Abstract
Purpose
To determine the ability of optic nerve head (ONH) parameters measured with spectral domain Cirrus™ HD-OCT to discriminate between normal and glaucomatous eyes and to compare them to the discriminating ability of peripapillary retinal nerve fiber layer (RNFL) thickness measurements performed with Cirrus™ HD-OCT.
Design
Evaluation of diagnostic test or technology.
Participants
Seventy-three subjects with glaucoma and one hundred and forty-six age-matched normal subjects.
Methods
Peripapillary ONH parameters and RNFL thickness were measured in one randomly selected eye of each participant within a 200×200 pixel A-scan acquired with Cirrus™ HD-OCT centered on the ONH.
Main Outcome Measures
ONH topographic parameters, peripapillary RNFL thickness, and the area under receiver operating characteristic curves (AUCs).
Results
For distinguishing normal from glaucomatous eyes, regardless of disease stage, the six best parameters (expressed as AUC) were vertical rim thickness (VRT, 0.963), rim area (RA, 0.962), RNFL thickness at clock-hour 7 (0.957), RNFL thickness of the inferior quadrant (0.953), vertical cup-to-disc ratio (VCDR, 0.951) and average RNFL thickness (0.950). The AUC for distinguishing between normal and eyes with mild glaucoma was greatest for RNFL thickness of clock-hour 7 (0.918), VRT (0.914), RA (0.912), RNFL thickness of inferior quadrant (0.895), average RNFL thickness (0.893) and VCDR (0.890). There were no statistically significant differences between AUCs for the best ONH parameters and RNFL thickness measurements (p > 0.05).
Conclusions
Cirrus™ HD-OCT ONH parameters are able to discriminate between eyes that are normal from those with glaucoma or even mild glaucoma. There is no difference in the ability of ONH parameters and RNFL thickness measurement, as measured with Cirrus™ OCT, to distinguish between normal and glaucomatous eyes.
Introduction
Evaluation of the optic nerve head (ONH) and retinal nerve fiber layer (RNFL) is a crucial step in diagnosing and monitoring glaucoma. However, diagnosing glaucoma based on ONH appearance alone can be challenging, particularly in early stages of the disease when changes to the ONH are subtle and not distinctly abnormal. The diagnosis can also be difficult due to wide variations in ONH anatomy in both normal and glaucomatous eyes. Additionally, studies have shown that photography has a low to medium interobserver agreement1–3 and suffers from the inability to detect diffuse RFNL loss. Lastly, standard automated perimetry can only detect visual field loss after substantial loss of retinal ganglion cells axons.4,5 To overcome these drawbacks, various computerized imaging modalities have been developed in recent years that provide objective and reproducible quantitative measurements of RNFL thickness and ONH anatomy. One of these modalities is optical coherence tomography (OCT). OCT has rapidly emerged as a widely used imaging system in ophthalmology where it is mainly used for diagnosing and monitoring glaucoma and retinal diseases. OCT may be particularly valuable in glaucoma detection and monitoring through identification of subtle RNFL or ONH changes over time.
The accuracy of RNFL thickness and ONH measurements to differentiate normal from glaucomatous eyes has been investigated for time domain (TD) OCT technology using Stratus™ OCT (Carl Zeiss Meditec, Inc., Dublin, California).6–13 Given its recent introduction, such reports are limited for RNFL thickness and ONH parameters measured by spectral domain OCT (SD-OCT). SD-OCT is a new system for obtaining high resolution cross-sectional image and quantitative assessment of the retina and optic nerve. This technology is faster and capable of producing three-dimensional volumetric measurements. Although serial RNFL thickness measurements may turn out to be useful for the longitudinal monitoring of glaucoma,14,15 continuous thinning of the RNFL during glaucoma progression may result in decrease of RNFL signal intensity, which in turn may render the posterior RNFL border more difficult to delineate and may therefore lead to inaccurate thickness measurement.16 On the contrary, ONH parameters may be easier to determine with SD-OCT given the high contrast between the non-reflective vitreous and the inner-limiting membrane and the ability of SD-OCT to delineate the end of Bruch's membrane,17 thereby defining a stable reference plane from which to measure the neuroretinal rim. Thus, ONH measurements derived from SD-OCT devices may prove to be more accurate and reproducible in evaluating glaucoma patients. The purpose of the current study was to compare the ability of peripapillary RNFL thickness and ONH parameters measured with Cirrus HD-OCT (Carl Zeiss Meditec, Inc., Dublin, California) instrument to discriminate between normal and glaucomatous eyes, with particular emphasis on mild glaucoma.
Materials and Methods
Participants
This study was approved by the Human Subject Research Office of the University of Miami Miller School of Medicine Institutional Review Board (IRB) and adhered to the Declaration of Helsinki and Health Insurance Portability and Accountability Act regulations, with informed consent obtained from all participants. IRB approval was also obtained at each institution participating in normative data collection.
Cirrus HD-OCT (Carl Zeiss Meditec, Inc., Dublin, California) optic disc scans of 73 glaucoma outpatients at the glaucoma clinic of the Anne Bates Leach Eye Hospital, Department of Ophthalmology, University of Miami Miller School of Medicine and similar scans of 146 age-matched normal subjects from the Carl Zeiss Meditec normative database were used for this study. Each glaucoma patient was matched to two normal subjects ± 5 years of age. Eligibility of all participants including normal subjects was determined through a screening eye examination, which included visual acuity (VA) and intraocular pressure (IOP) measurements, slit-lamp and fundus examinations, and visual field assessment with the Humphrey Visual Field Analyzer (Carl Zeiss Meditec, Inc., Dublin, California) using the Swedish Interactive Threshold Algorithm (SITA) Standard 24-2 program.
