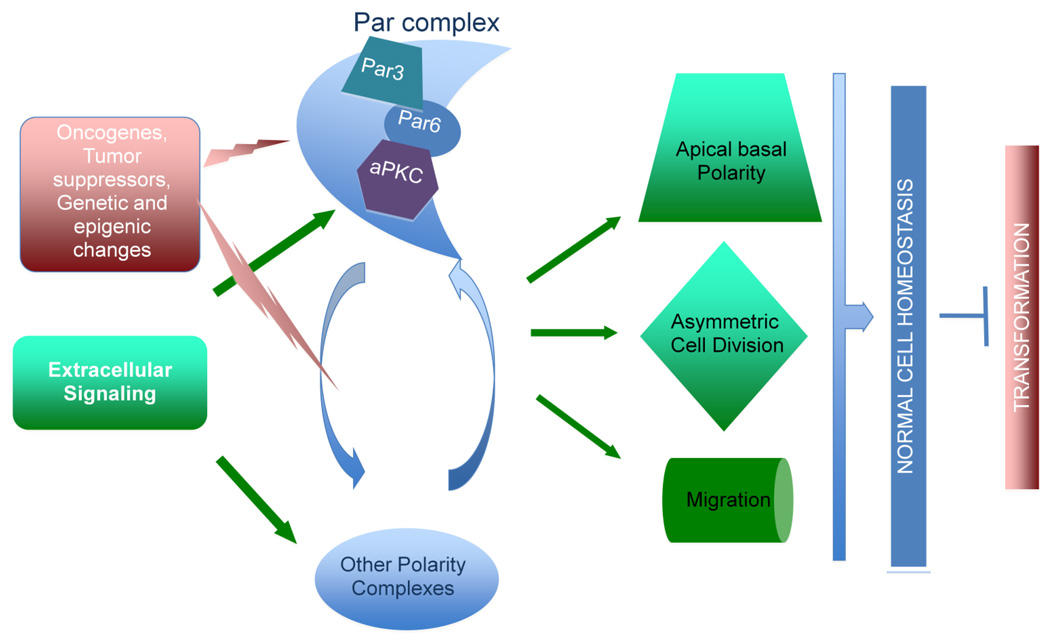
In the past 20 years, the discovery and characterization of the molecular machinery that controls cellular polarization has enabled us to achieve a better understanding of many biological processes. Spatial asymmetry or establishment of cell polarity, during embryogenesis, epithelial morphogenesis, neuronal differentiation, and migration of fibroblasts and T-cells are thought to rely on a small number of evolutionarily conserved proteins and pathways. Correct polarization is crucial for normal cell physiology and tissue homeostasis, and is lost in cancer. Thus, cell polarity signaling is likely to play an important role in tumor progression. Recent findings have identified a regulator of cell polarity, the Par complex, as an important signaling node in tumorigenesis. In normal cell types, the Par complex is part of the molecular machinery that regulates cell polarity and maintains normal cell homeostasis. As such, the polarity regulators are proposed to play a tumor suppressor role, consistent with the loss of polarity genes associated with hyperproliferation in Drosophila. However, recent studies showing that some members of this complex also display pro-oncogenic activities suggest a more complex regulation of the polarity machinery during cellular transformation. Here we examine the existing data about the different functions of the Par complex. We discuss how spatial restriction, binding partners, and substrate specificity determine the signaling properties of Par complex proteins. A better understanding of these processes will likely shed some light on how the Par complex can switch from a normal polarity regulation function to promotion of transformation downstream of oncogenes.
Polarity and cancer progression
Normal epithelial cells in glandular tissues are characterized by a defined organization, displaying an asymmetric distribution of proteins along the internal apical-basal axis. During morphogenesis cells undergo a profound reorganization of the cytoskeleton, organelles, membranes and other cellular components to create an internal axis of asymmetry. This property, known as apical-basal polarity, is part of a differentiation program that determines cell behavior. Intact cell organization is not only required for proper organ function, but may also dictate the growth state of the cell, as well as its survival. While many studies have characterized the molecular pathways that provide proliferation and survival downstream of oncogenic signaling (Hanahan and Weinberg, 2000), neither the contribution of cell organization and polarity nor the mechanisms by which oncogenes deregulate tissue organization during transformation have been characterized. Histologically, a strong correlation between malignancy and loss of epithelial organization has been documented for almost all types of tumors. This suggests that understanding the molecular mechanisms that regulate cell and tissue organization, as well as how they are targeted during transformation will give important insights into tumor development.
Polarization is also required for other biological processes that require spatial asymmetry, such as stem cell division, and cell migration in different contexts (Dow and Humbert, 2007; Lee and Vasioukhin, 2008; Morrison and Kimble, 2006). This suggests that polarity signaling may also be in play during more advanced stages of oncogenesis that involve invasive and migratory processes. The novel findings about the potential importance of stem cell regulation, and of epithelial-to-mesenchymal transition (both of which require spatial asymmetry and polarized signaling) (Hugo et al., 2007)(see Moreno-Bueno et al, this issue), as cancer related processes, indicate as well that polarity regulation may be involved in multiple aspects of oncogenesis.
Polarization is regulated by an extensive intracellular signaling network, that is only recently beginning to be understood (Bilder et al., 2003; Goldstein and Macara, 2007; Macara, 2004; Martin-Belmonte and Mostov, 2008a), and whose role in oncogenesis remains elusive. This is due, in part, to the lack of appropriate models to recreate and study cellular organization of glandular structures in vitro. Traditional in vitro approaches of monolayer cell culture do not recreate the interactions observed in the three-dimensional (3D) space of a complex organ. Moreover, cancer-derived cell lines have usually lost their ability to maintain structural and functional properties of the organ they are derived from. Studies pioneered by Mina Bissell using three-dimensional tissue cultured systems (Petersen et al., 1992) are overcoming these constraints and showing how epithelial organization can modulate the outcome of oncogenic signaling, and how oncogenes must deregulate organization to successfully transform cells in a three-dimensional environment, as occurs in vivo (Debnath et al., 2003; Hebner et al., 2008; Muthuswamy et al., 2001; Underwood et al., 2006; Weaver et al., 1996; Xiang and Muthuswamy, 2006). These studies provide the conceptual framework as well as the experimental tools to discover the molecular pathways that deregulate cell organization during transformation.
The molecular machinery that creates and maintains polarity is well conserved among species, and common to all different types of polarization. Polarity is achieved by the concerted action of three protein complexes that interplay with each other and with the structural components of the cytoskeleton and intercellular junctions in the case of apical-basal polarity) (Assémat et al., 2008; Dow and Humbert, 2007; Etienne-Manneville and Hall, 2003b). The Scribble complex, the Crumbs complex and the Par complex, define the basolateral domain, the apical domain, and the apical-lateral border respectively. Some of the proteins in these complexes are implicated in oncogenesis, and have been identified as a novel type of tumor suppressor (Bilder, 2004; Lee and Vasioukhin, 2008). Here we will describe the multiple aspects of Par complex signaling and how it interplays with oncogenic pathways.
Par complex is a crucial regulator of apical-basal polarity
The Par proteins (name derived from ‘partitioning defective’) were first identified in a C. elegans screen for mutants that were defective in anterior-posterior partitioning of proteins in the early embryo. (Kemphues et al., 1988). Since then, the six Par proteins have been found in almost every organism from C. elegans to Xenopus to mammals (reviewed in (Goldstein and Macara, 2007). The Par complex includes two of these Par proteins, Par3 and Par6, the serine/threonine kinase aPKC and small GTPases, such as Cdc42 or Rac1 (Joberty et al., 2000) (Johnson, 1999; Lin et al., 2000).
The initial characterization of the Par complex in C. elegans showed its involvement in asymmetric cell division at the one-cell stage of the embryo (Kemphues, 2000; Kemphues et al., 1988). Par complex is required for the spatial restriction of the cytoskeleton and the asymmetry of the spindle (Munro, 2006; Nance et al., 2003), as well as for the unequal distribution of cell fate determinants between daughter cells. This was shown to be dependent on the localization of Par complex components to asymmetric membrane domains of the dividing cell. The subsequent discovery of Par homologues in other organisms, as well as the description of their roles in numerous cell polarization processes, has established asymmetrical spatial restriction as the conserved Par complex function.
Genetic studies in D.melanogaster have established a prominent role for the Par complex in regulating the formation and maintenance of apical basal polarity in epithelial cells (Hirose et al., 2002; Hutterer et al., 2004; Rolls et al., 2003; Tepass et al., 2001). In mammals, the Par complex is also required for apical-basal polarity, but multiple isoforms of Par6 (Gao and Macara, 2004; Noda et al., 2001) and aPKC (Izumi et al., 1998) have been described which may exhibit functional redundancy. Much work is currently devoted to map the interactions and functions of the Par complex in establishing and maintaining cell polarity (Lin et al., 2000) (Hirose et al., 2002; Izumi et al., 1998; Nagai-Tamai et al., 2002; Qiu et al., 2000) (Figure 1). Biochemical and cell biological studies in mammalian cells are beginning to provide insights into how the Par complex is restricted to the apical-basal border of polarized epithelia, and how it promotes spatial asymmetry of the cytoskeleton and secretory organelles of the cells. (Goldstein and Macara, 2007; Wodarz and Nathke, 2007) (Bilder et al., 2003).
