Abstract
Background
This study examined gender differences in collagen regulation during rodent experimental abdominal aortic aneurysm (AAA) formation.
Methods
Infrarenal aortas of male and female rats were perfused with elastase or saline (control). Aortic diameters were measured at baseline (day 0), and on post-operative days 7 and 14. TGF-β1, collagen subtypes I and III, and MMP-13 (collagenase) expression and/or protein levels from aortic tissue were determined by RT-PCR and Western Blotting. Aortic tissue was stained for total collagen, neutrophils, and macrophages using immunohistochemistry on days 4 and 7.
Results
Seven and 14 days following perfusion, aortic diameter increased significantly in elastase-perfused males compared with females (P<0.001 for each). Four and 7 days post-perfusion, significantly more neutrophils and macrophages were present in elastase-perfused males compared with females. Seven days post-perfusion, protein levels of TGF-β1 were lower in males compared with females (P=0.04). Type I collagen levels also decreased on days 7 (P<0.001) and 14 (P=0.002), and type III collagen levels decreased on days 7 (P<0.001) and 14 (P<0.001), in males compared with females. By Masson's Trichrome, less adventitial collagen was present in the elastase-perfused males compared with females. MMP-13 expression (P<0.001) and protein levels (P=0.006) in elastase-perfused males were higher than females on day 14.
Conclusions
This study documents a decrease in types I and III collagen with a concurrent increase in MMP-13 following elastase perfusion in males compared with females. These data suggest that alterations in extracellular matrix collagen turnover may be responsible for altered AAA formation between genders.
Keywords: Aorta, aneurysm, collagen, estrogen, MMP-13, gender differences
Introduction
Abdominal aortic aneurysms (AAAs) are the 10th leading cause of death of men in the U.S. and the 14th leading cause of overall death in the US (1). Uncontrollable risk factors include age (risk increases with increasing age) and gender (2, 3), with males developing AAAs four times more often than females (4, 5). In contrast, women have a higher risk of rupture and increased morbidity following elective AAA repair (6). The mechanical and molecular mechanisms behind this observation are unknown.
Although the pathogenesis of AAAs is not entirely understood, a few distinguishing characteristics of AAAs include: significant inflammation; complex atherosclerosis; and changes in the levels, production, and degradation of extracellular matrix (ECM) proteins. It is clear that aortic elastin concentration is markedly reduced with a loss of elastic lamellae in AAAs (7, 8, 9). However, the concentration of collagen in AAAs may be decreased, unchanged, or increased, compared with normal aortic tissue (7, 10, 11).
Dobrin et al. demonstrated that treating explanted arteries in vitro, from various species, with collagenase resulted in artery rupture. In contrast, treating explanted arteries with elastase resulted in varying degrees of arterial dilation (12). In addition, a rodent model in which the infrarenal aorta is perfused with porcine pancreatic elastase, leading to infrarenal aortic aneurysms has been developed (13). In this model, there is an initial influx of inflammatory cells which degrade the aortic ECM and subsequently leads to aneurysm formation over seven to 14 days.
Much of the recent research on AAA pathogenesis has focused on matrix degrading enzymes, rather than the substrate ECM, believing the ECM to be a passive substrate. The present study examines gender differences in matrix properties that may provide insight into the higher prevalence of AAAs in males compared with females, and may allow for a better understanding of the role collagen plays during experimental AAA formation.
Materials and Methods
Adult male and female Sprague Dawley rats, weighing 190–210 grams were obtained from Charles River Laboratories (Wilmington, MA). Surgical procedures and experiments were approved by the University of Michigan Universal Committee on the Use and Care of Animals, per Protocol #8833.
Rodent Elastase Perfusion AAA Model
Adult male and female Sprague Dawley rats (n = 6 per group) were anesthetized with 2–3% isoflurane with 100% oxygen. Using sterile techniques, a ventral midline incision was made, and the infrarenal abdominal aorta was isolated. Briefly, the aorta was exposed from the iliac bifurcation (distal) to the level of the left renal vein (proximal), and all side branches within the exposed length of the aorta were tied off with 7-0 silk suture (Ethicon Inc., Somerville, NJ) to ensure pressurization of the aorta. Using 4-0 cotton suture (Genzyme Corp., Fall River, MA) and a hyfits clip, temporary distal and proximal aortic control was obtained. A 30 gauge needle was used to make an aortotomy just proximal of the iliac bifurcation. A polyethylene-10 (PE-10) tube was inserted through the aortotomy, and either saline (control) or pancreatic porcine elastase (6 units/mL; Lot Number 083K7655; Sigma, St. Louis, MO) was infused into the isolated infrarenal aorta for 30 minutes as previously described (13). Following perfusion, a 10-0 Nylon Monofilament Suture (Surgical Specialties Corp., Reading, PA) was used to repair the aortotomy in the aorta.
Using video micrometry, aortic diameters (proximal, mid, and distal) were measured pre- and post-perfusion. Images were taken using a Spot Insight Color Optical Camera (Diagnostic Instruments, Sterling Heights, MI) attached to the operating microscope (Nikon, Melville, NY), and aortic diameters were measured using Image Pro Express Software (Media Cybernetics Inc., Silver Spring, MD). 3-0 Vicryl Suture (Ethicon Inc., Somerville, NJ) was used to close the abdomen and the rats were allowed to recover. Seven or 14 days post-perfusion, rats were anesthetized, infrarenal aortas were exposed, and the aortic diameters were measured prior to aortic explantation. The aortas were then harvested for further analyses.
Messenger RNA (mRNA) Extraction and Reverse Transcription
Established TRIzol reagent extraction techniques were used to extract mRNA for real time reverse transcription polymerase chain reaction (RT-PCR). Briefly, TRIzol Reagent with glycogen (5 mg/mL) was added to explanted aortic tissue, and the tissue homogenized. Chloroform (+99%) was then added to the homogenized tissue, vortexed, centrifuged, and the supernatant washed with isopropanol (+99%). The resulting solution was centrifuged and the mRNA pellet resuspended in distilled water. The mRNA was then transcribed into complementary DNA (cDNA) using standard reagents in a GeneAmp 2400 PCR System.
RT-PCR
mRNA expression of collagen I, collagen III, and MMP-13 (collagenase) was compared with that of β-actin, a housekeeping gene. RT-PCR primers for these genes were designed using NCBI (www.ncbi.nih.gov/) to obtain the appropriate mRNA reference sequence and Primer Premier Software to search for sense and anti-sense primer sequences. Alternatively, previously designed primers were also used (14, 15, 16, 17). SYBR Green I, an intercalating dye which binds to all double-stranded DNA, was used to detect and quantify RT-PCR product. The Cepheid Smart Cycler System was used to amplify the target DNA, and obtain SYBR Green and melt curves for analyses.