Inclusion criteria for normal subjects were IOP ≤ 21 mmHg, absence of ONH abnormalities suggestive of glaucoma (cup-to-disc ratio ≥ 0.5 in either eye, cup-to disc ratio asymmetry ≥ 0.2, optic disc hemorrhage, or focal thinning of the rim) as seen in fundus photographs, and a normal visual field. Glaucoma subjects were included if they had a definitive diagnosis of any form of glaucoma based on the most recent exam (within 12 months of enrollment date), characteristic glaucomatous visual field loss, and supporting ONH abnormality. The minimal abnormality for a visual field defect included a glaucoma hemifield test (GHT) outside normal limits, a pattern standard deviation (PSD) with a p-value < 5%, or a cluster of 3 or more points in the pattern deviation plot in a single hemifield (superior or inferior) with p-values < 5%, one of which must have a p-value < 1%.18 Glaucomatous patients were classified into three groups of disease severity based on the visual field mean deviation (MD): mild = MD ≥ −6 dB, moderate = −6 dB > MD ≥ −12 dB, and severe = MD < −12 dB.19
The other inclusion criteria for both normal subjects and glaucoma patients were age ≥ 18 years old, best-corrected VA ≥ 20/40, refractive error < 5 diopters of sphere or 3 diopters of cylinder, no history of retinal disease such as macular degeneration, diabetic retinopathy or retinal detachment, or optic nerve disease including non-glaucomatous optic neuropathy, and no ocular surgery within one month prior to enrollment date. Glaucoma suspects were also excluded. Only one randomly selected eye from each participant was included in the study.
OCT imaging
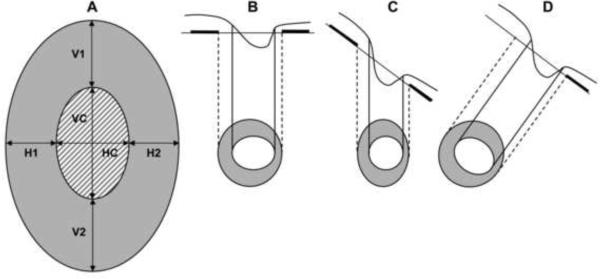
The qualifying eye of each participant was dilated with tropicamide 1% and phenylephrine 2.5% eye drops 10–15 min prior to the scanning. All scans were acquired with a Cirrus HD-OCT (version 3.0.0.64) using the Optic Disc Cube 200×200 protocol. This protocol is designed to position the cube scan on the ONH and to be primarily used for glaucoma analysis. Unlike Stratus OCT where peripapillary RNFL thickness and ONH parameters are measured through two different acquisition protocols, Cirrus HD-OCT uses the same protocol and scan to measure both. After the subject was seated and properly aligned, the iris was brought into view using the mouse-driven alignment system and the line scanning ophthalmoscopic image was focused by adjusting for refractive error. The ONH was then centered on the live image, after which the centering (Z-offset) and enhancement (polarization) were optimized. The laser then scanned over the 6 mm × 6 mm area, capturing a cube of data consisting of 200 A-scans from 200 linear B-scans (40,000 points) in about 1.5 seconds (27,000 A-scans/sec). Only good quality scans, defined as scans with signal strength ≥ 6, without RNFL discontinuity or misalignment, involuntary saccade or blinking artifacts and absence of RNFL algorithm segmentation failure, were used for analysis. No cases were removed due to ONH algorithm failure. Blinking was indicated by a straight horizontal black line across the fundus OCT image whereas involuntary saccade artifacts present as breaks in the vessels within 1.73 mm radius around the ONH or breaks or shifting on the ONH. To achieve these measurements, the Cirrus HD-OCT algorithms automatically identify the center of the optic disc and create an artificial B-scan in the shape of a circle with 3.46 mm diameter around it. The anterior and posterior margins of the RNFL are delineated and thickness determined from the data 256 A-scan samples along the path of the artificial B-scan. The system calculates the RNFL thickness at each point on the circle and generates the overall average RNFL thickness, RNFL thickness of each quadrant (temporal, superior, nasal and inferior) sector and individual RNFL thickness of all 12 clock-hour sectors. As illustrated in Figure 1A, the ONH parameters that were analyzed were the disc area, rim area, vertical rim thickness (VRT: the total rim thickness, in microns, measured in the vertical meridians), horizontal rim thickness (HRT: the total rim thickness, in microns, measured in the horizontal meridians), cup-to-disc area ratio (CDR: ratio of cup area to disc area), vertical cup-to-disc ratio (VCDR: ratio of vertical line through the cup center to the same vertical line extending to the disc margin), horizontal cup-to disc ratio (HCDR: ratio of the horizontal line through the cup center to the same line extending to the disc margin), and cup volume. Measurements of these parameters were automatically generated by a Carl Zeiss Meditec ONH analysis algorithm developed for Cirrus HD-OCT (version 5.0 software) and involved no user interaction. The algorithm identifies the termination of Bruch's membrane as the disc edge. The rim width around the entire circumference of the optic disc is then determined by measuring the thickness of the neuro-retinal tissue in the optic nerve as it turns to exit through the opening in Bruch's membrane. Measured within three-dimensional volume, this constitutes a single area measure. With this method, measurements remain unaffected despite changes if the same disc is viewed from a different angle caused by entering the pupil at a different location (Figures 1B, 1C, 1D). Additionally, the disc and rim area measurements correspond to the anatomy as would be viewed along the axis of the nerve exit. In contrast, when the ONH exit is excessively oblique or in extremely tilted discs, areas determined from ophthalmoscopic examination, photographs or other imaging techniques will be foreshortened, difficult to quantify, or erroneously quantified. Measuring the neuro-retinal rim area in the plane of the ONH addresses the foreshortening and ties the results to the anatomy.
