Abstract
In his clonal selection theory, Frank Macfarlane Burnet predicted that autoreactive lymphocytes are deleted to prevent autoimmunity. This and other principles of lymphocyte behavior outlined by Burnet guided many studies that lead to our current understanding of thymic selection. Thus, when the genetic mutation responsible for Autoimmune Polyendocrinopathy Syndrome type 1 (APS-1) was mapped to the AIRE gene, and Aire was found to be highly expressed in thymic epithelium, studying the role of Aire in negative selection made sense in the context of modern models of thymic selection. We now know Aire is a transcription factor required for the expression of many tissue specific antigens (TSAs) in the thymus. In the absence of functional Aire, human patients and mice develop multi-organ autoimmune disease due to a defect in thymic negative selection. In addition to its role in the thymus, recent work in our lab suggests that extrathymic Aire-expressing cells plays an important role in the clonal deletion of autoreactive CD8+ T cells. In this review, we summarize the latest studies on thymic and peripheral Aire expressing cells, as well as other TSA-expressing stromal cell populations in peripheral lymphoid organs. We also discuss theoretical differences in thymic and peripheral Aire function that warrant further studies.
The clonal selection theory foreshadows mechanisms of central tolerance
In this special feature of Immunology and Cell Biology, we honor the 50th anniversary of Frank Macfarlane Burnet’s Nobel prize by appreciating how his ground-breaking clonal selection theory is still relevant to cutting-edge discoveries in immunology today. An example of Burnet’s revolutionary insight is illustrated in the following passage he wrote over 50 years ago in The clonal selection theory of acquired immunity: “Suppose that at the appropriate stage of development a limited genetic determinant carrying the coding responsible for globulin pattern releases control in such a fashion that purely random arrangements are allowed which will be different at each replication. Then at a later state … contact with any determinant associated with a body component … results in the elimination of cells carrying such sites, and if all such clones are eliminated full tolerance is established.” 1 Burnet published this model of lymphocyte tolerance in 1958, 30 years before empirical evidence for the existence of the T cell receptor (TCR) “globulin pattern,”2 “random arrangements” via Rag1 and Rag2,3, 4 and the elimination of autoreactive cells5, 6 were generated. After seminal studies by Jacques Miller in the early 1960s demonstrated that the thymus played an important role in the development of lymphocytes,7 Burnet further hypothesized that tolerance was controlled by the thymus through deletional or functional inactivation mechanisms.8 In the late ‘80s, experimental proof of thymic negative selection was provided by the identification of superantigen-mediated deletion of thymocytes expressing particular Vβ TCRs 5 and the observation of antigen-specific deletion of thymocytes in T cell receptor transgenic mice.9, 10, 11 These studies demonstrated that T cells expressing high affinity TCRs for ligand in the thymus were deleted. Although thymic negative selection of self-reactive T cells had thus become an accepted mechanism of tolerance, many questions remained as to the scope of ‘self’ that was regulated in the thymus. That is, it was not clear how or if TCRs specific for non-ubiquitous, tissue-restricted antigens (TSAs) were exposed to such antigens in the thymus.
A clearer picture of thymic expression of self antigens began to emerge in the 1990s, led by the Hanahan lab who demonstrated that expression of insulin and insulin promoter-driven transgenes could be detected in the thymus.12 Such expression in transgenic systems was clearly demonstrated to impose strong central tolerance. These findings suggested that the thymus may harbor the ability to express self-antigens that are tissue-specific for organs other than the thymus (i.e. insulin being mainly restricted to the pancreas). The extent of such TSA expression in the thymus was later shown to be broad, and TSA expression was mapped to thymic medullary epithelial cells (mTECs).13–15 Part of the molecular explanation of how this seemingly ectopic TSA expression occurs in the thymus came through the identification of the Autoimmune Regulator (Aire) gene and its function. Aire was found to control the expression of many TSAs in the thymus, and was thus shown to be necessary for the proper negative selection of TSA-specific thymocytes. The following is a more detailed discussion of how Aire contributes to clonal selection and self tolerance in the thymus and peripheral lymphoid organs.
