that can amplify adaptive T cell mediated immune responses against pathogens 83. Thus peripheral blood monocytes in PBC are overly sensitive to infectious stimuli resulting in hyper-secretion of pro-inflammatory cytokines; the relevant mechanisms are unknown but might relate to the higher frequency of recurrent Gram-negative bacterial infections (e.g. urinary tract infections) in PBC. In other words both B cells and monocytes constantly exposed to bacterial products from portal blood could participate in the modulation of adaptive cellular immune response and possibly also in its priming.
NKT cells are now implicated in autoimmunity84 as innate effector cells which are regulated by self and non-self glycolipid antigens presented by the antigen-presenting molecule CD1d, allowing for a rapid NKT cells production of effector cytokines and chemokines, thus modulating both innate and adaptive immune responses. The involvement of NKT cells in the pathogenesis of PBC was suggested by our study reporting a higher than normal frequency of CD1d-restricted NKT cells in PBC patients and, as for autoreactive T cells, CD1d-restricted NKT cells were more frequent in liver than peripheral blood 26. Chuang et al. found likewise an increased number of CD1d-restricted NKT cells in the liver of a mouse model for PBC transgenic for directed expression of a dominant-negative form of TGF-beta receptor type II (dnTGFβRII). Such CD1d-restricted NKT cells in the liver had an increased IFN-γ production after exposure to α-galactosylceramide (α GalCer) and there was a decreased hepatic lymphoid cell infiltration and milder cholangitis than in controls 84 Innate immune system hyper-responsiveness is probably insufficient per se for disruption of immune tolerance, might well participate in the initiation and/or perpetuation of autoimmune injury. Thus Mattner et al demonstrated that, in a murine model of PBC (discussed below), N. aromaticivorans induced autoreactive AMAs and T cell-mediated autoimmunity against small bile ducts via NKT-dependent mechanisms 85.
Environmental agents and PBC autoantigens
Environmental agents, chemical compounds or infecting microbes, are suspected to be involved into the breakdown of tolerance, through molecular mimicry and cross reactivity mechanisms 86. This idea is supported by the less than complete genetic evidence including ~60% concordance rate for PBC in monozygotic twins 87 and insufficient associations in PBC patients from genome wide association studies 88, 89. Thus a mimotope carried by a microbe or a neo-antigen generated by xenobiotic-modified self-antigen that mimics mitochondrial proteins may activate autoreactive lymphocytes that have “leaked out” into the peripheral repertoire.. The process becomes self-perpetuating because of the presence of cross reactive unmodified self-antigens on cholangiocytes surface.
Similar to the majority of autoimmune diseases and their specific autoantibodies, the pathogenetic role of serum AMA remains debated and data questioned the sequence of the immunodominant B cell epitope and the role of the lysine-lipoylated motif in the PBC B cell response 90. Ultimately, only the recapitulation of an AMA-mediated injury in an animal model will provide definitive evidence of the autoantibody role in PBC pathology 91. However, the study of the immunodominant T cell epitope, PDC-E2 163–176, has provided important evidence on pathogenesis of PBC, based on the reactivity of cloned PDC-E2 163–176-specific T cell lines. Thus, for this peptide, the contact residues with T cell receptors (TCRs) are 168 EIExDK173 so that microbial proteins, whether or not related to PDC-E2, that have an ExDK sequence are potentially capable of recognition by autoreactive T cells. Interestingly, this activating PDC-E2 peptide was not lipoylated at K173 and conservative substitutions at position 173 did not abrogate T cell response, indicating that the lysine-lipoylated motif is a minor role participant in T cell responsiveness. However glutamic acid (E)170 is crucial to T cell recognition as its substitution abrogates reactivities. Considering the proximity of E170 to K173 (Figure 2), we hypothesize that the customary glutathionylation of the lysine-lipoyl residue at position 173 can mask or alter the exposure of E170 so as to abrogate contact between this residue and CDR3 of the TCR. Of note, this mechanism can be considered as an “immunologic defense” in most cell types with the exception of cholangiocytes 17. The experimental evidence illustrated thus far (along with the demonstration of autoantibody reactivity with lipoic acid) has obvious fascinating implications in terms of autoantigen selection. Indeed, one of the major issues in classical immunology is what makes an antigen an autoantigen. Indeed, this commonly takes place for intracellular enzymes (as in the case of PBC) and cell surface receptors. While molecular mimicry and epitope spreading (discussed below) cannot be overlooked, additional mechanisms have been sought and currently imply the structural features of antigens, particularly flexibility, as well illustrated by the three major type 1 diabetes autoantigens 92, 93. Other factors may also include dysfunctions of vitamin D 94 based on genetic polymorphisms 95 or other modulators 96.
Infectious agents
The question of an environmental factor initiating PBC 97, this is supported by the incomplete concordance among monozygotic twins 87, there are reported instances of non familial clustering 98–100, and a claimed changing risk of PBC in individuals moving from high risk to lower risk locations (i.e. geoepidemiology) 101, 102 103, 104. Infectious agents are the obvious choice for environmental candidates and support for an infectious hypothesis is garnered from data that lipopolysaccharide (LPS), a specific component of gram negative bacterial cell wall, injected into mice either alone or in combination with PDC-E2 induce the appearance of portal lymphocytic infiltration and cholangiocyte degeneration as seen in the human PBC liver. Further, lipoteichoic acid (LTA), the gram positive cell wall component, has been detected in PBC liver samples around damaged bile ducts, and serum levels of LTA-specific IgA are significantly higher in PBC than in normals 105, and bacterial DNA containing unmethylated CpG motifs triggers a PDC-specific Th1 response in PBMC from mice immunized with PDC 106. Finally, Th17 cells that are important components of the mucosal host defense system against infections (although also involved in the pathogenesis of various autoimmune diseases 107), are constituents of the periductular infiltrates in human PBC 108, 109. However, the identity of the suspected pathogen (if such does exist) and the exact initiating mechanisms remain unrecognized.
Among mechanisms proposed to explain just how an infectious agent could contribute to the onset of an autoimmune disease in genetically susceptible subjects, molecular mimicry remains popular, having been reported for many microbes 110. Shared sequences between human and microbial proteins can disrupt immune tolerance by inducing cross reactive antibodies or effector T cells and/or by promoting epitope spreading 111, although this has been refuted in PBC 112. Other non-exclusive mechanisms involve superantigen polyclonal activation of T cells, i.e. staphylococcal enterotoxins 113, mouse mammary tumor virus antigens 114 and viral polyclonal activation of B cells, i.e. Epstein Barr virus 115, IgA production, and Th17 differentiation.
We may summarize that numerous specific infectious agents, mainly bacteria, but also viruses, parasites, and fungi, have been suspected in PBC 116, but these studies have failed to demonstrate any clear association of a microbial agent with the disease and the evidence at best is only circumstantial, such as linear or conformational mimicry between microbial proteins and human mitochondrial antigens.
Xenobiotics
The other environmental factor seriously proposed for is constituted by foreign chemicals i.e. xenobiotics that can modify a defined self or non-self protein so as to cause a change in its molecular structure that enhances immunogenicity. This has been proposed for numerous autoimmune diseases and is consistent with the observed geoepidemiological gradient 117–119 and is supported by a number of epidemiology studies as previously discussed. One particular example is development of autoantibodies in subjects to halothane, a previously used inhalatory anesthetic that can induce antibodies reactive with lipoylated PDC-E2 120. As stated above, lipoic acid attaches to only a very limited number of proteins, yet is a critical component of the PDC-E2 epitope 17. The PDC-E2 structure exposes lipoic acid at the exterior of the protein complex making it accessible to chemical modification 29. The role of xenobiotics in PBC is supported by serum reactivity against specific organic compounds with structures similar to lipoic acid 121; further, two of these compounds (6-bromohexanoate and 2-octynoic acid) are capable of inducing AMA and PBC-like liver lesions in guinea pigs 122 and NOD.1101 123 or C57BL/6 124 mice, respectively. The ability of N. aromaticivorans to metabolize chemical compounds might link xenobiotics and bacteria in the etiology of PBC, as discussed above.
The 2-OADC antigens undergo several post-translational modifications endogenously, and such changes may alter the epitope regions of the proteins. Nevertheless, external influences can also contribute to protein alterations and neo-antigen formation 51. Of note, the liver is constantly exposed to chemicals derived from the gut through the portal circulation to be metabolized, activated, or excreted in the bile, and there is evidence that xenobiotics can modify mitochondrial proteins. Thus Long and colleagues in 2001 demonstrated that specific organic structures attached to the mitochondrial antigens were recognized by PBC sera with a higher affinity than the native forms of such antigens 125 suggesting that an organic compound may serve as a mimotope for an autoantigen; one such halogenated compound induced AMA production in rabbits with no requirement for the peptide backbone of PDC-E2 28 albeit without producing liver lesions (possibly due to latency of disease expression), and antibodies disappeared when the stimulus was discontinued 30. In another study induction of PBC-like liver lesions did follow during a longer follow-up in guinea pigs 122 while yet a further study reported two new xenobiotic-induced PBC murine models based on the immunization of NOD.1101 123 or C57BL/6124 mice with 2-octynoic acid. Both models illustrated breakdown of tolerance in the absence of exposure to PDC-E2 but there was no progression to liver disease. Utilizing a different approach, we next demonstrated that 2-nonynoic acid is capable of being recognized by PBC sera with high affinity 121, of interest since this non-naturally occurring compound is known to be found in several cosmetic products including nail polish and their frequent use among women could contribute to female predominant PBC 126, 127.