Figure 1.
Illustration of Cirrus HD-OCT Optic Nerve Head (ONH) Parameters (1A) and Determination of ONH Edge and Rim Width (1B, 1C and 1D). Figure 1A shows a sketch of an optic disc, as presented in the 2-dimensional en face view. The shaded region represents the neuro-retinal rim area (mm2), the patterned region is the area of the cup (mm2); the total area of the optic disc is the area of the rim plus the area of the cup (mm2). The cup-to-disc ratio (CDR) is given by the square-root of the ratio of the area of the cup to the area of the optic disc. The vertical cup-to-disc ratio (VCDR) is the ratio of the cup diameter to the disc diameter in the vertical meridian; VC/(VC+V1+V2). The horizontal cup-to-disc ratio (HCDR) is the ratio of the cup diameter to the disc diameter in the horizontal meridian; HC/(HC+H1+H2). Vertical rim thickness (VRT) is the disc diameter minus the cup diameter in the vertical meridian, or simply V1+V2, expressed in microns. Horizontal rim thickness is the disc diameter minus the cup diameter in the horizontal meridian; H1+H2 (microns). Cup volume (mm3) is a 3-dimensional measurement defined as the volume between a plane created by the cup outline at the vitreous interface and the posterior surface of the ONH. Figure 1B displays the disc perpendicular to viewing angle, where the optic disc and cup area are calculated in the plane of the ONH. The disc and rim areas are the same in Figures 1C and 1D. If the same disc is viewed from a different angle after entering the pupil from a different point, the displayed cup and disc are shortened as displayed in Figure 1C. However, the measurements are unaffected if they are calculated in the plane of the optic as in Figure 1B (Figure 1D).
Statistical analysis
The statistical software SPSS version 17.0 (SPSS Inc., Chicago, IL) was used for statistical analyses. Mean values of peripapillary RNFL thickness and ONH parameters were compared between normal and glaucomatous eyes using the Student-t test for independent samples. One-way analysis of variance (ANOVA) followed by the Tukey post-hoc test was used for multiple comparisons. Receiver operating characteristics (ROC) curves were used to describe the ability of each parameter to differentiate between normal and glaucomatous eyes and between glaucoma severity subgroups. P-values < 0.05 were considered statistically significant. The ROC curve plots the proportion of false positives (1-specificity) against the proportion of true positives (sensitivity). It is a useful way of showing the tradeoff between sensitivity and specificity of a given test or measure. The diagnostic performance of the test is then judged by its closeness to the upper left corner of the graph or the left-hand and the top border of the ROC space, which is assessed quantitatively by reporting the areas under receiver operating characteristics (AUCs). The AUC measures a test's diagnostic ability, that is, its power to correctly classify those with and without the disease. An AUC of 1 (100% sensitivity and 100% specificity) represents a perfect test, while an AUC = 0.5 indicates a completely worthless test. For this study the AUC was classified as follows:20 0.9 – 1 = excellent, 0.80 – 0.89 = good, 0.70 – 0.79 = fair, 0.60 – 0.69 = poor and 0.50 – 0.59 = worthless test. Significant differences between AUCs were assessed by the method described by Hanley and McNeil.21 AUCs were compared using MedCalc version 11.1.0 (MedCalc Software bvba, Mariakerke, Belgium).
Results
The participants' demographic and clinical characteristics are displayed in Table 1. The mean age for glaucoma patients and normal subjects was similar, p = 0.37. The visual field MD was −10.4 ± 8.47 dB for the entire group of glaucoma patients, with significant differences between severity subgroups (p < 0.001).
Table 1.
Demographic and Clinical Characteristics of Study Participants
| Parameters | Normal | All Glaucoma | Mild Glaucoma | Moderate Glaucoma | Severe Glaucoma | p- value |
|---|---|---|---|---|---|---|
| Sample size | 146 | 73 | 31 | 14 | 28 | |
| Male/female | 66/80 | 37/36 | 18/13 | 4/10 | 15/13 | 0.53 |
| Age* in years (SD) | 58.9 (11.4) | 60.4 (10.9) | 58.8 (11.0) | 59.7 (8.7) | 62.4 (10.8) | 0.37 |
| Age range (min–max) | 31–84 | 31–81 | 31–81 | 46–71 | 40–81 | |
| Right/left eye | 81/65 | 29/44 | 14/17 | 6/8 | 9/19 | 0.04 |
Value are presented as mean; SD = standard deviation; VF MD = visual field mean deviation
RNFL and ONH Variables in Normal and Glaucomatous Eyes
Mean values of RNFL thickness parameters in normal and glaucomatous eyes are presented in Table 2 (available at http://aaojournal.org). There were statistically significant differences (p < 0.05) between normal and all glaucomatous eyes and between normal eyes and eyes with mild glaucoma for average RNFL thickness, RNFL thickness in all four quadrants and individual clock-hour sectors, except for the clock-hour directly temporal (9 in right eye, 3 in the left eye). Normal eyes differed from moderate and severe glaucoma in the average and all sectors, except for clock-hours 9 and 3. Thickness of RNFL at the clock-hour 8 also did not differ between normals eyes and eyes with moderate glaucoma. The comparison between eyes with mild and those with moderate glaucoma showed significant differences in thickness of the RNFL only for average RNFL, in the inferior quadrant, and at 6 and 7 clock-hours. None of the RFNL parameters, except RNFL thickness of the superior quadrant (p = 0.037), showed a statistically significant difference when eyes with moderate glaucoma were compared to those with severe glaucoma.