Figure 1. The Par complex and normal polarized signaling.
Par complex activity is involved in multiple polarization processes that are part of normal cell physiology. The complex integrates extracellular stimuli into the polarized cell program and interprets spatial cues to maintain differentiated homeostasis. It has been shown that some oncogenes can directly recruit or deregulate the Par complex to promote oncogenesis by two mechanisms. Disruption of normal polarity functions can induce transformation, and also Par complex components can participate in oncogenic signaling pathways. This oncogenic role is dictated by qualitative and quantitative changes in the complex composition and localization.
In mammals the apical-lateral border is structurally defined by the tight junctions, a specialized type of intercellular adhesion complex (see Tsukita et al., this issue). Mature tight junctions enforce structural epithelial polarity by forming a physical barrier that prevents intermixing of membrane proteins between apical and basolateral surface. The components of the tight junctions, namely occludins, claudins, nectins and JAM (Junction Adhesion Molecule) proteins, form a tight intercellular seal that prevents uncontrolled paracellular leakage of fluids in and out of the luminal space (Ebnet et al., 2008; Fleming et al., 2000). Par3, a multiple PDZ (Postsynaptic density, Disc Large, ZO-1) domain containing protein, associates with these junctions via binding to JAM (Ebnet et al., 2003; Ebnet et al., 2001) and nectin-1/3 (Suzuki and Ohno, 2006). Par3 in turn is able to promote junction assembly by cofilin-mediated regulation of actin dynamics (Chen and Macara, 2006). Thus, Par3 provides anchorage to assemble the Par complex at the apical-lateral border, by binding Par6 and recruiting Par6 associated proteins.
Par6 is a scaffolding protein that contains a PDZ, a PB1 (Phox Bem1) and a semi-CRIB (Cdc42 Rac Interacting Binding) domain. These provide direct interaction with at least three other members of the Par complex, aPKC, Cdc42/Rac and Par3 (Noda et al., 2001). aPKC interacts with the PB1 domain of Par6, and while this interaction tethers the kinase to tight junctions in polarizing epithelia, it is also thought to inhibit the activity of aPKC. The Par6-aPKC complex is localized to the tight junctions by the interaction with Par3 (Bilder, 2003; Hirose et al., 2002). This spatial restriction is reinforced by a feedback mechanism where Par3 is phosphorylated by aPKC, and this phosphorylation maintains Par3 at tight junctions (Izumi et al., 1998; Macara, 2004). Binding of Cdc42 to this maturing Par complex via binding to semiCRIB and PDZ domains of Par6 is thought to be the key event that activates aPKC at the apical border (Chen and Macara, 2006; Etienne-Manneville, 2004; Joberty et al., 2000). Thus, aPKC is asymmetrically localized by the scaffolding Par proteins, but its activity is modulated by GTPase binding. Recent studies in keratinocytes as well as in neurons suggest that in addition to Cdc42, other GTPases such as Rac1 also function as modulators of Par complex activity. Consistent with this notion, Par3 interacts with Tiam1, a Rac GEF, and this interaction is important for tight junction formation and epithelial morphogenesis (Chen and Macara, 2005; Mertens et al., 2005).
Yet another layer of complexity to the Par complex activity is provided by upstream regulation by phosphoinositides. Cdc42 can be recruited to the apical surface by PIP2 (Phosphoinositol biphosphate) generated by PTEN (Phosphatase and TENsin homolog) (Figure 1) (Etienne-Manneville, 2004; Garrard et al., 2003; Johnson, 1999; Kay and Hunter, 2001; Lin et al., 2000; Peterson et al., 2004; Yamanaka et al., 2001). It seems likely that phosphoinositide signaling is involved in different aspects of polarity formation, as it was recently shown that PTEN can bind Par3 and this is required for tight junction formation (Feng et al., 2008; Martin-Belmonte et al., 2007; Martin-Belmonte and Mostov, 2008b; Wu et al., 2007). Moreover, Par6 and Par3 can also interact via their PDZ domains with phospholipase C beta (PLCβ), thus regulating other enzymatic activities that are important in phosphoinositide signaling (Cai et al., 2005).
The role of aPKC activity during establishment of polarity has been a subject of intense study, leading to the identification of several different substrates, of which many are polarity regulators. aPKC phosphorylation of polarity regulators outside the Par complex provides the necessary crosstalk to establish asymmetric membrane domains. Similar to its interacton with Par3, aPKC-mediated phosphorylation is required for the correct localization and function of the Crumbs complex (Hurd et al., 2003; Lemmers et al., 2004; Wang et al., 2004). Both PALS1 (Protein associated with lin 7 1) and the apical transmembrane protein Crumbs, members of the Crumbs complex directly bind Par6. (Hurov and Piwnica-Worms, 2007; Hurov et al., 2004; Sotillos et al., 2004; Suzuki et al., 2004). The mechanism of action of Crumbs complex remains largely unknown, but it relies on aPKC phosphorylation and Par6 binding that provide apical restriction. Concomitantly, the interaction with Crumbs complex upregulates aPKC activity and inhibits the function of basolateral regulators such as the Scribble complex at the apical space. Recent data has described the specific binding of the Crumbs complex to cytoskeleton motor protein dynein (Horne-Badovinac and Bilder, 2008; Li et al., 2008). This may be a crucial initiating event that provides apical restriction and enables the synergy between Crumbs complex and aPKC to direct the apical domain formation.
aPKC controls the establishment of the basolateral domain by excluding components of lateral membrane from the apical surface. For example, aPKC phosphorylates Lgl to exclude it from the apical membrane and restrict it to the lateral domain (Betschinger et al., 2005; Betschinger et al., 2003; Plant et al., 2003; Yamanaka and Ohno, 2008). Interestingly, Lgl and Par3 interact with aPKC in a mutually exclusive manner (Hutterer et al., 2004) suggesting that aPKC may exist in functionally distinct complexes to regulate establishment of apical-basal polarity. This may be mediated by different scaffolding systems. The functional and physical interaction between aPKC and Lgl is mediated by a novel aPKC activating protein, p32. p32 is required for proper establishment of apical-basal polarity in MDCK cells probably through its ability to regulate aPKC activity and facilitate phosphorylation of Lgl2 (Bialucha et al., 2007). Recently, a novel regulator of aPKC activity, the intersectin homologue Dap160, has been reported to bind both Par6 and aPKC as a positive regulator of the kinase’s activity and localization. This interaction is important for neuroblast polarity and cell cycle progression (Chabu and Doe, 2008).
Although this and other points of interplay between polarity regulator complexes are only beginning to be understood, our current knowledge already highlights the fine tuned nature of polarity signaling. The versatility of Par complex signaling extends to other polarization processes (Figure 1), such as polarized cell migration, which can also play a role in malignant progression (see Etienne-Manneville, this issue).
Par complex is involved in polarized cell migration
The role of Par complex in migratory processes relies on its spatial control of actomyosin fiber activity, and of GTPase activity. Studies describing wound-induced migration in astrocytes and fibroblasts, as well as activated T-cells, demonstrated that the core Par6-aPKC module coordinates the stepwise events leading to polarized movement (Gerard et al., 2007; Ludford-Menting et al., 2005). In these systems, the asymmetry is dictated by integrin ligation at the leading edge that recruits Cdc42 and activates Par6-bound aPKC (Etienne-Manneville and Hall, 2001). The spatially restricted aPKC activity at the leading edge phosphorylates GSK-3β at an inhibitory serine, detaching it from its substrate adenomatous polyposis coli (APC) (Etienne-Manneville and Hall, 2003a; Farooqui et al., 2006). APC is then free to bind the plus end of microtubules and anchor them at the cell membrane via binding to Dlg1 (Etienne-Manneville et al., 2005). This anchorage will promote the formation of a polarized cytoskeleton and relocalize the microtubule organization center (MTOC), Golgi and other cellular components towards the direction of migration (Gomes et al., 2005). GSK-3β phosphorylation may not be the only mechanism relevant for MTOC. Wnt5A signaling can promote and interaction between Dishevelled 2 and aPKC at the leading edge of a migrating cell, suggesting a novel link between wnt signaling and polarized cell migration involving Par6-aPKC-Cdc42 complex. Cdc42 participates in polarized migration by regulating Par6 and aPKC (Anderson et al., 2008) and also by directly controlling actin polymerization and MTOC reorientation through Par-independent mechanisms (Cau and Hall, 2005).