Collagen Extraction
Using modified methods of Keeley et al., collagen was extracted from aortic tissue (18). Briefly, aortic tissue was minced and defatted in a chloroform-methanol solution at 20°C for 16 hours, and washed in acetone. The tissue was then homogenized in a 0.5 M sodium chloride (NaCl) solution and stirred in the NaCl solution at 4°C for 16 hours, during which time the salt-soluble proteins were extracted out of the tissue. The remaining pellet was washed with a 0.5 M acetic acid solution and stirred in the acetic acid solution at 4°C for 16 hours, during which time the acid-soluble proteins were extracted out of the pellet. The pellet was stirred in a 2.0 M magnesium chloride (MgCl2) solution made in 0.05 M sodium acetate buffer at 4°C for 16 hours, centrifuged, and the resulting pellet washed in a 0.5 M acetic acid solution. Next, the pellet was stirred in a pepsin solution (1 mg pepsin per 1 mL 0.5 M acetic acid solution) at 4°C for 72 hours, and centrifuged. The collagen was precipitated from the supernatant by adding solid NaCl, redissolved in a 0.1 M acetic acid solution, and the resulting pepsin-soluble extract stored at −20°C.
Western Blotting
Western Blotting was performed as described by Eagleton et al. (19). Transforming growth factor beta 1 (TGF-β1), collagen types I and III, and MMP-13 levels were quantified via Western Blotting (max optical density (OD)/μg protein) from normalized protein samples, using the bicinchoninic acid assay. Samples were separated on standard sodium dodecyl sulfate polyacrylamide gel electrophoresis (SDS-PAGE) gels, and proteins were transferred to a nitrocellulose membrane (Bio-Rad Laboratories, Hercules, CA). The membrane was blocked with 5% dry milk made in 1× Tween-Tris Buffered Saline (T-TBS), incubated with the appropriate primary antibody, followed by incubation with a secondary antibody that binds to the primary antibody. Primary antibodies for rat TGF-β1 1:2000 (Cell Signaling, Danvers, MA), type I collagen 1:5000 (Biodesign Intl., Saco, ME), type III collagen 1:5000 (Santa Cruz Biotechnology, Inc., Santa Cruz, CA), and MMP-13 1:5000 (Neomarkers, Fremont, CA); and anti-rabbit and anti-goat secondary antibodies (Calbiochem, La Jolla, CA) were used for the Western Blotting. Protein bands were visualized using an ECL chemiluminescence detection kit.
Histology
Aortic tissue was fixed in 4% paraformaldehyde for 20 hours, transferred to and stored in 70% ethanol, and subsequently embedded in paraffin for immunohistochemistry. The sections were stained for total collagen with Masson's Trichrome Staining.
For neutrophil staining, aortic sections were deparaffinized in xylene and rehydrated in graded alcohols. Blocking buffer was applied to prevent non-specific binding. A rabbit anti rat neutrophil primary antibody 1:250 (Accurate Chemical and Scientific Corporation, Westbury, NY) was used for staining; followed by an anti-rabbit immunoglobulin G (IgG) biotinylated secondary antibody and avidin-biotin complex-AP reagent available in the Rabbit IgG Alkaline Phosphatase ABC Kit (Vector Laboratories, Burlingame, CA). Sections were then visualized using the Vector Red Alkaline Phosphatase Substrate Kit I (Vector Laboratories) and counterstained with Hematoxylin QS (Vector Laboratories). Neutrophils were quantified and are presented as “average cells per high power field (hpf).”
For macrophage staining, aortic sections were deparaffinized in xylene and rehydrated in graded alcohols. Heat induced epitope retrieval was performed using 10 mM sodium citrate, pH 6.0, in a microwave. The sections were then incubated with 3% hydrogen peroxide in methanol to block endogenous peroxidase activity, followed by a blocking buffer to prevent non-specific binding. A mouse anti rat CD68 primary antibody 1:100 (Serotec, Raleigh, NC) was used for staining; followed by an anti mouse IgG biotinylated secondary antibody and an avidin and biotinylated horseradish peroxidase (avidin-biotin-HRP) complex, available in the Mouse IgG Elite Vectastain ABC Kit (Vector Laboratories). Sections were then visualized using a DAB Peroxidase Substrate Kit (Vector Laboratories) and counterstained with Hematoxylin QS (Vector Laboratories). Macrophages were quantified and are presented as “average cells per hpf.”
Densitometry
Western Blots were imaged with a FOTO/Analyst charge-coupled device (CCD) Camera (Fotodyne, Hartland, WI) and the optical densities of the bands measured with GEL-Pro Analyzer, Software Version 3.1 (Media Cybernetics, Silver Springs, MD).
Total Protein Assay
Total protein levels (μg protein/mL) were quantified using the Bicinchoninic Acid Assay Kit (Pierce, Rockford, IL). This data was used to normalize between protein samples for Western Blotting.
Data Analysis
Statistical analyses were performed using Sigma Stat 2.03 (Systat Software Inc., Point Richmond, CA). Quantitative results were analyzed by unpaired two-tailed Student's t-test and two-way ANOVA with Bonferroni post tests, where a P value less than 0.05 was considered statistically significant.
Results
No Differences in Aortic Diameter, Collagen and MMP-13 Levels in Baseline Males versus Females
There were no significant differences in pre-perfusion aortic diameter between baseline male and female rats. There were no differences in expression and levels of types I collagen and III collagen, and MMP-13 by RT-PCR and Western Blotting, respectively, in baseline male and female aortas. There were also no differences in total collagen by Masson's Trichrome Staining (data not shown).
Males Develop Larger AAAs Compared with Females
Experimental AAA Formation
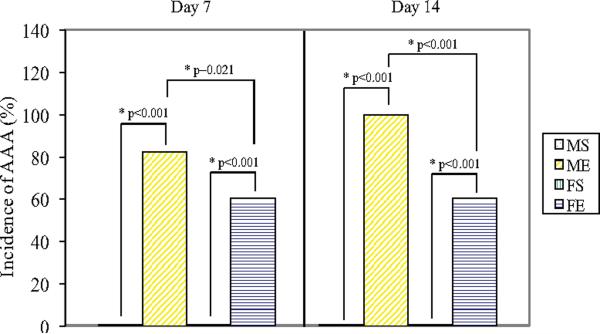
Mean percent increase in aortic diameter in male aortas was significantly greater than females 7 days (males: 178±23% versus females: 107±7%, P<0.001, Figure 1A) and 14 days (males: 299±32% versus females: 117±18%, P<0.001) following elastase perfusion. There was also a higher incidence of AAAs, defined as an increase in aortic diameter of 100% or greater, in males compared with females 7 and 14 days following elastase perfusion (P=0.021 and P<0.001, respectively, Figure 1B). No AAAs occurred in male or female saline controls.