Table 3 (available at: http://aaojournal.org) shows values of ONH measurements. The comparisons between normal and all glaucomatous eyes, between normal eyes and eyes with mild glaucoma, and between normal eyes and eyes with moderate or severe glaucoma all yielded statistically significant differences (p < 0.001) for all parameters, except for disc area, for which differences were observed only between normal eyes and eyes with moderate glaucoma. No differences were found between eyes with mild and those with moderate glaucoma with regard to CDR, HCDR and HRT. Mildly and moderately severe groups were marginally different with respect to rim area (p = 0.045). In addition, none of the ONH parameters demonstrated a significant difference between moderately and severely affected eyes.
Receiver Operating Characteristics in Distinguishing Groups
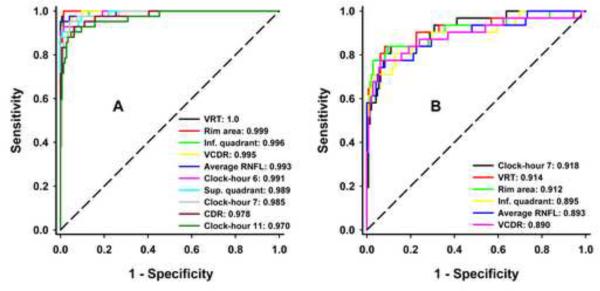
The AUCs of each peripapillary RNFL and ONH parameter for discriminating between healthy and glaucomatous eyes and between eyes at different stages of glaucoma were also calculated (Table 4, available at: http://aaojournal.org). For distinguishing normal from glaucomatous eyes, regardless of disease stage, on the basis of RNFL thickness, the clock-hour 7/5 (lower temporal in both eyes), inferior quadrant, overall average, superior quadrant, clock-hour 11/1 (upper temporal in both eyes) and clock-hour 6 were all excellent (AUCs between 0.923 and 0.957). With regard to ONH parameters, VRT, rim area, VCDR, CDR and HRT outperformed all other ONH parameters (AUCs between 0.901 and 0.963), with the two first seemingly somewhat better, but without a statistically demonstrable difference from the others (Table 5, available at: http://aaojournal.org). When eyes with mild glaucoma were removed from analysis, good to excellent ability to discriminate normal from eyes with moderate to severe glaucoma was observed for all parameters (AUCs = 0.8–1.0), except for disc area, RNFL thickness of the nasal quadrant, clock-hours 3, 8 and 9 (AUCs = 0.456–0.76). ROC curves of the ten parameters that best discriminate normal eyes from eyes with moderate to severe glaucoma are plotted in Figure 2A.
Figure 2.
Receiver Operator Characteristic Curves of the Best Parameters for Discriminating between Normal and Eyes with Moderate to Severe Glaucoma (2A) and of the Overall Best Six Parameters for Discriminating between Normal and Eyes with Mild Glaucoma (2B). VRT - Vertical Rim Thickness, Inf. - Inferior, VCDR - Vertical Cup-to-Disc Ratio, RNFL - Retinal Nerve Fiber Layer, Sup. - Superior, CDR - Cup-to-Disc Ratio, HCDR - Horizontal Cup-to-Disc Ratio.
The best discriminants between healthy and eyes with mild glaucoma, including both RNFL and ONH measurements were RNFL thickness of clock-hour 7/5 sector (lower temporal in both eyes), VRT, rim area, inferior quadrant RNFL, average RNFL thickness and VCDR (AUCs between 0.890 and 0.918). There were no significant differences between AUCs that best differentiate normal from glaucomatous eyes (Table 5A) or healthy from eyes with mild glaucoma (Table 5B, Figure 2B). The ability to discriminate between moderate and severe glaucoma only ranged from fair to poor, with the best parameters being RNFL thickness of the superior quadrant, RNFL thickness of clock-hour 1/11 (upper nasal in both eyes), rim area, average RNFL thickness, RNFL thickness of clock-hour 6 and RNFL thickness of clock-hour 11/1 (upper temporal in both eyes), with AUCs ranging from 0.786 to 0.668.
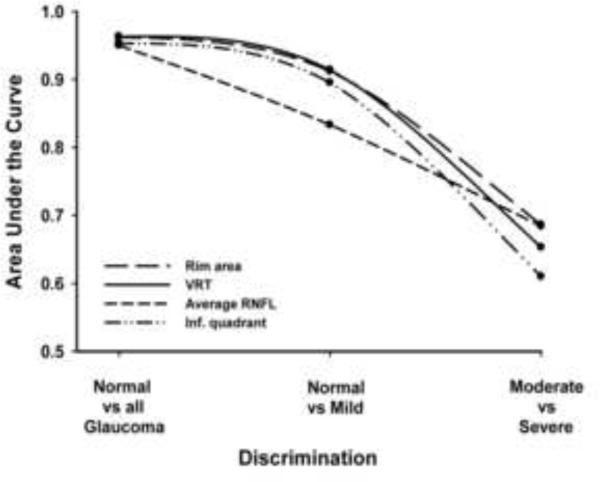
The AUCs of most parameters decreased gradually from discriminating between normal and all glaucomatous eyes (discrimination 1) to discriminating between normal from eyes with mild glaucoma (discrimination 2) and moderate from severe glaucoma (discrimination 3). Figure 3 illustrates the trends of AUCs for VRT, rim area, inferior quadrant RNFL and average RNFL throughout these three discrimination levels. Statistically significant differences were observed for all best six parameters (VRT, rim area, inferior quadrant RNFL, VCDR, average RNFL and RNFL at clock-hour 7) when comparing AUCs at discrimination levels 2 and 3, all p < 0.05.