The role of thymic Aire in central tolerance
Aire was originally identified through studies of a human autoimmune syndrome called Autoimmune Polyglandular Syndrome Type 1 (APS-1). Patients with this disorder develop spontaneous autoimmune infiltrates and autoantibodies to multiple organs, and inherit the disease in a monogenic autosomal recessive fashion.16 Through genetic linkage studies on families with APS-1, AIRE was identified as the defective gene linked to this disorder.17, 18 Aire is a 545 amino acid protein with nuclear localization sequences (NLS), two plant homeodomain (PHD) zinc fingers, a caspase recruitment domain (CARD)/homogeneously staining region (HSR), a SAND domain, and a proline-rich region (PRR) which are structures found in other transcription factors (Figure 1).19 Thus, since Aire expression is high in mTECs, it was hypothesized that Aire may be a transcription factor required for the expression of TSAs within mTECs. To test this hypothesis, an Aire knock-out (KO) mouse model was generated.15 Like APS-1 patients, Aire KO mice developed multi-organ autoimmunity.15 Microarray analysis of sorted mTECs from wild type (WT) and Aire KO mice demonstrated that Aire was required for the expression of a wide array of TSAs within mTECs.15 Thymic transfer experiments demonstrated the importance of Aire-dependent TSA expression in mTECs in the promotion of immune tolerance; i.e. the transfer of Aire-deficient thymic stroma into FoxN1-deficient, athymic hosts is sufficient for the transmission of autoimmunity.15 TCR transgenic models of negative selection have also been found to have Aire-dependency for some model self-antigens,20, 21 again bolstering a role of Aire in thymic negative selection. Although it has been reported that Aire has no dramatic effect on the overall frequency and function of regulatory T cells21–23 some transgenic mouse models demonstrate that Aire-expressing mTECs can be potent inducers of regulatory T cells.24, 25 Given these paradoxical reports, further study will be needed to clarify the role of Aire in the generation the thymically-derived regulatory T cells.
Figure 1. Functional domains in Aire that are common to other transcription factors.
Aire contains a CARD/HSR domain, nuclear localization sequences (NLS), a SAND domain, two PHD zinc finger domains, and a PRR which are structures found in other transcription factors.
Another line of studies on Aire have led to the suggestion that Aire may participate in other activities beyond TSA expression in mTECs, including the promotion of apoptosis in mTECs.26 This is an attractive model, as this could help promote the antigen processing and presentation of Aire-driven TSAs by nearby thymic dendritic cells. Another study has suggested that thymic epithelial cells may use the process of autophagy to help load the MHC class II pathway which plays an important role in positive and negative selection of T cells.27 Although both direct presentation of TSAs by mTECs and handoff of TSAs to nearby DCs were shown to contribute to CD4+ T cell selection in the thymus in vivo, it is not clear whether one pathway is dominant over the other.25, 28 In addition to a potential role in apoptosis, other groups have suggested that Aire may also play a role in improving the ability of mTECs to attract and interact with thymocytes, but the details of which determinants are involved in this potential activity of Aire remain unclear.21, 23, 29
Given that Aire promotes the expression of such a wide array of TSAs in mTECs, the nature of this unusual expression pattern has been extensively studied and a number of interesting observations have been made. Many of the Aire-induced TSAs are clustered in their genomic locations; however, there are examples of individual genes within a cluster of Aire-regulated genes that are not targeted by Aire.30, 31 Single cell analysis of Aire-expressing mTECs has shown that TSA targets are expressed in a stochastic and mono-allelic fashion.14, 31 Thus, Aire-targeted TSAs seem to be transcribed differently than they are in the peripheral organ in which they are expressed.32 One obvious difference between thymic and tissue expression of TSAs is that the level of TSA expression within mTECs is generally low when compared to the peripheral organ associated with a given TSA.14, 33 This suggests that negative selection requires a remarkably low level of antigen expression. It also appears that the array of genes Aire induces varies by cell type. Comparison of Aire activity when ectopically expressed in various cell types shows that Aire has the propensity for promoting TSA expression in a variety of contexts, but a different set of TSAs are activated in each case.34–36 Taken together, this data suggests that Aire is likely utilizing a cell-specific transcriptional milieu to activate target genes in a manner that is fundamentally different from that used by standard DNA-binding transcription factors.
So what are the molecular underpinnings of how Aire promotes the promiscuous expression of so many TSAs? Given the observations outlined above, it is possible that Aire is not a traditional DNA-binding transcription factor. A major advance in our understanding of how Aire functions was made when the first PHD zinc finger domain of Aire was demonstrated to bind to unmethlyated lysine at position 4 of H3 histone (H3K4).37–39 Though there are exceptions, the methylation status of histone tails correlates with the transcriptional activity at that genomic locus. For instance, tri-methylated H3K4 is frequently observed at sites of active transcription and is part of the epigenetic code regulating gene transcription.40, 41 The propensity of Aire to bind unmethylated H3K4 indicates that Aire is likely using this epigenetic mechanism to help recruit itself to inactive chromatin.
