Abstract
Background
We tested the hypothesis that changes in our transplant practice have improved outcomes over the last decade. To explore correlates of improved outcomes, we analyzed the frequency and severity of graft-versus-host disease and hepatic, renal, pulmonary and infectious complications.
Methods
During 1993–1997 and 2003–2007, 1418 and 1148 patients received their first allogeneic transplants at our Center. Outcome measures included non-relapse mortality, recurrent malignancy, overall mortality, and the frequency and severity of major complications across this decade. Components of the Pretransplant Assessment of Mortality (PAM) score were used in regression models to adjust for severity of illness at the time of transplantation.
Results
In comparing outcomes during 1993–1997 and 2003–2007, we observed statistically significant decreases in the hazards of day -200 non-relapse mortality (by 60%), overall non-relapse mortality (by 52%), relapse or progression of malignancy (by 21%), and overall mortality (by 41%), after adjusting for components of the PAM score. Similar results were seen when the analyses were confined to patients receiving myeloablative conditioning therapy. We found statistically significant declines in the risk of more severe GVHD, disease caused by infections (viral, bacterial, and fungal), and damage to the liver, kidneys, and lungs.
Conclusions
We document a substantial reduction in the hazard of death related to allogeneic hematopoietic cell transplantation as well as improved long-term survival over the last decade. Improved outcomes appear to be related to reductions in organ damage, infection, and severe acute GVHD.
Keywords: Marrow transplantation, hematopoietic cell transplantation, liver disease, kidney disease, pulmonary disease, infectious complications, graft-vs.-host disease, ursodiol, mortality, survival, comparative outcomes research
Introduction
Infections, graft-vs.-host disease (GVHD), and liver, kidney, and pulmonary complications have caused significant mortality after allogeneic hematopoietic cell transplantation since the inception of this procedure 40 years ago.1 Recent changes in practice have decreased organ toxicity.2–5 Improved prevention and treatment strategies have decreased the severity of acute GVHD.6–9 Control of infectious complications has improved since development of molecular methods for detection of viral and fungal infection, pre-emptive treatments, introduction of new antifungal agents, and prevention of nosocomial infection.10–13
To examine the hypothesis that changes in the care of transplant patients have improved outcomes, we compared the rates of non-relapse mortality, recurrent malignancy, and overall mortality in two large cohorts of our patients, from 1993–1997 and 2003–2007. To explore correlates of improved outcomes, we analyzed the frequency and severity of acute GVHD and hepatic, renal, pulmonary and infectious complications across this decade.
Methods
Patient selection
All recipients of first allogeneic transplantation from 1993–1997 and 2003–2007 were evaluated under an Institutional Review Board-approved protocol.
Technique of allogeneic transplantation
All patients received a conditioning regimen followed by infusion of donor cells. Although these regimens varied, the myeloablative conditioning regimens generally contained high-dose cyclophosphamide with busulfan or 12–13.2 Gy total body irradiation.1 Reduced-intensity regimens contained 2–3 Gy total body irradiation with or without fludarabine.14 Recipients were given immunosuppressive drugs, usually a calcineurin inhibitor plus methotrexate or mycophenolate mofetil to prevent GVHD. Prophylaxis for infections included low-dose acyclovir, trimethoprim/sulfamethoxazole or dapsone, an antifungal agent (fluconazole in both periods, anti-mold drugs for patients with pre-transplant mold infection), pre-emptive therapy with ganciclovir for patients with CMV antigenemia or DNAemia, and antibiotics for patients with neutropenia. Ursodiol was given to all patients as prophylaxis against cholestasis, beginning in 2003.15
Clinical assessments and definition of terms
Outcome measures
Non-relapse mortality was defined as death after transplant that was not preceded by recurrent or progressive malignancy. Data for mortality, non-relapse mortality, and relapse reflect events as of the date of last contact before the database was locked on January 12, 2010.
Complications involving the liver, kidneys, and lungs through day 100
Liver and kidney injury were assessed by total serum bilirubin and creatinine concentrations. Severity of liver injury and liver GVHD were defined by the peak bilirubin concentration.16 Acute kidney injury was scored as either a two-fold or three-fold or greater increase in baseline serum creatinine.3 Lung injury was defined by the need for diagnostic bronchoscopy and development of respiratory failure. Evaluation of pulmonary abnormalities included computed tomography to evaluate radiographic abnormalities and pulmonary consultation to determine whether fiberoptic bronchoscopy was indicated.17 Respiratory failure was defined as the need for >24 hours of mechanical ventilation for a non-elective reason.18
Viral, bacterial, and fungal infections through day 100
Cytomegalovirus infection was defined as the presence of viral pp65 antigen or DNA in plasma19; CMV disease was dysfunction of an organ infected by CMV.20 Patients with one or more positive blood cultures for gram-negative organisms were considered to have gram-negative bacteremia.21 Invasive fungal infections were classified by international consensus criteria.22 Only fungal infections that were proven or probable were included in this analysis.
Acute GVHD
The peak stage of gut and liver GVHD and the peak severity of acute GVHD were graded by PJM. according to the extent of rash, total serum bilirubin, the presence of upper gastrointestinal symptoms, and daily stool volume.16 Grades 2, 3, and 4 GVHD respectively indicate mild, moderate and severe peak GVHD manifestations (Supplementary Table 1).