In addition, the Par6-aPKC complex at the leading edge of a migrating cell recruits the E3 ubiquitin ligase Smurf 1 and targets RhoA for degradation (Nakayama et al., 2008; Nishimura et al., 2005; Pegtel et al., 2007; Wang et al., 2003). Once the protrusion is formed, active Par3 can bind Tiam1/Tiam2 and also modulate Rac1 activity in lamelipodial extension, in a similar fashion to the process of dendritic spine maturation (Zhang and Macara, 2006). Intriguingly, in this system, dendritic spine genesis is also initially regulated by Par6-mediated regulation of RhoA signaling. Par6 can recruit p190 RhoGAP and downregulate RhoA specifically at the membrane location where spines are being formed (Zhang and Macara, 2008). Par3 in turn plays a role in spine maturation by restricting the positive regulator Rac’s activity via sequestering its GEFs Tiam1/Tiam2. Thus, Par3 and Par6 play complementary roles to achieve spatiotemporal control of GTPase signaling during polarized cell migration.
The common theme arising from different polarization mechanism suggests that the Par complex should be viewed as a central node that integrates various signaling inputs into the program of cell polarity (Figure 1). However, much work is needed to decipher both upstream regulators and downstream effectors of the Par complex that may link this signaling module to other intracellular and extracellular signaling events.
Par complex interplays with normal signaling pathways
The extensive interplay between the Par complex and other signaling systems such as small GTPases (Etienne-Manneville and Hall, 2002) or phosphoinositide signaling (Martin-Belmonte and Mostov, 2007) suggests that the Par complex can change composition dynamically to modulate the outcome of extracellular inputs as well. (Bose and Wrana, 2006). This relies on the ability of Par3 and Par6 to bind different adaptors and transmit their inputs to aPKC-mediated phosphorylation of distinct substrates, or downstream effectors. New aPKC modulators, such as Dap60/intersectin, may represent an interesting connection between the Par complex and other pathways such as growth factor or Ras signaling (Tong et al., 2000; Wang et al., 2005)
On top of protein composition, cellular context also regulates Par complex activity. For example, we have discussed above how aPKC-directed phosphorylation of GSK-3β determines the directionality of migration in astrocytes and fibroblasts (Etienne-Manneville and Hall, 2001; Etienne-Manneville and Hall, 2003a; Etienne-Manneville et al., 2005; Farooqui et al., 2006). However, in three-dimensionally organized kidney epithelial cells, aPKC phosphorylation of GSK-3β functions to inhibit apoptosis in polarized cells (Kim et al., 2007). In myoblasts, insulin signaling is negatively regulated by Par6-aPKC. This is likely due to an insulin-induced, Par6-aPKC-dependent inhibition of both Akt and Insulin receptor substrate 1 (Moeschel et al., 2004; Weyrich et al., 2004; Weyrich et al., 2007).
In addition, Par complex members also display independent signaling activities outside of the complex and are likely to have cell-type specific functions. aPKC interacting domains, AIDs, has been reported in other proteins such as p62, that can tether aPKC activity to other signaling networks such as NF-κB and c-Jun N-terminal kinase (JNK) pathways (Moscat and Diaz-Meco, 2000; Moscat et al., 2006). However, the precise role aPKC plays in NF-κB and JNK pathways remains to be understood. If aPKC activity has different roles within and outside the Par complex, quantitative regulation of Par6 binding is likely to determine also the signaling outcomes of extracellular stimuli. Par6 defines aPKC signaling pathways, and this could be of importance in the case of genetic imbalance of one of the components of this network.
Par complex and regulation of oncogenic and tumor suppressor pathways
Interestingly, we have recently shown that Par6β is genetically amplified in breast tumors (Nolan et al., 2008). Overexpression of Par6β in normal human mammary epithelial cells induces activation of MAPK signaling and promotes growth factor independent proliferation. This function of Par6 is dependent on Cdc42 and aPKC interaction, and represents a new signaling role for the Par complex activity in regulating cancer related processes (Figure 1).
The fact that the Par complex provides quantitative regulation, selectivity and spatial restriction for aPKC activity is remarkable in view of the recent reports proposing an oncogenic role for deregulated aPKC. Studies in Drosophila have identified aPKC activation and erroneous localization as factors that promote tumor growth (Eder et al., 2005; Grifoni et al., 2007; Lee et al., 2006). aPKC activity has also been placed downstream of other transformation pathways, where it can participate in estrogen receptor-dependent stabilization of the oncogenic coactivator SRC-3 (Steroid Receptor Coactivator)(Yi et al., 2008). Moreover, aPKC isoforms have been implicated in human and mouse malignancies of ovarian, head and neck, lung, breast, liver, and colon origin (Eder et al., 2005; Fields and Regala, 2007; Kojima et al., 2008; Regala et al., 2005; Stallings-Mann et al., 2006; Zhang et al., 2006). The hyperactivation or mislocalization of the kinase affects tumor growth, motility, and proliferation in cell lines(Donson et al., 2000; Fields et al., 2003; Murray et al., 2004; Sun et al., 2005) (Cohen et al., 2006; Kuribayashi et al., 2007). This suggests that failure to curb aPKC by normal polarized activity of the Par complex may unleash its pro-oncogenic potential (Figure 1).
Members of the Par complex may also regulate other known tumor suppressor pathways. The von Hippel-Lindau factor VHL is an E3 ubiquitin ligase that participates in proliferation control by regulating hypoxia-induced factor (HIF) levels (Kuehn et al., 2007; Kurban et al., 2006). But VHL has also been shown to interact directly and induce degradation of aPKC (Okuda et al., 1999; Okuda et al., 2001). Interestingly, VHL regulates establishment of cell-cell junctions and cell polarity in a HIF1a-independent manner (Calzada et al., 2006; Ji and Burk, 2008), suggesting that both events may be related. Further analysis will be required to determine if the ability of VHL to regulate aPKC relates to its effects on cell-cell junctions and cell polarity. The aPKC connection is thus revealing the mechanisms by which VHL, and other proteins, function as tumor suppressors. As discussed above, PTEN, another tumor suppressor, also plays an important role in regulating the correct localization of the Par complex.
Loss of function mutations in other polarity regulators belonging to the Scrib/Lgl/Dlg group have been shown to act as tumor suppressor genes in Drosophila, promoting tumor growth and neoplasia (Bilder, 2004) demonstrating that cell polarity functions as a tumor suppressor factor. Interestingly, the tumorigenic effects of Lgl loss in Drosophila can be rescued by inactivating aPKC. Likewise, aPKC activity can be oncogenic when localized at the cortical surface (Grifoni et al., 2007). This is of importance considering the studies that suggest that Lgl is often lost in human malignancies (Kuphal et al., 2006; Schimanski et al., 2005; Tsuruga et al., 2007). The quantitative regulation of the Lgl-aPKC interaction is thus likely to shift the balance between normal and aberrant signaling. This, combined with the evidence supporting an oncogenic role for aPKC in mammalian systems and human tumors, suggests that whereas cell organization is a tumor suppressive factor, the Par complex can have wider implication in cancer and play an oncogenic role. The oncogenic role of aPKC seems to result from a combination of organization loss and diversion of aPKC activity to other signaling pathways. Taken together, these findings put the molecular machinery that controls cellular organization at the crossroads of normal tissue homeostasis and tumor progression. Due to the plastic nature of its interactions, the precise role of the Par complex in transformation is likely to depend on its ability to interact with different oncogenic stimuli.
Par complex as an effector of oncogenic signaling
Many studies have positioned the Par complex both downstream of tumor suppressor pathways as well as a target of oncogenic signaling (Wodarz and Nathke, 2007). The interaction between the Par complex and GTPase signaling has traditionally been regarded as a connection to oncogenic signaling. Par6 and aPKC have been shown to be required for transformation downstream of constitutively active Rac1 and Cdc42 (Noda et al., 2001), suggesting that they can modulate oncogenic stimuli. Moreover, Par 6 has been shown to bind the new small GTPases Rin and Rit, (Hoshino et al., 2005). This may provide a novel link between the Par complex and oncogenesis, because Par6 interaction with Rin and Rit potentiates transformation downstream of Rac and Cdc42 (Figure 1).
The Par complex also functions downstream of oncogenic signaling pathways. Wang et al. demonstrated how TGFβR type I can interact and phosphorylate Par6 at tight junctions, and this phosphorylation results in Par6 binding to Smurf1 to catalyze the degradation of RhoA (Wang et al., 2006). Interestingly, Par6-mediated degradation of RhoA is necessary for tight junction dissolution and progression to the epithelial-to-mesenchymal transition (EMT) pathway (Figure 1) (Ozdamar et al., 2005). In endocardial cells, Par6 is also a requirement downstream of TGFβ induction of EMT (Townsend et al., 2008).