In addition to this epigenetic strategy, a number of other mechanisms have been proposed to help explain Aire’s unique transcriptional activity. These mechanisms include the promotion of RNA elongation through interactions with pTEFb,42 complexing with the nuclear matrix,19 and binding to CREB-binding protein19 and DNA-PK.43 A recent study used a large scale proteomics approach to identify an array of potential Aire binding partners.36 Here the authors transfected a tagged version of Aire into 293 cells (which do not normally express Aire) and performed pull-down experiments to identify and characterize Aire-associated proteins. The identified proteins were confirmed in co-immunoprecipitation experiments and their biological relevance to TSA expression was tested through knockdown assays. These experiments allowed the authors to identify a number of binding partners in different known functional pathways. First, the authors found that Aire was part of a complex of proteins involved in the DNA-damage response, including DNA-PK, PARP-1, TOP2, FACT, and Ku. To further extend the relevance of some of the interactions, the authors demonstrated that mTECs deficient in DNA-PK have a reduction in the TSA expression of Aire target genes. A second functional complex mapping to Aire protein interactions involved factors associated with pre-mRNA splicing. The effect of this interaction appears to result in the increase of spliced mRNA of Aire-regulated target genes. This study provides a likely foundation of the mechanistic tools used by Aire to drive the expression of such a wide variety of genes; however, more study will be needed to confirm that these pathways are relevant to Aire-expressing cells in vivo.
The role of extra-thymic Aire in peripheral tolerance
Significant evidence demonstrates that normal thymic deletion of autoreactive T cells is an incomplete process. For example, depletion of FoxP3+ regulatory T cells in healthy adult mice unleashes the function of autoreactive T cells that have escaped negative selection, resulting in the development of lethal autoimmunity.44 Also, immunizing healthy mice with autoantigens can break tolerance. By injecting healthy mice with some self antigens, such as myelin basic protein in adjuvant, quiescent autoreactive T cells that have escaped deletion are primed and cause autoimmune attack on the tissues expressing these proteins.45 Marrack et al. argue that “leaky” central tolerance benefits the host by generating a larger T cell repertoire for peripheral immunity to infection. 46 Given that a significant number of autoreactive T cells evade thymic deletion, mechanisms of peripheral tolerance must exist to keep these cells in check.
A novel mechanism of peripheral self tolerance was revealed with studies of Aire in peripheral lymphoid organs.13, 15, 47 Although it was initially assumed that dentritic cells (DCs) were the source of peripheral Aire expression – early studies of Aire in the periphery were focused on DC function48 – current work shows that Aire expression in the lymph nodes and spleen derives from radio-resistant cells in the secondary lymphoid organs and not CD11c+ DCs.47 By generating reporter mice that express GFP under the control of the Aire locus, our lab was able to identify this unique population of extrathymic Aire-expressing cells (eTACs).34
Like Aire expressed in mTECs, Aire in secondary lymphoid organs is required for the expression of many TSAs.34 In order to test the ability of eTACs to induce tolerance to T cells, the islet specific glucose-6-phosphatase (IGRP) antigen was targeted to these cells by engineering a transgene that expressed IGRP under the control of the Aire locus, which was called the Aire-driven IGRP (Adig) mouse model.34 IGRP is not normally expressed in the thymus or secondary lymphoid organs of wild type mice. However, expression of Aire-driven IGRP was detected in Aire+ mTECs and eTACs of the Adig mouse.34 When IGRP-specific CD8+ T cells were transferred into the Adig mouse, eTACs stimulated IGRP-specific CD8+ cell proliferation and death by directly presenting IGRP to these T cells.34 The Adig mouse demonstrates that, like Aire expressing mTECs, eTACs can help to impose tolerance by deleting autoreactive CD8+ T cells.34
Other TSA-expressing, CD45-negative cell subsets in lymph nodes have been described that do not express Aire. For example, a mouse model which expresses a transgene consisting of a truncated OVA (tOVA) antigen under the control of the intestinal fatty acid binding protein (iFABP) promoter, expresses OVA in the intestinal epithelium as well as the stromal cells of peripheral lymph nodes.47, 49 When CD8+ OVA-specific TCR transgenic T cells are injected into iFABP-tOVA mice, the T cells proliferate and are eventually deleted.47 Thus, like eTACs, non-Aire-expressing stromal cell subsets can present TSAs to autoreactive CD8+ T cells, causing the deletion of these potentially harmful cells.