Cumulatively, the xenobiotic theory in PBC induction is fascinating and may well fit into the previously illustrated view that conformational changes of self-antigens may provide an efficient way to overcome the numerous checkpoints 128 for ‘forbidden’ clones to survive and expand to ultimately produce the orchestrated autoimmune response based on cellular and humoral responses 129.
The genetics of PBC
Not mutually exclusive with regard to environmental factors leading to PBC, the challenge to identify susceptibility gene(s) that predispose to the development of the diseases is still open. Of interest, a most recent multi-center study reported of the first genome-wide association study and identified interleukin 12 and its relative receptor as susceptibility genes for PBC 130. These data were later confirmed by our group in an independent cohort of Italian patients and controls as well as in a meta-analysis with Northern American subjects 131. Among significantly associated genes we recapitulated the proposed importance of HLA and STAT4 genes.
The majority of previous studies not only have been derived solely from case-control designs 132 but were also limited by poor control matching criteria and sample size or selection. A plethora of association studies have been conducted (reviewed elsewhere), mainly focused on immune genes that affect the immune system belonging to both the HLA family and non-HLA immune modulators genes, including CTLA-4, IL-1, IL-10 and vitamin D-receptor 132, 133.
Pertinent to the individual susceptibility to PBC, several sex-related factors appear to increase the risk of developing the disease, mostly by means of reproductive life variables. Among these are the role of pregnancies 134, 135, contraceptives, estrogen replacement treatments 126, and recurrent vaginitis 136 but the mechanisms remain to be elucidated. However, the novel hypothesis of sex chromosome-related effects on PBC appears promising 137, and may possibly operate via gene dosage or epigenetic changes 138 which appear to be common to autoimmunity in general 139–142.
Of interest to the individual genetic susceptibility to PBC is the observation that several animal models have been recently proposed based on a determined genetic background. Indeed, two animal models, i.e. dnTGFβRII and IL-2Rα knockout mouse, point out the possible crucial role of Tregs deficiency in loss of immune tolerance with consequent development of autoimmune response against PDC-E2 in PBC. The selective deficiency of the TGFβR-signaling pathway exclusively in T lymphocytes accounts for major impairments of peripheral tolerance as Treg cells depend on TGFβ for their regulatory activity, triggering the emergence of tissue-specific autoreactive effector T cells 143. A mouse deficient for IL2 receptor α (IL-2R α), which is highly expressed on Tregs developed 100% AMA positivity against PDC-E2, 80% ANA positivity, and lymphocyte infiltration around the portal tracts associated with cholangiocyte injury 144. These data illustrate the important relationships between Tregs and the appearance of autoimmune portal tract pathology and serum AMA. An additional animal model is a variant of the non obese diabetic (NOD) mouse model (NOD.c3c4) manifesting autoimmune cholestasis and PBC-specific serology, showing AMA positivity of 50%–60% and ANA positivity of 80%–90%. This mouse is protected from diabetes by B6/B10 regions on chromosomes 3 and 4 that contain B6/B10 insulin-dependent diabetes (Idd) loci. Histologically, it presents lymphocyte infiltration around portal tracts with chronic nonsuppurative destructive cholangitis and epithelioid granuloma formations; nevertheless, the morphological features of bile ducts differ somewhat from those in human PBC 145. Based on these animal models we may surmise that PBC susceptibility should be seen as a tolerance dysregulation secondary to a permissive background (as in the NOD model) or the lack of T regulatory mechanisms (as in the mouse deficient for IL2 receptor α on which an environmental insult could rapidly establish an autoimmune reaction 123, 124.
One cumulative theory has been recently proposed 128 and is based on the frequency of germline gene mutation, multiplied over the large number of known ‘tolerance/autoimmunity’ genes which are well illustrated in the numerous genome-wide association studies. These would result in a high frequency of deleterious mutations in a heterozygous state on which an autoimmune-prone phenotype could ensue either in the event of homozygosity of one or another such genes, if a somatic mutation occurred, or via a chromosome-specific haploinsufficiency, as well illustrated for the X chromosome 138. Further, most autoimmune diseases manifest a striking prevalence in older ages and the observed disease latency (in the case of PBC represented by the long latency between AMA appearance and disease manifestations) suggests that successive mutations may need to occur in a stepwise stochastic manner for the autoimmune phenotype to become apparent. Similar stochastic events may take place also at different levels, including the exposure to specific environmental factors or the interaction between different cell types in the maintenance of immune tolerance. This is supported by most recent evidence obtained in inflammatory bowel diseases 146 where genes and infections concur to the development of Crohn’s disease in an animal model 147. There are multiple other environmental agents and factors that have been incriminated in other autoimmune diseases, including the potential role of nucleic acids as adjuvants that have not yet been studied in PBC, but which should be addressed as possible modulators of pathology, including epigenetic influences, commensal microbiota, nanoparticles, ultraviolet light, tobacco smoke, nutrition and stress 148–156.
Linking AMA in the pathogenesis of PBC
In contrast to classic systemic autoimmune diseases, PBC is highly tissue specific with the cells of the small intrahepatic bile ducts as the primary target. However, apparently paradoxically, the autoantigens of PBC, PDC-E2, OGDC-E2, and BCOADC-E2, are ubiquitous mitochondrial proteins in all nucleated cells and hence seen as “non-traditional” at least as compared with the “traditional” autoantigens of thyrogastric organ-specific autoimmunity---this is possibly resolved as discussed below by the unique immunopathological characteristics of cholangiocytes.
However, first of all, staining of small bile ducts with a panel of monoclonal antibodies against the mitochondrial autoantigens has demonstrated that some give an intense staining at the apical surface of the cells lining the bile duct lumen, and this is specific for PBC liver157, 158 versus other liver pathology. This apical staining is seen only with selected PDC-E2 specific monoclonal antibodies and that distinct epitopes could identified with such monoclonal antibodies, leading to the hypothesis that a PDC-E2-mimicking (and thus cross-reactive with AMA protein is recognized by the human autoantibodies 157–159. Others have suggested that the apical staining is due to immune complexes of IgA AMA and PDC-E2 160, 161. While a solid proteomic confirmation of this critical issue is awaited, it does appear that cholangiocytes are not just an innocent bystander in PBC pathology, but rather are active participants. They may be involved in biliary and mucosal secretory transfer including that of dimeric IgA 162–164. In particular, experimental data 161 suggest that PDC-E2-specific IgA may enter bile duct cells via a poly-Ig receptor (pIgR) and complex with PDC-E2, thereby potentially contributing to pathology. Anti-PDC-E2 IgA antibody titer in PBC sera directly correlates with the level of caspase activation 54 and suggest that during transcytosis through pIgR+ cells, exposure to PDC-E2-specific dimeric IgA can result in the initiation of caspase activation. Based on the presence of dimeric AMA-IgA in biliary and mucosal secretions 165, constant transcytosis may render the exposed cells more susceptible to apoptosis, thus producing the bile duct damage. The apoptosis of biliary epithelial cells in PBC warrants further discussion and may be proved to be crucial for immune tolerance breakdown 40, 62, as illustrated in other experimental settings 166. It was first reported that PDC-E2 remains intact and retains its immunogenicity during cholangiocyte apoptosis, due to a cell-specific lack of glutathionylation of biliary epithelial cells 50. The intact PDC-E2 in apoptotic fragments could be uptaken by local antigen presenting cells and transferred to regional lymph nodes for priming of cognate T cells thus initiating PBC. A major contribution came from Lleo and colleagues who most recently demonstrated that PDC-E2 is found in the blebs of human intrahepatic bile duct cells undergoing apoptosis 40. More importantly, this phenomenon was not observed in other epithelial cell lines and appears to confirm the importance of apoptosis in the perpetuation of the autoimmune injury 62 as well as the view that PBC bile duct cells are not unique 48. This is indeed an attractive possibility, and data supporting such antigen presentation have been recently proposed 41 to ultimately support that PBC autoantigens (both nuclear and mitochondrial) are indeed ‘traditional’.