EMT is thought to play a role in late stage cancer progression to malignant disease (Huber et al., 2005)(see Moreno-Bueno et al, this issue), and therefore this signaling pathway is consistent with an oncogenic role for Par6. But the Par complex role in EMT is not limited to Par6 regulation of RhoA. Recent studies suggest that in parallel to Par6 binding and phosphorylation, TGFβ also downregulates Par3 expression (Wang et al., 2008) and induces translocation of Par6 from tight junctions to the cytoplasm. Overexpression of Par3 inhibits TGFβ-induced loss of E-cadherin and EMT, suggesting that TGFβ remodels the Par complex from one that regulates normal polarity to one that promotes transformation, by changing the binding partners, composition and localization of Par6-aPKC.
This paradigm is supported by ErbB2 mediated regulation of the Par complex. ErbB2 is a receptor tyrosine kinase that plays a relevant oncogenic role in breast cancer (Yarden, 2001; Yarden and Sliwkowski, 2001). ErbB2 is amplified in 25–30% of breast cancers and correlates with poor prognosis (Slamon, 1987; Slamon et al., 1987; Slamon et al., 1989). Seeking to identify new mediators and cooperating factors required for ErbB2 oncogenesis, we developed a 3D system to study the effects of ErbB2 activation in normal, organized mammary epithelial cells (Muthuswamy et al., 2001).The study of oncogenic signaling using three dimensional culture systems for normal mammary epithelial cells revealed that intact cell polarity and organization act as checkpoints during transformation. Oncogenes such as ErbB2 are able to overcome these check points and transform organization-competent cells, in clear distinction to other oncogenes such as ErbB1. Therefore, ErbB2 must use unique mechanisms that enable disruption of cellular organization. These mechanisms are being elucidated, and we discovered that they are dependent on regulation of the Par complex activity (Aranda et al., 2006). The ability of ErbB2 to transform organized epithelial cells is dependent on its ability to interact with Par6-aPKC and recruit both proteins away from Par3 and the apical-basal border. Thus, ErbB2 likely disrupts normal polarity signaling by inactivating the Par3-Par6-aPKC node. Moreover, functionally blocking Par6-aPKC interaction impairs ErbB2’s ability to induced neoplastic, aberrant growth, suggesting that ErbB2 recruitment of Par6-aPKC to its own activated complex is required for downstream signaling (Figure 1). This is independent of proliferative signaling, and only partially related to cell survival, suggesting a novel signaling pathway downstream of oncogenes such as ErbB2.
Disruption of cell polarity and tissue organization is an important event in the initiation and progression of tumorigenesis, and therefore alteration of the Par complex, intrinsic or induced by oncogenes, can potentially play a crucial role in the development of tumors in normal organized tissues in vivo (Figure 1). It is possible that novel mediators are required to divert the Par complex to an oncogenic role, either by modulating aPKC activity or by recruiting it to oncogenic complexes. Elucidating the mechanisms for oncogene-mediated recruitment and the effectors of Par6-aPKC in this context will shed light about the oncogenic role of Par complex in cancer.
Conclusions and perspectives
The Par complex had been traditionally involved in different types of polarization, from embryonic development to epithelial morphogenesis to migration. Recent studies are showing that this protein complex can play different functions provided by the combinatorial nature of the signaling elicited by it. The Par complex is emerging as a crucial signaling module that integrates external and internal inputs with different polarization processes. The outcome contributes to the homeostasis of normal cells and prevents aberrant, disorganized growth or movement. Consistent with this, studies depicting the stepwise process of tumorigenesis have shown that deregulation of Par complex activity is a key factor for initiation of transformation. However, recent data has uncovered a pro-oncogenic role for aPKC, and for the Par6-aPKC module downstream of oncogenic signaling. Thus, during transformation, the Par complex is likely to be affected in two opposite ways: negative regulation of its polarization activity and recruitment as a positive mediator for oncogenic pathways.
The ability of Par6 and Par3 to scaffold adaptors and effectors to activate many different pathways provides spatial regulation, as well as quantitative activation and substrate specificity for the enzymatic activity of the complex. It will be important to achieve a systematic characterization of the binding partners that determine the outcome of Par complex activity. One of the aspects that are likely to provide better insights is the study of Par complex interplay with GTPase signaling. New data are also strengthening the connection between the Par complex and diverse aspects of GTPase signaling, suggesting that Par6, and Par3, can also modulate GTPase activity as a downstream effector of polarized signaling. What determines the binding of a specific GTPase, or the recruitment of GEF and GAP factors to Par6 and Par3 is a question that promises exciting answers.
From the expanding Par complex signaling network, a common node arises. Regulation of aPKC phosphorylation activity is likely to be the main downstream effect of the Par complex. The activity of this Ser/Thr kinase seems increasingly like a double-edged sword: it is required for normal polarization but it is also a positive regulator of oncogenic signaling, and can drive transformation on its own. Recent findings linking aPKC to tumorigenesis are expanding our view of the polarity machinery from a potential tumor suppressor factor into a more dynamic pathway deeply engraved in many aspects of oncogenic signaling. Targeting aPKC may represent a novel interesting approach to stop tumor progression, even at an early stage. The use of an aPKC inhibitor that selectively targets Par6-aPKC interaction shows promising results in ovarian carcinoma (Fields et al., 2007; Jin et al., 2008; Regala et al., 2008; Stallings-Mann et al., 2006) and spearheads the many therapeutic opportunities that Par complex may offer. More interesting targets are bound to be identified as oncogenesis-specific Par complex partners are being discovered.
The work describing the role of Par complex in oncogenesis expands our vision of how, and why, cellular transformation takes place. It also highlights the need to parse our genetic data and redraw our signaling pathways to keep discovering novel aspects that may help us understand and stop tumor progression.
Acknowledgements
The authors want to thank members of the Muthuswamy laboratory for support and Lukas E Dow for helpful insights.
References
- Anderson DC, Gill JS, Cinalli RM, Nance J. Polarization of the C. elegans Embryo by RhoGAP-Mediated Exclusion of PAR-6 from Cell Contacts. Science. 2008;320:1771–1774. doi: 10.1126/science.1156063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aranda V, Haire T, Nolan ME, Calarco JP, Rosenberg AZ, Fawcett JP, et al. Par6-aPKC uncouples ErbB2 induced disruption of polarized epithelial organization from proliferation control. Nat Cell Biol. 2006;8:1235–1245. doi: 10.1038/ncb1485. [DOI] [PubMed] [Google Scholar]
- Assémat E, Bazellières E, Pallesi-Pocachard E, Le Bivic A, Massey-Harroche D. Polarity complex proteins. Biochim Biophys Acta. 2008;1778:614–630. doi: 10.1016/j.bbamem.2007.08.029. [DOI] [PubMed] [Google Scholar]
- Bachmann A, Schneider M, Theilenberg E, Grawe F, Knust E. Drosophila Stardust is a partner of Crumbs in the control of epithelial cell polarity. Nature. 2001;414:638–643. doi: 10.1038/414638a. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Eisenhaber F, Knoblich JA. Phosphorylation-induced autoinhibition regulates the cytoskeletal protein Lethal (2) giant larvae. Curr Biol. 2005;15:276–282. doi: 10.1016/j.cub.2005.01.012. [DOI] [PubMed] [Google Scholar]
