Abstract
BACKGROUND AND OBJECTIVES:
Fluorosis has become an endemic problem worldwide. Fluoride has its effect on various organs, including the reproductive system, although there are controversial reports over it. Thus, the present study was designed to study the effect of sodium fluoride (NaF) exposure for different durations.
MATERIALS AND METHODS:
30 healthy rabbits were divided into three equal groups. Group I was fed on a standard diet for 30 days, Group II was fed on sodium fluoride (20 mg/kg body weight) for 30 days and Group III was fed on sodium fluoride (20 mg/kg body weight) for 60 days. Sperm count, motility, progressive motility and weight of testis and epididymis were measured and compared with the control group.
RESULTS:
We observed that in the group exposed to NaF for 30 days (Group II), there was a significant decrease in all the parameters except the testicular weight and in the group on exposure of 60 days (Group III), there was a significant decrease in all the parameters when compared with the control group (Group I). On applying the Tukey post-hoc test between Groups II and III, we observed that there was a significant decrease in the sperm count while the other parameters showed a non-significant decrease. Testicular histology was confirmatory for the above findings.
CONCLUSION:
The present study demonstrates that fluoride hampers the reproductive functions of male rabbits and is proportional to the duration of fluoride exposure.
Keywords: Fluoride, sperm count, testis
INTRODUCTION
Humans are exposed to sodium fluoride (NaF) from a number of sources, including water, medicines, pesticides, insecticides, fertilizer residues, dental restorative materials, dental products (tooth pastes and mouth rinses), pediatric supplements, beverages prepared with fluoridated water and food.[1] Fluorosis is an endemic public health problem in nearly 22 nations around the world. The World Health Organization (WHO) guideline is that 1.5 ppm of fluoride is the desirable upper limit in drinking water. The magnitude of problem of fluorosis in India is roughly estimated to be of 66.62 million people at risk.[2] The recommended levels of fluoride in drinking water are 0.5–0.8 mg/L. Fluoride levels above 1.5 mg/L may lead to dental fluorosis while levels above 3–6 mg/L during the life time may lead to skeletal fluorosis.[3] Involvement of the reproductive organs due to fluorosis in animals had also been studied extensively. Messar et al., reported that the low levels of fluoride in food rendered mice infertile, while a high-fluoride diet improved their fertility.[3] Chinoy and Sequeira reported that sodium fluoride treatment in mice caused an alteration in the histology of reproductive organs and morphology of sperm and induced biochemical changes.[4] These reports were contradicted by Tao and Suttie, whose experiments showed that fluoride did not play any essential role in reproduction.[5] Because of these controversial reports, we intended to assess the effect of fluoride on the rabbit reproductive system.
MATERIALS AND METHODS
The study was conducted in the Department of Physiology in collaboration with the Department of Pathology after approval by the Institutional Animal Ethical Committee. Thirty healthy, adult New Zealand white male rabbits were used for the study. All the rabbits were of the same age group, with a weight range of 1.5–2.5 kg. The rabbits were housed in a well-ventilated animal house and caged separately, at a temperature of 29-32°C and exposed to 10–12 h of daylight. They received food and water ad libitum. Sodium fluoride (Ranbaxy Laboratories, Chandigarh, India) was given using a feeding tube attached to a hypodermic needle in the dose of 20 mg/kg body weight/day.[6] The rabbits were divided into three equal groups of 10 each.
Group I: (Untreated control) The rabbits were maintained on a standard diet and water ad libitum for 30 days and were sacrificed on the 31st day.
Group II: The rabbits were given sodium fluoride (NaF) for 30 days and were sacrificed on the 31st day.
Group III: The rabbits were given sodium fluoride (NaF) for 60 days and were sacrificed on the 61st day.
The control and the test group rabbits were anesthetized with intravenous injection of Urethane (0.5–1.5 g/kg of body weight). Incision was given on the scrotum of the rabbit and the testis and the epididymis were carefully exposed, removed and were subjected to the following physiological and histopathological studies:
Epididymal sperm count
From each separated epididymis, the cauda part was removed and placed in a beaker containing 1 mL physiological saline solution. Each section was quickly macerated with a pair of sharp scissors and left for a few minutes to liberate its spermatozoa into the saline solution. Sperm count was performed under the microscope using the improved Neubauer chamber (Fein-Optik, Blankenburg, Germany).[7]
Sperm motility and progressive sperm motility
A drop of semen was placed on the slide and two drops of warm 2.9% sodium citrate was added. The slide was covered with a cover slip and examined under the microscope using ×40 objective for sperm motility. Sperm motility and progressive motility were determined.