Figure 1.
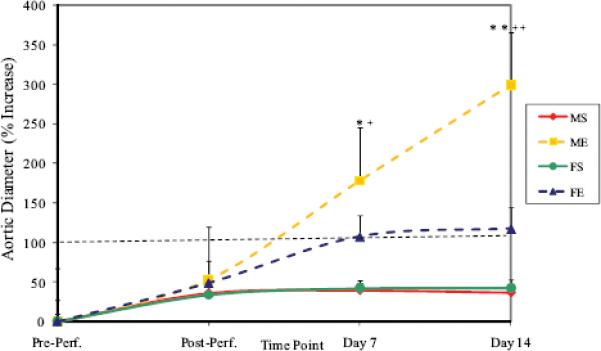
(A) Percent increase in aortic diameter in male and female aortas 7 and 14 days following perfusion. Elastase-perfused male aortas had significantly larger AAAs compared with saline control male aortas on Days 7 and 14 (*P=0.005, **P<0.001, respectively). Elastase-perfused male aortas had significantly larger AAAs compared with elastase-perfused female aortas on Days 7 and 14 (+P<0.001, ++P<0.001, respectively). M=Male, F=Female, S=Saline, E=Elastase. (B) Incidence of AAAs 7 and 14 days following perfusion. Males had significantly higher incidence of AAAs (defined as 100% increase in diameter) compared with females on Days 7 and 14 (P=0.021 and P<0.001, respectively). There were no AAAs in saline controls in either gender. M=Male, F=Female, S=Saline, E=Elastase.
Neutrophil and Macrophage Staining
Since inflammation peaks in the model in the aortic wall between days 4 and 7 (20), neutrophils and macrophages were quantified 4 and 7 days post-perfusion. Four days post-perfusion, significantly more neutrophils were present in elastase-perfused males and females compared with saline controls (P<0.001 and P<0.001, respectively, Table 1A). On post-perfusion day 4, significantly more neutrophils were present in elastase-perfused males compared with elastase-perfused females (P=0.031). Seven days post-perfusion, significantly more neutrophils were present in elastaseperfused males and females compared with saline controls (P<0.001 and P=0.011, respectively, Table 1B). On post-perfusion day 7, significantly more neutrophils were present in elastase-perfused males compared with elastase-perfused females (P=0.005). Likewise, there were significantly more macrophages in elastase-perfused males compared with saline controls on days 4 and 7 post-perfusion (P=0.014 and P<0.001, respectively, Table 1A, Table 1B). Similarly, significantly more macrophages were present in males compared with females 4 and 7 days following elastase perfusion (P=0.049 and P=0.002, respectively). Representative images of neutrophil and macrophage stained sections 4 days post-perfusion are shown in Figures 2 and 3, respectively. Fourteen days post-perfusion, there were few neutrophils and macrophages present in all groups (data not shown).
Table 1A.
Average neutrophils and macrophages per hpf, 4 days post-perfusion.
| Group | Average Neutrophils (hpf ± SEM)†‡ | Average Macrophages (hpf ± SEM)†‡ |
|---|---|---|
| Male Saline | 1±0 | 2±1 |
| Male Elastase | 14±5* | 12±4* |
| Female Saline | 0±0 | 1±0 |
| Female Elastase | 5±1* | 5±1* |
P values < 0.05 for elastase-perfused males compared with elastase-perfused females. See text for details.
High power field (hpf)
Standard error of the mean (SEM)
Table 1B.
Average neutrophils and macrophages per hpf, 7 days post-perfusion.
| Group | Average Neutrophils (hpf ± SEM) | Average Macrophages (hpf ± SEM) |
|---|---|---|
| Male Saline | 0±0 | 1±0 |
| Male Elastase | 41±5* | 18±1* |
| Female Saline | 0±0 | 0±0 |
| Female Elastase | 19±6* | 12±1* |
P values < 0.05 for elastase-perfused males compared with elastase-perfused females. See text for details.
† High power field (hpf)
‡ Standard error of the mean (SEM)
Figure 2.
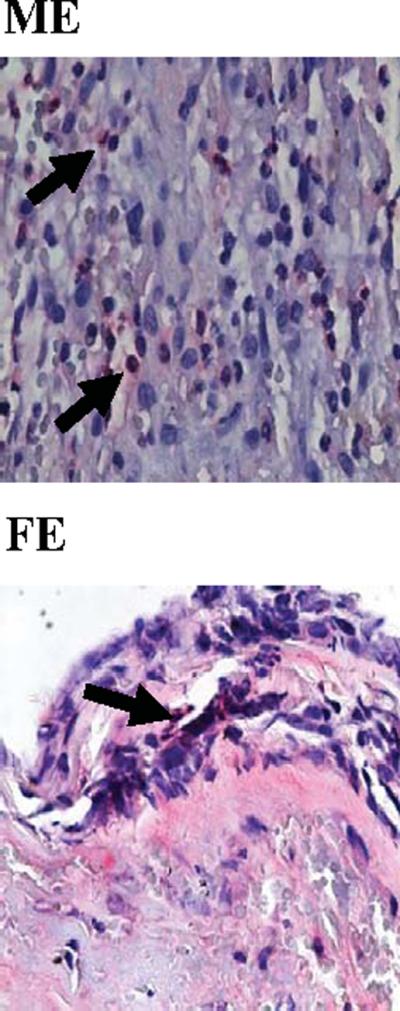
Representative neutrophil stained aortic sections, 4 days following elastase perfusion. 80×. M=Male, F=Female, E=Elastase.
Figure 3.