Figure 3.
Trend of the Ability of Vertical Rim Thickness (VRT), Rim Area, Average Retinal Nerve Fiber Layer (RNFL), and RNFL Thickness of the Inferior (Inf) Quadrant to Discriminate between Normal and Glaucomatous Eyes, Normal and Mild Glaucoma, and Between Moderate and Severe Glaucoma.
Discussion
The results of the current study indicated that both ONH and RNFL parameters as measured by HD-OCT are useful for distinguishing not only healthy from glaucomatous eyes in general, but also healthy from mildly diseased eyes. The results also showed, as might be predicted, a gradual decrease in performance of both RNFL and ONH parameters when they were successively tested for their ability to discriminate between normal eyes and those with moderate to severe glaucoma, between normal eyes and glaucomatous eyes irrespective of disease stage, between normal eyes and those with mild glaucoma, and between eyes with moderate and eyes with severe glaucoma.
When RNFL and ONH parameters were taken separately, in decreasing order of AUC values, RNFL thickness of clock-hour 7, inferior quadrant, and average RNFL thickness were the three RNFL-related parameters with the highest discriminating performance both between healthy and glaucomatous eyes irrespective of disease severity and between normal eyes and eyes with mild glaucoma. These observations are similar to those of Schuman,22 who compared the performance of macular and peripapillary RNFL measured by Cirrus HD-OCT and Stratus OCT to detect glaucoma, to test the hypothesis that the former will perform better than the latter. With regard to Cirrus HD-OCT RNFL parameters, he found that RNFL thickness best discriminated between glaucoma and normals using the inferior quadrant, the average RNFL thickness, clock hour 7, and the superior quadrant (AUC = 0.737–0.77). Interestingly, the two instruments showed similar glaucoma discriminating ability despite higher reproducibility for Cirrus HD-OCT and apparently larger Stratus OCT AUCs. Previous studies on the ability of ONH and RNFL parameters to differentiate normal from glaucomatous eyes used Stratus OCT or other imaging technologies. Although RNFL thickness measurements provided by Stratus OCT and Cirrus HD-OCT are not interchangeable due to systematic differences between the two,23 the current findings will nevertheless be compared to those of previous studies using Stratus OCT. Kanamori et al.24 also reported that RNFL thickness of clock-hour 7 and inferior quadrant as well as the average RNFL thickness were the best parameters, but their AUCs were smaller than ours, presumably due to different study populations. Manassakorn et al.11 compared the performance of peripapillary RNFL thickness and ONH parameters measured by Stratus OCT for glaucoma detection in 42 healthy and 65 glaucomatous eyes including 44 with early disease. They found that RNFL thickness at clock-hour 7, inferior quadrant, clock-hour 6, and average RNFL were best at discriminating between normals and the entire glaucomatous group. When they only considered eyes with mild glaucoma, the only change in the ranking was a switch between RNFL thickness at clock-hour 6 and average RNFL thickness. In a recent similar study by Yüksel et al.12 that included 81 healthy eyes, 68 eyes with mild, 72 eyes with moderate and 73 eyes with severe glaucoma, RNFL thickness of the inferior quadrant (AUC = 0.74) and average RNFL thickness (AUC = 0.74) were the best parameters and performed equally in discriminating between normal eyes and those with mild glaucoma, followed by the RNFL thickness of the superior quadrant (AUC = 0.68). Similar findings were reported by Bourne et al.25 after comparing the diagnostic accuracy of RNFL measurements by OCT 2000 and Stratus OCT. Chen and Huang,6 without evaluating the performance of quadrant sectors, reported somewhat different results from ours and other reports in that healthy eyes and those with mild glaucoma were best differentiated by average RNFL thickness, thickness of the clock-hour 4 and 3 sectors. However, when they tested these variables in another study comprising 100 normal and 89 eyes with mild glaucoma, RNFL thickness of the inferior quadrant was the best discriminant, followed by average RNFL thickness and RNFL thickness of clock-hour 7.9 Budenz et al.26 in a study including 18 mild, 21 moderate and 24 severe glaucoma subjects based on visual field damage reported that the RNFL thickness of the inferior quadrant, average RNFL thickness, and RNFL thickness of the superior quadrant had the highest AUCs of 0.97, 0.97 and 0.95, respectively. Interestingly, they also found that the sensitivity for detecting early glaucoma was 89% (95% CI: 74–100%) for RNFL thickness of one or more quadrants, 83% (66–100%) for the RNFL thickness of one or more clock-hour sectors, and 78% (59–97%) for average RNFL thickness outside 5% normal limits. For Medeiros et al.,27 RNFL of the inferior quadrant and average RNFL thickness had the same largest AUC (0.91), but the former had a slightly higher sensitivity (71% vs. 65%) for a similar specificity (96% vs. 95%). RNFL thickness of clock-hour 6 sector was third in their study. These three parameters were also the best discriminants between healthy and mildly diseased eyes in another study,7 but in a different order; that is RNFL thickness of clock-hour 6 and inferior quadrant sectors, and average RNFL thickness. For Badala et al.28 the parameters that best differentiated normal from