Weight of the testis and the epididymis
The weight of the testis and the epididymis of all the groups was taken.
Histopathology of the testis
The testis was removed after being freed from the surrounding tissue. The tissue was kept in a 10% neutral formalin solution for fixation. After 1 week, the tissue was washed for 24 h under running tap water, then dehydrated through ascending grades of alcohol, cleared in xylene and embedded and blocked in paraffin. Sections of 4–5-µm thickness were taken and stained with hematoxylin and eosin and were examined under the microscope.
Statistical analysis
The values of different parameters like sperm count, sperm motility, progressive motility and weight of the testis and epididymis were compared using one way ANOVA with post-hoc Tukey test and the statistical values were expressed as mean ± SD [Table 1].
Table 1.
Comparison of epididymal sperm count, motility and progressive motility of sperm, weights of testis and epididymis among Group I (control), Group II (fed on NaF for 30 days) and Group III (fed on NaF for 60 days) using ANOVA and post-hoc Tukey test
| Parameters | Group I Mean ± SD | Group II Mean ± SD | Group III Mean ± SD | ANOVA “F”-value | P-value |
|---|---|---|---|---|---|
| Sperm count × 106/epididymis | 162 ± 10.52 | 111.75 ± 17.48* | 91.5 ± 11.25†,‡ | 72.777 | 0.000 |
| Sperm motility (%) | 72.1 ± 3.28 | 59.1 ± 10.28* | 55.9 ± 7.24† | 13.053 | 0.000 |
| Progressive motility of sperm (%) | 58.6 ± 6.02 | 47.1 ± 11.44* | 39 ± 7.02† | 13.436 | 0.000 |
| Testicular weight (g) | 3.22 ± 0.46 | 2.86 ± 0.37* | 2.63 ± 0.42† | 4.931 | 0.015 |
| Epididymal weight (g) | 0.63 ± 0.21 | 0.46 ± 0.06* | 0.45 ± 0.05† | 5.835 | 0.008 |
Post-hoc Tukey test (P < 0.05) comparison between Groups I and II;
Post-hoc Tukey test (P < 0.05) comparison between Groups I and III;
Post-hoc Tukey test (P < 0.05) comparison between Groups II and III
RESULTS
On comparing the three groups using one way ANOVA, there was a statistically significant difference among the three groups [Table 1]. On multiple comparisons using Tukey’s post-hoc test, we observed that in the group exposed to NaF for 30 days (Group II), there was a significant decrease in all the parameters except the testicular weight and in the group on exposure of 60 days (Group III), there was a significant decrease in all the parameters when compared with the control group (Group I). On applying the Tukey post-hoc test between Groups II and III, we observed that there was a significant decrease in the sperm count while the other parameters showed a non-significant decrease.
Histopathology of the testis
Group I [Figure 1]: Histology of the testis showed normal spermatogenesis with different stages of differentiation and maturation. The spermatozoa were in groups attached to the inner aspect of the lumen of the seminiferous tubules.
Figure 1.
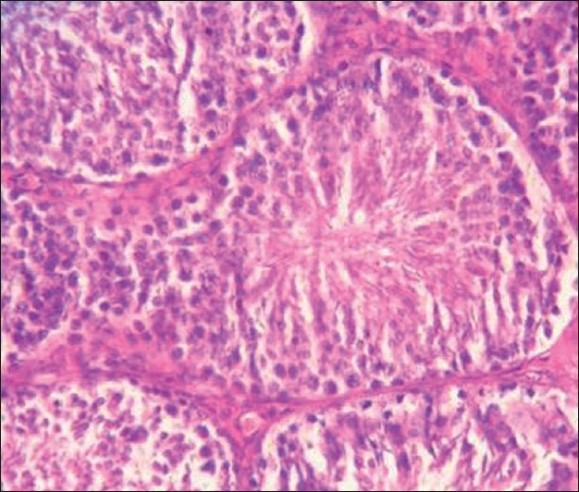
Histopathology of the testis in rabbits fed on a standard diet
Group II [Figure 2]: There was a lack of differentiation and maturation of spermatocytes and there was marked infiltration in the interstitial area of the seminiferous tubules. No mature spermatozoa were seen in the lumens of the seminiferous tubules.
Figure 2.