- Betschinger J, Mechtler K, Knoblich JA. The Par complex directs asymmetric cell division by phosphorylating the cytoskeletal protein Lgl. Nature. 2003;422:326–330. doi: 10.1038/nature01486. [DOI] [PubMed] [Google Scholar]
- Bhat MA, Izaddoost S, Lu Y, Cho KO, Choi KW, Bellen HJ. Discs Lost, a novel multi-PDZ domain protein, establishes and maintains epithelial polarity. Cell. 1999;96:833–845. doi: 10.1016/s0092-8674(00)80593-0. [DOI] [PubMed] [Google Scholar]
- Bialucha CU, Ferber EC, Pichaud F, Peak-Chew SY, Fujita Y. p32 is a novel mammalian Lgl binding protein that enhances the activity of protein kinase Czeta and regulates cell polarity. J Cell Biol. 2007;178:575–581. doi: 10.1083/jcb.200612022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilder D. PDZ domain polarity complexes. Curr Biol. 2003;13:R661–R662. doi: 10.1016/s0960-9822(03)00599-2. [DOI] [PubMed] [Google Scholar]
- Bilder D. Epithelial polarity and proliferation control: links from the Drosophila neoplastic tumor suppressors. Genes Dev. 2004;18:1909–1925. doi: 10.1101/gad.1211604. [DOI] [PubMed] [Google Scholar]
- Bilder D, Schober M, Perrimon N. Integrated activity of PDZ protein complexes regulates epithelial polarity. Nat Cell Biol. 2003;5:53–58. doi: 10.1038/ncb897. [DOI] [PubMed] [Google Scholar]
- Bose R, Wrana JL. Regulation of Par6 by extracellular signals. Curr Opin Cell Biol. 2006;18:206–212. doi: 10.1016/j.ceb.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Cai Y, Stafford LJ, Bryan BA, Mitchell D, Liu M. G-protein-activated phospholipase C-beta, new partners for cell polarity proteins Par3 and Par6. Oncogene. 2005;24:4293–4300. doi: 10.1038/sj.onc.1208593. [DOI] [PubMed] [Google Scholar]
- Calzada MJ, Esteban MA, Feijoo-Cuaresma M, Castellanos MC, Naranjo-Suarez S, Temes E, et al. von Hippel-Lindau tumor suppressor protein regulates the assembly of intercellular junctions in renal cancer cells through hypoxia-inducible factor-independent mechanisms. Cancer Res. 2006;66:1553–1560. doi: 10.1158/0008-5472.CAN-05-3236. [DOI] [PubMed] [Google Scholar]
- Cau J, Hall A. Cdc42 controls the polarity of the actin and microtubule cytoskeletons through two distinct signal transduction pathways. J Cell Sci. 2005;118:2579–2587. doi: 10.1242/jcs.02385. [DOI] [PubMed] [Google Scholar]
- Chabu C, Doe CQ. Dap160/intersectin binds and activates aPKC to regulate cell polarity and cell cycle progression. Development. 2008;135:2739–2746. doi: 10.1242/dev.024059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 controls tight junction assembly through the Rac exchange factor Tiam1. Nat Cell Biol. 2005 doi: 10.1038/ncb1226. [DOI] [PubMed] [Google Scholar]
- Chen X, Macara IG. Par-3 mediates the inhibition of LIM kinase 2 to regulate cofilin phosphorylation and tight junction assembly. J Cell Biol. 2006;172:671–678. doi: 10.1083/jcb.200510061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen EE, Lingen MW, Zhu B, Zhu H, Straza MW, Pierce C, et al. Protein kinase C zeta mediates epidermal growth factor-induced growth of head and neck tumor cells by regulating mitogen-activated protein kinase. Cancer Res. 2006;66:6296–6303. doi: 10.1158/0008-5472.CAN-05-3139. [DOI] [PubMed] [Google Scholar]
- Debnath J, Muthuswamy SK, Brugge JS. Morphogenesis and oncogenesis of MCF-10A mammary epithelial acini grown in three-dimensional basement membrane cultures. Methods. 2003;30:256–268. doi: 10.1016/s1046-2023(03)00032-x. [DOI] [PubMed] [Google Scholar]
- Donson AM, Banerjee A, Gamboni-Robertson F, Fleitz JM, Foreman NK. Protein kinase C zeta isoform is critical for proliferation in human glioblastoma cell lines. J Neurooncol. 2000;47:109–115. doi: 10.1023/a:1006406208376. [DOI] [PubMed] [Google Scholar]
- Dow LE, Humbert PO. Polarity regulators and the control of epithelial architecture, cell migration, and tumorigenesis. Int Rev Cytol. 2007;262:253–302. doi: 10.1016/S0074-7696(07)62006-3. [DOI] [PubMed] [Google Scholar]
- Ebnet K, Aurrand-Lions M, Kuhn A, Kiefer F, Butz S, Zander K, et al. The junctional adhesion molecule (JAM) family members JAM-2 and JAM-3 associate with the cell polarity protein PAR-3: a possible role for JAMs in endothelial cell polarity. J Cell Sci. 2003;116:3879–3891. doi: 10.1242/jcs.00704. [DOI] [PubMed] [Google Scholar]
- Ebnet K, Iden S, Gerke V, Suzuki A. Regulation of epithelial and endothelial junctions by PAR proteins. Front Biosci. 2008;13:6520–6536. doi: 10.2741/3172. [DOI] [PubMed] [Google Scholar]
- Ebnet K, Suzuki A, Horikoshi Y, Hirose T, Meyer Zu Brickwedde MK, Ohno S, et al. The cell polarity protein ASIP/PAR-3 directly associates with junctional adhesion molecule (JAM) EMBO J. 2001;20:3738–3748. doi: 10.1093/emboj/20.14.3738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eder AM, Sui X, Rosen DG, Nolden LK, Cheng KW, Lahad JP, et al. Atypical PKCiota contributes to poor prognosis through loss of apical-basal polarity and cyclin E overexpression in ovarian cancer. Proc Natl Acad Sci USA. 2005;102:12519–12524. doi: 10.1073/pnas.0505641102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Etienne-Manneville S. Cdc42--the centre of polarity. J Cell Sci. 2004;117:1291–1300. doi: 10.1242/jcs.01115. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Integrin-mediated activation of Cdc42 controls cell polarity in migrating astrocytes through PKCzeta. Cell. 2001;106:489–498. doi: 10.1016/s0092-8674(01)00471-8. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Rho GTPases in cell biology. Nature. 2002;420:629–635. doi: 10.1038/nature01148. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cdc42 regulates GSK-3beta and adenomatous polyposis coli to control cell polarity. Nature. 2003a;421:753–756. doi: 10.1038/nature01423. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Hall A. Cell polarity: Par6, aPKC and cytoskeletal crosstalk. Curr Opin Cell Biol. 2003b;15:67–72. doi: 10.1016/s0955-0674(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Etienne-Manneville S, Manneville JB, Nicholls S, Ferenczi MA, Hall A. Cdc42 and Par6-PKCzeta regulate the spatially localized association of Dlg1 and APC to control cell polarization. J Cell Biol. 2005;170:895–901. doi: 10.1083/jcb.200412172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farooqui R, Zhu S, Fenteany G. Glycogen synthase kinase-3 acts upstream of ADP-ribosylation factor 6 and Rac1 to regulate epithelial cell migration. Exp Cell Res. 2006;312:1514–1525. doi: 10.1016/j.yexcr.2006.01.018. [DOI] [PubMed] [Google Scholar]
- Feng W, Wu H, Chan LN, Zhang M. Par-3-mediated junctional localization of the lipid phosphatase PTEN is required for cell polarity establishment. J Biol Chem. 2008 doi: 10.1074/jbc.M802482200. [DOI] [PubMed] [Google Scholar]
- Fields AP, Frederick LA, Regala RP. Targeting the oncogenic protein kinase Ciota signalling pathway for the treatment of cancer. Biochem Soc Trans. 2007;35:996–1000. doi: 10.1042/BST0350996. [DOI] [PubMed] [Google Scholar]
- Fields AP, Murray NR, Gustafson WC. Characterization of the role of protein kinase C isozymes in colon carcinogenesis using transgenic mouse models. Methods Mol Biol. 2003;233:539–553. doi: 10.1385/1-59259-397-6:539. [DOI] [PubMed] [Google Scholar]
- Fields AP, Regala RP. Protein kinase C iota: human oncogene, prognostic marker and therapeutic target. Pharmacol Res. 2007;55:487–497. doi: 10.1016/j.phrs.2007.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleming TP, Papenbrock T, Fesenko I, Hausen P, Sheth B. Assembly of tight junctions during early vertebrate development. Semin Cell Dev Biol. 2000;11:291–299. doi: 10.1006/scdb.2000.0179. [DOI] [PubMed] [Google Scholar]
- Gao L, Macara IG. Isoforms of the polarity protein par6 have distinct functions. J Biol Chem. 2004;279:41557–41562. doi: 10.1074/jbc.M403723200. [DOI] [PubMed] [Google Scholar]
