Abstract
Background
Impaired walking limits function after spinal cord injury (SCI), but training-related improvements are possible even in people with chronic motor incomplete SCI.
Objective
The objective of this study was to compare changes in walking speed and distance associated with 4 locomotor training approaches.
Design
This study was a single-blind, randomized clinical trial.
Setting
This study was conducted in a rehabilitation research laboratory.
Participants
Participants were people with minimal walking function due to chronic SCI.
Intervention
Participants (n=74) trained 5 days per week for 12 weeks with the following approaches: treadmill-based training with manual assistance (TM), treadmill-based training with stimulation (TS), overground training with stimulation (OG), and treadmill-based training with robotic assistance (LR).
Measurements
Overground walking speed and distance were the primary outcome measures.
Results
In participants who completed the training (n=64), there were overall effects for speed (effect size index [d]=0.33) and distance (d=0.35). For speed, there were no significant between-group differences; however, distance gains were greatest with OG. Effect sizes for speed and distance were largest with OG (d=0.43 and d=0.40, respectively). Effect sizes for speed were the same for TM and TS (d=0.28); there was no effect for LR. The effect size for distance was greater with TS (d=0.16) than with TM or LR, for which there was no effect. Ten participants who improved with training were retested at least 6 months after training; walking speed at this time was slower than that at the conclusion of training but remained faster than before training.
Limitations
It is unknown whether the training dosage and the emphasis on training speed were optimal. Robotic training that requires active participation would likely yield different results.
Conclusions
In people with chronic motor incomplete SCI, walking speed improved with both overground training and treadmill-based training; however, walking distance improved to a greater extent with overground training.
It has been estimated that 12,000 people in the United States sustain spinal cord injury (SCI) each year.1 The loss of motor function associated with SCI often produces severe limitations on mobility and self-care. However, because of advances in the medical management of acute SCI, more people now retain or recover some motor function.2 Preserved motor function improves the possibility that these people may be able to walk at least short distances. The ability to stand and walk through a narrow entryway or to negotiate confined spaces inaccessible to a wheelchair provides opportunities to participate in activities that might otherwise be inaccessible.
The early 1990s ushered in a time of great interest in treadmill-based locomotor training for people with SCI. In a study with a small sample of participants, Barbeau and Blunt3 described treadmill-based locomotor training with a harness-lift system to provide partial body-weight support (BWS). The approach was hailed as one of the first models of rehabilitation to be grounded in a scientific basis, as earlier work had demonstrated that, with BWS, animals with complete spinal cord transection could step on a treadmill. In animals with complete spinal cord transection, movement of the limbs below the level of transection is observed only in response to afferent input. For example, when afferent input is supplied by a moving treadmill, the spinal central pattern–generating circuitry is activated and evokes innate rhythmic behaviors, such as stepping (for a review, see Bouyer4). The moving surface of the treadmill supplies biomechanical assistance for the stance phase of stepping and neural assistance for initiation of the swing phase. During the stance phase, the treadmill belt assists with moving the limb into extension and promotes hip extension during terminal stance. During terminal stance, excitation of stretch receptors in the hip flexor muscles interacts with the spinal central pattern–generating circuitry, promoting termination of the stance phase and initiation of the swing phase.5 On the basis of this evidence, it may seem logical to suppose that treadmill-based locomotor training offers advantages over overground locomotor training for people with SCI.
During treadmill-based locomotor training, stepping can be assisted in various ways, such as the manual assistance of a trainer, the mechanical assistance of a robotic gait orthosis, or electrical stimulation. Each approach has particular advantages. A trainer providing manual assistance can encourage the individual to voluntarily generate the portions of the cycle that he or she is able to control but can assist with portions over which the individual has less control. This approach likely balances voluntary effort and external assistance consistent with the “assist-as-needed” concept, which is thought to promote motor learning.6,7 Although robotic assistance may be configured to provide assistance as needed, it also can be used to provide maximal assistance to produce kinematically appropriate limb movement. In this mode, the device provides passive mechanical guidance, thereby eliciting precise gait-specific proprioceptive input—information that is thought to facilitate motor learning by contributing to the development of an accurate internal representation for the movement experience.8 Finally, electrical stimulation can be used to produce a flexion withdrawal response for stepping. It has been suggested that this approach targets the spinal circuitry,9 as the flexor reflex afferents are believed to be part of the pattern-generating circuitry underlying locomotor output.10–13
Thus far, there is no consensus about the best approach for locomotor training after SCI. A systematic review concluded that there is insufficient evidence from randomized clinical trials to conclude that any approach improves walking function more than another approach.14 In the largest randomized trial of locomotor training in people with SCI, treadmill-based locomotor training with manual assistance and BWS was compared with conventional overground locomotor training in participants with acute SCI. The investigators concluded that there was little difference in outcomes related to walking speed and distance between treadmill-based training and overground training for people with acute SCI.15 Although no differences between treadmill-based training and overground training were observed in people with acute SCI, it could be argued that training in the overground environment provides an experience that most closely resembles the functional task of walking. Overground locomotor training allows people to learn how to generate and control the forces necessary to initiate stepping, to move their bodies overground, and to practice and improve performance.
The objective of this study was to determine whether there are differences in walking speed and distance outcomes associated with 4 locomotor training approaches in people with chronic SCI. Earlier studies of locomotor training in people with SCI were restricted to comparisons of 2 groups; therefore, differences in study inclusion criteria, training duration, and training dosage limited the ability to make meaningful comparisons of approaches. All 4 approaches that we compared incorporated BWS but differed in the type of assistance provided for stepping (ie, manual, electrical stimulation, or mechanical) and the environment in which training was performed (ie, treadmill or overground). The approaches were intended to emphasize particular aspects of the various mechanisms that contribute to locomotion.