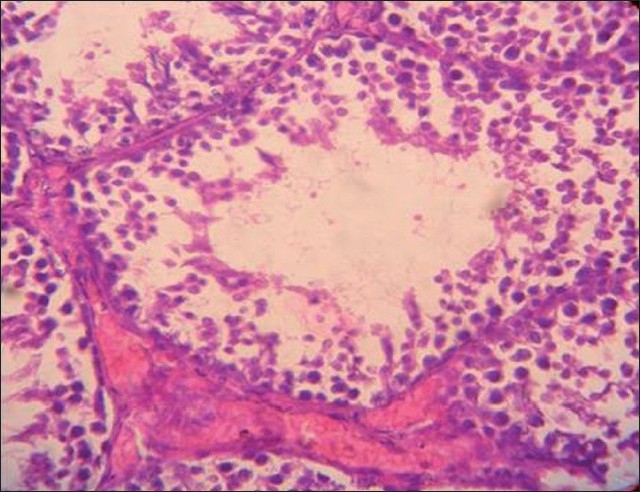
Histopathology of the testis in rabbits fed on NaF (20 mg/ kg) for 30 days
Group III [Figure 3]: There was a marked atrophy and necrosis of the seminiferous tubules and no normal spermatocytes or spermatids were seen. There was complete cessation of spermatogenesis and the seminiferous tubules were devoid of spermatozoa. This group also showed that there was sloughing off of the spermeogenic cells in the luminal region of the seminiferous tubules of the testis, leading to disorganization of their epithelium.
Figure 3.
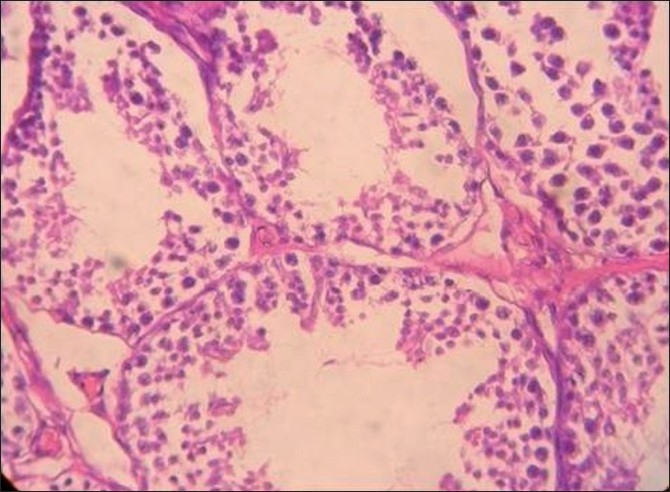
Histopathology of the testis in rabbits fed on NaF (20 mg/ kg) for 60 days
DISCUSSION
Animal studies had shown contradictory reports regarding the effect of fluorosis on the reproductive system. Therefore, the present study was performed to establish the link between fluoride and its effect on sperm count, motility, progressive motility, weight of testis, weight of epididymis and histopathology of the testis of age and body weight matched groups.[6]
Effect on sperm count
It was observed that in comparison with the control group, there was a significant decrease in the epididymal sperm count when NaF was given for 30 days to rabbits and a greater decrease when NaF was given for 60 days. Hence, the present study proved that higher the fluoride exposure, higher the effect on sperm counts. Similar results had been observed in rats, mice and rabbits in studies performed earlier.[8–10]
The effect of fluoride toxicity on spermatogenesis could be because fluoride reduces the testosterone levels and by reducing the testicular zinc levels, it impairs angiotensin-converting enzyme (ACE) activity and hence causes inhibition of spermatogenesis.[1] Apart from the direct effect on the levels of testosterone, fluoride inhibits androgen receptor (AR) mRNA expression in Sertoli cells and causes a decrease in AR through which testosterone acts.[11] Wan et al., reported that epidermal growth factor (EGF) and its receptor (EGFR), which plays an important role in male reproductive functions of rat, were significantly decreased in Leydig cells, spermatogonia and spermatocytes on exposure to 68 ppm fluoride for 10 days.[12] Other factors responsible for the arrest of spermatogenesis might be the lack of available proteins necessary for cell division, growth and differentiation of germ cells. In a study, disruption of normal cell cycle and apoptosis was indicated at doses of 200–30 mg/L of fluoride, which may be attributed to the blockage of the G1 phase of the cell cycle. Another mechanism of apoptosis is increased levels of oxidants, which damages the DNA.[13]
Effect on motility
In the present study, there was a significant decrease in sperm motility and progressive motility in the group fed with fluoride 20 mg/kg for 30 days and 60 days as compared with the control. Huang et al., also reported a significant reduction in the sperm motility of mice fed on 100, 200 and 300 mg NaF/L for 8 weeks as compared with the control group.[13] Similar results were shown in rats and mice in many other studies.[9,14–16] One important study demonstrated that human spermatozoa lost their motility in vitro in the presence of 250 mM fluoride within 20 mins of exposure and, similarly, fluoride (30 mM) made the bull sperms immotile within 2 min at 30°C in vitro.[8]
The mechanism by which fluoride affects sperm motility has not been clearly elucidated. However, it has been postulated that fluoride could act directly on the motile apparatus without affecting other metabolic systems.[17] One mechanism could be decline in the fructose level, which provides energy for motility, in the seminal vesicle and vas deferens due to alteration in carbohydrate.[8,17] Fluoride may also act by inhibiting many enzymes: the first mode of action is that fluoride binds with cofactors like Mg, Ca, Zn and Se and thus inhibits glycolysis, respiration and motility of sperms, or it might also form insoluble complexes with magnesium or phosphates in enzymes like enolase and acid and alkaline phosphatase.[17] Another reason for decreased sperm motility was decreased level of androgen carrier proteins involved in sperm motility.[18] Furthermore, structural defects were observed in the flagellum, acrosome and nucleus of spermatids and epididymal spermatozoa of fluoride-treated rabbits (10 mg/kg/day for 18 months) leading to abnormal motility.[19]