Statistical analyses
The probability of overall survival was estimated by the method of Kaplan and Meier. Probabilities of non-relapse mortality and relapse were summarized using cumulative incidence estimates23, where relapse was viewed as a competing risk for non-relapse mortality, and non-relapse mortality a competing risk for relapse. Jaundice, GVHD, and doubling and tripling of baseline serum creatinine were compared between cohorts by logistic regression. Time to engraftment was defined as the first of three consecutive days of absolute neutrophil count (ANC) >500 cells/mm3. Mean times to engraftment as well as mean peak total serum bilirubin and creatinine values were compared using linear regression. Average daily total serum bilirubin and creatinine values were modeled with generalized estimating equations24. Cox regression compared the hazards of failure for all other endpoints. Non-relapse mortality failures beyond day 200 were considered non-failures and censored at day 200 for day -200 non-relapse mortality. Non-failures for non-relapse mortality at any time, relapse and overall mortality were censored at date of last contact. Failures within the first 100 days were considered for infectious and pulmonary complications; failures beyond this time were censored at day 100 and treated as non-failures. Components of the Pretransplant Assessment of Mortality (PAM) score25 (Supplementary Table 2) were used in regression models to adjust for severity of illness at the time of transplantation, with the exception of the conditioning-regimen component. All PAM components were treated as categorical variables, with a category for missing data included for each component. Additional adjustment for comorbidities as captured by the Hematopoietic Cell Transplant Comorbidity Index (HCT-CI)26 (Supplementary Table 3) was made among a subset of 1000 patients (409 from 1993–1997, 591 from 2003–2007) who had been previously scored by MLS. Two-sided p-values were estimated from the Wald test; no adjustments were made for multiple comparisons.
Results
Patient characteristics
Table 1 and Supplementary Table 4 display demographic, disease, and transplant characteristics, including components of the PAM score contained in regression models. Reflecting the greater use of peripheral blood hematopoietic cells, the adjusted average time to engraftment was 1.83 days less (p<0.001) in 2003–2007 among all patients and 1.62 days less (p<0.001) among those receiving myeloablative regimens.
Table 1.
Patient Characteristics. Additional patient characteristics can be found in Supplementary Table 4.
| Characteristic | 1993–1997 (N=1418) | 2003–2007 (N=1148) | p-VALUEd |
|---|---|---|---|
| Median age (range)25 | 37.4 (0.6–67.8) | 47.2 (0.4–78.9) | <0.001 |
| Diagnosis | <0.001 | ||
| Aplastic anemia | 46 (3%) | 39 (3%) | |
| Acute lymphocytic leukemia | 188 (13%) | 166 (14%) | |
| Acute myeloid leukemia | 352 (25%) | 459 (40%) | |
| Chronic lymphocytic leukemia | 15 (1%) | 32 (3%) | |
| Chronic myeloid leukemia | 463 (33%) | 104 (9%) | |
| Hodgkin’s lymphoma | 18 (1%) | 3 (<1%) | |
| Myelodysplastic syndrome | 174 (12%) | 230 (20%) | |
| Multiple myeloma | 56 (4%) | 3 (<1%) | |
| Non-Hodgkin’s lymphoma | 71 (5%) | 60 (5%) | |
| Other | 35 (2%) | 52 (5%) | |
| Disease Severity25 | <0.001 | ||
| Low | 433 (31%) | 174 (15%) | |
| Intermediate | 427 (30%) | 622 (54%) | |
| High | 558 (39%) | 352 (31%) | |
| Donor25 | <0.001 | ||
| HLA-identical sibling | 625 (44%) | 443 (39%) | |
| Mismatched sibling or non-sibling relative | 200 (14%) | 29 (4%) | |
| Unrelated | 593 (42%) | 676 (59%) | |
| Stem Cell Source | <0.001 | ||
| Bone marrow | 1240 (87%) | 227 (20%) | |
| Peripheral blood hematopoietic cells | 158 (11%) | 871 (76%) | |
| Bone marrow and peripheral blood cells | 11 (1%) | 1 (<1%) | |
| Cord blood | 9 (1%) | 49 (4%) | |
| Conditioning Intensity | <0.001 | ||
| Reduced-intensity | 1 (<1%) | 257 (22%) | |
| Myeloablativea | 427 (30%) | 774 (67%) | |
| High-dose myeloablativeb | 990 (70%) | 117 (10%) | |
| GVHD prophylaxisc | <0.001 | ||
| CNI+MTX or TMTX | 1258 (89%) | 643 (56%) | |
| CNI+MMF | 1 (<1%) | 242 (21%) | |
| CNI Alone | 64 (5%) | 46 (4%) | |
| Other | 95 (7%) | 217 (19%) | |
Myeloablative regimens included cyclophosphamide plus total body irradiation ≤12 Gy; targeted busulfan plus cyclophosphamide; and fludarabine plus busulfan or treosuflan.
High-dose myeloablative regimens included cyclophosphamide plus total body irradiation >12 Gy; busulfan, cyclophosphamide, and total body irradiation; BCV; and non-targeted busulfan plus cyclophosphamide.
CNI, calcineurin inhibitor; MTX, methotrexate; TMTX, trimetrexate; MMF, mycophenolate mofetil
By two-sample t-test for age; chi square test for all other comparisons.