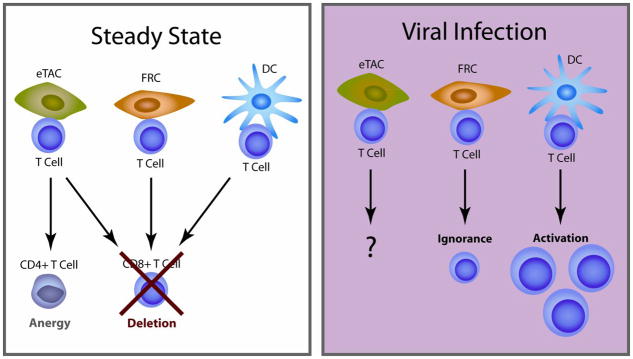
In both the Adig and iFABP-tOVA mice, antigen expression is driven by a transgene. Thus, it is possible that antigen-specific CD8+ cells were deleted due to engagement of non-physiologically high levels of antigen expression. However, Nichols et al. recently showed that a stromal cell subset in lymph nodes expresses the TSA, tyrosinase, and can present this antigen to tyrosinase-specific CD8+ T cells.50 In this model system the endogenous expression level of tyrosinase in these stromal cells was enough to delete tyrosinase-specific CD8+ T cells, providing evidence that physiological expression of TSAs by stromal cells can activate and delete antigen-specific CD8+ T cells, similar to transgenic TSA expression.50 Overall, the existence of different TSA-expressing CD45-negative cell subsets in peripheral lymphoid organs suggests that this class of cells provides a significant survival advantage; the evidence thus far demonstrates that eTACs and TSA-expressing stromal cell subsets play an important role in the maintenance of peripheral self tolerance by deleting autoreactive CD8+ T cells (Figure 2).
Figure 2. Model of T cell activation by APC under steady state conditions or during a viral infection.
Under steady state conditions, CD8+ T cells are deleted by eTACs, FRCs, and hematopoetic DC, while CD4+ T cells proliferate and become anergic. Upon TLR3 engagement by virus-derived, double stranded RNA, FRCs may decrease self antigen presentation while hematopoetic DCs mature to activate T cells.
There are several properties of both eTACs and non-Aire expressing, TSA-expressing stromal cells which make them well suited to play an important role in peripheral tolerance. First, location of these cells in the lymph nodes is ideal for tolerizing autoreactive T cells. Naïve T cells constitutively traffic through lymph nodes but generally do not traffic through tissues under normal circumstances.51 Thus naïve autoreactive T cells may be tolerized in secondary lymphoid organs and not at the site of the tissue itself. Second, eTACs express a different set of tissue restricted antigens than Aire expressing mTECs; Aire regulates 163 genes in eTACs and 1835 genes in mTECs, but there are only 7 overlapping genes that are controlled by both peripheral and thymic Aire.34 By expressing a different set of TSAs in the periphery, eTACs may tolerize antigen-specific T cells that were not deleted by Aire-expressing mTECs.
The Adig model system demonstrates that eTACs and other TSA expressing stromal cell subsets in peripheral lymphoid organs can function to delete naïve, autoreactive CD8+ T cells in a manner similar to that which Aire-expressing mTECs delete autoreactive thymocytes. Although Aire-expressing mTECs and eTACs present different self antigens, are their functions otherwise redundant? Are there differences between how eTACs and Aire-expressing mTECs delete autoreactive T cells? Thus far, the characterization of eTACs was guided by studies of Aire expressing mTECs, thereby biasing findings to those which are similar to thymic selection. The following is a discussion of features unique to eTACs which may allow this APC population to provide functions distinct from Aire expressing mTECs.