Organ weights
In our study, there was a significant decrease in the epididymal weight in Groups II and III as compared with the control while there was a significant decrease in the testicular weight in Group III only, indicating that a higher level of NaF is needed to affect testicular weight than epididymal weight. In other words, we can conclude that fluoride affects epididymal weight earlier than testicular weight.
Similarly, in another study, rabbits fed on fluoride were having a significant decrease in epididymal weight.[20] Also, the weight of the cauda epididymis in fluoride-treated (10 mg/kg for 30 days) mice declined significantly compared with the control groups.[9] However, no significant difference was observed in the mean testicular weights of rats fed on 100 and 300 ppm fluoride for 12 weeks when compared with the control. It might be because of the lower fluoride concentration.[16] Our findings differed from those of Ghosh et al., who reported an increase in the relative testis weight of rats as compared with the control with fluoride treatment (20 mg/kg for 29 days), which might had been due to a compensatory change or due to fluid accumulation in the testis.[21]
Testicular histology
Figures 1–3 show that longer the fluoride exposure, higher was the damage to the reproductive system.
There was a lack of differentiation and maturation of spermatocytes, with no mature spermatozoa seen in the lumens in Group II [Figure 2] and complete cessation of spermatogenesis in Group III [Figure 3] as compared with normal spermatogenesis in the control group [Figure 1]. Thus, our results were similar to a study that showed that 30 days of treatment with sodium fluoride (10 mg/kg body weight) to mice resulted in sloughing off of the spermatogenic cells in the luminal regions of the seminiferous tubules of the testis, leading to disorganization of their epithelium, which caused a complete absence of spermatogenesis in the testis.[18] Also, direct injection of sodium fluoride (50 µg/50 µl) into the vas deferens resulted in spermatogenic arrest, the absence of spermatozoa in the seminiferous tubules, a decreased sperm count in the cauda epididymis and a decrease in fertility,[22] while Sprando et al., showed that rats fed on a 250 ppm fluoride for 10 weeks showed no distinguishable change in testicular histology from their control group.[1] This might be due to a lower level of fluoride. Susheela et al., in their study on rabbits found that there was disruption of spermatogenic cells in the seminiferous tubules that were degenerated and devoid of spermatozoa. In animals treated for 18 or 29 months, loss of cilia on the epithelial cells lining the lumen of the ductuli efferentes of the caput epididymidis and of the stereocilia on the epithelial cells lining the lumen of the vas deferens was also observed. Similarly, in our study, there was a lack of spermatogenesis, as evident on testicular histology.[10]
CONCLUSION
Fluoride definitely has adverse effects on the reproductive functions of male rabbits, and these effects are more pronounced with the increasing duration of exposure.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared
REFERENCES
- 1.Sprando RL, Collins TF, Black T, Olejnik N, Rorie J. Testing the potential of sodium fluoride to affect spermatogenesis: A morphometric study. Food Chem Toxicol. 1998;36:1117–24. doi: 10.1016/s0278-6915(98)00085-4. [DOI] [PubMed] [Google Scholar]