Outcome measures: Non-relapse mortality, relapse, and overall mortality
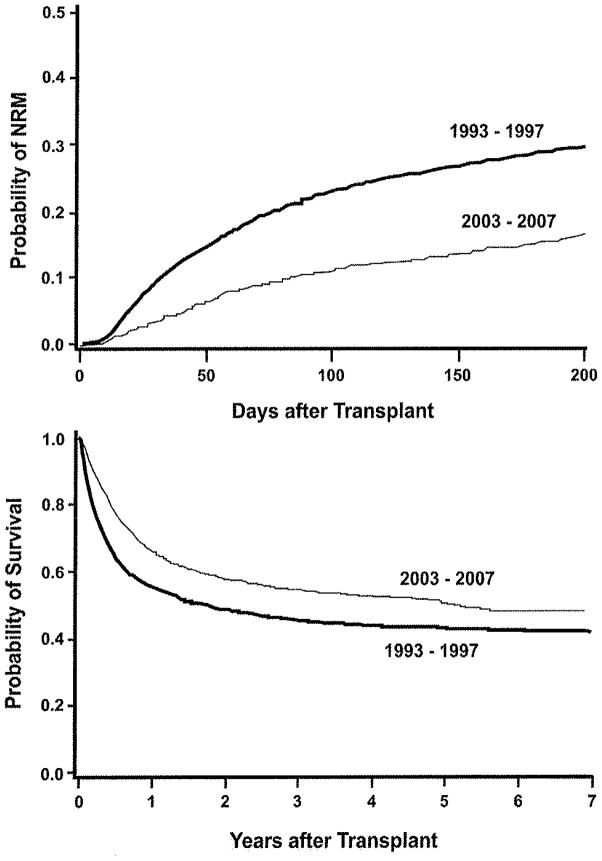
From the 1993–1997 period to the 2003–2007 period, statistically significant decreases were seen in the hazards of day-200 non-relapse mortality (by 60%), overall non-relapse mortality (by 52%), relapse or progression of malignancy (by 21%), and overall mortality (by 41%) (Table 2). The probabilities of day-200 non-relapse mortality and overall survival are shown in Figure 1. Among patients who had received myeloablative regimens, statistically significant reductions were seen in the hazards of day-200 non-relapse mortality, overall non-relapse mortality, relapse, and overall mortality by 56%, 52%, 18%, and 39%, respectively (Table 2). Improvements in outcomes were consistent among various subgroups. For the diagnoses ALL, AML, CML, and MDS, HRs for day-200 non-relapse mortality were 0.62, 0.38, 0.60, and 0.42, respectively; for overall mortality, HRs were 0.67, 0.63, 0.67, and 0.65, respectively. Average PAM scores for patients receiving myeloablative regimens were 16.3 during 1993–1997 vs. 17.3 during 2003–2007 vs. 22.1 in patients receiving reduced-intensity regimens. For patients with “low” PAM (scores <18, the median PAM), the HR for day-200 non-relapse mortality in the two periods was 0.41 and for overall mortality was 0.77. For patients with “high” PAM, the HR for day-200 non-relapse mortality was 0.36 and for overall mortality was 0.51. The HR for day-200 non-relapse mortality among patients transplanted from a matched-sibling donor was 0.45, from a non-sibling relative or mismatched-sibling donor was 0.35, and from an unrelated donor was 0.35; for overall mortality, HRs were 0.72, 0.47, and 0.52, respectively. Among CMV-positive recipients, the HR for day-200 non-relapse mortality was 0.43 and for overall mortality was 0.61, while for CMV-negative patients, HRs were 0.34 and 0.55, respectively.
Table 2.
Comparison of outcomes, organ dysfunction, infection, and acute GVHD after transplant between two eras.
| Event | Number (%) Failures Among All Patients | Adjusted Hazard/Odds (Ratioa(95% Confidence Interval, p-value) | ||
|---|---|---|---|---|
| 1993–97 (n=1418) | 2003–07 (n=1148) | All Patients | Patients who received myeloablative conditioning therapy | |
| Outcomes | ||||
| Day-200 non-relapse mortality | 419(30%) | 186(16%) | 0.40 (0.32–0.49, p<0.001) | 0.44 (0.36–0.54, p<0.001) |
| Overall non-relapse mortality | 580(41%) | 297 (26%) | 0.48 (0.40–0.57, p<0.001) | 0.48 (0.40–0.58, p< 0.001) |
| Relapse or progression | 379 (27%) | 302 (26%) | 0.79 (0.66–0.94, p=0.008) | 0.82 (0.68–0.99, p=0.04) |
| Overall morality | 891 (63%) | 545 (47%) | 0.59 (0.52–0.67, p<0.001) | 0.61 (0.53–0.69, p<0.001) |
| Liver dysfunction through day 100 | ||||
| Peak total serum bilirubin ≥ 4 mg/dLb | 677 (48%) | 232 (20%) | 0.26 (0.21–0.32, p<0.001) | 0.28 (0.23–0.35, p<0.001) |
| Peak total serum bilirubin ≥ 10 mg/dLb | 287 (20%) | 64 (6%) | 0.22 (0.16–0.30, p<0.001) | 0.24 (0.17–0.33, p<0.001) |
| Stage 3–4 liver GVHDc | 165(12%) | 25 (2%) | 0.15 (0.09–0.24, p<0.001) | 0.18 (0.11–0.29, p<0.001) |
| Stage 4 liver GVHDc | 78 (6%) | 2(<1%) | 0.03 (0.01–0.12, p<0.001) | 0.04 (0.01–0.17, p<0.001) |
| Acute Kidney Injury through day 100 | ||||
| Creatinine 2-times baseline | 710(50%) | 384 (33%) | 0.47 (0.39–0.56, p<0.001) | 0.46 (0.38–0.56, p<0.001) |
| Creatinine 3-times baseline | 257(18%) | 115(10%) | 0.48 (0.37–0.64, p<0.001) | 0.51 (0.38–0.68, p<0.001) |
| Dialysis | 112(7.9%) | 58 (5.0%) | 0.62 (0.42–0.90, p=0.01) | 0.72 (0.49–1.07, p=0.10) |