On the basis of our earlier study,9 we hypothesized that a treadmill-based training approach that incorporated electrical stimulation to evoke a flexion withdrawal reflex would be associated with the greatest improvements in both walking speed and walking distance. This hypothesis was based on our supposition that a combination of the flexion withdrawal response (thought to be involved with the spinal locomotor pattern-generating circuitry)10–13 and the moving treadmill would provide optimal drive to the spinal locomotor centers, resulting in superior gains. Preliminary results from an interim data analysis have been published elsewhere.16
Method
Design Overview
This study was a single-blind, randomized clinical trial.
Setting and Participants
The study took place at The Miami Project to Cure Paralysis, Miller School of Medicine, University of Miami. Participant enrollment commenced May 2002, and the final participant completed training in December 2008. People with chronic (≥1 year) SCI were recruited. Eligibility criteria for the study included American Spinal Injury Association Impairment Scale classification C or D,17 injury at or above T10, ability to take at least 1 step with 1 leg, and ability to rise to a standing position with, at most, moderate assistance (50% effort) from 1 other person. Exclusion criteria were current orthopedic problems, history of cardiac condition, or radiographic evidence of hip pathology that could be aggravated by training. All participants gave written and verbal informed consent according to the protocol of a study approved by the Human Studies Research Office, Miller School of Medicine, University of Miami.
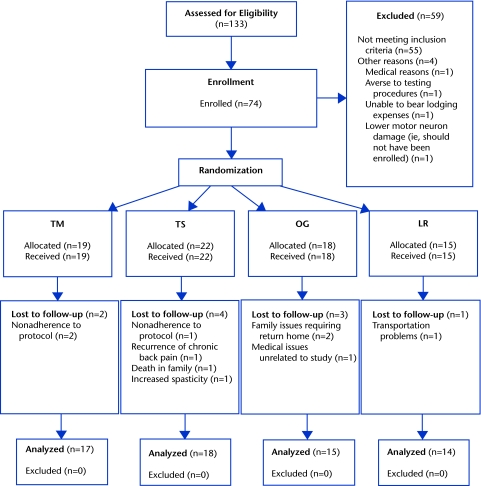
The research volunteer registry at The Miami Project to Cure Paralysis was the primary means of recruitment. Of the 3,396 entries available at the start of the study, 802 (23.6%) were identified as possibly being suitable for inclusion on the basis of queries about injury level, American Spinal Injury Association Impairment Scale classification, and assistive devices used for walking. A total of 133 registrants who were contacted expressed interest in being screened. Twenty-two people who sent video recordings and 33 people who were screened on site did not meet the inclusion criteria. Seventy-eight people provided consent to participate; 4 were excluded for various reasons before randomization. Ten people did not complete the training and were lost to follow-up. The breakdown by group is shown in the CONSORT (Consolidated Standards of Reporting Trials) diagram (Fig. 1).
Figure 1.
Flow of participants through the locomotor training study. LR=treadmill-based training with robotic assistance, OG=overground training with electrical stimulation, TM=treadmill-based training with manual assistance, TS=treadmill-based training with electrical stimulation.
Randomization and Interventions
Participants were stratified into 1 of 4 strata on the basis of pretraining Lower Extremity Motor Scores (LEMS)17: stratum 1=LEMS of 1 to 10, stratum 2=LEMS of 11 to 21, stratum 3=LEMS of 22 to 32, and stratum 4=LEMS of 32 to 40. Stratified randomization was used to assign participants to 1 of 4 BWS training groups: (1) treadmill-based training with manual assistance (TM), (2) treadmill-based training with stimulation (Digitimer DS7AH*) (TS), (3) overground training with stimulation (WalkAide stimulator†) (OG), or (4) treadmill-based training with robotic assistance (Lokomat robotic gait orthosis‡) (LR). Participants trained 5 days per week for 12 weeks (target=60 sessions). The mean number of training sessions completed was 49 (SD=7, range=27–58).
For all training approaches, BWS was provided by a harness and overhead lift. Our goal was to impose the maximum lower-extremity weight-bearing load during walking. Excessive knee flexion during the stance phase (ie, >∼40°) or toe dragging during the swing phase indicated that BWS was insufficient and cued the trainers to increase BWS. Except for the first week of training, BWS was maintained at or below 30%. This level of support has been shown to be associated with gait kinematics resembling those of unsupported walking.18 Higher levels of support in the overground condition are associated with participants having difficulty with forward progression.9
The form of assistance provided for stepping was specific to each training approach. For the TM approach, unilateral or bilateral manual assistance for stepping was provided in accordance with published guidelines.19 Participants were encouraged to step voluntarily to the extent possible. For the TS approach, bilateral stimulation to the common peroneal nerves was used to elicit a flexor reflex response. Stimulation was manually triggered to coincide with the onset of stepping. The intensity was adjusted to elicit a robust response, and pulse parameters were adjusted during the training session to manage habituation. For the OG approach, portable electrical stimulators were secured to the upper calf in an electrode position identical to that used in the TS approach. To the extent possible, stimulus pulse parameters were similar for the TS and OG approaches. However, the intensity supplied by the portable device used in the OG approach was adjusted to produce ankle dorsiflexion during the swing phase. For the LR approach, participants were secured to the robotic gait orthosis according to manufacturer instructions. The device was used to impose a kinematically consistent gait pattern; guidance forces were set at 100% to provide maximal assistance throughout the step cycle. Although participants were encouraged to “walk with the robot,” no attempt was made to monitor the forces of the interaction between the participant and the device.
Participants in all groups were encouraged to walk as fast as possible without regard for the duration of their walking bouts and were allowed to rest as needed. Participants were asked to rate their level of perceived exertion on a 15-point Borg scale (6 [rest]–20 [exhaustion]) after each walking bout and were encouraged to aim for an exertion level of at least 13 (moderate). For participants in the treadmill-based training groups, the treadmill speed was increased at the start of each session to the level at which stepping quality began to degrade and then was reduced to the level at which stepping quality was acceptable. Speed was increased periodically during the sessions to determine whether the participants could tolerate a faster speed. Participants in the OG group were encouraged to walk as fast as possible around a 24.4-m (80-ft) track over which a mobile, motorized BWS lift was suspended.