Concluding remarks
Based on the data discussed, PBC can be sustained as a model autoimmune disease in which there is loss of immune tolerance to the mitochondrially located PDC-E2 autoantigen with the emergence of autoreactive populations of T and B lymphocytes (forbidden clones in the old parlance). Among many likely contributors to PBC pathogenesis is a strong genetic predisposition, not yet sufficiently dissected out, and environmental influences among which some could determine the structure and flexibility of the autoantigen, and others (life-style factors) could impact more on immune function itself 126. Particularly promising are current studies on a cholangiocyte-specific form of apoptotic degradation of the PDC-E2 autoantigen which in our view could blur distinctions between the “traditional” autoantigens of the organ-specific autoimmune diseases and the “non-traditional” autoantigens of PBC, chiefly PDC-E2 40, 41. Further, we may expect that additional unsuspected factors will be investigated in the pathogenesis of PBC. These may include the recently suggested immunomodulatory effects of the gut microbiota 167 as intestinal adsorption capacity was already found impaired in patients with PBC 168. Clearly there are many avenues still wide open to would-be investigators of the pathogenesis of PBC.
Acknowledgments
Funding provided by National Institutes of Health grant DK39588.
Bibliography
- 1.Atassi MZ, Casali P. Molecular mechanisms of autoimmunity. Autoimmunity. 2008;41(2):123–32. doi: 10.1080/08916930801929021. [DOI] [PubMed] [Google Scholar]
- 2.Mackay IR. Autoimmunity since the 1957 clonal selection theory: a little acorn to a large oak. Immunol Cell Biol. 2008;86(1):67–71. doi: 10.1038/sj.icb.7100135. [DOI] [PubMed] [Google Scholar]
- 3.Cohn M, Mitchison NA, Paul WE, Silverstein AM, Talmage DW, Weigert M. Reflections on the clonal-selection theory. Nat Rev Immunol. 2007;7(10):823–30. doi: 10.1038/nri2177. [DOI] [PubMed] [Google Scholar]
- 4.Neuberger MS. Antibody diversification by somatic mutation: from Burnet onwards. Immunol Cell Biol. 2008;86(2):124–32. doi: 10.1038/sj.icb.7100160. [DOI] [PubMed] [Google Scholar]
- 5.Reuben A. The serology of the Addison-Gull syndrome. Hepatology. 2003;37(1):225–8. doi: 10.1002/hep.510370134. [DOI] [PubMed] [Google Scholar]
- 6.Ahrens EH, Jr, Payne MA, Kunkel HG, Eisenmenger WJ, Blondheim SH. Primary biliary cirrhosis. Medicine (Baltimore) 1950;29(4):299–364. doi: 10.1097/00005792-195012000-00002. [DOI] [PubMed] [Google Scholar]
- 7.Walker JG, Doniach D, Roitt IM, Sherlock S. Serological Tests in Diagnosis of Primary Biliary Cirrhosis. Lancet. 1965;39:827–31. doi: 10.1016/s0140-6736(65)91372-3. [DOI] [PubMed] [Google Scholar]
- 8.Gershwin ME, Mackay IR, Sturgess A, Coppel RL. Identification and specificity of a cDNA encoding the 70 kd mitochondrial antigen recognized in primary biliary cirrhosis. J Immunol. 1987;138(10):3525–31. [PubMed] [Google Scholar]
- 9.Fussey SP, Guest JR, James OF, Bassendine MF, Yeaman SJ. Identification and analysis of the major M2 autoantigens in primary biliary cirrhosis. Proc Natl Acad Sci U S A. 1988;85(22):8654–8. doi: 10.1073/pnas.85.22.8654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mackay IR. Travels and travails of autoimmunity: a historical journey from discovery to rediscovery. Autoimmun Rev. 2010;9(5):A251–8. doi: 10.1016/j.autrev.2009.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Tamby MC, Chanseaud Y, Guillevin L, Mouthon L. New insights into the pathogenesis of systemic sclerosis. Autoimmun Rev. 2003;2(3):152–7. doi: 10.1016/s1568-9972(03)00004-1. [DOI] [PubMed] [Google Scholar]
- 12.Zieve GW, Khusial PR. The anti-Sm immune response in autoimmunity and cell biology. Autoimmun Rev. 2003;2(5):235–40. doi: 10.1016/s1568-9972(03)00018-1. [DOI] [PubMed] [Google Scholar]
- 13.Reed LJ, Hackert ML. Structure-function relationships in dihydrolipoamide acyltransferases. J Biol Chem. 1990;265(16):8971–4. [PubMed] [Google Scholar]
- 14.Thekkumkara TJ, Pons G, Mitroo S, Jentoft JE, Patel MS. Molecular biology of the human pyruvate dehydrogenase complex: structural aspects of the E2 and E3 components. Ann N Y Acad Sci. 1989;573:113–29. doi: 10.1111/j.1749-6632.1989.tb14990.x. [DOI] [PubMed] [Google Scholar]
- 15.Neagle J, De Marcucci O, Dunbar B, Lindsay JG. Component X of mammalian pyruvate dehydrogenase complex: structural and functional relationship to the lipoate acetyltransferase (E2) component. FEBS Lett. 1989;253(1–2):11–5. doi: 10.1016/0014-5793(89)80919-6. [DOI] [PubMed] [Google Scholar]
- 16.Howard MJ, Fuller C, Broadhurst RW, Perham RN, Tang JG, Quinn J, et al. Three-dimensional structure of the major autoantigen in primary biliary cirrhosis. Gastroenterology. 1998;115(1):139–46. doi: 10.1016/s0016-5085(98)70375-0. [DOI] [PubMed] [Google Scholar]
- 17.Bruggraber SF, Leung PS, Amano K, Quan C, Kurth MJ, Nantz MH, et al. Autoreactivity to lipoate and a conjugated form of lipoate in primary biliary cirrhosis. Gastroenterology. 2003;125(6):1705–13. doi: 10.1053/j.gastro.2003.09.034. [DOI] [PubMed] [Google Scholar]
- 18.Meda F, Zuin M, Invernizzi P, Vergani D, Selmi C. Serum autoantibodies: a road map for the clinical hepatologist. Autoimmunity. 2008;41(1):27–34. doi: 10.1080/08916930701619227. [DOI] [PubMed] [Google Scholar]
- 19.Shimoda S, Van de Water J, Ansari A, Nakamura M, Ishibashi H, Coppel RL, et al. Identification and precursor frequency analysis of a common T cell epitope motif in mitochondrial autoantigens in primary biliary cirrhosis. J Clin Invest. 1998;102(10):1831–40. doi: 10.1172/JCI4213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shimoda S, Harada K, Niiro H, Yoshizumi T, Soejima Y, Taketomi A, et al. Biliary epithelial cells and primary biliary cirrhosis: the role of liver-infiltrating mononuclear cells. Hepatology. 2008;47(3):958–65. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 21.Shimoda S, Ishikawa F, Kamihira T, Komori A, Niiro H, Baba E, et al. Autoreactive T-cell responses in primary biliary cirrhosis are proinflammatory whereas those of controls are regulatory. Gastroenterology. 2006;131(2):606–18. doi: 10.1053/j.gastro.2006.05.056. [DOI] [PubMed] [Google Scholar]
- 22.Shimoda S, Miyakawa H, Nakamura M, Ishibashi H, Kikuchi K, Kita H, et al. CD4 T-cell autoreactivity to the mitochondrial autoantigen PDC-E2 in AMA-negative primary biliary cirrhosis. J Autoimmun. 2008;31(2):110–5. doi: 10.1016/j.jaut.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 23.Shimoda S, Nakamura M, Ishibashi H, Hayashida K, Niho Y. HLA DRB4 0101-restricted immunodominant T cell autoepitope of pyruvate dehydrogenase complex in primary biliary cirrhosis: evidence of molecular mimicry in human autoimmune diseases. J Exp Med. 1995;181(5):1835–45. doi: 10.1084/jem.181.5.1835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Shimoda S, Nakamura M, Ishibashi H, Kawano A, Kamihira T, Sakamoto N, et al. Molecular mimicry of mitochondrial and nuclear autoantigens in primary biliary cirrhosis. Gastroenterology. 2003;124(7):1915–25. doi: 10.1016/s0016-5085(03)00387-1. [DOI] [PubMed] [Google Scholar]
- 25.Shimoda S, Nakamura M, Shigematsu H, Tanimoto H, Gushima T, Gershwin ME, et al. Mimicry peptides of human PDC-E2 163–176 peptide, the immunodominant T-cell epitope of primary biliary cirrhosis. Hepatology. 2000;31(6):1212–6. doi: 10.1053/jhep.2000.8090. [DOI] [PubMed] [Google Scholar]