| Pulmonary complications through day 100 | ||||
| Bronchoscopy | 272(19%) | 242(21%) | 0.91 (0.75–1.12, p=0.38) | 0.90 (0.73–1.12, p=0.34) |
| Respiratory Failure | 211 (15%) | 131(11%) | 0.64 (0.49–0.82, p=0.001) | 0.69 (0.53–0.90, p=0.007) |
| Infections through day 100 | ||||
| CMV infectiond | 420 (57%) | 419 (63%) | 1.02 (0.87–1.20, p=0.77) | 1.04 (0.88–1.23, p=0.63) |
| CMV diseased | 62 (8%) | 33 (5%) | 0.52 (0.32–0.85, p=0.009) | 0.53 (0.31–0.89, p=0.02) |
| Gram-negative bacteremia | 213 (15%) | 129 (11%) | 0.61 (0.48–0.79, p<0.001) | 0.57 (0.44–0.75, p<0.001)i |
| Invasive mold infection | 125 (9%) | 80 (7%) | 0.49 (0.35–0.71, p<0.001) | 0.55 (0.38–0.78, p<0.001) |
| Invasive Candida infection | 99 (7%) | 10 (1%) | 0.12 (0.06–0.25, p<0.001) | 0.15 (0.08–0.29, p<0.001) |
| Acute GVHD | ||||
| Grades 2–4 | 1076 (77%) | 815 (71%) | 0.61 (0.50–0.75, p<0.001) | 0.66 (0.53–0.82, p<0.001) |
| Grades 3–4 | 421 (30%) | 161 (14%) | 0.33 (0.26–0.42, p<0.001) | 0.33 (0.26–0.42, p<0.001) |
| Grade 4 | 102 (7%) | 27 (2%) | 0.31 (0.18–0.51, p<0.001) | 0.30 (0.18–0.53, p<0.001) |
| Stage 2–4 gut GVHDe | 231 (17%) | 119 (10%) | 0.53 (0.40–0.70, p<0.001) | 0.52 (0.39–0.70, p<0.001) |
| Stage 3–4 gut GVHDe | 141 (10%) | 73 (6%) | 0.53 (0.37–0.75, p<0.001) | 0.55 (0.38–0.79, p=0.001) |
Change over the decade is expressed as a hazard ratio (HR) or odds ratio (OR), as calculated by regression models adjusted for age, donor, disease severity, and baseline values for serum creatinine, ALT, FEV1, and DLCO (see Methods).
Conversion of total serum bilirubin to SI units: 1 mg/dL=17.1 μmol/L
Liver stage 1, total serum bilirubin 2–2.9 mg/dL; stage 2, 3–5.9 mg/dL; stage 3, 6–14.9 mg/dL; stage 4, ≥15 mg/dL (1 mg/dL=17.1 μmol/L).
Among CMV-seropositive patients.
Gut stage 1, diarrhea 500–999 mL/day or biopsy-proven upper gut involvement; stage 2, diarrhea 1000–1499 mL/day; stage 3, diarrhea 1500–1999 mL/day; stage 4, diarrhea > 2000 mL or severe abdominal pain with or without ileus.
Figure 1.
Probability of non-relapse mortality (NRM) by day 200 (upper panel) and overall survival (lower panel) during two time periods. Patients alive beyond seven years are censored at 7 years for graphical purposes only.
Among the subset of 1000 patients (39%) who had previously been scored for HCT-CI, average scores were 1.26 among patients who received myeloablative regimens during 1993–1997 and 1.54 during 2003–2007. Among patients who received reduced-intensity regimens, the average HCT-CI was 2.31. After further adjusting the PAM-adjusted mortality models for HCT-CI, the HRs for day-200 non-relapse mortality, overall non-relapse mortality, and overall mortality were lower by 1.7%, 0.9%, and 1.1%, respectively. Moreover, both PAM and HCT-CI were associated with each outcome even after adjustment for the other (p<0.001 for each).
Complications associated with mortality: organ dysfunction, infection, and acute GVHD
Liver disease
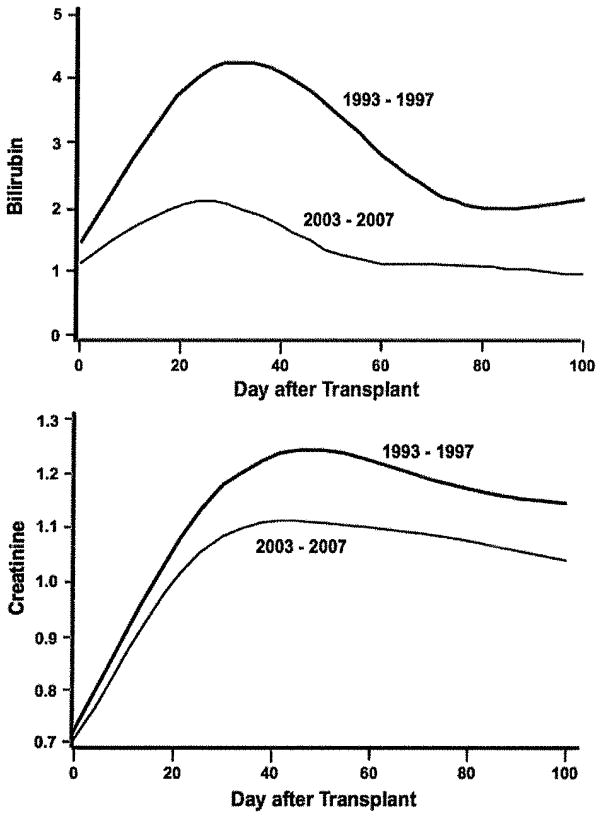
From 1993–1997 to 2003–2007, the odds of jaundice were statistically significantly reduced by over 70% (Table 2). The magnitude of the reduction was similar for patients who received only myeloablative regimens (Table 2). The average peak serum bilirubin in the earlier era was 7.6 mg/dL compared to 3.3 mg/dL in the later era (adjusted mean difference, 4.4 mg/dL, p<0.001). Figure 2 shows fitted average daily serum bilirubin values for each era. After adjustment, the estimated modeled difference in average daily serum bilirubin was 1.4 mg/dL (p<0.001).