early glaucoma using Stratus OCT were average RNFL thickness (AUC = 0.96), RNFL thickness of the inferior quadrant (AUC = 0.95) and clock-hour 7 (AUC = 0.93) sectors, the same parameters as in the present study, but with a different ranking. Wollstein et al.15 performed a study comparing peripapillary RNFL thickness, macular RNFL thickness, and ONH parameters. They reported not only that the RNFL and ONH parameters performed better than macular ones, but also that average RNFL thickness and RNFL thickness of the inferior quadrant had the same highest AUC of 0.94, but average RNFL thickness was more sensitive (84.6% vs. 80.8%) at 95% specificity. Unlike most studies where RNFL thickness of the inferior quadrant or RNFL thickness of individual clock-hours within this quadrant are found to be the best for detection of early glaucoma, Nouri-Mahdavi29 observed a predominance of RFNL parameters of the superior quadrant. From our results and those derived from the use of Stratus OCT, it appears that RNFL thickness of the inferior quadrant, individual RNFL thickness of clock-hour 7 and 6, average RNFL thickness sectors have the highest diagnostic accuracy to distinguish between normal and eyes with mild glaucoma, despite variations in the ranking across studies. It is possible that OCT could more easily detect and measure RNFL changes in the vertical than the horizontal axis since, anatomically, the superior and inferior regions of the ONH have thicker RNFL bundles than the temporal and nasal sectors. The RNFL findings also corroborate the observation that glaucomatous changes to the ONH often start in the superior or inferior poles of the ONH.30
Software for analysis of the ONH with Cirrus HD-OCT has just recently been developed and was made available for this study. The ONH parameters' AUCs for discriminating between healthy and eyes with mild glaucoma were highest for VRT (0.914), rim area (0.912) and VCDR (0.890). They have a sensitivity of 83.9% for VRT and 77.4% for rim area and VCDR, with specificities of 91.8%, 97.3% and 93.8%, respectively. This may be the first study using the new software for Cirrus HD-OCT optic disc analysis, and the number of reports available for Stratus OCT ONH analysis in glaucoma is far fewer than that on RNFL thickness. The lack of enthusiasm for using ONH parameters measured by Stratus OCT probably stems from the low reproducibility of ONH scans31 and weaknesses of the Stratus OCT ONH analysis algorithm (misidentification of the optic disc margin and vitreoretinal boundary and sometimes inclusion of non-optic disk tissue such as vitreous tuft as rim tissue),32 which require manual correction. The diagnostic accuracy of ONH parameters to detect mild glaucoma was examined using Stratus OCT by Leung et al.10 in a study comprising 41 normal and 30 eyes with early glaucoma. VRT (AUC = 0.968), VCDR (AUC = 0.962), and CDR (AUC = 0.960) had the highest ability to distinguish the two groups of eyes. In a Stratus OCT study by Medeiros et al.27 that included 78 normal and 88 glaucomatous eyes (61 mild, 15 moderate and 12 severe), CDR, VCDR, horizontal integrated rim with (HIRW) and rim area had the same best AUC of 0.88 each, with sensitivities of 69, 65, 55 and 51%, respectively for the same specificity of 95%. However, the report failed to mention whether the discrimination was done between normal and eyes with all glaucoma combined or those with mild glaucoma only. Wollstein et al.,15 using a prototype OCT unit, reported that the best ONH discriminants were rim area (AUC = 0.97), HIRW (AUC = 0.96), vertical integrated rim area (VIRA, AUC = 0.95) and CDR (0.94). These AUC values were similar to those reported by Leung et al.,10 but higher than those from most studies, likely because their study eyes included those with moderate and severe glaucoma. A study using Stratus OCT by Deleon-Ortega and colleagues7 reported AUCs of 0.854 for VIRA, 0.850 for HIRW and 0.844 for cup area. Manassakorn et al.11 found that the ONH parameters with highest ability to distinguish normal from eyes with mild glaucoma were VCDR, HIRW and VIRA, with AUCs of 0.89 for the first and 0.88 for the last two parameters. These authors also employed Stratus OCT. More recently, similar AUCs were reported by Yüksel et al.12 for whom cup area (AUC = 0.83), VIRA (AUC = 0.82) and CDR (AUC = 0.82) best discriminated normal from eyes with mild glaucoma using Stratus OCT. Good discrimination was also reported using the stratus OCT for rim volume and VIRA by Sihota et al.,33 with AUCs of 0.889 and 0.835, respectively. In a cross-sectional prospective study performed by Anton and colleagues34 to assess the usefulness of ONH and RNFL to discriminate between ocular hypertensive (n = 95), glaucomatous (n = 79) and normal (n = 55) eyes, HIRW was the single ONH with the highest AUC (0.850). The lowest ability of ONH parameters to distinguish normal from mildly affected eyes using Stratus OCT was reported by Chen and Huang6 and Huang and Chen.9 The largest AUCs of the best discriminants in these two studies were respectively 0.728 and 0.724 for VCDR, 0.711 and 0.724 for CDR, and 0.691 and 0.707 for rim area, with sensitivities not exceeding 60.5% in either study. Altogether, previous reports using Stratus OCT show that VIRA, rim area, and VCDR appear to be the most frequent among the best three ONH parameters, but studies have yet to find a single consistent ONH parameter to be used for glaucoma detection and progression.