Participants were allowed to use handrails and assistive devices for balance as needed. Participants in the treadmill-based training groups were encouraged to swing their arms and were discouraged from bearing weight through the upper extremities. However, they were allowed to use handrails for balance.20 Participants in the OG group were allowed to use their preferred upper-extremity assistive devices. No attempt was made to advance participants to less supportive assistive devices. Participants who wore lower-extremity orthotic devices performed the initial training sessions without the devices, and the investigator (E.C.F.F.) made a clinical judgment regarding the safety of allowing the participants to continue training without the orthotic devices. If participants were unable to maintain a safe position of the lower-extremity joints (eg, exhibiting excessive supination or inadequate dorsiflexion), the orthotic devices were used during training in addition to the form of assistance used for the assigned training approach. Additional details regarding the training protocol are available in a preliminary report.16
Outcome Measures and Follow-up
Primary outcome measures.
Overground walking ability was evaluated over a short distance (10 m) to assess walking speed and over a longer distance to assess functional walking capacity (distance traversed in 2 minutes). Walking tests were performed before and after training, without BWS or assistance for stepping. Participants used the assistive or orthotic devices (or both) with which they were most comfortable. For both the test of speed and the test of distance, participants were given the instruction to “walk at your fastest comfortable speed.” The tests were scheduled on different days to minimize the effects of fatigue.
Short-distance overground walking speed was calculated from the average walking speed in 5 trials, captured as the participant walked over a 10-m path, the central 6 m of which was a calibrated motion capture area (Vicon Peak 8-camera 3-dimensional system§); complete methods related to kinematic data capture are described elsewhere.21 Participants were allowed to rest as needed between trials. Functional walking capacity was assessed with a 2-minute walk test. The distance traversed was determined from a video record obtained as participants walked around a 24.4-m (80-ft) oblong track with demarcations indicating 1.5-m (5-ft) increments. The people who captured and analyzed the kinematic and video data used to calculate walking speed and distance were unaware of training group assignments.
Secondary outcome measure.
Lower-extremity motor scores17 were used as a measure of the strength (force-generating capacity) of the right and left lower extremities.
Follow-up.
When we initiated the clinical trial, we had no plans for follow-up testing. Once the trial was under way, we recognized the value of examining whether training effects were retained after the completion of the study. Therefore, we performed follow-up testing for a convenience sample of 10 participants who were classified as showing improvement at the conclusion of training (ie, walking speed increased by at least 0.05 m/s; see “Data Analysis” section) and who were able to return for testing at least 6 months later.
Data Analysis
Sample size calculations were based on the assumption of a large effect size for an analysis of variance (ANOVA) comparing pretest-posttest intervention changes in walking speed for the 4 training groups. On the basis of this assumption, a sample size of 16 participants per group was required to achieve a power of 0.8. All data were managed with Microsoft Excel∥ and analyzed with SAS version 9.1.3.# The demographic and clinical characteristics of participants lost to follow-up were compared with those of participants completing the study by use of Student t-test and chi-square statistics. The baseline characteristics of the participants assigned to the 4 intervention groups were compared by use of ANOVA and chi-square analysis. The pretest-posttest intervention changes for the 4 intervention groups were compared by use of a repeated-measures ANOVA to examine both the time × group interaction and the time effect. When the time × group interaction was significant, pair-wise contrast analysis was used. When the time effect was significant, paired t tests were used to examine pretest-posttest intervention changes by group. The effect size index (d) was calculated for speed, distance, and LEMS for each intervention group by dividing the mean change score for each group by the standard deviation of the baseline score for that group.
The proportion of participants in each training group whose change in 10-m walking speed was 0.05 m/s or greater was determined. This degree of change in speed was considered the minimally important difference (MID) on the basis of earlier studies of community-dwelling people with SCI22 and older people who were healthy.23 The MID for walking distance was calculated to be 4 m on the basis of a test-retest reliability value of .9724 and a baseline standard deviation of 23.9 (for participants in the present study) for the 2-minute walk test distance. Participants were classified as showing improvement in either walking speed or walking distance if they met or exceeded the respective MID values.
For the purposes of data analysis, participants were categorized as “more impaired” or “less impaired” on the basis of LEMS; if the LEMS were greater than 15 for at least 1 leg and greater than or equal to 10 for the other leg, then the participant was categorized as less impaired. All other participants were categorized as more impaired. This categorization was based on earlier findings from our laboratory16 and others indicating that there is a strong relationship between LEMS and walking ability.25–27 Previous literature also indicated that people with at least 1 strong leg have the potential to achieve functional walking.27 Chi-square analysis was used to compare the proportions of participants in the intervention groups who showed improvements, by LEMS impairment group.
We used a repeated-measures ANOVA followed by paired t tests to examine the retention of improvement in a convenience sample of 10 participants who were classified as showing improvement in walking speed at the conclusion of training and who were available for follow-up testing at least 6 months later. A correlation coefficient was calculated to examine the relationship between the time since the conclusion of training and the retention of improvement.
Role of the Funding Source
Funding support for this study was provided by National Institutes of Health grant R01HD41487 (to Dr Field-Fote) and The Miami Project to Cure Paralysis.