Figure 2.
Display of daily total serum bilirubin (top panel) and serum creatinine (lower panel) values from day 0 to day 100 in mg/dL, during two eras. The lines represent fitted cubic spline curves of the observed data. Conversion to SI units: For total serum bilirubin, 1 mg/dL=17.1 μmol/L and for serum creatinine, 1 mg/dL=88.4 μmol/L.
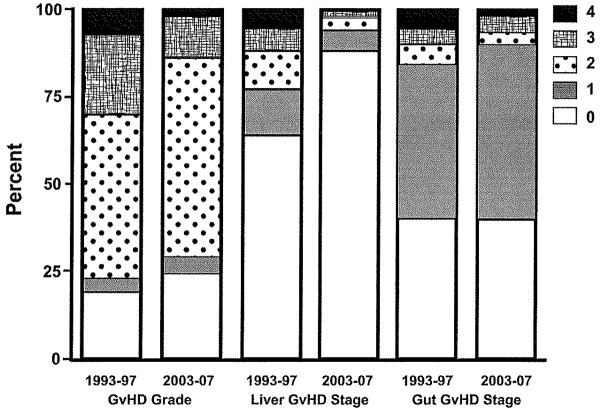
The recent reduction in the intensity of conditioning (Table 1) did not solely explain the reduction in liver injury. The average peak bilirubin value after high-dose myeloablative regimens in 1993–1997 was 4.1 mg/dL compared to 1.5 mg/dL in 2003–2007. Patients who received lower dose myeloablative regimens had average peak bilirubin values of 3.1 mg/dL and 1.8 mg/dL, respectively, in the two periods. Patients who received a reduced-intensity regimen in 2003–2007 had a mean peak bilirubin value of 1.8 mg/dL. Stage 3–4 liver GVHD was seen in 11.9% of patients in 1993–1997 versus 2.1% in 2003–2007. There were 78 cases of stage 4 liver GVHD in the earlier cohort, and only 2 during 2003–2007 (Figure 3). We demonstrated a positive correlation between total serum bilirubin and day-200 non-relapse mortality for patients in the 1993–1997 cohort.27 A similar positive correlation was seen in the 2003–2007 cohort (data not shown).
Figure 3.
Distribution of the overall grade of acute GVHD and the stage of liver and gastrointestinal GVHD16 in two eras.
Renal injury
During the decade, the odds of acute kidney injury were statistically significantly reduced. The magnitude of reduction was similar for patients who received only myeloablative regimens (Table 2). Figure 2 (bottom panel) shows fitted average daily serum creatinine values to day 100. The adjusted, modeled average difference in daily serum creatinine was 0.13 mg/dL (p<0.001) (1 mg/dL = 88.4 μmol/L). The average peak creatinine in 1993–1997 was 2.06 compared to 1.67 mg/dL in 2003–2007, and the adjusted difference was 0.53 mg/dL (p<0.001).
Pulmonary complications
The hazard of manifesting a condition that required bronchoscopic evaluation in 2003–2007 was similar to that in 1993–1997, both among all patients and among only those who received myeloablative conditioning therapy (Table 2). The hazard of respiratory failure had declined by 36% in the 2003–2007 cohort relative to the earlier cohort; a similar decline was seen when the analysis was confined lo patients receiving myeloablative regimens (Table 2).
Infections
Although the rate of CMV reactivation remained stable across the decade, the hazard of early CMV disease was reduced during 2003–2007 by 48% when all CMV-seropositive patients are considered, and by 47% when CMV-seropositive patients receiving myeloablative regimens are considered (Table 2). The hazards of developing bacteremia with a gram-negative organism declined by 39%, invasive mold infection by 51%, and invasive candidal infection by 88% between the two periods. The magnitude of declines in the hazard of these infections was similar among patients who received myeloablative conditioning regimens.
Acute GVHD
The percentage of patients with mild, moderate, and severe acute GVHD declined over the decade, with a 67% decrease in the odds of developing grade 3–4 GVHD (Table 2). We found statistically significant reductions in the frequency of stage 3–4 gut and especially stage 3–4 liver GVHD in the 2003–2007 period (Table 2, Figure 3). The reduction in the odds of developing grade 3–4 GVHD was consistently seen across different donor types: odds ratios were 0.35, 0.11, and 0.33 for patients who had a matched-sibling donor, a non-sibling relative or mismatched-sibling donor, or an unrelated donor, respectively.
Discussion
We document a substantial reduction in the hazard of mortality related to allogeneic transplantation and improved long-term survival over two time periods a decade apart. We also saw declines in the hazard or probability of almost every transplant complication that we examined. In these analyses, we adjusted our models for individual components of the previously validated PAM score25 and, when available, HCT-CI scores.26 On average, older and sicker patients with more advanced disease were coming to transplant during 2003–2007. In a subset of patients scored for HCT-CI, further adjustment for HCT-CI changed the MRs for mortality outcomes by less than 2%. Both scores provide important prognostic factors in this population.