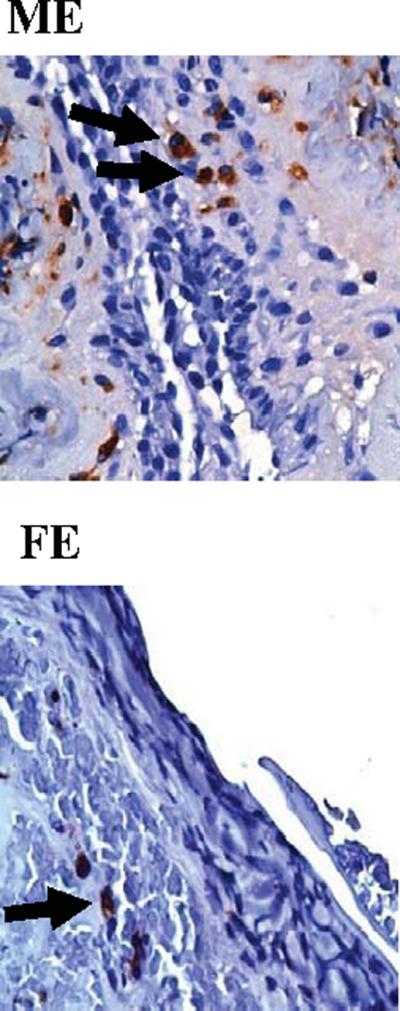
Representative macrophage stained aortic sections, 4 days following elastase perfusion. 80×. M=Male, F=Female, E=Elastase.
TGF-β1 Protein Levels
Seven days post-perfusion, TGF-β1 protein levels were significantly lower in elastase-perfused male aortas compared with females 7 days following perfusion (P=0.040, Figure 4).
Figure 4.
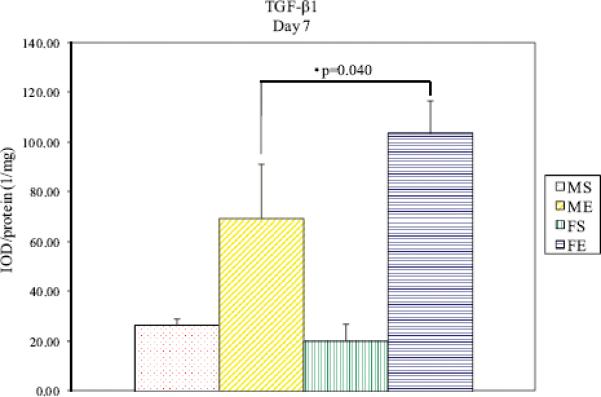
TGF-β1 protein levels in male and female aortas 7 days following perfusion. Elastase-perfused male aortas had significantly lower TGF-β1 levels compared with elastase-perfused female aortas on Day 7 (P=0.040).
Type I, III Collagen Protein Levels
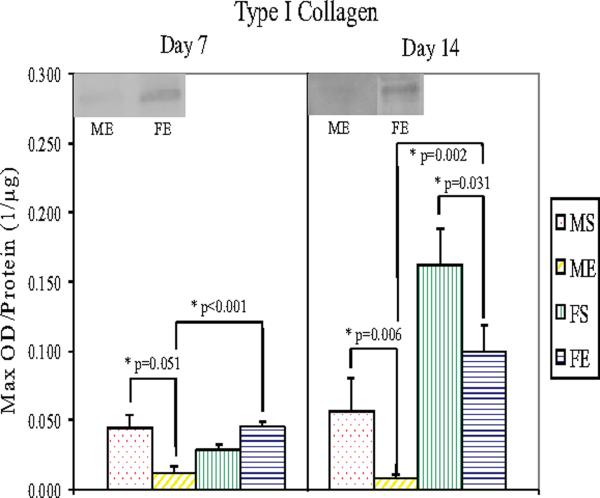
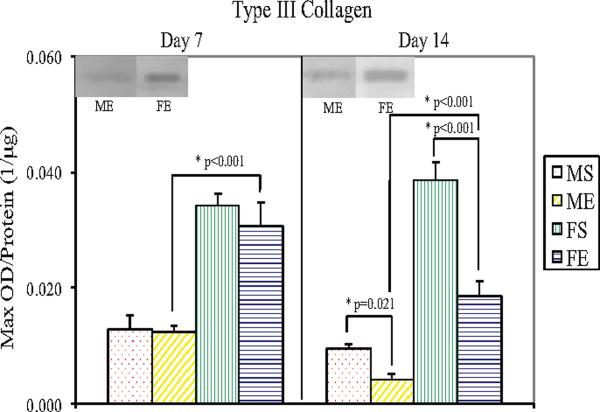
Western Blotting showed significantly lower type I collagen levels in male (P=0.006) and female (P=0.031) elastase-perfused aortas compared with saline controls 14 days following perfusion (Figure 5). Seven and 14 days following elastase perfusion, type I collagen levels decreased 72% (P<0.001) and 91% (P=0.002), respectively, in male aortas compared with female aortas. Type III collagen levels were significantly lower in male (P=0.021) and female (P<0.001) elastase-perfused aortas compared with saline controls 14 days following perfusion (Figure 6). Seven and 14 days following elastase perfusion, type III collagen levels decreased 58% (P<0.001) and 77% (P<0.001), respectively, in male aortas compared with female aortas.
Figure 5.
Type I Collagen protein levels in male and female aortas 7 and 14 days following perfusion. Elastase-perfused male aortas had significantly lower collagen I levels compared with saline control male aortas on Days 7 and 14 (P=0.051 and P=0.006, respectively). On Day 14, elastase-perfused female aortas had significantly lower collagen I levels compared with saline control female aortas (P=0.031). Collagen I levels in elastase-perfused male aortas were significantly lower than in elastase-perfused female aortas on Days 7 and 14 (P<0.001 and P=0.002, respectively).
Figure 6.
Type III Collagen protein levels in male and female aortas 7 and 14 days following perfusion. Elastase-perfused male aortas had significantly lower collagen III levels compared with saline control male aortas on Day 14 (P=0.021). On Day 14, elastase-perfused female aortas had significantly lower collagen III levels compared with saline control female aortas (P<0.001). Collagen III levels in elastase-perfused male aortas were significantly lower than in elastase-perfused female aortas on Days 7 and 14 (P<0.001 for each day).
Masson's Trichrome Collagen Staining
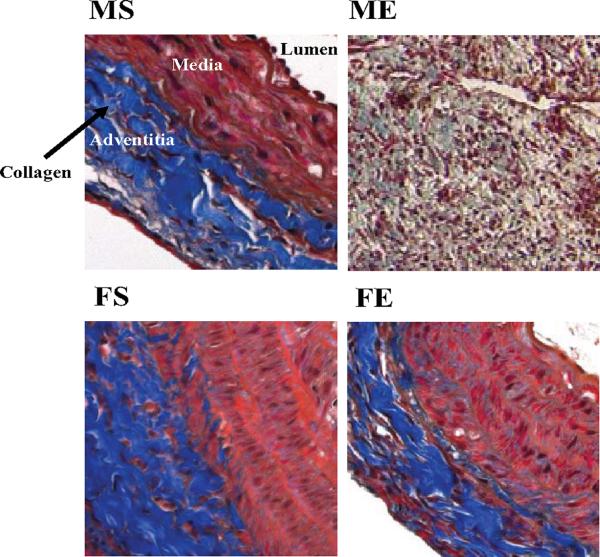
By immunohistochemistry, total collagen in the adventitia was shown to decrease in the elastase-perfused male aortas compared with elastase-perfused female aortas 7 and 14 days following perfusion. Representative Masson's Trichrome stained aortic sections 7 days following perfusion are shown in Figure 7.