Immunofluorescent staining shows that eTACs are found primarily in the B cell-T cell boundary of T cell zones of peripheral lymphoid organs, in close proximity to CD11c+ APCs.34 Two-photon imaging experiments show that autoreactive T cells can make stable contacts with eTACs in the B cell-T cell boundary of the T cell zone, the same microenvironment in which antigen-specific T cells have been shown to be primed by LPS-activated DCs.34, 52 The proximity of eTACs to sites of T cell priming may allow them to serve a unique function in shaping the fate of TCR-triggered T cells. In other words, perhaps unlike Aire expressing mTECs, eTACs can receive cues from and/or play an important role in local immune responses. In line with this hypothesis, Fletcher et al. have shown that some non-Aire-expressing stromal cell subsets in the lymph node can present TSAs and potentially respond to viral infections through TLR3 signaling.49 Interestingly, one of the TLR3-expressing stromal cell subsets, fibroblastic reticular cells (FRCs), can stimulate and delete autoreactive CD8+ T cells.47, 49 However, upon stimulation with TLR3 agonist, FRCs decrease TSA expression, thus losing the ability to stimulate autoreactive T cell proliferation (Figure 2).49 It is thought that TLR3 agonist decreases TSA expression on FRCs to reduce the chance of stimulating autoreactive T cells and prevent the development of autoimmunity during clearance of double-stranded RNA viral infections.49 eTACs do not express TLR3, 7, or 849, but it is not known whether they express other receptors that can detect the presence of foreign microorganisms or endogenous ‘stress’ ligands. That is, studies of infection models could be used to determine whether like FRCs, eTACs can be directly stimulated through innate receptors like TLRs, NLRs, and Rig-like helicases, and/or APC-derived ‘stress’ ligands like inflammatory cytokines, heat-shock proteins, etc. Unlike the thymus, secondary lymphoid organs are well suited for sensing pathogenic processes in the periphery and organizing appropriate immune responses to these processes. Therefore, it would not be surprising if a feature of eTACs that distinguishes them from Aire-expressing mTECs is the ability to somehow adapt to changing environments caused by infection or inflammation.
Although autoreactive CD8+ thymocytes and mature CD8+ T cells are similarly deleted by mTECs and eTACs, respectively,21, 34 our lab has recently found that CD4+ T cells respond differently. While CD4+ thymocytes are deleted or shunted toward the FoxP3+ Treg lineage after interacting with Aire expressing mTECs,21, 24, 25 mature CD4+ T cells are triggered to proliferate and become functionally inert FoxP3-negative cells after interacting with eTACs [Gardner, Metzger, and Anderson, unpublished observations]. The difference in reactivity of CD4+ thymocytes and mature CD4+ T cells to Aire expressing mTECs and eTACs demonstrates that at least some of the functions of thymic and peripheral Aire are not redundant. Further studies of autoreactive CD4+ T cell reactivity to Aire expressing APC may be able to address the following questions: 1) Are the different effects of peripheral vs. thymic Aire on CD4+ T cell function T cell or APC intrinsic? and 2) What are the unique signals that control CD4+ T cell deletion vs. anergy?
A better understanding of the commonalities and differences between Aire-expressing mTECs and eTACs is needed to delineate the evolutionary pressure for peripheral Aire expression. The study of peripheral Aire’s effect on T cells, particularly CD4+ T cells, in infection models will be able to better define the role of eTACs.
Concluding Remarks
Burnet’s prediction that autoreactive lymphocytes are deleted to prevent autoimmunity guided many studies that lead to our current understanding of thymic selection. Thus, when the genetic mutation responsible for APS-1 was mapped to the AIRE gene, and Aire was found to be highly expressed in thymic epithelium, studying the role of Aire in negative selection made sense in the context of modern models of thymic selection. More recent insights into the function of Aire in peripheral lymphoid organs have extended our understanding of peripheral tolerance. The fact that the newly defined mechanisms of Aire-dependent tolerance still fits into the framework of the clonal selection theory is an illustration of how Burnet’s ground-breaking ideas can still be used as a guideline for cutting-edge discoveries made today.
Acknowledgments
This work was supported by the JDRF, NIH, Helmsley Foundation, and Burroughs Wellcome Fund. We would also like to thank Una Fan for creating the figure illustrations and Adam Savage, Kellsey Johannes, and Eileen McMahon for helpful comments on this manuscript. The authors have no conflict of interest to disclose.