Several changes in our transplant practice appear to have contributed to improved outcomes. We now treat patients with co-morbid medical conditions with less toxic conditioning regimens. This shift in conditioning regimen intensity resulted from data showing that higher dose regimens resulted in more organ damage, without the commensurate benefit of a reduced risk of recurrent malignancy and from data showing that graft-vs.-tumor activity of donor cells can have a dominant role in eliminating malignant cells.14 Lower dose myeloablative regimens were those that limited the dose of total body irradiation, substituted fludarabine for cyclophosphamide, and personalized cyclophosphamide dosing—based on data showing that aberrant metabolism of cyclophosphamide and high TBI exposures were factors leading to fatal hepatic sinusoidal obstruction syndrome and multi-organ failure.2, 28, 29
Despite a greater frequency of use of peripheral blood hematopoietic cells instead of marrow during 2003–2007, the odds of developing grade 3–4 GVHD decreased by 67% over the decade, partly because of ursodiol’s effect on GVHD-related cholestasis and the near disappearance of stage 4 liver GVHD.15 The role of more accurate HLA matching of unrelated donors in improving outcomes cannot be readily ascertained from these data, which show that the reduction in severe GVHD was similar in both matched sibling and unrelated donor transplants. GVHD prophylaxis did not change substantially over the decade, but our approach to treatment of GVHD did change. By 2003, two syndromes of gastrointestinal GVHD were apparent, one affecting mostly the upper gut (anorexia, nausea, vomiting, satiety) and the other mostly the mid-gut (diarrhea, abdominal pain, bleeding).30 The upper gut syndrome occurs more frequently, seldom progresses to grade 4 GVHD, responds to prednisone therapy, and has a better prognosis.6, 8 Our past practice of treating all patients with acute GVHD with prednisone at 2 mg/kg/day was abandoned in favor of therapy based on clinical manifestations and the risk of mortality.7 This change in treatment philosophy was also prompted by data showing that the risks of CMV, fungal, and bacterial infections were significantly related to prednisone dose.31–34 During 2003–2007, most patients with the upper gut GVHD syndrome were initially treated with prednisone 1 mg/kg/day plus a topically-active glucocorticoid7, 8, reducing average prednisone exposure by 48%.7
Greater use of peripheral blood donor cells resulted in significantly faster neutrophil engraftment35 and earlier recovery of immunity against fungal and bacterial infections.36 The decreased hazard of gram-negative bacteremia and fungemia might be related to less gut toxicity from conditioning regimens, less frequent multi-organ failure, and fewer patients developing mid-gut GVHD. Antibacterial prophylaxis in patients with neutropenia shifted from cephalosporins to quinolones over the decade. Antifungal prophylaxis with fluconazole was used during 1993–1997; with the advent of fungal antigen testing and new antifungal drugs, patients with positive blood tests or pulmonary nodules were more likely to receive mold-active azoles (itraconazole, voriconazole) or an echinocandin. Pre-emptive antiviral therapy is now based on a more sensitive diagnostic test for CMV viremia.12, 19
The decrease in the degree of jaundice can be traced to less-intense conditioning regimens, less frequent bacteremia and GVHD, and use of ursodiol to prevent cholestasis. During 2003–2007, patients at risk for fatal sinusoidal obstruction syndrome37 were conditioned with fludarabine-busulfan or reduced-intensity regimens or personalized doses of CY, based on therapeutic drug monitoring, instead of high-dose cyclophosphamide and TBI.14, 28, 29 The adoption of ursodiol prophylaxis was based on data showing that ursodiol improved liver tests in patients with GVHD, decreased the frequency of jaundice, and improved survival after transplant.15, 38
The decline in the frequency of renal dysfunction and respiratory failure is intertwined with the significantly lower frequency of higher dose myeloablative regimens, sinusoidal obstruction syndrome, gram-negative bacteremia, invasive mold infections, and avoidance of amphotericin products. The decreased frequency of more severe GVHD may also have affected renal and pulmonary function for the better, as both the kidneys and the lungs are affected by the inflammatory milieu of acute GVHD.39, 40
In conclusion, the data show clear improvement in transplant outcomes over the decade. The data also indicate areas of transplant biology and patient care where research is needed to achieve further progress, specifically GVHD and graft-vs.-tumor effects, immunologic tolerance, infection management, and recurrent malignancy.
Supplementary Material
Acknowledgments
Our research was supported by grants from the National Institutes of Health (CA18029, CA15704, CA78902, HL36444, HL088201, HL088021, HL096831, DK063038). We are grateful to the many physicians, nurses, physician assistants, pharmacists, and support staff who cared for our patients during this decade, and to the patients who allowed us to care for them and who participated in our ongoing clinical research. We also acknowledge help with data abstraction provided by David Myerson, Gary Schoch, Chris Davis, Margaret Au, Miwa Sakai Vernon, and Emily Pao.