- 26.Kita H, Naidenko OV, Kronenberg M, Ansari AA, Rogers P, He XS, et al. Quantitation and phenotypic analysis of natural killer T cells in primary biliary cirrhosis using a human CD1d tetramer. Gastroenterology. 2002;123(4):1031–43. doi: 10.1053/gast.2002.36020. [DOI] [PubMed] [Google Scholar]
- 27.Kita H, Matsumura S, He XS, Ansari AA, Lian ZX, Van de Water J, et al. Quantitative and functional analysis of PDC-E2-specific autoreactive cytotoxic T lymphocytes in primary biliary cirrhosis. J Clin Invest. 2002;109(9):1231–40. doi: 10.1172/JCI14698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Leung PS, Quan C, Park O, Van de Water J, Kurth MJ, Nantz MH, et al. Immunization with a xenobiotic 6-bromohexanoate bovine serum albumin conjugate induces antimitochondrial antibodies. J Immunol. 2003;170(10):5326–32. doi: 10.4049/jimmunol.170.10.5326. [DOI] [PubMed] [Google Scholar]
- 29.Walden HR, Kirby JA, Yeaman SJ, Gray J, Jones DE, Palmer JM. Xenobiotic incorporation into pyruvate dehydrogenase complex can occur via the exogenous lipoylation pathway. Hepatology. 2008;48(6):1874–84. doi: 10.1002/hep.22540. [DOI] [PubMed] [Google Scholar]
- 30.Amano K, Leung PS, Xu Q, Marik J, Quan C, Kurth MJ, et al. Xenobiotic-induced loss of tolerance in rabbits to the mitochondrial autoantigen of primary biliary cirrhosis is reversible. J Immunol. 2004;172(10):6444–52. doi: 10.4049/jimmunol.172.10.6444. [DOI] [PubMed] [Google Scholar]
- 31.Amano K, Leung PS, Rieger R, Quan C, Wang X, Marik J, et al. Chemical xenobiotics and mitochondrial autoantigens in primary biliary cirrhosis: identification of antibodies against a common environmental, cosmetic, and food additive, 2-octynoic acid. J Immunol. 2005;174(9):5874–83. doi: 10.4049/jimmunol.174.9.5874. [DOI] [PubMed] [Google Scholar]
- 32.Quinn J, Diamond AG, Palmer JM, Bassendine MF, James OF, Yeaman SJ. Lipoylated and unlipoylated domains of human PDC-E2 as autoantigens in primary biliary cirrhosis: significance of lipoate attachment. Hepatology. 1993;18 (6):1384–91. [PubMed] [Google Scholar]
- 33.Li M, Eastman CJ, Boyages SC. Iodine induced lymphocytic thyroiditis in the BB/W rat: early and late immune phenomena. Autoimmunity. 1993;14(3):181–7. doi: 10.3109/08916939309077364. [DOI] [PubMed] [Google Scholar]
- 34.Maczek C, Neu N, Wick G, Hala K. Target organ susceptibility and autoantibody production in an animal model of spontaneous autoimmune thyroiditis. Autoimmunity. 1992;12(4):277–84. doi: 10.3109/08916939209148470. [DOI] [PubMed] [Google Scholar]
- 35.Many MC, Maniratunga S, Denef JF. The non-obese diabetic (NOD) mouse: an animal model for autoimmune thyroiditis. Exp Clin Endocrinol Diabetes. 1996;104 (Suppl 3):17–20. doi: 10.1055/s-0029-1211673. [DOI] [PubMed] [Google Scholar]
- 36.Sternthal E, Like AA, Sarantis K, Braverman LE. Lymphocytic thyroiditis and diabetes in the BB/W rat. A new model of autoimmune endocrinopathy. Diabetes. 1981;30(12):1058–61. doi: 10.2337/diab.30.12.1058. [DOI] [PubMed] [Google Scholar]
- 37.Wick G, Brezinschek HP, Hala K, Dietrich H, Wolf H, Kroemer G. The obese strain of chickens: an animal model with spontaneous autoimmune thyroiditis. Adv Immunol. 1989;47:433–500. doi: 10.1016/s0065-2776(08)60666-5. [DOI] [PubMed] [Google Scholar]
- 38.Padgett KA, Selmi C, Kenny TP, Leung PS, Balkwill DL, Ansari AA, et al. Phylogenetic and immunological definition of four lipoylated proteins from Novosphingobium aromaticivorans, implications for primary biliary cirrhosis. J Autoimmun. 2005;24(3):209–19. doi: 10.1016/j.jaut.2005.01.012. [DOI] [PubMed] [Google Scholar]
- 39.Macdonald P, Palmer J, Kirby JA, Jones DE. Apoptosis as a mechanism for cell surface expression of the autoantigen pyruvate dehydrogenase complex. Clin Exp Immunol. 2004;136(3):559–67. doi: 10.1111/j.1365-2249.2004.02484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Lleo A, Selmi C, Invernizzi P, Podda M, Coppel RL, Mackay IR, et al. Apotopes and the biliary specificity of primary biliary cirrhosis. Hepatology. 2009;49(3):871–9. doi: 10.1002/hep.22736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lleo A, Bowlus CL, Yang GX, Invernizzi P, Podda M, Van de Water J, et al. Biliary apotopes and anti-mitochondrial antibodies activate innate immune responses in primary biliary cirrhosis. Hepatology. 2010 doi: 10.1002/hep.23783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Neuberger J. Liver transplantation for primary biliary cirrhosis: indications and risk of recurrence. J Hepatol. 2003;39(2):142–8. doi: 10.1016/s0168-8278(03)00283-6. [DOI] [PubMed] [Google Scholar]
- 43.Jacob DA, Neumann UP, Bahra M, Langrehr JM, Neuhaus P. Liver transplantation for primary biliary cirrhosis: influence of primary immunosuppression on survival. Transplant Proc. 2005;37(4):1691–2. doi: 10.1016/j.transproceed.2005.03.130. [DOI] [PubMed] [Google Scholar]
- 44.Cha S, Leung PS, Gershwin ME, Fletcher MP, Ansari AA, Coppel RL. Combinatorial autoantibodies to dihydrolipoamide acetyltransferase, the major autoantigen of primary biliary cirrhosis. Proc Natl Acad Sci U S A. 1993;90(6):2527–31. doi: 10.1073/pnas.90.6.2527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ichiki Y, Shimoda S, Hara H, Shigematsu H, Nakamura M, Hayashida K, et al. Analysis of T-cell receptor beta of the T-cell clones reactive to the human PDC-E2 163–176 peptide in the context of HLA-DR53 in patients with primary biliary cirrhosis. Hepatology. 1997;26(3):728–33. doi: 10.1053/jhep.1997.v26.pm0009303504. [DOI] [PubMed] [Google Scholar]
- 46.Shigematsu H, Shimoda S, Nakamura M, Matsushita S, Nishimura Y, Sakamoto N, et al. Fine specificity of T cells reactive to human PDC-E2 163–176 peptide, the immunodominant autoantigen in primary biliary cirrhosis: implications for molecular mimicry and cross-recognition among mitochondrial autoantigens. Hepatology. 2000;32(5):901–9. doi: 10.1053/jhep.2000.18714. [DOI] [PubMed] [Google Scholar]
- 47.Chen QY, Rowley MJ, Mackay IR. Anti-idiotypic antibodies to anti-PDC-E2 in primary biliary cirrhosis and normal subjects. Hepatology. 1999;29(3):624–31. doi: 10.1002/hep.510290344. [DOI] [PubMed] [Google Scholar]
- 48.Shimoda S, Harada K, Niiro H, Yoshizumi T, Soejima Y, Taketomi A, et al. Biliary epithelial cells and primary biliary cirrhosis: The role of liver-infiltrating mononuclear cells. Hepatology. 2008;47(3):958–965. doi: 10.1002/hep.22102. [DOI] [PubMed] [Google Scholar]