References
- 1.Burnet FM. The clonal selection theory of acquired immunity. Nashville, Tennessee: Vanderbilt University Press; 1958. [Google Scholar]
- 2.Hedrick SM, Cohen DI, Nielsen EA, Davis MM. Isolation of cDNA clones encoding T cell-specific membrane-associated proteins. Nature. 1984;308:149–53. doi: 10.1038/308149a0. [DOI] [PubMed] [Google Scholar]
- 3.Schatz DG, Oettinger MA, Baltimore D. The V(D)J recombination activating gene, RAG-1. Cell. 1989;59:1035–48. doi: 10.1016/0092-8674(89)90760-5. [DOI] [PubMed] [Google Scholar]
- 4.Oettinger MA, Schatz DG, Gorka C, Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990;248:1517–23. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- 5.Kappler JW, Roehm N, Marrack P. T cell tolerance by clonal elimination in the thymus. Cell. 1987;49:273–80. doi: 10.1016/0092-8674(87)90568-x. [DOI] [PubMed] [Google Scholar]
- 6.Starr TK, Jameson SC, Hogquist KA. Positive and negative selection of T cells. Annu Rev Immunol. 2003;21:139–76. doi: 10.1146/annurev.immunol.21.120601.141107. [DOI] [PubMed] [Google Scholar]
- 7.Miller JF. Immunological function of the thymus. Lancet. 1961;2:748–9. doi: 10.1016/s0140-6736(61)90693-6. [DOI] [PubMed] [Google Scholar]
- 8.Burnet M. Role of the thymus and related organs in immunity. Br Med J. 1962;2:807–11. doi: 10.1136/bmj.2.5308.807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pircher H, Burki K, Lang R, Hengartner H, Zinkernagel RM. Tolerance induction in double specific T-cell receptor transgenic mice varies with antigen. Nature. 1989;342:559–61. doi: 10.1038/342559a0. [DOI] [PubMed] [Google Scholar]
- 10.Kisielow P, Bluthmann H, Staerz UD, Steinmetz M, von Boehmer H. Tolerance in T-cell-receptor transgenic mice involves deletion of nonmature CD4+8+ thymocytes. Nature. 1988;333:742–6. doi: 10.1038/333742a0. [DOI] [PubMed] [Google Scholar]
- 11.Murphy KM, Heimberger AB, Loh DY. Induction by antigen of intrathymic apoptosis of CD4+CD8+TCRlo thymocytes in vivo. Science. 1990;250:1720–3. doi: 10.1126/science.2125367. [DOI] [PubMed] [Google Scholar]
- 12.Smith KM, Olson DC, Hirose R, Hanahan D. Pancreatic gene expression in rare cells of thymic medulla: evidence for functional contribution to T cell tolerance. Int Immunol. 1997;9:1355–65. doi: 10.1093/intimm/9.9.1355. [DOI] [PubMed] [Google Scholar]
- 13.Zuklys S, Balciunaite G, Agarwal A, Fasler-Kan E, Palmer E, Hollander GA. Normal thymic architecture and negative selection are associated with Aire expression, the gene defective in the autoimmune-polyendocrinopathy-candidiasis-ectodermal dystrophy (APECED) J Immunol. 2000;165:1976–83. doi: 10.4049/jimmunol.165.4.1976. [DOI] [PubMed] [Google Scholar]
- 14.Derbinski J, Schulte A, Kyewski B, Klein L. Promiscuous gene expression in medullary thymic epithelial cells mirrors the peripheral self. Nat Immunol. 2001;2:1032–9. doi: 10.1038/ni723. [DOI] [PubMed] [Google Scholar]
- 15.Anderson MS, Venanzi ES, Klein L, Chen Z, Berzins SP, Turley SJ, et al. Projection of an immunological self shadow within the thymus by the aire protein. Science. 2002;298:1395–401. doi: 10.1126/science.1075958. [DOI] [PubMed] [Google Scholar]
- 16.Perheentupa J. Autoimmune polyendocrinopathy--candidiasis--ectodermal dystrophy (APECED) Horm Metab Res. 1996;28:353–6. doi: 10.1055/s-2007-979814. [DOI] [PubMed] [Google Scholar]
- 17.An autoimmune disease, APECED, caused by mutations in a novel gene featuring two PHD-type zinc-finger domains. Nat Genet. 1997;17:399–403. doi: 10.1038/ng1297-399. [DOI] [PubMed] [Google Scholar]
- 18.Nagamine K, Peterson P, Scott HS, Kudoh J, Minoshima S, Heino M, et al. Positional cloning of the APECED gene. Nat Genet. 1997;17:393–8. doi: 10.1038/ng1297-393. [DOI] [PubMed] [Google Scholar]
- 19.Peterson P, Org T, Rebane A. Transcriptional regulation by AIRE: molecular mechanisms of central tolerance. Nat Rev Immunol. 2008;8:948–57. doi: 10.1038/nri2450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Liston A, Lesage S, Wilson J, Peltonen L, Goodnow CC. Aire regulates negative selection of organ-specific T cells. Nat Immunol. 2003;4:350–4. doi: 10.1038/ni906. [DOI] [PubMed] [Google Scholar]