Footnotes
Disclosure: Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Appelbaum FR, Forman SJ, Negrin RS, Blume KG. Thomas’ Hematopoietic Cell Transplantation. 4. Oxford, UK: Wiley-Blackwell Publishing; 2009. [Google Scholar]
- 2.McDonald GB, Slattery JT, Bouvier ME, et al. Cyclophosphamide metabolism, liver toxicity, and mortality following hematopoietic stem cell transplantation. Blood. 2003;101:2043–8. doi: 10.1182/blood-2002-06-1860. [DOI] [PubMed] [Google Scholar]
- 3.Hingorani SR, Guthrie K, Batchelder A, et al. Acute renal failure after myeloablative hematopoietic cell transplant: incidence and risk factors. Kidney International. 2005;67:272–7. doi: 10.1111/j.1523-1755.2005.00078.x. [DOI] [PubMed] [Google Scholar]
- 4.Clark JG, Madtes DK, Martin TR, Hackman RC, Farrand AL, Crawford SW. Idiopathic pneumonia after bone marrow transplantation: cytokine activation and lipopolysaccharide amplification in the bronchoalveolar compartment. Critical Care Medicine. 1999;27:1800–6. doi: 10.1097/00003246-199909000-00016. [DOI] [PubMed] [Google Scholar]
- 5.Patriarca F, Skert C, Sperotto A, et al. Incidence, outcome, and risk factors of late-onset noninfectious pulmonary complications after unrelated donor stem cell transplantation. Bone Marrow Transplantation. 2004;33:751–8. doi: 10.1038/sj.bmt.1704426. [DOI] [PubMed] [Google Scholar]
- 6.Martin PJ, McDonald GB, Sanders JE, et al. Increasingly frequent diagnosis of acute graft-versus-host disease after allogeneic hematopoietic cell transplantation. Biology of Blood and Marrow Transplantation. 2004;10:320–7. doi: 10.1016/j.bbmt.2003.12.304. [DOI] [PubMed] [Google Scholar]
- 7.Mielcarek M, Storer BE, Boeckh M, et al. Initial therapy of acute graft-versus-host disease with low dose prednisone does not compromise patient outcomes. Blood. 2009;113:2888–94. doi: 10.1182/blood-2008-07-168401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hockenbery DM, Cruickshank S, Rodell TC, et al. A randomized, placebo-controlled trial of oral beclomethasone dipropionate as a prednisone-sparing therapy for gastrointestinal graft-versus-host disease. Blood. 2007;109:4557–63. doi: 10.1182/blood-2006-05-021139. [DOI] [PubMed] [Google Scholar]
- 9.Lee SJ, Klein J, Haagenson M, et al. High-resolution donor-recipient HLA matching contributes to the success of unrelated donor marrow transplantation. Blood. 2007;110:4576–83. doi: 10.1182/blood-2007-06-097386. [DOI] [PubMed] [Google Scholar]
- 10.Upton A, Kirby KA, Carpenter P, Boeckh M, Marr KA. Invasive aspergillosis following hematopoietic cell transplantation: outcomes and prognostic factors associated with mortality. Clinical Infectious Diseases. 2007;44:531–40. doi: 10.1086/510592. [DOI] [PubMed] [Google Scholar]
- 11.Yokoe D, Casper C, Dubberke E, et al. Infection prevention and control in health-care facilities in which hematopoietic cell transplant recipients are treated. Bone Marrow Transplantation. 2009;44:495–507. doi: 10.1038/bmt.2009.261. [DOI] [PubMed] [Google Scholar]
- 12.Boeckh M, Bowden RA, Gooley T, Myerson D, Corey L. Successful modification of a pp65 antigenemia-based early treatment strategy for prevention of CMV disease in allogeneic marrow transplant recipients (letter) Blood. 1999;93:1781–2. [PubMed] [Google Scholar]
- 13.Nakamae H, Kirby KA, Sandmaier BM, et al. Effect of conditioning regimen intensity on CMV infection in allogeneic hematopoietic cell transplantation. Biology of Blood & Marrow Transplantation. 2009;15:694–703. doi: 10.1016/j.bbmt.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sandmaier BM, Storb R. Reduced-intensity conditioning followed by hematopoietic cell transplantation for hematologic malignancies. 4. Oxford, UK: Wiley-Blackwell; 2009. pp. 1043–58. [Google Scholar]
- 15.Ruutu T, Eriksson B, Remes K, et al. Ursodeoxycholic acid for the prevention of hepatic complications in allogeneic stem cell transplantation. Blood. 2002;100:1977–83. doi: 10.1182/blood-2001-12-0159. [DOI] [PubMed] [Google Scholar]
- 16.Przepiorka D, Weisdorf D, Martin P, et al. 1994 Consensus Conference on Acute GVHD Grading. Bone Marrow Transplant. 1995;15:825–8. [PubMed] [Google Scholar]
- 17.Chien JW, Sakai M, Gooley TA, Schoch HG, McDonald GB. Influence of oral beclomethasone dipropionate on early non-infectious pulmonary outcomes after allogeneic hematopoietic cell transplantation: results from two randomized trials. Bone Marrow Transplantation. 2010;45:317–24. doi: 10.1038/bmt.2009.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parimon T, Madtes DK, Au DH, Clark JG, Chien JW. Pretransplant lung function, respiratory failure, and mortality after stem cell transplantation. American Journal of Respiratory & Critical Care Medicine. 2005;172:384–90. doi: 10.1164/rccm.200502-212OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Boeckh M, Huang M, Ferrenberg J, et al. Optimization of quantitative detection of cytomegalovirus DNA in plasma by real-time PCR. Journal of Clinical Microbiology. 2004;42:1142–8. doi: 10.1128/JCM.42.3.1142-1148.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Ljungman P, Griffiths P, Paya C. Definitions of cytomegalovirus infection and disease in transplant recipients. Clinical Infectious Diseases. 2002;34:1094–7. doi: 10.1086/339329. [DOI] [PubMed] [Google Scholar]