- 49.Borchers AT, Shimoda S, Bowlus C, Keen CL, Gershwin ME. Lymphocyte recruitment and homing to the liver in primary biliary cirrhosis and primary sclerosing cholangitis. Semin Immunopathol. 2009;31(3):309–22. doi: 10.1007/s00281-009-0167-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Odin JA, Huebert RC, Casciola-Rosen L, LaRusso NF, Rosen A. Bcl-2-dependent oxidation of pyruvate dehydrogenase-E2, a primary biliary cirrhosis autoantigen, during apoptosis. J Clin Invest. 2001;108(2):223–32. doi: 10.1172/JCI10716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Mao TK, Davis PA, Odin JA, Coppel RL, Gershwin ME. Sidechain biology and the immunogenicity of PDC-E2, the major autoantigen of primary biliary cirrhosis. Hepatology. 2004;40(6):1241–8. doi: 10.1002/hep.20491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacobson EM, Huber A, Tomer Y. The HLA gene complex in thyroid autoimmunity: from epidemiology to etiology. J Autoimmun. 2008;30(1–2):58–62. doi: 10.1016/j.jaut.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Sasaki M, Ikeda H, Nakanuma Y. Activation of ATM signaling pathway is involved in oxidative stress-induced expression of mito-inhibitory p21WAF1/Cip1 in chronic non-suppurative destructive cholangitis in primary biliary cirrhosis: an immunohistochemical study. J Autoimmun. 2008;31(1):73–8. doi: 10.1016/j.jaut.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 54.Matsumura S, Van De Water J, Leung P, Odin JA, Yamamoto K, Gores GJ, et al. Caspase induction by IgA antimitochondrial antibody: IgA-mediated biliary injury in primary biliary cirrhosis. Hepatology. 2004;39(5):1415–22. doi: 10.1002/hep.20175. [DOI] [PubMed] [Google Scholar]
- 55.Allina J, Hu B, Sullivan DM, Fiel MI, Thung SN, Bronk SF, et al. T cell targeting and phagocytosis of apoptotic biliary epithelial cells in primary biliary cirrhosis. J Autoimmun. 2006;27(4):232–41. doi: 10.1016/j.jaut.2006.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ballardini G, Mirakian R, Bianchi FB, Pisi E, Doniach D, Bottazzo GF. Aberrant expression of HLA-DR antigens on bileduct epithelium in primary biliary cirrhosis: relevance to pathogenesis. Lancet. 1984;2(8410):1009–13. doi: 10.1016/s0140-6736(84)91108-5. [DOI] [PubMed] [Google Scholar]
- 57.Van den Oord JJ, Sciot R, Desmet VJ. Expression of MHC products by normal and abnormal bile duct epithelium. J Hepatol. 1986;3(3):310–7. doi: 10.1016/s0168-8278(86)80483-4. [DOI] [PubMed] [Google Scholar]
- 58.Ayres RC, Neuberger JM, Shaw J, Joplin R, Adams DH. Intercellular adhesion molecule-1 and MHC antigens on human intrahepatic bile duct cells: effect of pro-inflammatory cytokines. Gut. 1993;34(9):1245–9. doi: 10.1136/gut.34.9.1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Nishimoto H, Yamada G, Mizuno M, Tsuji T. Immunoelectron microscopic localization of MHC class 1 and 2 antigens on bile duct epithelial cells in patients with primary biliary cirrhosis. Acta Med Okayama. 1994;48(6):317–22. doi: 10.18926/AMO/31096. [DOI] [PubMed] [Google Scholar]
- 60.Tsuneyama K, Van de Water J, Leung PS, Cha S, Nakanuma Y, Kaplan M, et al. Abnormal expression of the E2 component of the pyruvate dehydrogenase complex on the luminal surface of biliary epithelium occurs before major histocompatibility complex class II and BB1/B7 expression. Hepatology. 1995;21 (4):1031–7. [PubMed] [Google Scholar]
- 61.Nelson JB, O’Hara SP, Small AJ, Tietz PS, Choudhury AK, Pagano RE, et al. Cryptosporidium parvum infects human cholangiocytes via sphingolipid-enriched membrane microdomains. Cell Microbiol. 2006;8(12):1932–45. doi: 10.1111/j.1462-5822.2006.00759.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lleo A, Selmi C, Invernizzi P, Podda M, Gershwin ME. The consequences of apoptosis in autoimmunity. J Autoimmun. 2008;31(3):257–62. doi: 10.1016/j.jaut.2008.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muratori P, Granito A, Ferri S, Pappas G, Volta U, Menichella R, et al. Multiple nuclear dots and rim-like/membranous IgG isotypes in primary biliary cirrhosis. Autoimmunity. 2009;42(3):224–7. doi: 10.1080/08916930802709133. [DOI] [PubMed] [Google Scholar]
- 64.Invernizzi P, Selmi C, Ranftler C, Podda M, Wesierska-Gadek J. Antinuclear antibodies in primary biliary cirrhosis. Semin Liver Dis. 2005;25(3):298–310. doi: 10.1055/s-2005-916321. [DOI] [PubMed] [Google Scholar]
- 65.Granito A, Yang WH, Muratori L, Lim MJ, Nakajima A, Ferri S, et al. PML nuclear body component Sp140 is a novel autoantigen in primary biliary cirrhosis. Am J Gastroenterol. 2010;105(1):125–31. doi: 10.1038/ajg.2009.596. [DOI] [PubMed] [Google Scholar]
- 66.Janka C, Selmi C, Gershwin ME, Will H, Sternsdorf T. Small ubiquitin-related modifiers: A novel and independent class of autoantigens in primary biliary cirrhosis. Hepatology. 2005;41(3):609–16. doi: 10.1002/hep.20619. [DOI] [PubMed] [Google Scholar]
- 67.Worman HJ. Nuclear envelope protein autoantigens in primary biliary cirrhosis. Hepatol Res. 2007;37 (Suppl 3):S406–11. doi: 10.1111/j.1872-034X.2007.00227.x. [DOI] [PubMed] [Google Scholar]
- 68.Nakamura M, Kondo H, Mori T, Komori A, Matsuyama M, Ito M, et al. Anti-gp210 and anti-centromere antibodies are different risk factors for the progression of primary biliary cirrhosis. Hepatology. 2007;45(1):118–27. doi: 10.1002/hep.21472. [DOI] [PubMed] [Google Scholar]
- 69.Invernizzi P, Podda M, Battezzati PM, Crosignani A, Zuin M, Hitchman E, et al. Autoantibodies against nuclear pore complexes are associated with more active and severe liver disease in primary biliary cirrhosis. J Hepatol. 2001;34(3):366–72. doi: 10.1016/s0168-8278(00)00040-4. [DOI] [PubMed] [Google Scholar]
- 70.Zuchner D, Sternsdorf T, Szostecki C, Heathcote EJ, Cauch-Dudek K, Will H. Prevalence, kinetics, and therapeutic modulation of autoantibodies against Sp100 and promyelocytic leukemia protein in a large cohort of patients with primary biliary cirrhosis. Hepatology. 1997;26(5):1123–30. doi: 10.1002/hep.510260506. [DOI] [PubMed] [Google Scholar]
- 71.Makinen D, Fritzler M, Davis P, Sherlock S. Anticentromere antibodies in primary biliary cirrhosis. Arthritis Rheum. 1983;26(7):914–7. doi: 10.1002/art.1780260714. [DOI] [PubMed] [Google Scholar]
- 72.Lan RY, Cheng C, Lian ZX, Tsuneyama K, Yang GX, Moritoki Y, et al. Liver-targeted and peripheral blood alterations of regulatory T cells in primary biliary cirrhosis. Hepatology. 2006;43(4):729–37. doi: 10.1002/hep.21123. [DOI] [PubMed] [Google Scholar]
- 73.Nakamura M, Komori A, Ito M, Kondo H, Aiba Y, Migita K, et al. Predictive role of anti-gp210 and anticentromere antibodies in long-term outcome of primary biliary cirrhosis. Hepatol Res. 2007;37 (Suppl 3):S412–9. doi: 10.1111/j.1872-034X.2007.00244.x. [DOI] [PubMed] [Google Scholar]
- 74.Wesierska-Gadek J, Penner E, Battezzati PM, Selmi C, Zuin M, Hitchman E, et al. Correlation of initial autoantibody profile and clinical outcome in primary biliary cirrhosis. Hepatology. 2006;43(5):1135–44. doi: 10.1002/hep.21172. [DOI] [PubMed] [Google Scholar]
- 75.Invernizzi P, Crosignani A, Battezzati PM, Covini G, De Valle G, Larghi A, et al. Comparison of the clinical features and clinical course of antimitochondrial antibody-positive and -negative primary biliary cirrhosis. Hepatology. 1997;25 (5):1090–5. doi: 10.1002/hep.510250507. [DOI] [PubMed] [Google Scholar]
- 76.Zuin M, Giorgini A, Selmi C, Battezzati PM, Cocchi CA, Crosignani A, et al. Acute liver and renal failure during treatment with buprenorphine at therapeutic dose. Dig Liver Dis. 2008 doi: 10.1016/j.dld.2007.12.014. [DOI] [PubMed] [Google Scholar]
- 77.Selmi C, Lleo A, Pasini S, Zuin M, Gershwin ME. Innate immunity and primary biliary cirrhosis. Curr Mol Med. 2009;9(1):45–51. doi: 10.2174/156652409787314525. [DOI] [PubMed] [Google Scholar]
- 78.Chuang YH, Lian ZX, Tsuneyama K, Chiang BL, Ansari AA, Coppel RL, et al. Increased killing activity and decreased cytokine production in NK cells in patients with primary biliary cirrhosis. J Autoimmun. 2006;26(4):232–40. doi: 10.1016/j.jaut.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 79.Kaplan MM, Gershwin ME. Primary biliary cirrhosis. N Engl J Med. 2005;353(12):1261–73. doi: 10.1056/NEJMra043898. [DOI] [PubMed] [Google Scholar]