- 2.Susheela AK. Treatise on Fluorosis. 3rd ed. Delhi: Published by Fluorosis Research and Rural Development Foundation; 2006. pp. 1–119. [Google Scholar]
- 3.Messer HH, Armstrong WD, Singer L. Fertility impairment in mice on a low fluoride intake. Science. 1972;177:893–4. doi: 10.1126/science.177.4052.893. [DOI] [PubMed] [Google Scholar]
- 4.Chinoy NJ, Sequeira E. Effects of fluoride on the histoarchitecture of reproductive organs of the male mouse. Reprod Toxicol. 1989;3:261–7. doi: 10.1016/0890-6238(89)90020-8. [DOI] [PubMed] [Google Scholar]
- 5.Tao S, Suttie JW. Evidence for a lack of an effect of dietary fluoride level on reproduction in mice. J Nutr. 1976;106:1115–22. doi: 10.1093/jn/106.8.1115. [DOI] [PubMed] [Google Scholar]
- 6.Chinoy NJ, Sequeira E, Narayana MV. Effects of vitamin C and calcium on the reversibility of fluoride induced alterations in spermatozoa of rabbit. Fluoride. 1991;24:29–39. [Google Scholar]
- 7.Saalu LC, Oluyemi KA, Omotuyi IO. Tocopherol (vitamin E) attenuates the testicular toxicity associated with experimental cryptorchidism in rats. Afr J Biotechnol. 2007;6:1373–7. [Google Scholar]
- 8.Chinoy NF, Narayana MV, Dalal V, Rawat M, Patel D. Amelioration of fluoride toxicity in some accessory reproductive glands and spermatozoa of rat. Fluoride. 1995;28:75–86. [Google Scholar]
- 9.Chinoy NJ, Sharma A. Amelioration of fluoride toxicity by vitamins E and D in reproductive functions of male mice. Fluoride. 1998;31:203–16. [Google Scholar]
- 10.Susheela AK, Kumar A. A study of the effect of high concentration of fluoride on the reproductive organs of male rabbit, using light and scanning microscopy. J Reprod Fertil. 1991;92:353–60. doi: 10.1530/jrf.0.0920353. [DOI] [PubMed] [Google Scholar]
- 11.Huang C, Yang H, Niu R, Sun Z, Wang J. Effect of sodium fluoride on androgen receptor expression in male mice. Fluoride. 2008;41:10–7. [Google Scholar]
- 12.Wan S, Zhang J, Wang J. Fluoride-induced changes in the expression of epidermal growth factor and its receptor in testicular tissues of young male rats. Fluoride. 2006;39:121–5. [Google Scholar]
- 13.Huang C, Niu R, Wang J. Toxic effects of sodium fluoride on reproductive function in male mice. Fluoride. 2007;40:162–8. [Google Scholar]
- 14.Narayana MV, Chinoy NJ. Reversible effects of sodium fluoride ingestion on spermatozoa of the rat. Int J Fertil Menopausal Stud. 1994;39:337–46. [PubMed] [Google Scholar]
- 15.Narayana MV, Chinoy NJ. Effect of fluoride on rat testicular steroidogenesis. Fluoride. 1994;27:7–12. [Google Scholar]
- 16.Bataineh HN, Nusier M. Impact of 12-week ingestion of sodium fluoride on aggression, sexual behavior and fertility in adult male rats. Fluoride. 2006;39:293–301. [Google Scholar]
- 17.Zakrzewska H, Udala J, Blaszczyk B. In vitro influence of sodium fluoride on ram semen quality and enzyme activities. Fluoride. 2002;35:153–60. [Google Scholar]
- 18.Chinoy NJ, Shukla S, Walimbe AS, Bhattacharya S. Fluoride toxicity on rat testis and cauda epididymal tissue components and its reversal. Fluoride. 1997;30:41–50. [Google Scholar]
- 19.Kumar A, Susheela AK. Ultrastructural studies of spermiogenesis in rabbit exposed to chronic fluoride toxicity. Int J Fertil Menopausal Stud. 1994;39:164–71. [PubMed] [Google Scholar]
- 20.Kumar A, Susheela AK. Effects of chronic fluoride toxicity on the morphology of ductus epididymis and the maturation of spermatozoa of rabbit. Int J Exp Pathol. 1995;76:1–11. [PMC free article] [PubMed] [Google Scholar]
- 21.Ghosh D, Das S, Sarkar S, Maiti R, Jana D, Das UB. Testicular toxicity in sodium fluoride treated rats: Association with oxidative stress. Reprod Toxicol. 2002;16:385–90. doi: 10.1016/s0890-6238(02)00038-2. [DOI] [PubMed] [Google Scholar]
- 22.Chinoy NJ, Rao MV, Narayana MV, Neelakanta E. Microdose vasal injection of sodium fluoride in the rat. Reprod Toxicol. 1991;5:505–12. doi: 10.1016/0890-6238(91)90022-8. [DOI] [PubMed] [Google Scholar]