Results
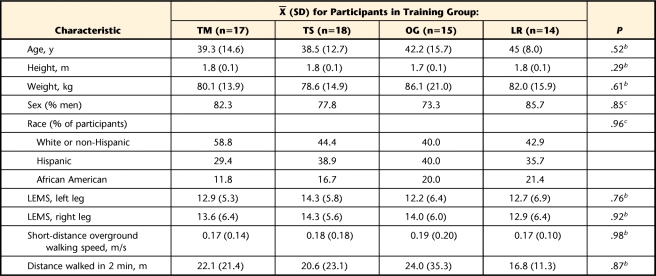
Sixty-four of the 74 participants who were randomly assigned to training groups completed the study (86% completion rate). Therefore, because data following training were not available for all of the participants, analysis was performed on protocol rather than intention to treat. The age, height, weight, and LEMS of the 10 participants who did not complete training or follow-up testing were similar to those of the participants who completed the study. However, the 10 participants who did not complete the study walked more slowly (0.06 versus 0.18 m/s; P<.0001) and walked shorter distances (5.16 versus 21.0 m; P<.0001) before training than did the participants who completed the study. There were no significant differences among the 4 training groups in demographic characteristics, baseline LEMS, 10-m walking speed, or 2-minute walk test distance (Tab. 1).
Table 1.
Baseline Equivalence of Groupwise Demographic Characteristicsa
TM=treadmill-based training with manual assistance, TS=treadmill-based training with electrical stimulation, OG=overground training with electrical stimulation, LR=treadmill-based training with robotic assistance, LEMS=Lower Extremity Motor Score.
b As determined with analysis of variance.
c As determined with chi-square analysis.
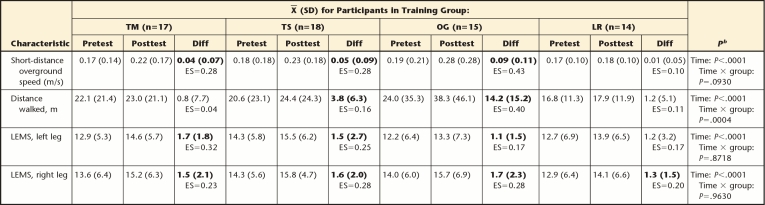
Overall, there was a significant time effect of training on walking speed (P<.0001) (Tab. 2). The improvement in walking speed was statistically significant for the OG, TS, and TM groups but not for the LR group. The effect size was moderate (d=0.43) for the OG group and small for both the TS group and the TM group (d=0.28). Although the groups had different pretest-posttest intervention changes in walking speed, the time × group interaction approached but did not reach statistical significance (P=.0930).
Table 2.
Pretest-Posttest Intervention Change by Training Groupa
TM=treadmill-based training with manual assistance, TS=treadmill-based training with electrical stimulation, OG=overground training with electrical stimulation, LR=treadmill-based training with robotic assistance, Diff=mean difference between pretest and posttest scores, LEMS=Lower Extremity Motor Score, ES=effect size (mean change score/standard deviation of pretest). Bolded Diff values are statistically different from 0.
b As determined with repeated-measures analysis of variance.
Overall, there also was a time effect of training on walking distance (P<.0001) (Tab. 2). The increase in walking distance was statistically significant for the OG and TS groups but not for the TM and LR groups. The effect size was moderate for the OG group (d=0.40) and small for the TS group (d=0.16). The time × group interaction was statistically significant (P=.0004), and post hoc testing indicated that the increase in the OG group was significantly greater than that in any treadmill-based training group (P≤.01) (Tab. 2).
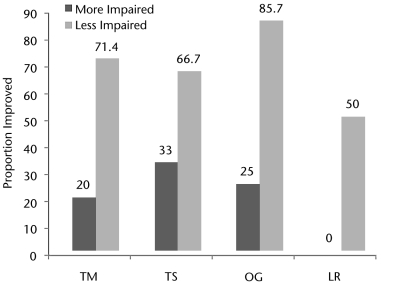
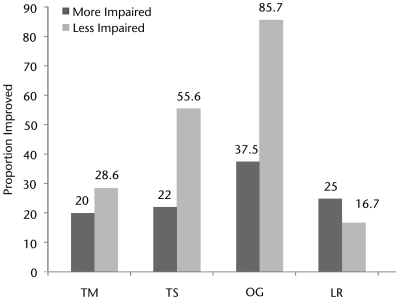
The LEMS of participants in all 4 training groups increased by 8% to 13% after the intervention. There were no significant between-groups differences in changes in LEMS for the right leg (P=.9630) or the left leg (P=.7818) (Tab. 2). Generally, compared with participants who were more impaired, a larger proportion of participants who were less impaired met or exceeded the MID for both walking speed (P<.0001) (Fig. 2) and walking distance (P=.0611) (Fig. 3). Among participants who were less impaired, a larger proportion of participants in the OG group than in the treadmill-based training groups met or exceeded the MID for both walking speed and walking distance (Figs. 2 and 3). However, among participants who were more impaired, the TS group had the largest proportion of participants who met or exceeded the MID for speed, whereas the OG group had the largest proportion of participants who met or exceeded the MID for distance.
Figure 2.
Proportions of “less impaired” and “more impaired” participants in each training group who achieved a minimally important difference in walking speed. Dark gray bars represent participants classified as more strength impaired. Light gray bars represent participants classified as less strength impaired. Values represent the proportion of participants in a category whose walking speed had improved by at least 0.05 m/s after training. LR=treadmill-based training with robotic assistance, OG=overground training with electrical stimulation, TM=treadmill-based training with manual assistance, TS=treadmill-based training with electrical stimulation.
Figure 3.
Proportions of less impaired and more impaired participants in each training group achieving a minimally important difference in walking distance. Dark gray bars represent participants classified as more strength impaired. Light gray bars represent participants classified as less strength impaired. Values represent the proportion of participants in a category whose walking distance on the 2-minute walk test had improved by at least 4 m after training. LR=treadmill-based training with robotic assistance, OG=overground training with electrical stimulation, TM=treadmill-based training with manual assistance, TS=treadmill-based training with electrical stimulation.