- 80.Pares A, Caballeria L, Rodes J, Bruguera M, Rodrigo L, Garcia-Plaza A, et al. Long-term effects of ursodeoxycholic acid in primary biliary cirrhosis: results of a double-blind controlled multicentric trial. UDCA-Cooperative Group from the Spanish Association for the Study of the Liver. J Hepatol. 2000;32(4):561–6. doi: 10.1016/s0168-8278(00)80216-0. [DOI] [PubMed] [Google Scholar]
- 81.Kikuchi K, Lian ZX, Yang GX, Ansari AA, Ikehara S, Kaplan M, et al. Bacterial CpG induces hyper-IgM production in CD27(+) memory B cells in primary biliary cirrhosis. Gastroenterology. 2005;128(2):304–12. doi: 10.1053/j.gastro.2004.11.005. [DOI] [PubMed] [Google Scholar]
- 82.Moritoki Y, Lian ZX, Wulff H, Yang GX, Chuang YH, Lan RY, et al. AMA production in primary biliary cirrhosis is promoted by the TLR9 ligand CpG and suppressed by potassium channel blockers. Hepatology. 2007;45(2):314–22. doi: 10.1002/hep.21522. [DOI] [PubMed] [Google Scholar]
- 83.Mao TK, Lian ZX, Selmi C, Ichiki Y, Ashwood P, Ansari AA, et al. Altered monocyte responses to defined TLR ligands in patients with primary biliary cirrhosis. Hepatology. 2005;42(4):802–8. doi: 10.1002/hep.20859. [DOI] [PubMed] [Google Scholar]
- 84.Chuang YH, Lian ZX, Yang GX, Shu SA, Moritoki Y, Ridgway WM, et al. Natural killer T cells exacerbate liver injury in a transforming growth factor beta receptor II dominant-negative mouse model of primary biliary cirrhosis. Hepatology. 2008;47(2):571–80. doi: 10.1002/hep.22052. [DOI] [PubMed] [Google Scholar]
- 85.Mattner J, Savage PB, Leung P, Oertelt SS, Wang V, Trivedi O, et al. Liver autoimmunity triggered by microbial activation of natural killer T cells. Cell Host Microbe. 2008;3(5):304–15. doi: 10.1016/j.chom.2008.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Selmi C. The worldwide gradient of autoimmune conditions. Autoimmun Rev. 2010;9(5):A247–50. doi: 10.1016/j.autrev.2010.02.004. [DOI] [PubMed] [Google Scholar]
- 87.Selmi C, Mayo MJ, Bach N, Ishibashi H, Invernizzi P, Gish RG, et al. Primary biliary cirrhosis in monozygotic and dizygotic twins: genetics, epigenetics, and environment. Gastroenterology. 2004;127(2):485–92. doi: 10.1053/j.gastro.2004.05.005. [DOI] [PubMed] [Google Scholar]
- 88.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Gu X, et al. Primary biliary cirrhosis associated with HLA, IL12A, and IL12RB2 variants. N Engl J Med. 2009;360(24):2544–55. doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Liu X, Invernizzi P, Lu Y, Kosoy R, Lu Y, Bianchi I, et al. Genome-wide meta-analyses identifies three loci associated with primary biliary cirrhosis. Nat Genet. 2010 doi: 10.1038/ng.627. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Braun S, Berg C, Buck S, Gregor M, Klein R. Catalytic domain of PDC-E2 contains epitopes recognized by antimitochondrial antibodies in primary biliary cirrhosis. World J Gastroenterol. 2010;16(8):973–81. doi: 10.3748/wjg.v16.i8.973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rieger R, Gershwin ME. The X and why of xenobiotics in primary biliary cirrhosis. J Autoimmun. 2007;28(2–3):76–84. doi: 10.1016/j.jaut.2007.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Plotz PH. The autoantibody repertoire: searching for order. Nat Rev Immunol. 2003;3(1):73–8. doi: 10.1038/nri976. [DOI] [PubMed] [Google Scholar]
- 93.Arafat Y, Fenalti G, Whisstock JC, Mackay IR, Garcia de la Banda M, Rowley MJ, et al. Structural determinants of GAD antigenicity. Mol Immunol. 2009;47(2–3 ):493–505. doi: 10.1016/j.molimm.2009.08.022. [DOI] [PubMed] [Google Scholar]
- 94.Arteh J, Narra S, Nair S. Prevalence of vitamin D deficiency in chronic liver disease. Dig Dis Sci. 2010;55(9):2624–8. doi: 10.1007/s10620-009-1069-9. [DOI] [PubMed] [Google Scholar]
- 95.Tanaka A, Nezu S, Uegaki S, Kikuchi K, Shibuya A, Miyakawa H, et al. Vitamin D receptor polymorphisms are associated with increased susceptibility to primary biliary cirrhosis in Japanese and Italian populations. J Hepatol. 2009;50(6):1202–9. doi: 10.1016/j.jhep.2009.01.015. [DOI] [PubMed] [Google Scholar]
- 96.Peters MG, Di Bisceglie AM, Kowdley KV, Flye NL, Luketic VA, Munoz SJ, et al. Differences between Caucasian, African American, and Hispanic patients with primary biliary cirrhosis in the United States. Hepatology. 2007;46(3):769–75. doi: 10.1002/hep.21759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Selmi C, Cocchi CA, Zuin M, Gershwin ME. The chemical pathway to primary biliary cirrhosis. Clin Rev Allergy Immunol. 2009;36(1):23–9. doi: 10.1007/s12016-008-8089-7. [DOI] [PubMed] [Google Scholar]
- 98.Abu-Mouch S, Selmi C, Benson GD, Kenny TP, Invernizzi P, Zuin M, et al. Geographic clusters of primary biliary cirrhosis. Clin Dev Immunol. 2003;10:127–31. doi: 10.1080/10446670310001626526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Ala A, Stanca CM, Bu-Ghanim M, Ahmado I, Branch AD, Schiano TD, et al. Increased prevalence of primary biliary cirrhosis near Superfund toxic waste sites. Hepatology. 2006;43(3):525–31. doi: 10.1002/hep.21076. [DOI] [PubMed] [Google Scholar]
- 100.Prince MI, Chetwynd A, Diggle P, Jarner M, Metcalf JV, James OF. The geographical distribution of primary biliary cirrhosis in a well-defined cohort. Hepatology. 2001;34(6):1083–8. doi: 10.1053/jhep.2001.29760. [DOI] [PubMed] [Google Scholar]
- 101.Watson RG, Angus PW, Dewar M, Goss B, Sewell RB, Smallwood RA. Low prevalence of primary biliary cirrhosis in Victoria, Australia. Melbourne Liver Group Gut. 1995;36(6):927–30. doi: 10.1136/gut.36.6.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Anand AC, Elias E, Neuberger JM. End-stage primary biliary cirrhosis in a first generation migrant south Asian population. Eur J Gastroenterol Hepatol. 1996;8 (7):663–6. [PubMed] [Google Scholar]
- 103.Invernizzi P. Geoepidemiology of autoimmune liver diseases. J Autoimmun. 2010;34 (3):J300–6. doi: 10.1016/j.jaut.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 104.Youinou P, Pers JO, Gershwin ME, Shoenfeld Y. Geo-epidemiology and autoimmunity. J Autoimmun. 2010;34(3):J163–7. doi: 10.1016/j.jaut.2009.12.005. [DOI] [PubMed] [Google Scholar]
- 105.Haruta I, Hashimoto E, Kato Y, Kikuchi K, Kato H, Yagi J, et al. Lipoteichoic acid may affect the pathogenesis of bile duct damage in primary biliary cirrhosis. Autoimmunity. 2006;39(2):129–35. doi: 10.1080/08916930600623841. [DOI] [PubMed] [Google Scholar]
- 106.Jones DE, Palmer JM, Burt AD, Walker C, Robe AJ, Kirby JA. Bacterial motif DNA as an adjuvant for the breakdown of immune self-tolerance to pyruvate dehydrogenase complex. Hepatology. 2002;36(3):679–86. doi: 10.1053/jhep.2002.35067. [DOI] [PubMed] [Google Scholar]
- 107.Lan RY, Salunga TL, Tsuneyama K, Lian ZX, Yang GX, Hsu W, et al. Hepatic IL-17 responses in human and murine primary biliary cirrhosis. J Autoimmun. 2009;32(1):43–51. doi: 10.1016/j.jaut.2008.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Rong G, Zhou Y, Xiong Y, Zhou L, Geng H, Jiang T, et al. Imbalance between T helper type 17 and T regulatory cells in patients with primary biliary cirrhosis: the serum cytokine profile and peripheral cell population. Clin Exp Immunol. 2009;156(2):217–25. doi: 10.1111/j.1365-2249.2009.03898.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Harada K, Shimoda S, Sato Y, Isse K, Ikeda H, Nakanuma Y. Periductal interleukin–17 production in association with biliary innate immunity contributes to the pathogenesis of cholangiopathy in primary biliary cirrhosis. Clin Exp Immunol. 2009;157(2):261–70. doi: 10.1111/j.1365-2249.2009.03947.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Van de Water J, Ishibashi H, Coppel RL, Gershwin ME. Molecular mimicry and primary biliary cirrhosis: premises not promises. Hepatology. 2001;33(4):771–5. doi: 10.1053/jhep.2001.23902. [DOI] [PubMed] [Google Scholar]