Figure 7.
Representative Masson's Trichrome stained aortic sections - 7 days following perfusion - showed decreased collagen in males compared with females. 20×. Collagen is shown in blue; non-collagen proteins are shown in pink and red.
MMP-13 Gene Expression and Protein Levels
There were no differences in MMP-13 gene expression on day 7 post-perfusion. However, by day 14, elastase-perfused males had significantly higher MMP-13 expression compared with elastase-perfused females following perfusion (males: 0.530±0.114 versus females: 0.148±0.0430, P<0.001, Figure 8A). Fourteen days post-perfusion, relative expression of MMP-13 to β-actin was significantly higher in elastase-perfused male aortas compared with male saline control aortas (male elastase: 0.530±0.114 versus male saline: 0.0874±0.0546, P<0.001). Western Blotting reflected elastase-perfused male aortas had significantly higher MMP-13 protein levels compared with elastase-perfused females 14 days following perfusion (P=0.006, Figure 8B).
Figure 8.
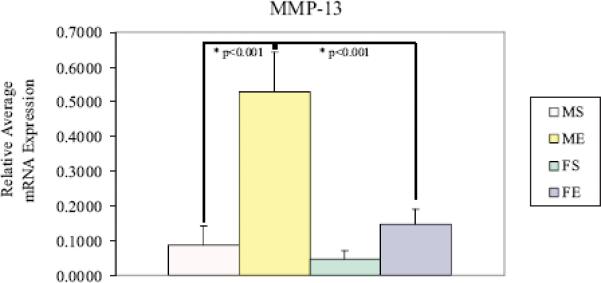
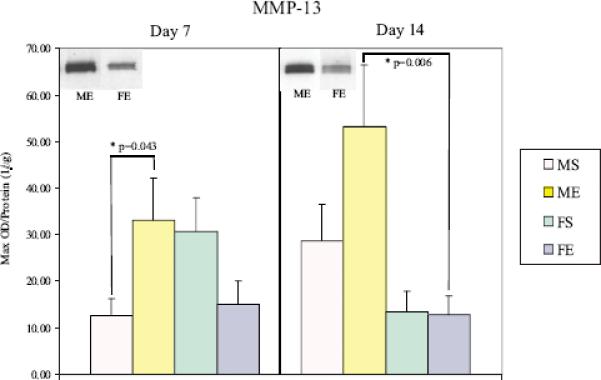
(A) MMP-13 mRNA expression in male and female aortas 14 days following perfusion. Elastase-perfused male aortas had significantly higher MMP-13 expression compared with saline controls (P<0.001). MMP-13 expression in elastase-perfused male aortas was significantly higher than in elastaseperfused female aortas (P<0.001). (B) MMP-13 protein levels in male and female aortas 7 and 14 days following perfusion. Elastase-perfused male aortas had significantly higher MMP-13 levels compared with saline controls on Day 7 (P=0.043). MMP-13 levels in elastase-perfused male aortas were significantly higher than in elastase-perfused female aortas on Day 14 (P=0.006).
Discussion
The current study confirms that male rats form larger experimental AAAs, and do so more frequently than female rats. This gender disparity in AAA formation is associated with an increase in leukocyte infiltration that precedes a decrease in TGF-β1, and collagen types I and III. These changes in the aortic wall occur in conjunction with an increase in MMP-13 in males.
Although the role of gender in AAA development has not been studied in detail, some data suggests that estrogen may be protective during AAA formation (21). In a previous study, Ailawadi et al. reported an estrogen-mediated reduction in macrophage secreted MMP-9 in female rats (4). It is also known that estrogen influences remodeling of elastin and collagen (22), and that type I collagen levels decreased in ovariectomized rats, but returned to baseline levels following treatment with estrogen (23). Our laboratory has previously conducted cross-over experiments in which we documented that male rats treated with estrogen have decreased AAA size, secondary to macrophage dependent MMP-9 down regulation (24). A study by Fischer showed that estradiol increases collagen and elastin degradation in rat aorta, resulting in a higher elastin to collagen ratio, thus decreasing stiffness of the vessel wall (25). This finding supports the well-established view and data that aneurysmal aortas are stiffer than normal aortas, and may provide a partial explanation for the role of estradiol in protecting the distensible female aorta from AAA formation.
While estrogen plays a protective role in some cardiovascular diseases (26), excessive testosterone may increase the risk of cardiovascular disease (27). In the angiotensin II rodent model of AAAs, androgens, rather than estrogen, seemed to mediate a higher incidence and severity of aneurysms as demonstrated by a reduction of AAA incidence in orchiectomized males compared with females (28).
As most recent studies have focused on the enzymes involved in degrading ECM proteins, it is now accepted that vessel wall remodeling is an active process involving the substrate (e.g. collagen), as well as the proteases degrading the substrate. Collagen types I and III are the primary structural proteins of the aorta (11), and primarily provide tensile strength. Collagen is composed of three intertwined helical strands and provides tensile strength. Unlike elastin, which is mostly produced during embryonic development and postnatal years, collagen continues to turnover throughout adult years (35, 36). Collagen degradation is known to result in a decrease in the tensile strength of the aorta, thereby weakening the aortic wall and eventually AAA formation (12, 30).
Numerous groups have examined the possible regulation of collagen by hormones in vivo and in vitro (31), but there is not complete agreement on the effects of estradiol. Previously, estradiol was shown to increase collagen in the uterus and other tissues (32). Estrogen also inhibits collagen destruction in the involuting rat uterus (33). Increased degradation of collagen in the presence of estradiol has been documented in atherosclerosis (34). Similar findings were reported in hypertension studies in which estradiol prevented or decreased collagen accumulation in rat aortas (35, 36), and surgical and hormonal gender manipulation experiments in which increases in collagen and elastin were associated with decreases in estrogen and possibly testosterone (37). Hormones may also affect collagen synthesis by becoming involved in vessel wall remodeling in response to injury (38).