- 21.Chien JW, Boeckh MJ, Hansen JA, Clark JG. Lipopolysaccharide binding protein promoter variants influence the risk for Gram-negative bacteremia and mortality after allogeneic hematopoietic cell transplantation. Blood. 2008;111:2462–9. doi: 10.1182/blood-2007-09-101709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Ascioglu S, Rex JH, de Pauw B, et al. Defining opportunistic invasive fungal infections in immunocompromised patients with cancer and hematopoietic stem cell transplants: an international consensus. Clinical Infectious Diseases. 2002;34:7–14. doi: 10.1086/323335. [DOI] [PubMed] [Google Scholar]
- 23.Gooley TA, Leisenring W, Crowley J, Storer BE. Estimation of failure probabilities in the presence of competing risks: new representations of old estimators. Statistics in Medicine. 1999;18:695–706. doi: 10.1002/(sici)1097-0258(19990330)18:6<695::aid-sim60>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 24.Liang K, Zeger S. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 25.Parimon T, Au DH, Martin PJ, Chien JW. A risk score for mortality after allogeneic hematopoietic cell transplantation. Annals of Internal Medicine. 2006;144:407–14. doi: 10.7326/0003-4819-144-6-200603210-00007. [DOI] [PubMed] [Google Scholar]
- 26.Sorror ML, Maris MB, Storb R, et al. Hematopoietic cell transplantation (HCT)-specific comorbidity index: a new tool for risk assessment before allogeneic HCT. Blood. 2005;106:2912–9. doi: 10.1182/blood-2005-05-2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gooley TA, Rajvanshi P, Schoch HG, McDonald GB. Serum bilirubin levels and mortality after myeloablative allogeneic hematopoietic cell transplantation. Hepatology. 2005;41:345–52. doi: 10.1002/hep.20529. [DOI] [PubMed] [Google Scholar]
- 28.de Lima M, Couriel D, Thall PF, et al. Once-daily intravenous busulfan and fludarabine: clinical and pharmacokinetic results of a myeloablative, reduced-toxicity conditioning regimen for allogeneic stem cell transplantation in AML and MDS. Blood. 2004;104:857–64. doi: 10.1182/blood-2004-02-0414. [DOI] [PubMed] [Google Scholar]
- 29.McCune JS, Batchelder AL, Guthrie KA, et al. Personalized dosing of cyclophosphamide in the Total Body Irradiation - cyclophosphamide conditioning regimen: A phase II trial in patients with hematologic malignancy. Clinical Pharmacology and Therapeutics. 2009;85:615–22. doi: 10.1038/clpt.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Strasser SI, McDonald GB. Gastrointestinal and hepatic complications. In: Appelbaum FR, Forman SJ, Negrin R, Blume KG, editors. Thomas’ Hematopoietic Cell Transplantation. 4. Oxford, UK: Wiley-Blackwell; 2009. pp. 1434–55. [Google Scholar]
- 31.Hakki M, Riddell SR, Storek J, et al. Immune reconstitution to cytomegalovirus after allogeneic hematopoietic stem cell transplantation: impact of host factors, drug therapy, and subclinical reactivation. Blood. 2003;102:3060–7. doi: 10.1182/blood-2002-11-3472. [DOI] [PubMed] [Google Scholar]
- 32.Marr KA, Carter RA, Boeckh M, Martin P, Corey L. Invasive aspergillosis in allogeneic stem cell transplant recipients: changes in epidemiology and risk factors. Blood. 2002;100:4358–66. doi: 10.1182/blood-2002-05-1496. [DOI] [PubMed] [Google Scholar]
- 33.Fukuda T, Boeckh M, Carter RA, et al. Risks and outcomes of invasive fungal infections in recipients of allogeneic hematopoietic stem cell transplants after nonmyeloablative conditioning. Blood. 2003;102:827–33. doi: 10.1182/blood-2003-02-0456. [DOI] [PubMed] [Google Scholar]
- 34.Narimatsu H, Matsumura T, Kami M, et al. Bloodstream infection after umbilical cord blood transplantation using reduced-intensity stem cell transplantation for adult patients. Biology of Blood & Marrow Transplantation. 2005;11:429–36. doi: 10.1016/j.bbmt.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 35.Stem Cell Trialists’ Collaborative, G. Allogeneic peripheral blood stem-cell compared with bone marrow transplantation in the management of hematologic malignancies: an individual patient data meta-analysis of nine randomized trials. Journal of Clinical Oncology. 2005;23:5074–87. doi: 10.1200/JCO.2005.09.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Storek J, Dawson MA, Storer B, et al. Immune reconstitution after allogeneic marrow transplantation compared with blood stem cell transplantation. Blood. 2001;97:3380–9. doi: 10.1182/blood.v97.11.3380. [DOI] [PubMed] [Google Scholar]
- 37.McDonald GB. Hepatobiliary complications of hematopoietic cell transplantation, 40 years on. Hepatology. 2010;51:1450–60. doi: 10.1002/hep.23533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fried RH, Murakami CS, Fisher LD, Wilison RA, Sullivan KM, McDonald GB. Ursodeoxycholic acid treatment of refractory chronic graft-versus-host disease of the liver. Ann Intern Med. 1992;116:624–9. doi: 10.7326/0003-4819-116-8-624. [DOI] [PubMed] [Google Scholar]
- 39.Hingorani SR, Seidel K, Lindner A, Aneja T, Schoch G, McDonald G. Albuminuria in hematopoietic cell transplantation patients: prevalence, clinical associations, and impact on survival. Biology of Blood & Marrow Transplantation. 2008;14:1365–72. doi: 10.1016/j.bbmt.2008.09.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Freudenberger TD, Madtes DK, Curtis JR, Cummings P, Storer BE, Hackman RC. Association between acute and chronic graft-versus-host disease and bronchiolitis obliterans organizing pneumonia in recipients of hematopoietic stem cell transplants. Blood. 2003;102:3822–8. doi: 10.1182/blood-2002-06-1813. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.