- 111.Agmon-Levin N, Katz BS, Shoenfeld Y. Infection and primary biliary cirrhosis. Isr Med Assoc J. 2009;11(2):112–5. [PubMed] [Google Scholar]
- 112.Dubel L, Tanaka A, Leung PS, Van de Water J, Coppel R, Roche T, et al. Autoepitope mapping and reactivity of autoantibodies to the dihydrolipoamide dehydrogenase-binding protein (E3BP) and the glycine cleavage proteins in primary biliary cirrhosis. Hepatology. 1999;29(4):1013–8. doi: 10.1002/hep.510290403. [DOI] [PubMed] [Google Scholar]
- 113.Fraser JD, Proft T. The bacterial superantigen and superantigen-like proteins. Immunol Rev. 2008;225:226–43. doi: 10.1111/j.1600-065X.2008.00681.x. [DOI] [PubMed] [Google Scholar]
- 114.Czarneski J, Rassa JC, Ross SR. Mouse mammary tumor virus and the immune system. Immunol Res. 2003;27(2–3):469–80. doi: 10.1385/IR:27:2-3:469. [DOI] [PubMed] [Google Scholar]
- 115.Kuppers R. B cells under influence: transformation of B cells by Epstein-Barr virus. Nat Rev Immunol. 2003;3(10):801–12. doi: 10.1038/nri1201. [DOI] [PubMed] [Google Scholar]
- 116.Selmi C, De Santis M, Cavaciocchi F, Gershwin ME. Infectious agents and xenobiotics in the etiology of primary biliary cirrhosis. Dis Markers. 2010 doi: 10.3233/DMA-2010-0746. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Cooper GS, Bynum ML, Somers EC. Recent insights in the epidemiology of autoimmune diseases: Improved prevalence estimates and understanding of clustering of diseases. J Autoimmun. 2009 doi: 10.1016/j.jaut.2009.09.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Tobon GJ, Youinou P, Saraux A. The environment, geo-epidemiology, and autoimmune disease: Rheumatoid arthritis. J Autoimmun. 2010;35(1):10–4. doi: 10.1016/j.jaut.2009.12.009. [DOI] [PubMed] [Google Scholar]
- 119.Burek CL, Talor MV. Environmental triggers of autoimmune thyroiditis. J Autoimmun. 2009;33(3–4):183–9. doi: 10.1016/j.jaut.2009.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Christen U, Jeno P, Gut J. Halothane metabolism: the dihydrolipoamide acetyltransferase subunit of the pyruvate dehydrogenase complex molecularly mimics trifluoroacetyl-protein adducts. Biochemistry. 1993;32(6):1492–9. doi: 10.1021/bi00057a013. [DOI] [PubMed] [Google Scholar]
- 121.Rieger R, Leung PS, Jeddeloh MR, Kurth MJ, Nantz MH, Lam KS, et al. Identification of 2-nonynoic acid, a cosmetic component, as a potential trigger of primary biliary cirrhosis. J Autoimmun. 2006;27(1):7–16. doi: 10.1016/j.jaut.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 122.Leung PS, Park O, Tsuneyama K, Kurth MJ, Lam KS, Ansari AA, et al. Induction of primary biliary cirrhosis in guinea pigs following chemical xenobiotic immunization. J Immunol. 2007;179(4):2651–7. doi: 10.4049/jimmunol.179.4.2651. [DOI] [PubMed] [Google Scholar]
- 123.Wakabayashi K, Yoshida K, Leung PS, Moritoki Y, Yang GX, Tsuneyama K, et al. Induction of autoimmune cholangitis in non-obese diabetic (NOD). 1101 mice following a chemical xenobiotic immunization. Clin Exp Immunol. 2009;155(3):577–86. doi: 10.1111/j.1365-2249.2008.03837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Wakabayashi K, Lian ZX, Leung PS, Moritoki Y, Tsuneyama K, Kurth MJ, et al. Loss of tolerance in C57BL/6 mice to the autoantigen E2 subunit of pyruvate dehydrogenase by a xenobiotic with ensuing biliary ductular disease. Hepatology. 2008;48(2):531–40. doi: 10.1002/hep.22390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Long SA, Quan C, Van de Water J, Nantz MH, Kurth MJ, Barsky D, et al. Immunoreactivity of organic mimeotopes of the E2 component of pyruvate dehydrogenase: connecting xenobiotics with primary biliary cirrhosis. J Immunol. 2001;167(5):2956–63. doi: 10.4049/jimmunol.167.5.2956. [DOI] [PubMed] [Google Scholar]
- 126.Gershwin ME, Selmi C, Worman HJ, Gold EB, Watnik M, Utts J, et al. Risk factors and comorbidities in primary biliary cirrhosis: a controlled interview-based study of 1032 patients. Hepatology. 2005;42(5):1194–202. doi: 10.1002/hep.20907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Prince MI, Ducker SJ, James OF. Case-control studies of risk factors for primary biliary cirrhosis in two United Kingdom populations. Gut. 2010;59(4):508–12. doi: 10.1136/gut.2009.184218. [DOI] [PubMed] [Google Scholar]
- 128.Goodnow CC. Multistep pathogenesis of autoimmune disease. Cell. 2007;130(1):25–35. doi: 10.1016/j.cell.2007.06.033. [DOI] [PubMed] [Google Scholar]
- 129.Gershwin ME, Ansari AA, Mackay IR, Nakanuma Y, Nishio A, Rowley MJ, et al. Primary biliary cirrhosis: an orchestrated immune response against epithelial cells. Immunol Rev. 2000;174:210–25. doi: 10.1034/j.1600-0528.2002.017402.x. [DOI] [PubMed] [Google Scholar]
- 130.Hirschfield GM, Liu X, Xu C, Lu Y, Xie G, Gu X, et al. Primary Biliary Cirrhosis Associated with HLA, IL12A, and IL12RB2 Variants. N Engl J Med. 2009 doi: 10.1056/NEJMoa0810440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Liu X, Invernizzi P, Lu Y, Kosoy R, Bianchi I, Podda M, et al. Genome-wide meta-analyses identify three loci associated with primary biliary cirrhosis. Nat Genet. 2010;42(8):658–60. doi: 10.1038/ng.627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Selmi C, Invernizzi P, Zuin M, Podda M, Gershwin ME. Genetics and geoepidemiology of primary biliary cirrhosis: following the footprints to disease etiology. Semin Liver Dis. 2005;25(3):265–80. doi: 10.1055/s-2005-916319. [DOI] [PubMed] [Google Scholar]
- 133.Invernizzi P, Gershwin ME. The genetic basis of primary biliary cirrhosis: premises, not promises. Gastroenterology. 2008;135(4):1044–7. doi: 10.1053/j.gastro.2008.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Borchers AT, Naguwa SM, Keen CL, Gershwin ME. The implications of autoimmunity and pregnancy. J Autoimmun. 2010;34(3):J287–99. doi: 10.1016/j.jaut.2009.11.015. [DOI] [PubMed] [Google Scholar]
- 135.Parikh-Patel A, Gold E, Utts J, Gershwin ME. The association between gravidity and primary biliary cirrhosis. Ann Epidemiol. 2002;12(4):264–72. doi: 10.1016/s1047-2797(01)00277-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Bogdanos D, Pusl T, Rust C, Vergani D, Beuers U. Primary biliary cirrhosis following Lactobacillus vaccination for recurrent vaginitis. J Hepatol. 2008;49 (3):466–73. doi: 10.1016/j.jhep.2008.05.022. [DOI] [PubMed] [Google Scholar]
- 137.Invernizzi P, Miozzo M, Battezzati PM, Bianchi I, Grati FR, Simoni G, et al. Frequency of monosomy X in women with primary biliary cirrhosis. Lancet. 2004;363(9408):533–5. doi: 10.1016/S0140-6736(04)15541-4. [DOI] [PubMed] [Google Scholar]
- 138.Selmi C. The X in sex: how autoimmune diseases revolve around sex chromosomes. Best Pract Res Clin Rheumatol. 2008;22(5):913–22. doi: 10.1016/j.berh.2008.09.002. [DOI] [PubMed] [Google Scholar]