The mechanism(s) of estrogen protection in AAA formation is multifaceted; the following may contribute to its protective function. Estradiol increases vasodilation of the aorta via the nitric oxide synthase (NOS) pathway. This mechanism involves estrogen activating endothelial NOS (eNOS) by binding to estrogen receptor (ER) alpha in the endothelium, releasing NO and causing vasodilation. When activated (ER alpha in the endothelium and ER beta in the media), estrogen can change the expression of genes, such as eNOS and collagen, in vascular cells. Thus, estrogen increases vasodilation and inhibits the response of blood vessels to injury (39).
ER alpha and ER beta are potential mediators of estrogen action on inflammatory cells (40). Because of the presence of ER beta in the media, estrogen may be binding, thereby inhibiting proliferation of smooth muscle cells (SMCs), which are a source of cytokines and chemokines which attract inflammatory cells into the media (41). In the case of AAAs, estrogen may be inhibiting inflammatory cell recruitment and SMC production of MMPs, enzymes that destroy the aortic wall, and thereby limit the replacement of collagen by non-collagenous matrix proteins, contributing to disease progression.
Additionally, TGF-β1 protein levels were significantly lower in elastase-perfused male aortas compared with females 7 days following perfusion. Decreased TGF-β1 protein levels in males result in upregulation of inflammation, a decrease in collagen production, an increase in MMP-13, and an increase in incidence and size of AAAs. To compensate for the decreased, relatively low, TGF-β1 protein levels in males, we speculate that there is an increase in TGF-β1 expression in males compared with females (42). Xu et al. reported that enlargement of the abdominal aorta in a rat aortocaval fistula model was preceded by an increase in TGF-β1 expression (43).
An increase in TGF-β1 expression increased collagen synthesis in vascular SMCs (44), and reduced proteolytic degradation of matrix proteins (45), which is not consistent with the present finding that collagen levels are decreased and MMP-13 levels are higher in elastase-perfused males compared with females. In a recent study by Dai et al., overexpression of TGF-β1 in already-formed rodent AAAs resulted in aortic wall reconstruction (46). Others have reported similar findings, namely that TGF-β1 induces gene expression of collagen (47) and elastin (48), decreases inflammation, promotes vascular SMC growth (48, 49, 50), and inhibits MMP-dependent proteolysis (51).
Limitations exist in the current study. First, since the experiments were conducted primarily in rats, knockout animals were not available. Second, only the balance between collagen production and degradation was explored. Since the pathophysiology of AAAs is a complex process, multiple other mechanisms are most likely involved and could also play a role.
The present study verifies that male rats form larger experimental AAAs compared with females, and do so more frequently. This gender disparity in AAA formation is associated with an increase in leukocyte infiltration that precedes a decrease in TGF-β1 protein levels, decreases in collagen types I and III, and an increase in MMP-13. Documenting shifts in the dynamic enzymatic balance between vessel wall repair and destruction is critical in helping to better understand the complex pathophysiology that occurs in AAA formation.
Acknowledgments
Supported by: NIH KO8 (HL67885-02) (GRU), VonLeibig Award- Lifeline Foundation (GRU), NIH Cellular Biotchnology Training Program (GM008353) (BSC), Surgery Research Advisory Committee Grant (BSC), and the Jobst Foundation.
Abbreviations appearing in text (listed alphabetically)
- (AAA)
Abdominal aortic aneurysm
- (avidin-biotin-HRP)
Avidin and biotinylated horseradish peroxidase
- (cDNA)
Complementary DNA
- (eNOS)
Endothelial NOS
- (ER)
Estrogen receptor
- (ECM)
Extracellular matrix
- (hpf)
High power field
- (IgG)
Immunoglobulin G
- (MgCl2)
Magnesium chloride
- (MMP)
Matrix metalloproteinase
- (MMP-13, collagenase)
Matrix metalloproteinase 13
- (mRNA)
Messenger ribonucleic acid
- (NOS)
Nitric oxide synthase
- (RT-PCR)
Real time or Reverse transcription polymerase chain reaction
- (SMC)
Smooth muscle cell
- (NaCl)
Sodium chloride
- (SDS-PAGE)
Sodium dodecyl sulfate polyacrylamide gel electrophoresis
- (SEM)
Standard error of the mean
- (TGF-β1)
Transforming growth factor beta 1
- (T-TBS)
Tween-Tris Buffered Saline
References
- 1.Ailawadi G, Eliason JL, Upchurch GR., Jr Current concepts in the pathogenesis of abdominal aortic aneurysm. J Vasc Surg. 2003;38:584–8. doi: 10.1016/s0741-5214(03)00324-0. [DOI] [PubMed] [Google Scholar]
- 2.Vemuri C, Wainess RM, Dimick JB, Cowan JA, Jr, Henke PK, Stanley JC, et al. Effect of increasing patient age on complication rates following intact abdominal aortic aneurysm repair in the United States. J Surg Res. 2004;118:26–31. doi: 10.1016/j.jss.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 3.Ailawadi G, Eliason JL, Roelofs KJ, Sinha I, Hannawa KK, Kaldjian EP, et al. Gender differences in experimental aortic aneurysm formation. Arterioscler Thromb Vasc Biol. 2004;24:2116–22. doi: 10.1161/01.ATV.0000143386.26399.84. [DOI] [PubMed] [Google Scholar]
- 4.Singh K, Bonaa KH, Jacobsen BK, Bjork L, Solberg S. Prevalence of and risk factors for abdominal aortic aneurysms in a population-based study: The Tromso Study. Am J Epidemiol. 2001;154:236–44. doi: 10.1093/aje/154.3.236. [DOI] [PubMed] [Google Scholar]
- 5.Katz DJ, Stanley JC, Zelenock GB. Gender differences in abdominal aortic aneurysm prevalence, treatment, and outcome. J Vasc Surg. 1997;25:561–8. doi: 10.1016/s0741-5214(97)70268-4. [DOI] [PubMed] [Google Scholar]