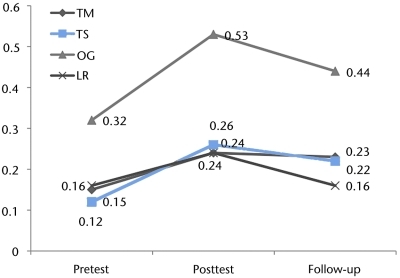
Ten participants whose improvement in 10-m walking speed met or exceeded the MID of 0.05 m/s during the intervention phase of the study were available for follow-up testing to assess the retention of training effects. These participants were tested an average of 20.3 months (SD=14.3 months) after the completion of training. Four of these participants had received the OG intervention, and 2 participants had received each of the other 3 interventions. The participants improved an average of 0.15 m/s (SD=0.09 m/s) by the conclusion of training. The participants declined an average of 0.06 m/s (SD=0.07 m/s) between the conclusion of training and follow-up testing. However, at follow-up, these participants were an average of 0.08 m/s (SD=0.07 m/s) faster than they had been before training (Fig. 4). There was no correlation between the time since the conclusion of training and the decline in walking speed (r=−.21, P=.55).
Figure 4.
Changes in walking speed overall and by training group. Data were obtained before training, at the conclusion of training, and at follow-up (retention) from 10 participants who had improved by the conclusion of training, overall and by training group. All 10 participants returned for follow-up retention testing at least 6 months (mean=20.3 months, SD=14.3 months) after completing training. LR=treadmill-based training with robotic assistance, OG=overground training with electrical stimulation, TM=treadmill-based training with manual assistance, TS=treadmill-based training with electrical stimulation.
Discussion
We had hypothesized that of the 4 training approaches, the TS approach would best activate the spinal locomotor circuitry and, therefore, would result in the greatest improvements in walking function. Our hypothesis was not supported. The OG approach was associated with significantly greater improvements in walking distance, and the effect size was greater for both speed and distance in the OG group than in any of the treadmill-based training groups. Furthermore, although the TS group had the largest proportion of participants who were more impaired and who met or exceeded the MID for walking speed, the OG group had the largest proportion of participants who were less impaired and who met or exceeded the MID for walking speed. In addition, the OG group had the largest proportion of participants who met the MID for walking distance among both participants who were less impaired and participants who were more impaired.
In both people with SCI and people with stroke, there is a strong relationship between walking distance and walking speed.27,28 However, it could be argued that the ability to enter a confined space such as a toilet stall or to maneuver around a kitchen while preparing a meal is likely to be limited more by the distance that an individual can walk than by walking speed. Given that the OG approach ordinarily is the least equipment- and personnel-intensive training option, it is clinically relevant that this approach was superior to the treadmill-based approaches in many ways.
We speculate that the reason why the OG approach was associated with greater improvements than the treadmill-based approaches may have been the requirements for walking after SCI. In the intact vertebrate nervous system, the spinal locomotor pattern generators are activated by the supraspinal centers (for a review, see Grillner et al29). Step initiation also is a function of the supraspinal centers.30 Damage to the spinal pathways means that the supraspinal centers have limited access to the spinal locomotor circuitry that contributes to proficient walking; it also indicates impaired conduction of signals related to descending drive for step initiation. Although treadmill walking takes advantage of the moving support surface to facilitate walking through afferent activation of the spinal locomotor centers31 (in effect substituting for the loss of supraspinal input), overground walking is distinguished by its greater demand for voluntary effort for step initiation and forward progression. Consequently, rather than focusing on “retraining the injured spinal cord”32 to improve overground walking function, it may be more valuable for people with SCI to focus on learning to execute the repetitive sequences of step initiation during overground walking and thereby to maximize supraspinal drive to the spinal locomotor circuitry.
The idea that people with SCI have impaired mechanisms of gait initiation is supported by earlier studies showing that even people with SCI who have good walking function (eg, able to take at least 10 steps with no assistance or assistive device) require significantly more time to initiate a voluntary step after an auditory cue than do people without a disability.33 In a recently published case series, a crossover design was used to compare outcomes associated with a skilled overground walking program and a treadmill-based locomotor training program.34 Although both types of training were effective at improving walking function, skilled overground walking was associated with greater gains in most of the outcome measures, including walking speed, walking endurance, balance confidence, and stair climbing.34 The overground walking program included elements that required continuous, active engagement for volitional movement, such as stepping over obstacles and walking up ramps. These findings lend support to the concept that voluntary effort and maximizing descending drive may be key considerations for locomotor training in people with SCI.
The findings related to improvements in lower-extremity strength across all groups and the lack of between-group differences in strength are noteworthy. These results are consistent with our previously reported findings, namely, that measures of gait quality (step length, cadence, and symmetry) improved to similar degrees in all groups, with no between-group differences.21 Although lower-extremity strength is correlated with walking speed in people with SCI16,25–27 and gait quality presumably influences walking function, strength and gait quality do not account for the larger improvements in walking function achieved by participants in the OG group. Alternatively, it is possible that LEMS used as measures of muscle strength are not sufficiently sensitive to detect training-related changes in muscle force-generating capacity that may contribute to improvements in walking function.
We found that, on average, participants who returned for follow-up testing still walked faster than they had before training but that their walking speed at follow-up had declined relative to their walking speed at the conclusion of training. There was no apparent relationship between time since training and decline in speed, suggesting that retention in participants tested 6 months after training was similar to that in participants tested 2.5 years after training. The sample of participants (n=10) who were able to return for follow-up retention testing was too small to allow an analysis of between-groups differences in retention. However, although the sample was small relative to the full study cohort, the mean time since training was more than 1.5 years, making this the largest data set for long-term retention of overground walking outcomes in participants with chronic SCI. Hicks et al35 assessed retention for up to 8 months in a sample of 13 participants who had chronic SCI and who had been trained for 12 to 15 months (144 sessions). However, because 11 of the participants in that study were unable to stand or walk at the start of training, overground walking outcomes were measured only with a categorical scale based on what assistive devices the participants required and whether they were able to walk fewer or more than 5 steps, and walking speed was assessed only on the treadmill with BWS. Additional research is needed to identify factors predicting the retention of improvements after interventions.