- 139.Svyryd Y, Molina GH, Vargas F, Sanchez-Guerrero J, Segovia DA, Mutchinick OM. X chromosome monosomy in primary and overlapping autoimmune diseases. Autoimmun Rev. 2010 doi: 10.1016/j.autrev.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 140.Invernizzi P, Pasini S, Selmi C, Gershwin ME, Podda M. Female predominance and X chromosome defects in autoimmune diseases. J Autoimmun. 2009;33(1):12–6. doi: 10.1016/j.jaut.2009.03.005. [DOI] [PubMed] [Google Scholar]
- 141.Persani L, Rossetti R, Cacciatore C, Bonomi M. Primary Ovarian Insufficiency: X chromosome defects and autoimmunity. J Autoimmun. 2009;33(1):35–41. doi: 10.1016/j.jaut.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 142.Sawalha AH, Harley JB, Scofield RH. Autoimmunity and Klinefelter’s syndrome: when men have two X chromosomes. J Autoimmun. 2009;33(1):31–4. doi: 10.1016/j.jaut.2009.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Oertelt S, Lian ZX, Cheng CM, Chuang YH, Padgett KA, He XS, et al. Anti-mitochondrial antibodies and primary biliary cirrhosis in TGF-beta receptor II dominant-negative mice. J Immunol. 2006;177(3):1655–60. doi: 10.4049/jimmunol.177.3.1655. [DOI] [PubMed] [Google Scholar]
- 144.Wakabayashi K, Lian ZX, Moritoki Y, Lan RY, Tsuneyama K, Chuang YH, et al. IL-2 receptor alpha(−/−) mice and the development of primary biliary cirrhosis. Hepatology. 2006;44(5):1240–9. doi: 10.1002/hep.21385. [DOI] [PubMed] [Google Scholar]
- 145.Irie J, Wu Y, Wicker LS, Rainbow D, Nalesnik MA, Hirsch R, et al. NOD. c3c4 congenic mice develop autoimmune biliary disease that serologically and pathogenetically models human primary biliary cirrhosis. J Exp Med. 2006;203(5):1209–19. doi: 10.1084/jem.20051911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Simmons A. Crohn’s disease: Genes, viruses and microbes. Nature. 2010;466(7307):699–700. doi: 10.1038/466699a. [DOI] [PubMed] [Google Scholar]
- 147.Cadwell K, Patel KK, Maloney NS, Liu TC, Ng AC, Storer CE, et al. Virus-plus-susceptibility gene interaction determines Crohn’s disease gene Atg16L1 phenotypes in intestine. Cell. 2010;141(7):1135–45. doi: 10.1016/j.cell.2010.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Arnson Y, Shoenfeld Y, Amital H. Effects of tobacco smoke on immunity, inflammation and autoimmunity. J Autoimmun. 2010;34(3):J258–65. doi: 10.1016/j.jaut.2009.12.003. [DOI] [PubMed] [Google Scholar]
- 149.Brooks WH, Le Dantec C, Pers JO, Youinou P, Renaudineau Y. Epigenetics and autoimmunity. J Autoimmun. 2010;34(3):J207–19. doi: 10.1016/j.jaut.2009.12.006. [DOI] [PubMed] [Google Scholar]
- 150.Chang C. The immune effects of naturally occurring and synthetic nanoparticles. J Autoimmun. 2010;34(3):J234–46. doi: 10.1016/j.jaut.2009.11.009. [DOI] [PubMed] [Google Scholar]
- 151.Hoffmann MH, Trembleau S, Muller S, Steiner G. Nucleic acid-associated autoantigens: pathogenic involvement and therapeutic potential. J Autoimmun. 2010;34(3):J178–206. doi: 10.1016/j.jaut.2009.11.013. [DOI] [PubMed] [Google Scholar]
- 152.Maverakis E, Miyamura Y, Bowen MP, Correa G, Ono Y, Goodarzi H. Light, including ultraviolet. J Autoimmun. 2010;34(3):J247–57. doi: 10.1016/j.jaut.2009.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Powell JJ, Faria N, Thomas-McKay E, Pele LC. Origin and fate of dietary nanoparticles and microparticles in the gastrointestinal tract. J Autoimmun. 2010;34 (3):J226–33. doi: 10.1016/j.jaut.2009.11.006. [DOI] [PubMed] [Google Scholar]
- 154.Round JL, O’Connell RM, Mazmanian SK. Coordination of tolerogenic immune responses by the commensal microbiota. J Autoimmun. 2010;34(3):J220–5. doi: 10.1016/j.jaut.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Selmi C, Tsuneyama K. Nutrition, geoepidemiology, and autoimmunity. Autoimmun Rev. 2010;9(5):A267–70. doi: 10.1016/j.autrev.2009.12.001. [DOI] [PubMed] [Google Scholar]
- 156.Stojanovich L. Stress and autoimmunity. Autoimmun Rev. 2010;9(5):A271–6. doi: 10.1016/j.autrev.2009.11.014. [DOI] [PubMed] [Google Scholar]
- 157.Migliaccio C, Nishio A, Van de Water J, Ansari AA, Leung PS, Nakanuma Y, et al. Monoclonal antibodies to mitochondrial E2 components define autoepitopes in primary biliary cirrhosis. J Immunol. 1998;161(10):5157–63. [PubMed] [Google Scholar]
- 158.Migliaccio C, Van de Water J, Ansari AA, Kaplan MM, Coppel RL, Lam KS, et al. Heterogeneous response of antimitochondrial autoantibodies and bile duct apical staining monoclonal antibodies to pyruvate dehydrogenase complex E2: the molecule versus the mimic. Hepatology. 2001;33(4):792–801. doi: 10.1053/jhep.2001.23783. [DOI] [PubMed] [Google Scholar]
- 159.Van de Water J, Turchany J, Leung PS, Lake J, Munoz S, Surh CD, et al. Molecular mimicry in primary biliary cirrhosis. Evidence for biliary epithelial expression of a molecule cross-reactive with pyruvate dehydrogenase complex-E2. J Clin Invest. 1993;91(6):2653–64. doi: 10.1172/JCI116504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Malmborg AC, Shultz DB, Luton F, Mostov KE, Richly E, Leung PS, et al. Penetration and co-localization in MDCK cell mitochondria of IgA derived from patients with primary biliary cirrhosis. J Autoimmun. 1998;11(5):573–80. doi: 10.1006/jaut.1998.0220. [DOI] [PubMed] [Google Scholar]
- 161.Fukushima N, Nalbandian G, Van De Water J, White K, Ansari AA, Leung P, et al. Characterization of recombinant monoclonal IgA anti-PDC-E2 autoantibodies derived from patients with PBC. Hepatology. 2002;36(6):1383–92. doi: 10.1053/jhep.2002.37140. [DOI] [PubMed] [Google Scholar]
- 162.Nishio A, Van de Water J, Leung PS, Joplin R, Neuberger JM, Lake J, et al. Comparative studies of antimitochondrial autoantibodies in sera and bile in primary biliary cirrhosis. Hepatology. 1997;25(5):1085–9. doi: 10.1002/hep.510250506. [DOI] [PubMed] [Google Scholar]
- 163.Reynoso-Paz S, Leung PS, Van De Water J, Tanaka A, Munoz S, Bass N, et al. Evidence for a locally driven mucosal response and the presence of mitochondrial antigens in saliva in primary biliary cirrhosis. Hepatology. 2000;31(1):24–9. doi: 10.1002/hep.510310106. [DOI] [PubMed] [Google Scholar]
- 164.Tanaka A, Nalbandian G, Leung PS, Benson GD, Munoz S, Findor JA, et al. Mucosal immunity and primary biliary cirrhosis: presence of antimitochondrial antibodies in urine. Hepatology. 2000;32(5):910–5. doi: 10.1053/jhep.2000.19254. [DOI] [PubMed] [Google Scholar]
- 165.Tanaka A, Nezu S, Uegaki S, Mikami M, Okuyama S, Kawamura N, et al. The clinical significance of IgA antimitochondrial antibodies in sera and saliva in primary biliary cirrhosis. Ann N Y Acad Sci. 2007;1107:259–70. doi: 10.1196/annals.1381.028. [DOI] [PubMed] [Google Scholar]
- 166.Allina J, Stanca CM, Garber J, Hu B, Sautes-Fridman C, Bach N, et al. Anti-CD16 autoantibodies and delayed phagocytosis of apoptotic cells in primary biliary cirrhosis. J Autoimmun. 2008;30(4):238–45. doi: 10.1016/j.jaut.2007.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Maslowski KM, Vieira AT, Ng A, Kranich J, Sierro F, Yu D, et al. Regulation of inflammatory responses by gut microbiota and chemoattractant receptor GPR43. Nature. 2009;461(7268):1282–6. doi: 10.1038/nature08530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Feld JJ, Meddings J, Heathcote EJ. Abnormal intestinal permeability in primary biliary cirrhosis. Dig Dis Sci. 2006;51(9):1607–13. doi: 10.1007/s10620-006-9544-z. [DOI] [PubMed] [Google Scholar]