- 6.Dimick JB, Stanley JC, Axelrod DA, Kazmers A, Henke PK, Jacobs LA, et al. Variation in death rate after abdominal aortic aneurysmectomy in the United States: impact of hospital volume, gender, and age. Ann Surg. 2002;235:579–85. doi: 10.1097/00000658-200204000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sumner DS, Hokanson DE, Strandness DE., Jr Stress-strain characteristics and collagen-elastin content of abdominal aortic aneurysms. Surg Gynecol Obstet. 1970;130:459–66. [PubMed] [Google Scholar]
- 8.White JV, Haas K, Phillips S, Comerota AJ. Adventitial elastolysis is a primary event in aneurysm formation. J Vasc Surg. 1993;17:371–81. doi: 10.1067/mva.1993.43023. [DOI] [PubMed] [Google Scholar]
- 9.Campa JS, Greenhalgh RM, Powell JT. Elastin degradation in abdominal aortic aneurysms. Atherosclerosis. 1987;65:13–21. doi: 10.1016/0021-9150(87)90003-7. [DOI] [PubMed] [Google Scholar]
- 10.Menashi S, Campa JS, Greenhalgh RM, Powell JT. Collagen in abdominal aortic aneurysm: typing, content, and degradation. J Vasc Surg. 1987;6:578–82. doi: 10.1067/mva.1987.avs0060578. [DOI] [PubMed] [Google Scholar]
- 11.Rizzo RJ, McCarthy WJ, Dixit SN, Lilly MP, Shively VP, Flinn WR, et al. Collagen types and matrix protein content in human abdominal aortic aneurysms. J Vasc Surg. 1989;10:365–73. doi: 10.1067/mva.1989.13151. [DOI] [PubMed] [Google Scholar]
- 12.Dobrin PB, Baker WH, Grey WC. Elastolytic and collagen-olytic studies of arteries: implications for the mechanical properties of aneurysms. Arch Surg. 1984;119:405–9. doi: 10.1001/archsurg.1984.01390160041009. [DOI] [PubMed] [Google Scholar]
- 13.Anidjar S, Salzmann JL, Gentric D, Lagneau P, Camilleri JP, Michel JB. Elastase-induced experimental aneurysms in rats. Circulation. 1990;82:973–81. doi: 10.1161/01.cir.82.3.973. [DOI] [PubMed] [Google Scholar]
- 14.Lu L, Saulis AS, Liu WR, Roy NK, Chao JD, Ledbetter S, et al. The temporal effects of anti-TGF-beta1, 2, and 3 monoclonal antibody on wound healing and hypertrophic scar formation. J Am Coll Surg. 2005;201:391–7. doi: 10.1016/j.jamcollsurg.2005.03.032. [DOI] [PubMed] [Google Scholar]
- 15.Paradis V, Dargere D, Bonvoust F, Vidaud M, Segarini P, Bedossa P. Effects and regulation of connective tissue growth factor on hepatic stellate cells. Lab Invest. 2002;82:767–74. doi: 10.1097/01.lab.0000017365.18894.d3. [DOI] [PubMed] [Google Scholar]
- 16.Ogata T, Miyauchi T, Sakai S, Irukayama-Tomobe Y, Goto K, Yamaguchi I. Stimulation of peroxisome-proliferator-activated receptor alpha (PPAR alpha) attenuates cardiac fibrosis and endothelin-1 production in pressure-overloaded rat hearts. Clin Sci (Lond) 2002;103(Suppl 48):284S–8S. doi: 10.1042/CS103S284S. [DOI] [PubMed] [Google Scholar]
- 17.Tsubota M, Sasano Y, Takahashi I, Kagayama M, Shimauchi H. Expression of MMP-8 and MMP-13 mRNAs in rat periodontium during tooth eruption. J Dent Res. 2002;81:673–8. doi: 10.1177/154405910208101004. [DOI] [PubMed] [Google Scholar]
- 18.Keeley FW, Morin JD, Vesely S. Characterization of collagen from normal human sclera. Exp Eye Res. 1984;39:533–42. doi: 10.1016/0014-4835(84)90053-8. [DOI] [PubMed] [Google Scholar]
- 19.Eagleton MJ, Peterson DA, Sullivan VV, Roelofs KJ, Ford JA, Stanley JC, et al. Nitric oxide inhibition increases aortic wall matrix metalloproteinase-9 expression. J Surg Res. 2002;104:15–21. doi: 10.1006/jsre.2002.6396. [DOI] [PubMed] [Google Scholar]
- 20.Hannawa KK, Eliason JL, Woodrum DT, Pearce CG, Roelofs KJ, Grigoryants V, et al. L-selectin-mediated neutrophil recruitment in experimental rodent aneurysm formation. Circulation. 2005;112:241–7. doi: 10.1161/CIRCULATIONAHA.105.535625. [DOI] [PubMed] [Google Scholar]
- 21.Bengtsson H, Sonesson B, Bergqvist D. Incidence and prevalence of AAA. Ann NY Acad Sci. 1996;800:1–24. doi: 10.1111/j.1749-6632.1996.tb33294.x. [DOI] [PubMed] [Google Scholar]
- 22.Fischer GM, Swain ML. Influence of contraceptive and other sex steroids on aortic collagen and elastin. Exp Mol Pathol. 1980;33:15–24. doi: 10.1016/0014-4800(80)90003-9. [DOI] [PubMed] [Google Scholar]
- 23.Pirila E, Ramamurthy N, Maisi P, McClain S, Kucine A, Wahlgren J, et al. Wound healing in ovariectomized rats: effects of chemically modified tetracycline (CMT-8) and estrogen on matrix metalloproteinases -8, -13 and type I collagen expression. Curr Med Chem. 2001;8:281–94. doi: 10.2174/0929867013373552. [DOI] [PubMed] [Google Scholar]
- 24.Cho BS, Woodrum DT, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR., Jr Differential regulation of aortic growth in male and female rodents is associated with AAA development. J Surg Res. 2008 doi: 10.1016/j.jss.2008.07.027. Epub ahead of print. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Fischer GM. In vivo effects of estradiol on collagen and elastin dynamics in rat aorta. Endocrinology. 1972;91:1227–32. doi: 10.1210/endo-91-5-1227. [DOI] [PubMed] [Google Scholar]
- 26.Squadrito F, Altavilla D, Campo GM, Arlotta M, Arcoraci V, Minutoli L, et al. 17Beta-oestradiol reduces cardiac leukocyte accumulation in myocardial ischaemia reperfusion injury in rat. Eur J Pharmacol. 1997;335:185–927. doi: 10.1016/s0014-2999(97)01201-6. [DOI] [PubMed] [Google Scholar]
- 27.Ling S, Dai A, Williams MR, Myles K, Dilley RJ, Komesaroff PA, et al. Testosterone enhances apoptosis-related damage in human vascular endothelial cells. Endocrinology. 2002;143:1119–25. doi: 10.1210/endo.143.3.8679. [DOI] [PubMed] [Google Scholar]
- 28.Henriques TA, Huang J, D'Souza SS, Daugherty A, Cassis LA. Orchidectomy, but not ovariectomy, regulates angiotensin II-induced vascular diseases in apolipoprotein E-deficient mice. Endocrinology. 2004;145:3866–72. doi: 10.1210/en.2003-1615. [DOI] [PubMed] [Google Scholar]