In summary, Cirrus HD-OCT ONH parameters, especially vertical rim thickness, rim area, and vertical cup to disc ratio, have excellent ability to discriminate between normal and eyes with even mild glaucoma. These ONH parameters appear to be as good as the best RNFL thickness parameters, including thickness at clock hour 7, inferior quadrant thickness, and average thickness. Although each parameter alone can successfully discriminate between eyes with glaucoma and healthy eyes, using the information from all parameters may be beneficial as this may increase the ability of early glaucoma detection.
Précis.
Optic nerve head parameters measured by Cirrus™ Spectral Domain Optical Coherence Tomography have excellent discriminating ability for glaucoma, performing at least as well as retinal nerve fiber layer thickness measurements using the same technology.
Supplementary Material
Acknowledgments
Financial Disclosure: Supported by an unrestricted grant to the Bascom Palmer Eye Institute, University of Miami Miller School of Medicine, from the Research to Prevent Blindness, Inc., New York, NY; an unrestricted grant from Carl Zeiss Meditec, Inc., Dublin, CA, and by Core Grant; NIH P30 EY014801 awarded to the Bascom Palmer Eye Institute by the National Institute of Health, Bethesda, MD.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Azuara-Blanco A, Katz LJ, Spaeth GL, et al. Clinical agreement among glaucoma experts in the detection of glaucomatous changes of the optic disk using simultaneous stereoscopic photographs. Am J Ophthalmol. 2003;136:949–50. doi: 10.1016/s0002-9394(03)00480-x. [DOI] [PubMed] [Google Scholar]
- 2.Coleman AL, Sommer A, Enger C, et al. Interobserver and intraobserver variability in the detection of glaucomatous progression of the optic disc. J Glaucoma. 1996;5:384–9. [PubMed] [Google Scholar]
- 3.Tielsch JM, Katz J, Quigley HA, et al. Intraobserver and interobserver agreement in measurement of optic disc characteristics. Ophthalmology. 1988;95:350–6. doi: 10.1016/s0161-6420(88)33177-5. [DOI] [PubMed] [Google Scholar]
- 4.Mikelberg FS, Yidegiligne HM, Schulzer M. Optic nerve axon count and axon diameter in patients with ocular hypertension and normal visual fields. Ophthalmology. 1995;102:342–8. doi: 10.1016/s0161-6420(95)31019-6. [DOI] [PubMed] [Google Scholar]
- 5.Sommer A, Katz J, Quigley HA, et al. Clinically detectable nerve fiber atrophy precedes the onset of glaucomatous field loss. Arch Ophthalmol. 1991;109:77–83. doi: 10.1001/archopht.1991.01080010079037. [DOI] [PubMed] [Google Scholar]
- 6.Chen HY, Huang ML. Discrimination between normal and glaucomatous eyes using Stratus optical coherence tomography in Taiwan Chinese subjects. Graefes Arch Clin Exp Ophthalmol. 2005;243:894–902. doi: 10.1007/s00417-005-1140-y. [DOI] [PubMed] [Google Scholar]
- 7.Deleon-Ortega JE, Arthur SN, McGwin G, Jr, et al. Discrimination between glaucomatous and nonglaucomatous eyes using quantitative imaging devices and subjective optic nerve head assessment. Invest Ophthalmol Vis Sci. 2006;47:3374–80. doi: 10.1167/iovs.05-1239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Greaney MJ, Hoffman DC, Garway-Heath DF, et al. Comparison of optic nerve imaging methods to distinguish normal eyes from those with glaucoma. Invest Ophthalmol Vis Sci. 2002;43:140–5. [PubMed] [Google Scholar]
- 9.Huang ML, Chen HY. Development and comparison of automated classifiers for glaucoma diagnosis using Stratus optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:4121–9. doi: 10.1167/iovs.05-0069. [DOI] [PubMed] [Google Scholar]
- 10.Leung CK, Chan WM, Hui YL, et al. Analysis of retinal nerve fiber layer and optic nerve head in glaucoma with different reference plane offsets, using optical coherence tomography. Invest Ophthalmol Vis Sci. 2005;46:891–9. doi: 10.1167/iovs.04-1107. [DOI] [PubMed] [Google Scholar]
- 11.Manassakorn A, Nouri-Mahdavi K, Caprioli J. Comparison of retinal nerve fiber layer thickness and optic disk algorithms with optical coherence tomography to detect glaucoma. Am J Ophthalmol. 2006;141:105–15. doi: 10.1016/j.ajo.2005.08.023. [DOI] [PubMed] [Google Scholar]
- 12.Yuksel N, Altintas O, Ozkan B, et al. Discriminating ability of optical coherence tomography data in staging glaucomatous damage. Can J Ophthalmol. 2009;44:297–307. doi: 10.3129/i09-020. [DOI] [PubMed] [Google Scholar]
- 13.Zangwill LM, Bowd C, Berry CC, et al. Discriminating between normal and glaucomatous eyes using the Heidelberg Retina Tomograph, GDx Nerve Fiber Analyzer, and optical coherence tomograph. Arch Ophthalmol. 2001;119:985–93. doi: 10.1001/archopht.119.7.985. [DOI] [PubMed] [Google Scholar]
- 14.Sehi M, Greenfield DS. Assessment of retinal nerve fiber layer using optical coherence tomography and scanning laser polarimetry in progressive glaucomatous optic neuropathy. Am J Ophthalmol. 2006;142:1056–9. doi: 10.1016/j.ajo.2006.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wollstein G, Schuman JS, Price LL, et al. Optical coherence tomography longitudinal evaluation of retinal nerve fiber layer thickness in glaucoma. Arch Ophthalmol. 2005;123:464–70. doi: 10.1001/archopht.123.4.464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ishikawa H, Piette S, Liebmann JM, Ritch R. Detecting the inner and outer borders of the retinal nerve fiber layer using optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2002;240:362–71. doi: 10.1007/s00417-002-0461-3. [DOI] [PubMed] [Google Scholar]
- 17.Downs JC, Yang H, Girkin C, et al. Three-dimensional histomorphometry of the normal and glaucomatous monkey optic nerve head: neural canal and subarachnoid space architecture. Invest Ophthalmol Vis Sci. 2007;48:3195–208. doi: 10.1167/iovs.07-0021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Budenz DL. Atlas of Visual Fields. Lippincott-Raven; Philadelphia, PA: 1997. pp. 143–5. [Google Scholar]
- 19.Hodapp E, Parrish RK, II, Anderson DR. Clinical Decisions in Glaucoma. Mosby; St. Louis, MO: 1993. pp. 52–61. [Google Scholar]
- 20.Tape TG. [Accessed April 25, 2010];Interpreting Diagnostic Tests. Available at: http://gim.unmc.edu/dxtests/Default.htm.