- Garrard SM, Capaldo CT, Gao L, Rosen MK, Macara IG, Tomchick DR. Structure of Cdc42 in a complex with the GTPase-binding domain of the cell polarity protein, Par6. EMBO J. 2003;22:1125–1133. doi: 10.1093/emboj/cdg110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerard A, Mertens AE, van der Kammen RA, Collard JG. The Par polarity complex regulates Rap1- and chemokine-induced T cell polarization. J Cell Biol. 2007;176:863–875. doi: 10.1083/jcb.200608161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein B, Macara IG. The PAR proteins: fundamental players in animal cell polarization. Dev Cell. 2007;13:609–622. doi: 10.1016/j.devcel.2007.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomes ER, Jani S, Gundersen GG. Nuclear movement regulated by Cdc42, MRCK, myosin, and actin flow establishes MTOC polarization in migrating cells. Cell. 2005;121:451–463. doi: 10.1016/j.cell.2005.02.022. [DOI] [PubMed] [Google Scholar]
- Grifoni D, Garoia F, Bellosta P, Parisi F, De D. aPKC zeta cortical loading is associated with Lgl cytoplasmic release and tumor growth in Drosophila …. Oncogene. 2007 doi: 10.1038/sj.onc.1210389. [DOI] [PubMed] [Google Scholar]
- Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- Hebner C, Weaver VM, Debnath J. Modeling morphogenesis and oncogenesis in three-dimensional breast epithelial cultures. Annu Rev Pathol. 2008;3:313–339. doi: 10.1146/annurev.pathmechdis.3.121806.151526. [DOI] [PubMed] [Google Scholar]
- Hirose T, Izumi Y, Nagashima Y, Tamai-Nagai Y, Kurihara H, Sakai T, et al. Involvement of ASIP/PAR-3 in the promotion of epithelial tight junction formation. J Cell Sci. 2002;115:2485–2495. doi: 10.1242/jcs.115.12.2485. [DOI] [PubMed] [Google Scholar]
- Horne-Badovinac S, Bilder D. Dynein regulates epithelial polarity and the apical localization of stardust A mRNA. PLoS Genet. 2008;4:e8. doi: 10.1371/journal.pgen.0040008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshino M, Yoshimori T, Nakamura S. Small GTPase proteins Rin and Rit Bind to PAR6 GTP-dependently and regulate cell transformation. J Biol Chem. 2005;280:22868–22874. doi: 10.1074/jbc.M411592200. [DOI] [PubMed] [Google Scholar]
- Huber MA, Kraut N, Beug H. Molecular requirements for epithelial-mesenchymal transition during tumor progression. Curr Opin Cell Biol. 2005;17:548–558. doi: 10.1016/j.ceb.2005.08.001. [DOI] [PubMed] [Google Scholar]
- Hugo H, Ackland ML, Blick T, Lawrence MG, Clements JA, Williams ED, et al. Epithelial--mesenchymal and mesenchymal--epithelial transitions in carcinoma progression. J Cell Physiol. 2007;213:374–383. doi: 10.1002/jcp.21223. [DOI] [PubMed] [Google Scholar]
- Hurd TW, Gao L, Roh MH, Macara IG, Margolis B. Direct interaction of two polarity complexes implicated in epithelial tight junction assembly. Nat Cell Biol. 2003;5:137–142. doi: 10.1038/ncb923. [DOI] [PubMed] [Google Scholar]
- Hurov J, Piwnica-Worms H. The Par-1/MARK family of protein kinases: from polarity to metabolism. Cell Cycle. 2007;6:1966–1969. doi: 10.4161/cc.6.16.4576. [DOI] [PubMed] [Google Scholar]
- Hurov JB, Watkins JL, Piwnica-Worms H. Atypical PKC phosphorylates PAR-1 kinases to regulate localization and activity. Curr Biol. 2004;14:736–741. doi: 10.1016/j.cub.2004.04.007. [DOI] [PubMed] [Google Scholar]
- Hutterer A, Betschinger J, Petronczki M, Knoblich JA. Sequential roles of Cdc42, Par-6, aPKC, and Lgl in the establishment of epithelial polarity during Drosophila embryogenesis. Dev Cell. 2004;6:845–854. doi: 10.1016/j.devcel.2004.05.003. [DOI] [PubMed] [Google Scholar]
- Izumi Y, Hirose T, Tamai Y, Hirai S, Nagashima Y, Fujimoto T, et al. An atypical PKC directly associates and colocalizes at the epithelial tight junction with ASIP, a mammalian homologue of Caenorhabditis elegans polarity protein PAR-3. J Cell Biol. 1998;143:95–106. doi: 10.1083/jcb.143.1.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji Q, Burk RD. Downregulation of integrins by von Hippel-Lindau (VHL) tumor suppressor protein is independent of VHL-directed hypoxia-inducible factor alpha degradation. Biochem Cell Biol. 2008;86:227–234. doi: 10.1139/o08-035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YT, Ying XX, Hu YH, Zou Q, Wang HY, Xu YH. aPKC inhibitors might be the sensitizer of chemotherapy and adoptive immunotherapy in the treatment of hASIPa-overexpressed breast cancer. Oncol Res. 2008;17:59–68. doi: 10.3727/096504008784523630. [DOI] [PubMed] [Google Scholar]
- Joberty G, Petersen C, Gao L, Macara IG. The cell-polarity protein Par6 links Par3 and atypical protein kinase C to Cdc42. Nat Cell Biol. 2000;2:531–539. doi: 10.1038/35019573. [DOI] [PubMed] [Google Scholar]
- Johnson DI. Cdc42: An essential Rho-type GTPase controlling eukaryotic cell polarity. Microbiol Mol Biol Rev. 1999;63:54–105. doi: 10.1128/mmbr.63.1.54-105.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay AJ, Hunter CP. CDC-42 regulates PAR protein localization and function to control cellular and embryonic polarity in C. elegans. Curr Biol. 2001;11:474–481. doi: 10.1016/s0960-9822(01)00141-5. [DOI] [PubMed] [Google Scholar]
- Kemphues K. PARsing embryonic polarity. Cell. 2000;101:345–348. doi: 10.1016/s0092-8674(00)80844-2. [DOI] [PubMed] [Google Scholar]
- Kemphues KJ, Priess JR, Morton DG, Cheng NS. Identification of genes required for cytoplasmic localization in early C. elegans embryos. Cell. 1988;52:311–320. doi: 10.1016/s0092-8674(88)80024-2. [DOI] [PubMed] [Google Scholar]
- Kim M, Datta A, Brakeman P, Yu W, Mostov KE. Polarity proteins PAR6 and aPKC regulate cell death through GSK-3beta in 3D epithelial morphogenesis. J Cell Sci. 2007;120:2309–2317. doi: 10.1242/jcs.007443. [DOI] [PubMed] [Google Scholar]
- Kojima Y, Akimoto K, Nagashima Y, Ishiguro H, Shirai S, Chishima T, et al. The overexpression and altered localization of the atypical protein kinase C lambda/iota in breast cancer correlates with the pathologic type of these tumors. Hum Pathol. 2008;39:824–831. doi: 10.1016/j.humpath.2007.11.001. [DOI] [PubMed] [Google Scholar]
- Kuehn EW, Walz G, Benzing T. Von hippel-lindau: a tumor suppressor links microtubules to ciliogenesis and cancer development. Cancer Res. 2007;67:4537–4540. doi: 10.1158/0008-5472.CAN-07-0391. [DOI] [PubMed] [Google Scholar]
- Kuphal S, Wallner S, Schimanski CC, Bataille F, Hofer P, Strand S, et al. Expression of Hugl-1 is strongly reduced in malignant melanoma. Oncogene. 2006;25:103–110. doi: 10.1038/sj.onc.1209008. [DOI] [PubMed] [Google Scholar]
- Kurban G, Hudon V, Duplan E, Ohh M, Pause A. Characterization of a von Hippel Lindau pathway involved in extracellular matrix remodeling, cell invasion, and angiogenesis. Cancer Res. 2006;66:1313–1319. doi: 10.1158/0008-5472.CAN-05-2560. [DOI] [PubMed] [Google Scholar]
- Kuribayashi K, Nakamura K, Tanaka M, Sato T, Kato J, Sasaki K, et al. Essential role of protein kinase C zeta in transducing a motility signal induced by superoxide and a chemotactic peptide, fMLP. J Cell Biol. 2007;176:1049–1060. doi: 10.1083/jcb.200607019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee CY, Robinson KJ, Doe CQ. Lgl, Pins and aPKC regulate neuroblast self-renewal versus differentiation. Nature. 2006;439:594–598. doi: 10.1038/nature04299. [DOI] [PubMed] [Google Scholar]
- Lee M, Vasioukhin V. Cell polarity and cancer-cell and tissue polarity as a non-canonical tumor suppressor. J Cell Sci. 2008;121:1141–1150. doi: 10.1242/jcs.016634. [DOI] [PubMed] [Google Scholar]