Restoration of Walking Ability Follows the Same Principles as Other Forms of Motor Learning
Our findings indicated that the attributes of overground training were superior to those of the other 3 approaches for promoting functional walking capacity. The fundamental principles of motor learning include intensive, repetitive training, task specificity, and the opportunity to solve problems.36–38 Our results indicated that the experiences necessary to improve walking function follow principles known to be true for learning other motor behaviors. Activity-dependent plasticity requires repetition and intensive practice.36 In people with subacute stroke, a comparison of overground training and treadmill-based training revealed that although walking speed improved to the same extent in both groups, there was a greater improvement in walking distance in the treadmill-based training group.39 The authors attributed these findings to the larger amount of practice obtained by the treadmill-based training group in the 30-minute training sessions.39 In the present study, although all 4 training approaches challenged the participants in terms of both intensity and amount of training, people who trained with the treadmill-based approaches walked at significantly faster training speeds16 and thus were likely to have taken more steps per session and to have more practice. Therefore, in the present study, it is likely that factors other than the amount of practice contributed to the greater improvements observed with the OG approach. Perhaps the elements that contribute to an optimal training regimen for people with hemiplegia caused by stroke are different from those for people with tetraplegia or paraplegia caused by SCI.
Although the kinematics and temporal aspects of walking on a treadmill are very similar to those of overground walking, from the perspective of task specificity there are clear differences between these 2 walking environments in terms of joint moments at the ankle, hip, and knee and differences in joint power at the knee and hip.40 Learning to generate and control these forces while walking on a treadmill may not transfer completely to the motor output that is necessary for overground walking. Lee and Hidler40 speculated that these differences may result in the development of motor strategies for treadmill walking that are different from those for overground walking. A study done to directly assess the extent of transfer of training from a split-belt treadmill to overground walking revealed only partial transfer of the pattern learned on the treadmill.41 It seems reasonable to conclude that the overground locomotor training task or environment best allows an individual to learn to generate and control the forces necessary to initiate stepping, to move the body overground, and to practice and improve performance. Along these lines, it could be argued that the participants trained with the OG approach had the advantage of training that closely mimicked the manner in which they were tested. However, given that the goal of locomotor training is to improve functional walking capacity in the real-world environment, overground walking speed and distance are the most salient outcome measures. Although it is likely that the results of the present study would have been different if treadmill walking speed and distance had been the outcomes of interest, the relevance of such measures to real-world function is questionable.
The motor learning needed for improved walking function appears to require more than simple repetition34 and benefits from the opportunity to make and correct errors.42 In the present study, the overground training environment likely provided greater variety in the walking task than did the more predictable treadmill training environment (especially with regard to the fixed, 100% assistance parameters that we used in the LR approach). Participants in the OG group needed to lift and advance their assistive devices and negotiate 4 curves on the 22.4-m track above which the BWS system was suspended. There is evidence from training studies of people with SCI43 and stroke,44 as well as from animal models,7 that variability is important for locomotor learning. Furthermore, there is evidence that after central nervous system injury, training in skilled behaviors such as walking or climbing in challenging environments (ie, ropes, rods, ladders, obstacles) is associated with changes in corticomotor neural structure, whereas no plastic effects are associated with treadmill training.45 We propose that, for walking function, the task specificity of training and the opportunity to solve the biomechanical and kinetic problems experienced in the overground condition were important factors contributing to the results associated with overground locomotor training.
Limitations
The present study had several limitations. First, we do not know whether the training dosage was optimal for improving walking speed and distance. The number of training sessions, the duration of each training session, and the training frequency were meant to represent a training dosage that would be challenging but tolerable for the target study population. This training dosage was based on clinical judgment in the absence of experimental evidence related to the optimal training dosage. Second, we focused on walking speed during training rather than on other aspects of walking, such as asking participants to focus on producing optimal kinematics. Although training at faster speeds has been shown to be associated with greater increases in walking function in people with stroke,46 training speed does not appear to be an important determinant of locomotor outcomes in people with SCI.16 Third, all but a few of the participants in the present study used a wheelchair as their primary means of mobility and therefore did not ambulate in the community. For this reason, the amount of change that is qualified as meaningful change in this group may be smaller than that necessary for meaningful change in people who use walking as their primary means of mobility. Fourth, the training parameters used in the robotic gait orthosis approach were configured to impose a kinematically appropriate gait pattern, and stepping proceeded regardless of whether participants contributed effort. Therefore, different results may be obtained if the training parameters require participants to actively generate stepping to assist the robotic device. A final limitation was that only a small proportion of participants were able to return for assessment of the retention of training effects. Unfortunately, because many participants resided outside the local area, traveling was financially burdensome. This limitation resulted in an incomplete assessment of the influence of the training approach on retention.
Conclusions
People with chronic motor incomplete SCI have the potential to increase their overground walking speed and walking distance in a specified time (ie, functional walking capacity). Overground locomotor training resulted in greater improvements in functional walking capacity than did treadmill-based training. Several characteristics of overground locomotor training likely contributed to the greater gains observed with this approach. These include greater specificity of training for the real-world task of walking, increased voluntary effort for step initiation and forward progression, and more opportunities for learning how to generate and control the forces required for walking overground. We suggest that in people with SCI and minimal walking ability, walking distance is arguably a better indicator of walking function than is walking speed. Further work is needed to determine the optimal training dosage.
The Bottom Line
What do we already know about this topic?
Prior studies have shown that body-weight–supported locomotor training can improve walking function in individuals with motor-incomplete spinal cord injury. However, it is unclear what approach to training results in the greatest improvements.
What new information does this study offer?
This study indicates that gains in walking speed achieved with locomotor training in the overground environment are equivalent to the gains made with treadmill-based training, and that the gains in walking distance achieved with overground training surpass those with treadmill-based training.
If you're a patient, what might these findings mean for you?
The findings of this study indicate that individuals with motor-incomplete spinal cord injury benefit from locomotor training, even years after injury, and that high-tech equipment is not needed to attain these benefits.