- 21.Anderson MS, Venanzi ES, Chen Z, Berzins SP, Benoist C, Mathis D. The cellular mechanism of Aire control of T cell tolerance. Immunity. 2005;23:227–39. doi: 10.1016/j.immuni.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 22.Daniely D, Kern J, Cebula A, Ignatowicz L. Diversity of TCRs on natural Foxp3+ T cells in mice lacking Aire expression. J Immunol. 184:6865–73. doi: 10.4049/jimmunol.0903609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kuroda N, Mitani T, Takeda N, Ishimaru N, Arakaki R, Hayashi Y, et al. Development of autoimmunity against transcriptionally unrepressed target antigen in the thymus of Aire-deficient mice. J Immunol. 2005;174:1862–70. doi: 10.4049/jimmunol.174.4.1862. [DOI] [PubMed] [Google Scholar]
- 24.Aschenbrenner K, D’Cruz LM, Vollmann EH, Hinterberger M, Emmerich J, Swee LK, et al. Selection of Foxp3+ regulatory T cells specific for self antigen expressed and presented by Aire+ medullary thymic epithelial cells. Nat Immunol. 2007;8:351–8. doi: 10.1038/ni1444. [DOI] [PubMed] [Google Scholar]
- 25.Hinterberger M, Aichinger M, da Costa OP, Voehringer D, Hoffmann R, Klein L. Autonomous role of medullary thymic epithelial cells in central CD4(+) T cell tolerance. Nat Immunol. 11:512–9. doi: 10.1038/ni.1874. [DOI] [PubMed] [Google Scholar]
- 26.Gray D, Abramson J, Benoist C, Mathis D. Proliferative arrest and rapid turnover of thymic epithelial cells expressing Aire. J Exp Med. 2007;204:2521–8. doi: 10.1084/jem.20070795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nedjic J, Aichinger M, Emmerich J, Mizushima N, Klein L. Autophagy in thymic epithelium shapes the T-cell repertoire and is essential for tolerance. Nature. 2008;455:396–400. doi: 10.1038/nature07208. [DOI] [PubMed] [Google Scholar]
- 28.Gallegos AM, Bevan MJ. Central tolerance to tissue-specific antigens mediated by direct and indirect antigen presentation. J Exp Med. 2004;200:1039–49. doi: 10.1084/jem.20041457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Yano M, Kuroda N, Han H, Meguro-Horike M, Nishikawa Y, Kiyonari H, et al. Aire controls the differentiation program of thymic epithelial cells in the medulla for the establishment of self-tolerance. J Exp Med. 2008;205:2827–38. doi: 10.1084/jem.20080046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Derbinski J, Gabler J, Brors B, Tierling S, Jonnakuty S, Hergenhahn M, et al. Promiscuous gene expression in thymic epithelial cells is regulated at multiple levels. J Exp Med. 2005;202:33–45. doi: 10.1084/jem.20050471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Johnnidis JB, Venanzi ES, Taxman DJ, Ting JP, Benoist CO, Mathis DJ. Chromosomal clustering of genes controlled by the aire transcription factor. Proc Natl Acad Sci U S A. 2005;102:7233–8. doi: 10.1073/pnas.0502670102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Villasenor J, Besse W, Benoist C, Mathis D. Ectopic expression of peripheral-tissue antigens in the thymic epithelium: probabilistic, monoallelic, misinitiated. Proc Natl Acad Sci U S A. 2008;105:15854–9. doi: 10.1073/pnas.0808069105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.DeVoss J, Hou Y, Johannes K, Lu W, Liou GI, Rinn J, et al. Spontaneous autoimmunity prevented by thymic expression of a single self-antigen. J Exp Med. 2006;203:2727–35. doi: 10.1084/jem.20061864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Gardner JM, Devoss JJ, Friedman RS, Wong DJ, Tan YX, Zhou X, et al. Deletional tolerance mediated by extrathymic Aire-expressing cells. Science. 2008;321:843–7. doi: 10.1126/science.1159407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Guerau-de-Arellano M, Mathis D, Benoist C. Transcriptional impact of Aire varies with cell type. Proc Natl Acad Sci U S A. 2008;105:14011–6. doi: 10.1073/pnas.0806616105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Abramson J, Giraud M, Benoist C, Mathis D. Aire’s partners in the molecular control of immunological tolerance. Cell. 140:123–35. doi: 10.1016/j.cell.2009.12.030. [DOI] [PubMed] [Google Scholar]