- Lemmers C, Michel D, Lane-Guermonprez L, Delgrossi MH, Medina E, Arsanto JP, et al. CRB3 binds directly to Par6 and regulates the morphogenesis of the tight junctions in mammalian epithelial cells. Mol Biol Cell. 2004;15:1324–1333. doi: 10.1091/mbc.E03-04-0235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z, Wang L, Hays TS, Cai Y. Dynein-mediated apical localization of crumbs transcripts is required for Crumbs activity in epithelial polarity. J Cell Biol. 2008;180:31–38. doi: 10.1083/jcb.200707007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin D, Edwards AS, Fawcett JP, Mbamalu G, Scott JD, Pawson T. A mammalian PAR-3-PAR-6 complex implicated in Cdc42/Rac1 and aPKC signalling and cell polarity. Nat Cell Biol. 2000;2:540–547. doi: 10.1038/35019582. [DOI] [PubMed] [Google Scholar]
- Ludford-Menting MJ, Oliaro J, Sacirbegovic F, Cheah ET, Pedersen N, Thomas SJ, et al. A network of PDZ-containing proteins regulates T cell polarity and morphology during migration and immunological synapse formation. Immunity. 2005;22:737–748. doi: 10.1016/j.immuni.2005.04.009. [DOI] [PubMed] [Google Scholar]
- Macara IG. Parsing the polarity code. Nat Rev Mol Cell Biol. 2004;5:220–231. doi: 10.1038/nrm1332. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Gassama A, Datta A, Yu W, Rescher U, Gerke V, et al. PTEN-mediated apical segregation of phosphoinositides controls epithelial morphogenesis through Cdc42. Cell. 2007;128:383–397. doi: 10.1016/j.cell.2006.11.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin-Belmonte F, Mostov K. Phosphoinositides control epithelial development. Cell Cycle. 2007;6:1957–1961. doi: 10.4161/cc.6.16.4583. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008a;20:227–234. doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Martin-Belmonte F, Mostov K. Regulation of cell polarity during epithelial morphogenesis. Curr Opin Cell Biol. 2008b doi: 10.1016/j.ceb.2008.01.001. [DOI] [PubMed] [Google Scholar]
- Mertens AE, Rygiel TP, Olivo C, van der Kammen R, Collard JG. The Rac activator Tiam1 controls tight junction biogenesis in keratinocytes through binding to and activation of the Par polarity complex. J Cell Biol. 2005;170:1029–1037. doi: 10.1083/jcb.200502129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moeschel K, Beck A, Weigert C, Lammers R, Kalbacher H, Voelter W, et al. Protein kinase C-zeta-induced phosphorylation of Ser318 in insulin receptor substrate-1 (IRS-1) attenuates the interaction with the insulin receptor and the tyrosine phosphorylation of IRS-1. J Biol Chem. 2004;279:25157–25163. doi: 10.1074/jbc.M402477200. [DOI] [PubMed] [Google Scholar]
- Morrison SJ, Kimble J. Asymmetric and symmetric stem-cell divisions in development and cancer. Nature. 2006;441:1068–1074. doi: 10.1038/nature04956. [DOI] [PubMed] [Google Scholar]
- Moscat J, Diaz-Meco MT. The atypical protein kinase Cs. Functional specificity mediated by specific protein adapters. EMBO Rep. 2000;1:399–403. doi: 10.1093/embo-reports/kvd098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moscat J, Rennert P, Diaz-Meco MT. PKCzeta at the crossroad of NF-kappaB and Jak1/Stat6 signaling pathways. Cell Death Differ. 2006;13:702–711. doi: 10.1038/sj.cdd.4401823. [DOI] [PubMed] [Google Scholar]
- Munro EM. PAR proteins and the cytoskeleton: a marriage of equals. Curr Opin Cell Biol. 2006;18:86–94. doi: 10.1016/j.ceb.2005.12.007. [DOI] [PubMed] [Google Scholar]
- Murray NR, Jamieson L, Yu W, Zhang J, Gokmen-Polar Y, Sier D, et al. Protein kinase Ciota is required for Ras transformation and colon carcinogenesis in vivo. J Cell Biol. 2004;164:797–802. doi: 10.1083/jcb.200311011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS. ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol. 2001;3:785–792. doi: 10.1038/ncb0901-785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagai-Tamai Y, Mizuno K, Hirose T, Suzuki A, Ohno S. Regulated protein-protein interaction between aPKC and PAR-3 plays an essential role in the polarization of epithelial cells. Genes Cells. 2002;7:1161–1171. doi: 10.1046/j.1365-2443.2002.00590.x. [DOI] [PubMed] [Google Scholar]
- Nakayama M, Goto TM, Sugimoto M, Nishimura T, Shinagawa T, Ohno S, et al. Rho-kinase phosphorylates PAR-3 and disrupts PAR complex formation. Dev Cell. 2008;14:205–215. doi: 10.1016/j.devcel.2007.11.021. [DOI] [PubMed] [Google Scholar]
- Nance J, Munro EM, Priess JR. C. elegans PAR-3 and PAR-6 are required for apicobasal asymmetries associated with cell adhesion and gastrulation. Development. 2003;130:5339–5350. doi: 10.1242/dev.00735. [DOI] [PubMed] [Google Scholar]
- Nishimura T, Yamaguchi T, Kato K, Yoshizawa M, Nabeshima Y, Ohno S, et al. PAR-6-PAR-3 mediates Cdc42-induced Rac activation through the Rac GEFs STEF/Tiam1. Nat Cell Biol. 2005;7:270–277. doi: 10.1038/ncb1227. [DOI] [PubMed] [Google Scholar]
- Noda Y, Takeya R, Ohno S, Naito S, Ito T, Sumimoto H. Human homologues of the Caenorhabditis elegans cell polarity protein PAR6 as an adaptor that links the small GTPases Rac and Cdc42 to atypical protein kinase C. Genes Cells. 2001;6:107–119. doi: 10.1046/j.1365-2443.2001.00404.x. [DOI] [PubMed] [Google Scholar]
- Nolan ME, Aranda V, Lee S, Lakshmi B, Basu S, Allred C, et al. The Polarity Protein Par6 Induces Cell Proliferation And Is Overexpressed In Breast Cancer. Cancer Res. 2008 doi: 10.1158/0008-5472.CAN-07-6567. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okuda H, Hirai S, Takaki Y, Kamada M, Baba M, Sakai N, et al. Direct interaction of the beta-domain of VHL tumor suppressor protein with the regulatory domain of atypical PKC isotypes. Biochem Biophys Res Commun. 1999;263:491–497. doi: 10.1006/bbrc.1999.1347. [DOI] [PubMed] [Google Scholar]
- Okuda H, Saitoh K, Hirai S, Iwai K, Takaki Y, Baba M, et al. The von Hippel-Lindau tumor suppressor protein mediates ubiquitination of activated atypical protein kinase C. J Biol Chem. 2001;276:43611–43617. doi: 10.1074/jbc.M107880200. [DOI] [PubMed] [Google Scholar]
- Ozdamar B, Bose R, Barrios-Rodiles M, Wang HR, Zhang Y, Wrana JL. Regulation of the polarity protein Par6 by TGFbeta receptors controls epithelial cell plasticity. Science. 2005;307:1603–1609. doi: 10.1126/science.1105718. [DOI] [PubMed] [Google Scholar]
- Pegtel DM, Ellenbroek SI, Mertens AE, van der Kammen RA, de Rooij J, Collard JG. The Par-Tiam1 complex controls persistent migration by stabilizing microtubule-dependent front-rear polarity. Curr Biol. 2007;17:1623–1634. doi: 10.1016/j.cub.2007.08.035. [DOI] [PubMed] [Google Scholar]
- Petersen OW, Ronnov-Jessen L, Howlett AR, Bissell MJ. Interaction with basement membrane serves to rapidly distinguish growth and differentiation pattern of normal and malignant human breast epithelial cells. Proc Natl Acad Sci U S A. 1992;89:9064–9068. doi: 10.1073/pnas.89.19.9064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson FC, Penkert RR, Volkman BF, Prehoda KE. Cdc42 regulates the Par-6 PDZ domain through an allosteric CRIB-PDZ transition. Mol Cell. 2004;13:665–676. doi: 10.1016/s1097-2765(04)00086-3. [DOI] [PubMed] [Google Scholar]
- Plant PJ, Fawcett JP, Lin DC, Holdorf AD, Binns K, Kulkarni S, et al. A polarity complex of mPar-6 and atypical PKC binds, phosphorylates and regulates mammalian Lgl. Nat Cell Biol. 2003;5:301–308. doi: 10.1038/ncb948. [DOI] [PubMed] [Google Scholar]
- Qiu RG, Abo A, Steven Martin G. A human homolog of the C. elegans polarity determinant Par-6 links Rac and Cdc42 to PKCzeta signaling and cell transformation. Curr Biol. 2000;10:697–707. doi: 10.1016/s0960-9822(00)00535-2. [DOI] [PubMed] [Google Scholar]
- Regala RP, Thompson EA, Fields AP. Atypical protein kinase C iota expression and aurothiomalate sensitivity in human lung cancer cells. Cancer Res. 2008;68:5888–5895. doi: 10.1158/0008-5472.CAN-08-0438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Regala RP, Weems C, Jamieson L, Khoor A, Edell ES. Atypical Protein Kinase Cι Is an Oncogene in Human Non-Small Cell Lung Cancer. Cancer Res. 2005 doi: 10.1158/0008-5472.CAN-05-2372. [DOI] [PubMed] [Google Scholar]