- 29.Johnson DJ, Robson P, Hew Y, Keeley FW. Decreased elastin synthesis in normal development and in long-term aortic organ and cell cultures is related to rapid and selective destabilization of mRNA for elastin. Circ Res. 1995;77:1107–13. doi: 10.1161/01.res.77.6.1107. [DOI] [PubMed] [Google Scholar]
- 30.Vorp DA, Raghavan ML, Muluk SC, Makaroun MS, Steed DL, Shapiro R, et al. Wall strength and stiffness of aneurysmal and nonaneurysmal abdominal aorta. Ann NY Acad Sci. 1996;800:274–6. doi: 10.1111/j.1749-6632.1996.tb33330.x. [DOI] [PubMed] [Google Scholar]
- 31.Beldekas JC, Smith B, Gerstenfeld LC, Sonenshein GE, Franzblau C. Effects of 17 beta-estradiol on the biosynthesis of collagen in cultured bovine aortic smooth muscle cells. Biochemistry. 1981;20:2162–7. doi: 10.1021/bi00511a014. [DOI] [PubMed] [Google Scholar]
- 32.Kao KT, Hitt WE, Bush AT, McGavack TH. Connective tissue XII. Stimulating effects of estrogens on collagen synthesis in rat uterine slices. Proc Soc Exp Biol Med. 1964;117:86–97. doi: 10.3181/00379727-117-29504. [DOI] [PubMed] [Google Scholar]
- 33.Woessner JF., Jr Inhibition by oestrogen of collagen breakdown in the involuting rat uterus. Biochem J. 1969;112:637–45. doi: 10.1042/bj1120637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Henneman DH. Effect of estrogen on in vivo and in vitro collagen biosynthesis and maturation in old and young female guinea pigs. Endocrinology. 1968;83:678–90. doi: 10.1210/endo-83-4-678. [DOI] [PubMed] [Google Scholar]
- 35.Fischer GM, Swain ML. Effect of sex hormones on blood pressure and vascular connective tissue in castrated and noncastrated male rats. Am J Physiol. 1977;232:H617–21. doi: 10.1152/ajpheart.1977.232.6.H617. [DOI] [PubMed] [Google Scholar]
- 36.Wolinsky H. Effects of estrogen and progestogen treatment on the response of the aorta of male rats to hypertension. Morphological and chemical studies. Circ Res. 1972;30:341–9. doi: 10.1161/01.res.30.3.341. [DOI] [PubMed] [Google Scholar]
- 37.Fischer GM, Swain ML. In vivo effects of sex hormones on aortic elastin and collagen dynamics in castrated and intact male rats. Endocrinology. 1978;102:92–7. doi: 10.1210/endo-102-1-92. [DOI] [PubMed] [Google Scholar]
- 38.Farhat MY, Lavigne MC, Ramwell PW. The vascular protective effects of estrogen. FASEB J. 1996;10:615–24. [PubMed] [Google Scholar]
- 39.Mendelsohn ME, Karas RH. The protective effects of estrogen on the cardiovascular system. N Engl J Med. 1999;340:1801–11. doi: 10.1056/NEJM199906103402306. [DOI] [PubMed] [Google Scholar]
- 40.Ashcroft GS, Greenwell-Wild T, Horan MA, Wahl SM, Ferguson MW. Topical estrogen accelerates cutaneous wound healing in aged humans associated with an altered inflammatory response. Am J Pathol. 1999;155:1137–46. doi: 10.1016/S0002-9440(10)65217-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Foegh ML, Asotra S, Howell MH, Ramwell PW. Estradiol inhibition of arterial neointimal hyperplasia after balloon injury. J Vasc Surg. 1994;19:722–6. doi: 10.1016/s0741-5214(94)70047-8. [DOI] [PubMed] [Google Scholar]
- 42.Sinha I, Cho BS, Roelofs KJ, Stanley JC, Henke PK, Upchurch GR. Female Gender Attenuates Cytokine and Chemokine Expression and Leukocyte Recruitment in Experimental Rodent Abdominal Aortic Aneurysms. Ann NY Acad Sci. 2006;1085:367–79. doi: 10.1196/annals.1383.027. [DOI] [PubMed] [Google Scholar]
- 43.Xu C, Lee S, Shu C, Masuda H, Zarins CK. Expression of TGF-beta1 and beta3 but not apoptosis factors relates to flow-induced aortic enlargement. BMC Cardiovasc Disord. 2002;2:11. doi: 10.1186/1471-2261-2-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Letterio JJ, Roberts AB. Regulation of immune responses by TGF-beta. Annu Rev Immunol. 1998;16:137–161. doi: 10.1146/annurev.immunol.16.1.137. [DOI] [PubMed] [Google Scholar]
- 45.Roberts AB, McCune BK, Sporn MB. TGF-beta: regulation of extracellular matrix. Kidney Int. 1992;41:557–9. doi: 10.1038/ki.1992.81. [DOI] [PubMed] [Google Scholar]
- 46.Dai J, Losy F, Guinault AM, Pages C, Anegon I, Desgranges P, et al. Overexpression of transforming growth factor-beta1 stabilizes already-formed aortic aneurysms: a first approach to induction of functional healing by endovascular gene therapy. Circulation. 2005;112:1008–15. doi: 10.1161/CIRCULATIONAHA.104.523357. [DOI] [PubMed] [Google Scholar]
- 47.Roberts AB, Sporn MB, Assoian RK, Smith JM, Roche NS, Wakefield LM, et al. Transforming growth factor type beta: rapid induction of fibrosis and angiogenesis in vivo and stimulation of collagen formation in vitro. Proc Natl Acad Sci USA. 1986;83:4167–71. doi: 10.1073/pnas.83.12.4167. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sauvage M, Hinglais N, Mandet C, Badier C, Deslandes F, Michel JB, et al. Localization of elastin mRNA and TGF-beta1 in rat aorta and caudal artery as a function of age. Cell Tissue Res. 1998;291:305–14. doi: 10.1007/s004410051000. [DOI] [PubMed] [Google Scholar]
- 49.Nabel EG, Shum L, Pompili VJ, Yang ZY, San H, Shu HB, et al. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci USA. 1993;90:10759–63. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Shah M, Revis D, Herrick S, Baillie R, Thorgeirson S, Ferguson M, et al. Role of elevated plasma transforming growth factor-beta1 levels in wound healing. Am J Pathol. 1999;154:1115–24. doi: 10.1016/s0002-9440(10)65364-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vaday GG, Schor H, Rahat MA, Lahat N, Lider O. Transforming growth factor-beta suppresses tumor necrosis factor alpha-induced matrix metalloproteinase-9 expression in monocytes. J Leukoc Biol. 2001;69:613–21. [PubMed] [Google Scholar]