- 21.Hanley JA, McNeil BJ. A method of comparing the areas under receiver operating characteristic curves derived from the same cases. Radiology. 1983;148:839–43. doi: 10.1148/radiology.148.3.6878708. [DOI] [PubMed] [Google Scholar]
- 22.Schuman JS. Spectral domain optical coherence tomography for glaucoma (an AOS thesis) Trans Am Ophthalmol Soc. 2008;106:426–58. [PMC free article] [PubMed] [Google Scholar]
- 23.Knight OJ, Chang RT, Feuer WJ, Budenz DL. Comparison of retinal nerve fiber layer measurements using time domain and spectral domain optical coherent tomography. Ophthalmology. 2009;116:1271–7. doi: 10.1016/j.ophtha.2008.12.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kanamori A, Nakamura M, Escano MF, et al. Evaluation of the glaucomatous damage on retinal nerve fiber layer thickness measured by optical coherence tomography. Am J Ophthalmol. 2003;135:513–20. doi: 10.1016/s0002-9394(02)02003-2. [DOI] [PubMed] [Google Scholar]
- 25.Bourne RR, Medeiros FA, Bowd C, et al. Comparability of retinal nerve fiber layer thickness measurements of optical coherence tomography instruments. Invest Ophthalmol Vis Sci. 2005;46:1280–5. doi: 10.1167/iovs.04-1000. [DOI] [PubMed] [Google Scholar]
- 26.Budenz DL, Michael A, Chang RT, et al. Sensitivity and specificity of the StratusOCT for perimetric glaucoma. Ophthalmology. 2005;112:3–9. doi: 10.1016/j.ophtha.2004.06.039. [DOI] [PubMed] [Google Scholar]
- 27.Medeiros FA, Zangwill LM, Bowd C, et al. Evaluation of retinal nerve fiber layer, optic nerve head, and macular thickness measurements for glaucoma detection using optical coherence tomography. Am J Ophthalmol. 2005;139:44–55. doi: 10.1016/j.ajo.2004.08.069. [DOI] [PubMed] [Google Scholar]
- 28.Badala F, Nouri-Mahdavi K, Raoof DA, et al. Optic disk and nerve fiber layer imaging to detect glaucoma. Am J Ophthalmol. 2007;144:724–32. doi: 10.1016/j.ajo.2007.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nouri-Mahdavi K, Hoffman D, Tannenbaum DP, et al. Identifying early glaucoma with optical coherence tomography. Am J Ophthalmol. 2004;137:228–35. doi: 10.1016/j.ajo.2003.09.004. [DOI] [PubMed] [Google Scholar]
- 30.Uchida H, Brigatti L, Caprioli J. Detection of structural damage from glaucoma with confocal laser image analysis. Invest Ophthalmol Vis Sci. 1996;37:2393–401. [PubMed] [Google Scholar]
- 31.Iliev ME, Meyenberg A, Garweg JG. Morphometric assessment of normal, suspect and glaucomatous optic discs with Stratus OCT and HRT II. Eye. 2006;20:1288–99. doi: 10.1038/sj.eye.6702101. [DOI] [PubMed] [Google Scholar]
- 32.Ortega JdeL, Kakati B, Girkin CA. Artifacts on the optic nerve head analysis of the optical coherence tomography in glaucomatous and nonglaucomatous eyes. J Glaucoma. 2009;18:186–91. doi: 10.1097/IJG.0b013e31818159cb. [DOI] [PubMed] [Google Scholar]
- 33.Sihota R, Sony P, Gupta V, et al. Comparing glaucomatous optic neuropathy in primary open angle and chronic primary angle closure glaucoma eyes by optical coherence tomography. Ophthalmic Physiol Opt. 2005;25:408–15. doi: 10.1111/j.1475-1313.2005.00304.x. [DOI] [PubMed] [Google Scholar]
- 34.Anton A, Moreno-Montanes J, Blazquez F, et al. Usefulness of optical coherence tomography parameters of the optic disc and the retinal nerve fiber layer to differentiate glaucomatous, ocular hypertensive, and normal eyes. J Glaucoma. 2007;16:1–8. doi: 10.1097/01.ijg.0000212215.12180.19. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.