Footnotes
Both authors provided concept/idea/research design and writing. Dr Roach provided data analysis. Dr Field-Fote provided project management, fund procurement, participants, and facilities/equipment. The authors acknowledge the contributions of the staff of the Neuromotor Rehabilitation Research Laboratory at The Miami Project to Cure Paralysis, especially Saumitra Sinha Ray, Kathleen Manella, Stephen Lindley, Rebecca Avshalom, and Lea Lenahan.
Funding support for this study was provided by National Institutes of Health grant R01HD41487 (to Dr Field-Fote) and The Miami Project to Cure Paralysis.
ClinicalTrials.gov registration: R01HD41487.
Digitimer Ltd, 37 Hydeway, Welwyn Garden City, Hertfordshire, United Kingdom.
Hanger Orthopedic Group Inc, Two Bethesda Metro Center, Suite 1200, Bethesda, MD 20814.
Hocoma AG, Industriestrasse 4, Zurich, Switzerland.
Peak Performance Technologies Inc, 7388 S Revere Pkwy, Suite 603, Englewood, CO 80112.
Microsoft Corp, One Microsoft Way, Redmond, WA 98052-6399.
SAS Institute Inc, 100 SAS Campus Dr, Cary, NC 27513-2414.
References
- 1. National Spinal Cord Injury Information Network Spinal cord injury facts and figures at a glance. National Spinal Cord Injury Statistical Center. Available at: https://www.nscisc.uab.edu/public_content/pdf/FactsApr09.pdf. Published April 2009 Accessed September 16, 2010
- 2. Tator CH, Duncan EG, Edmonds VE, et al. Changes in epidemiology of acute spinal cord injury from 1947 to 1981. Surg Neurol. 1993;40:207–215 [DOI] [PubMed] [Google Scholar]
- 3. Barbeau H, Blunt R. A novel interactive locomotor approach using body weight support to retrain gait in spastic paretic subjects. In: Wernig A. ed. Plasticity of Motoneuronal Connections. Amsterdam, the Netherlands: Elsevier Science Publishers; 1991:461–474 [Google Scholar]
- 4. Bouyer LJ. Animal models for studying potential training strategies in persons with spinal cord injury. J Neurol Phys Ther. 2005;29:117–125 [DOI] [PubMed] [Google Scholar]
- 5. Hiebert GW, Whelan PJ, Prochazka A, Pearson KG. Contribution of hind limb flexor muscle afferents to the timing of phase transitions in the cat step cycle. J Neurophysiol. 1996;75:1126–1137 [DOI] [PubMed] [Google Scholar]
- 6. Emken JL, Benitez R, Reinkensmeyer DJ. Human-robot cooperative movement training: learning a novel sensory motor transformation during walking with robotic assistance-as-needed. J Neuroeng Rehabil. 2007;4:8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Cai LL, Fong AJ, Otoshi CK, et al. Implications of assist-as-needed robotic step training after a complete spinal cord injury on intrinsic strategies of motor learning. J Neurosci. 2006;26:10564–10568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Wolpert DM, Miall RC. Forward models for physiological motor control. Neural Netw. 1996;9:1265–1279 [DOI] [PubMed] [Google Scholar]
- 9. Field-Fote E, Tepavac D. Combined use of body weight support, functional electric stimulation, and treadmill training to improve walking ability in individuals with chronic incomplete spinal cord injury. Arch Phys Med Rehabil. 2001;82:818–824 [DOI] [PubMed] [Google Scholar]
- 10. Bussel B. Late flexion reflex in paraplegic patients: evidence for a spinal stepping generator. Brain Res Bull. 1989;22:53–56 [DOI] [PubMed] [Google Scholar]
- 11. Bussel B, Roby-Brami A, Néris OR, Yakovleff A. Evidence for a spinal stepping generator in man: electrophysiological study. Acta Neurobiol Exp (Wars). 1996;56:465–468 [DOI] [PubMed] [Google Scholar]
- 12. Jankowska E, Jukes MG, Lund S, Lundberg A. The effect of DOPA on the spinal cord. 6. Half-centre organization of interneurones transmitting effects from the flexor reflex afferents. Acta Physiol Scand. 1967;70:389–402 [DOI] [PubMed] [Google Scholar]
- 13. Berkowitz A, Yosten GL, Ballard RM. Somato-dendritic morphology predicts physiology for neurons that contribute to several kinds of limb movements. J Neurophysiol. 2006;95:2821–2831 [DOI] [PubMed] [Google Scholar]
- 14. Mehrholz J, Kugler J, Pohl M. Locomotor training for walking after spinal cord injury. Cochrane Database Syst Rev. 2008;2:CD006676. [DOI] [PubMed] [Google Scholar]
- 15. Dobkin B, Apple D, Barbeau H, et al. Weight-supported treadmill vs over-ground training for walking after acute incomplete SCI. Neurology. 2006;66:484–493 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Field-Fote EC, Lindley SD, Sherman AL. Locomotor training approaches for individuals with spinal cord injury: a preliminary report of walking-related outcomes. J Neurol Phys Ther. 2005;29:127–137 [DOI] [PubMed] [Google Scholar]
- 17. American Spinal Injury Association/International Spinal Cord Society Standards for Neurological Classification of Spinal Cord Injury. Chicago, IL: American Spinal Injury Association; 2006 [Google Scholar]
- 18. Finch L. Influence of body weight support on normal human gait: development of a gait retraining strategy. Phys Ther. 1991;71:842–855 [DOI] [PubMed] [Google Scholar]
- 19. Behrman AL, Harkema SJ. Locomotor training after human spinal cord injury: a series of case studies. Phys Ther. 2000;80:688–700 [PubMed] [Google Scholar]
- 20. Hornby TG, Zemon DH, Campbell D. Robotic-assisted, body-weight-supported treadmill training in individuals following motor incomplete spinal cord injury. Phys Ther. 2005;85:52–66 [PubMed] [Google Scholar]