- 37.Org T, Chignola F, Hetenyi C, Gaetani M, Rebane A, Liiv I, et al. The autoimmune regulator PHD finger binds to non-methylated histone H3K4 to activate gene expression. EMBO Rep. 2008;9:370–6. doi: 10.1038/embor.2008.11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Koh AS, Kingston RE, Benoist C, Mathis D. Global relevance of Aire binding to hypomethylated lysine-4 of histone-3. Proc Natl Acad Sci U S A. 107:13016–21. doi: 10.1073/pnas.1004436107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chakravarty S, Zeng L, Zhou MM. Structure and site-specific recognition of histone H3 by the PHD finger of human autoimmune regulator. Structure. 2009;17:670–9. doi: 10.1016/j.str.2009.02.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Taverna SD, Ilin S, Rogers RS, Tanny JC, Lavender H, Li H, et al. Yng1 PHD finger binding to H3 trimethylated at K4 promotes NuA3 HAT activity at K14 of H3 and transcription at a subset of targeted ORFs. Mol Cell. 2006;24:785–96. doi: 10.1016/j.molcel.2006.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Sims RJ, 3rd, Millhouse S, Chen CF, Lewis BA, Erdjument-Bromage H, Tempst P, et al. Recognition of trimethylated histone H3 lysine 4 facilitates the recruitment of transcription postinitiation factors and pre-mRNA splicing. Mol Cell. 2007;28:665–76. doi: 10.1016/j.molcel.2007.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Oven I, Brdickova N, Kohoutek J, Vaupotic T, Narat M, Peterlin BM. AIRE recruits P-TEFb for transcriptional elongation of target genes in medullary thymic epithelial cells. Mol Cell Biol. 2007;27:8815–23. doi: 10.1128/MCB.01085-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Liiv I, Rebane A, Org T, Saare M, Maslovskaja J, Kisand K, et al. DNA-PK contributes to the phosphorylation of AIRE: importance in transcriptional activity. Biochim Biophys Acta. 2008;1783:74–83. doi: 10.1016/j.bbamcr.2007.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nat Immunol. 2007;8:191–7. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 45.Bailey SL, Schreiner B, McMahon EJ, Miller SD. CNS myeloid DCs presenting endogenous myelin peptides ‘preferentially’ polarize CD4+ T(H)-17 cells in relapsing EAE. Nat Immunol. 2007;8:172–80. doi: 10.1038/ni1430. [DOI] [PubMed] [Google Scholar]
- 46.Pullen AM, Marrack P, Kappler JW. The T-cell repertoire is heavily influenced by tolerance to polymorphic self-antigens. Nature. 1988;335:796–801. doi: 10.1038/335796a0. [DOI] [PubMed] [Google Scholar]
- 47.Lee JW, Epardaud M, Sun J, Becker JE, Cheng AC, Yonekura AR, et al. Peripheral antigen display by lymph node stroma promotes T cell tolerance to intestinal self. Nat Immunol. 2007;8:181–90. doi: 10.1038/ni1427. [DOI] [PubMed] [Google Scholar]
- 48.Ramsey C, Hassler S, Marits P, Kampe O, Surh CD, Peltonen L, et al. Increased antigen presenting cell-mediated T cell activation in mice and patients without the autoimmune regulator. Eur J Immunol. 2006;36:305–17. doi: 10.1002/eji.200535240. [DOI] [PubMed] [Google Scholar]
- 49.Fletcher AL, Lukacs-Kornek V, Reynoso ED, Pinner SE, Bellemare-Pelletier A, Curry MS, et al. Lymph node fibroblastic reticular cells directly present peripheral tissue antigen under steady-state and inflammatory conditions. J Exp Med. 207:689–97. doi: 10.1084/jem.20092642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Nichols LA, Chen Y, Colella TA, Bennett CL, Clausen BE, Engelhard VH. Deletional self-tolerance to a melanocyte/melanoma antigen derived from tyrosinase is mediated by a radio-resistant cell in peripheral and mesenteric lymph nodes. J Immunol. 2007;179:993–1003. doi: 10.4049/jimmunol.179.2.993. [DOI] [PubMed] [Google Scholar]
- 51.Weninger W, Carlsen HS, Goodarzi M, Moazed F, Crowley MA, Baekkevold ES, et al. Naive T cell recruitment to nonlymphoid tissues: a role for endothelium-expressed CC chemokine ligand 21 in autoimmune disease and lymphoid neogenesis. J Immunol. 2003;170:4638–48. doi: 10.4049/jimmunol.170.9.4638. [DOI] [PubMed] [Google Scholar]
- 52.Lindquist RL, Shakhar G, Dudziak D, Wardemann H, Eisenreich T, Dustin ML, et al. Visualizing dendritic cell networks in vivo. Nat Immunol. 2004;5:1243–50. doi: 10.1038/ni1139. [DOI] [PubMed] [Google Scholar]