- Rolls MM, Albertson R, Shih HP, Lee CY, Doe CQ. Drosophila aPKC regulates cell polarity and cell proliferation in neuroblasts and epithelia. J Cell Biol. 2003;163:1089–1098. doi: 10.1083/jcb.200306079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimanski CC, Schmitz G, Kashyap A, Bosserhoff AK, Bataille F, Schafer SC, et al. Reduced expression of Hugl-1, the human homologue of Drosophila tumour suppressor gene lgl, contributes to progression of colorectal cancer. Oncogene. 2005;24:3100–3109. doi: 10.1038/sj.onc.1208520. [DOI] [PubMed] [Google Scholar]
- Slamon DJ. Proto-oncogenes and human cancers. N Engl J Med. 1987;317:955–957. doi: 10.1056/NEJM198710083171509. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Clark GM, Wong SG, Levin WJ, Ullrich A, McGuire WL. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235:177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- Slamon DJ, Godolphin W, Jones LA, Holt JA, Wong SG, Keith DE, et al. Studies of the HER-2/neu proto-oncogene in human breast and ovarian cancer. Science. 1989;244:707–712. doi: 10.1126/science.2470152. [DOI] [PubMed] [Google Scholar]
- Sotillos S, Díaz-Meco MT, Caminero E, Moscat J, Campuzano S. DaPKC-dependent phosphorylation of Crumbs is required for epithelial cell polarity in Drosophila. J Cell Biol. 2004;166:549–557. doi: 10.1083/jcb.200311031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stallings-Mann M, Jamieson L, Regala RP, Weems C, Murray NR, Fields AP. A novel small-molecule inhibitor of protein kinase Ciota blocks transformed growth of non-small-cell lung cancer cells. Cancer Res. 2006;66:1767–1774. doi: 10.1158/0008-5472.CAN-05-3405. [DOI] [PubMed] [Google Scholar]
- Sun R, Gao P, Chen L, Ma D, Wang JM, Oppenheim JJ. … Kinase C ζ Is Required for Epidermal Growth Factor-Induced Chemotaxis of Human Breast Cancer Cells. Cancer Res. 2005 doi: 10.1158/0008-5472.CAN-04-1163. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Hirata M, Kamimura K, Maniwa R, Yamanaka T, Mizuno K, et al. aPKC acts upstream of PAR-1b in both the establishment and maintenance of mammalian epithelial polarity. Curr Biol. 2004;14:1425–1435. doi: 10.1016/j.cub.2004.08.021. [DOI] [PubMed] [Google Scholar]
- Suzuki A, Ohno S. The PAR-aPKC system: lessons in polarity. J Cell Sci. 2006;119:979–987. doi: 10.1242/jcs.02898. [DOI] [PubMed] [Google Scholar]
- Tepass U, Tanentzapf G, Ward R, Fehon R. Epithelial cell polarity and cell junctions in Drosophila. Annu Rev Genet. 2001;35:747–784. doi: 10.1146/annurev.genet.35.102401.091415. [DOI] [PubMed] [Google Scholar]
- Tong XK, Hussain NK, Adams AG, O'Bryan JP, McPherson PS. Intersectin can regulate the Ras/MAP kinase pathway independent of its role in endocytosis. J Biol Chem. 2000;275:29894–29899. doi: 10.1074/jbc.M004096200. [DOI] [PubMed] [Google Scholar]
- Townsend TA, Wrana JL, Davis GE, Barnett JV. Transforming growth factor-beta-stimulated endocardial cell transformation is dependent on Par6c regulation of RhoA. J Biol Chem. 2008;283:13834–13841. doi: 10.1074/jbc.M710607200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuruga T, Nakagawa S, Watanabe M, Takizawa S, Matsumoto Y, Nagasaka K, et al. Loss of Hugl-1 expression associates with lymph node metastasis in endometrial cancer. Oncol Res. 2007;16:431–435. doi: 10.3727/000000007783980855. [DOI] [PubMed] [Google Scholar]
- Underwood JM, Imbalzano KM, Weaver VM, Fischer AH, Imbalzano AN, Nickerson JA. The ultrastructure of MCF-10A acini. J Cell Physiol. 2006;208:141–148. doi: 10.1002/jcp.20639. [DOI] [PubMed] [Google Scholar]
- Wang HR, Ogunjimi AA, Zhang Y, Ozdamar B, Bose R, Wrana JL. Degradation of RhoA by Smurf1 ubiquitin ligase. Methods Enzymol. 2006;406:437–447. doi: 10.1016/S0076-6879(06)06032-0. [DOI] [PubMed] [Google Scholar]
- Wang HR, Zhang Y, Ozdamar B, Ogunjimi AA, Alexandrova E, Thomsen GH, et al. Regulation of cell polarity and protrusion formation by targeting RhoA for degradation. Science. 2003;302:1775–1779. doi: 10.1126/science.1090772. [DOI] [PubMed] [Google Scholar]
- Wang JB, Wu WJ, Cerione RA. Cdc42 and Ras cooperate to mediate cellular transformation by intersectin-L. J Biol Chem. 2005;280:22883–22891. doi: 10.1074/jbc.M414375200. [DOI] [PubMed] [Google Scholar]
- Wang Q, Hurd TW, Margolis B. Tight junction protein Par6 interacts with an evolutionarily conserved region in the amino terminus of PALS1/stardust. J Biol Chem. 2004;279:30715–30721. doi: 10.1074/jbc.M401930200. [DOI] [PubMed] [Google Scholar]
- Wang X, Nie J, Zhou Q, Liu W, Zhu F, Chen W, et al. Downregulation of Par-3 expression and disruption of Par complex integrity by TGF-beta during the process of epithelial to mesenchymal transition in rat proximal epithelial cells. Biochim Biophys Acta. 2008;1782:51–59. doi: 10.1016/j.bbadis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- Weaver VM, Fischer AH, Peterson OW, Bissell MJ. The importance of the microenvironment in breast cancer progression: recapitulation of mammary tumorigenesis using a unique human mammary epithelial cell model and a three-dimensional culture assay. Biochem Cell Biol. 1996;74:833–851. doi: 10.1139/o96-089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyrich P, Kapp K, Niederfellner G, Melzer M, Lehmann R, Haring HU, et al. Partitioning-defective protein 6 regulates insulin-dependent glycogen synthesis via atypical protein kinase C. Mol Endocrinol. 2004;18:1287–1300. doi: 10.1210/me.2003-0253. [DOI] [PubMed] [Google Scholar]
- Weyrich P, Neuscheler D, Melzer M, Hennige AM, Haring HU, Lammers R. The Par6alpha/aPKC complex regulates Akt1 activity by phosphorylating Thr34 in the PH-domain. Mol Cell Endocrinol. 2007;268:30–36. doi: 10.1016/j.mce.2007.01.011. [DOI] [PubMed] [Google Scholar]
- Wodarz A, Nathke I. Cell polarity in development and cancer. Nat Cell Biol. 2007 doi: 10.1038/ncb433. [DOI] [PubMed] [Google Scholar]
- Wu H, Feng W, Chen J, Chan LN, Huang S, Zhang M. PDZ domains of Par-3 as potential phosphoinositide signaling integrators. Mol Cell. 2007;28:886–898. doi: 10.1016/j.molcel.2007.10.028. [DOI] [PubMed] [Google Scholar]
- Xiang B, Muthuswamy SK. Using three-dimensional acinar structures for molecular and cell biological assays. Methods Enzymol. 2006;406:692–701. doi: 10.1016/S0076-6879(06)06054-X. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Horikoshi Y, Suzuki A, Sugiyama Y, Kitamura K, Maniwa R, et al. PAR-6 regulates aPKC activity in a novel way and mediates cell-cell contact-induced formation of the epithelial junctional complex. Genes Cells. 2001;6:721–731. doi: 10.1046/j.1365-2443.2001.00453.x. [DOI] [PubMed] [Google Scholar]
- Yamanaka T, Ohno S. Role of Lgl/Dlg/Scribble in the regulation of epithelial junction, polarity and growth. Front Biosci. 2008;13:6693–6707. doi: 10.2741/3182. [DOI] [PubMed] [Google Scholar]
- Yarden Y. Biology of HER2 and its importance in breast cancer. Oncology. 2001;61 Suppl 2:1–13. doi: 10.1159/000055396. [DOI] [PubMed] [Google Scholar]
- Yarden Y, Sliwkowski MX. Untangling the ErbB signalling network. Nat Rev Mol Cell Biol. 2001;2:127–137. doi: 10.1038/35052073. [DOI] [PubMed] [Google Scholar]
- Yi P, Feng Q, Amazit L, Lonard DM, Tsai SY, Tsai MJ, et al. Atypical protein kinase C regulates dual pathways for degradation of the oncogenic coactivator SRC-3/AIB1. Mol Cell. 2008;29:465–476. doi: 10.1016/j.molcel.2007.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Macara IG. The polarity protein PAR-3 and TIAM1 cooperate in dendritic spine morphogenesis. Nat Cell Biol. 2006;8:227–237. doi: 10.1038/ncb1368. [DOI] [PubMed] [Google Scholar]
- Zhang H, Macara IG. The PAR-6 polarity protein regulates dendritic spine morphogenesis through p190 RhoGAP and the Rho GTPase. Dev Cell. 2008;14:216–226. doi: 10.1016/j.devcel.2007.11.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang L, Huang J, Yang N, Liang S, Barchetti A, Giannakakis A, et al. Integrative genomic analysis of protein kinase C (PKC) family identifies PKCiota as a biomarker and potential oncogene in ovarian carcinoma. Cancer Res. 2006;66:4627–4635. doi: 10.1158/0008-5472.CAN-05-4527. [DOI] [PubMed] [Google Scholar]