- 21. Nooijen CF, Ter Hoeve N, Field-Fote EC. Gait quality is improved by locomotor training in individuals with SCI regardless of training approach. J Neuroeng Rehabil. 2009;6:36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Musselman KE. Clinical significance testing in rehabilitation research: what, why, and how? Phys Ther Rev. 2007;12:287–296 [Google Scholar]
- 23. Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54:743–749 [DOI] [PubMed] [Google Scholar]
- 24. Rossier P, Wade DT. Validity and reliability comparison of 4 mobility measures in patients presenting with neurologic impairment. Arch Phys Med Rehabil. 2001;82:9–13 [DOI] [PubMed] [Google Scholar]
- 25. Waters RL, Adkins R, Yakura J, Vigil D. Prediction of ambulatory performance based on motor scores derived from standards of the American Spinal Injury Association. Arch Phys Med Rehabil. 1994;75:756–760 [PubMed] [Google Scholar]
- 26. Zörner B, Blanckenhorn WU, Dietz V, et al. Clinical algorithm for improved prediction of ambulation and patient stratification after incomplete spinal cord injury. J Neurotrauma. 2010;27:241–252 [DOI] [PubMed] [Google Scholar]
- 27. Kim CM, Eng JJ, Whittaker MW. Level walking and ambulatory capacity in persons with incomplete spinal cord injury: relationship with muscle strength. Spinal Cord. 2004;42:156–162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Eng JJ, Chu KS, Dawson AS, et al. Functional walk tests in individuals with stroke: relation to perceived exertion and myocardial exertion. Stroke. 2002;33:756–761 [DOI] [PubMed] [Google Scholar]
- 29. Grillner S, Ekeberg Ö, El Manira A, et al. Intrinsic function of a neuronal network: a vertebrate central pattern generator. Brain Res Brain Res Rev. 1998;26:184–197 [DOI] [PubMed] [Google Scholar]
- 30. Gerasimenko YP, Makarovskii AN, Nikitin OA. Control of locomotor activity in humans and animals in the absence of supraspinal influences. Neurosci Behav Physiol. 2002;32:417–423 [DOI] [PubMed] [Google Scholar]
- 31. Shik ML, Orlovsky GN. Neurophysiology of locomotor automatism. Physiol Rev. 1976;56:465–501 [DOI] [PubMed] [Google Scholar]
- 32. Edgerton VR, Leon RD, Harkema SJ, et al. Retraining the injured spinal cord. J Physiol. 2001;533:15–22 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Chang HA, Chuang TY, Lee SJ, et al. Temporal differences in relative phasing of gait initiation and first step length in patients with cervical and lumbosacral spinal cord injuries. Spinal Cord. 2004;42:281–289 [DOI] [PubMed] [Google Scholar]
- 34. Musselman KE, Fouad K, Misiaszek JE, Yang JF. Training of walking skills overground and on the treadmill: case series on individuals with incomplete spinal cord injury. Phys Ther. 2009;89:601–611 [DOI] [PubMed] [Google Scholar]
- 35. Hicks AL, Adams MM, Martin GK, et al. Long-term body-weight-supported treadmill training and subsequent follow-up in persons with chronic SCI: effects on functional walking ability and measures of subjective well-being. Spinal Cord. 2005;43:291–298 [DOI] [PubMed] [Google Scholar]
- 36. Kleim JA, Jones TA. Principles of experience-dependent neural plasticity: implications for rehabilitation after brain damage. J Speech Lang Hear Res. 2008;51:S225–S239 [DOI] [PubMed] [Google Scholar]
- 37. Forrester LW, Hanley DF, Macko RF. Effects of treadmill exercise on transcranial magnetic stimulation-induced excitability to quadriceps after stroke. Arch Phys Med Rehabil. 2006;87:229–234 [DOI] [PubMed] [Google Scholar]
- 38. Mastos M, Miller K, Eliasson AC, Imms C. Goal-directed training: linking theories of treatment to clinical practice for improved functional activities in daily life. Clin Rehabil. 2007;21:47–55 [DOI] [PubMed] [Google Scholar]
- 39. Dean CM, Ada L, Bampton J, et al. Treadmill walking with body weight support in subacute non-ambulatory stroke improves walking capacity more than overground walking: a randomised trial. J Physiother. 2010;56:97–103 [DOI] [PubMed] [Google Scholar]
- 40. Lee SJ, Hidler J. Biomechanics of overground vs. treadmill walking in healthy individuals. J Appl Physiol. 2008;104:747–755 [DOI] [PubMed] [Google Scholar]
- 41. Reisman DS, Wityk R, Silver K, Bastian AJ. Split-belt treadmill adaptation transfers to overground walking in persons poststroke. Neurorehabil Neural Repair. 2009;23:735–744 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Emken J, Reinkensmeyer D. Robot-enhanced motor learning: accelerating internal model formation during locomotion by transient dynamic amplification. IEEE Trans Neural Syst Rehabil Eng. 2005;13:33–39 [DOI] [PubMed] [Google Scholar]
- 43. Jezernik S, Schärer R, Colombo G, Morari M. Adaptive robotic rehabilitation of locomotion: a clinical study in spinally injured individuals. Spinal Cord. 2003;41:657–666 [DOI] [PubMed] [Google Scholar]
- 44. Lewek MD, Cruz TH, Moore JL, et al. Allowing intralimb kinematic variability during locomotor training poststroke improves kinematic consistency: a subgroup analysis from a randomized clinical trial. Phys Ther. 2009;89:829–839 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Jones TA, Chu CJ, Grande LA, Gregory AD. Motor skills training enhances lesion-induced structural plasticity in the motor cortex of adult rats. J Neurosci. 1999;19:10153–10163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Pohl M, Mehrholz J, Ritschel C, Ruckriem S. Speed-dependent treadmill training in ambulatory hemiparetic stroke patients: a randomized controlled trial. Stroke. 2002;33:553–558 [DOI] [PubMed] [Google Scholar]