Abstract
Dietary freeze-dried black raspberries inhibit tumor induction by N-nitrosomethylbenzylamine in the rat esophagus, but the constituents responsible for this chemopreventive activity have not been identified. We fractionated freeze-dried black raspberries and used mouse epidermal JB6 Cl 41 cells stably transfected with either a nuclear factor kappa B (NFκB)- or an activator protein 1 (AP-1)-luciferase reporter, and treated with racemic anti-benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE), to assess the inhibitory effects of the fractions. The ethanol and water extracts of the freeze-dried black raspberries had inhibitory activity and these extracts were fractionated by HPLC to give several bioactive fractions. Further HPLC analysis yielded multiple subfractions, some of which inhibited BPDE-induced NFκB activity. Major constituents of the most active subfractions were identified by their spectral properties and in comparison with standards as cyanidin-3-O-glucoside, cyanidin 3-O-(2G-xylosylrutinoside) and cyanidin 3-O-rutinoside. Analysis of freeze-dried black raspberries indicated that these three components comprised ∼3.4% of the material by dry weight. Consistent with these results, standard cyanidin-3-O-glucoside and cyanidin chloride were also good inhibitors of BPDE-induced NFκB activity. The results of this study demonstrate that cyanidin glycosides of freeze-dried black raspberries are bioactive compounds which could account for at least some of the chemopreventive activity observed in animal models.
Introduction
Multiple epidemiologic studies show an inverse relationship between fruit consumption and cancer of the esophagus (1). An International Agency for Research on Cancer working group evaluated 16 case-control studies of fruit consumption and esophageal cancer and found a mean relative risk for high versus low consumption of 0.54, with a range 0.14–1.50. They concluded however that some of the results could have been due to confounding because of insufficient control for smoking and alcohol consumption and that the overall evidence of an association was limited (2). An earlier working group concluded that the evidence that diets high in vegetables and fruits decrease the risk of esophageal cancer is convincing (3). Based on these data, it is plausible that chemoprevention of esophageal cancer could be accomplished by dietary modification and/or use of agents found in fruits or vegetables. The development of chemopreventive agents against esophageal cancer is very important, as this disease has a poor prognosis, with a 5-year survival rate of only 14% (4).
In pursuit of this goal, Stoner and co-workers (5–8) have used the well-established N-nitrosomethylbenzylamine (NMBA) rat esophageal tumor model to evaluate numerous potential chemopreventive agents. Kresty et al. (9) demonstrated that freeze-dried black raspberries (Rubus occidentalis), administered in the diet at levels of 5 or 10%, significantly inhibited tumor induction in this model, in both the initiation and promotion/progression stages of carcinogenesis. Inhibition in the promotion/progression phase is of particular interest, as there are few chemopreventive agents with this activity in the rat esophagus. Freeze-dried black raspberries also inhibited tumors induced by carcinogens in the hamster oral cavity and rat colon (10,11).
The constituents of black raspberries responsible for the inhibition of tumorigenesis in the esophagus and other tissues have not been identified. Black raspberries are rich in nutrients and non-nutritive phytochemicals (9). Previous work focused on ellagic acid and chromatographic fractions of black raspberry extract. The amounts of ellagic acid in freeze-dried black raspberries were insufficient to explain the inhibitory activity against rat esophageal tumorigenesis (9,12,13). Huang et al. (14) used mouse epidermal JB6 Cl 41 cells stably transfected with either a nuclear factor kappa B (NFκB)- or an activator protein 1 (AP-1)-luciferase reporter to assess the effects of black raspberry fractions on NFκB and AP-1 activities induced by racemic anti-benzo[a]pyrene-7,8-diol-9,10-epoxide (BPDE). They demonstrated that polar constituents of black raspberry extract were able to inhibit NFκB and AP-1 activities. These findings are potentially important as NFκB and AP-1 play an important role in tumor promotion and carcinogenesis (15,16). Furthermore, recent studies suggest that extracts of fruits such as pomegranates and lingonberries modulate NFκB and AP-1 activity and are protective against tumor promotion (17,18). In the present study, we used the NFκB and AP-1 assays to identify anthocyanins as specific constituents of freeze-dried black raspberries with inhibitory properties that are potentially responsible for the chemopreventive activity of this fruit. There is considerable evidence in the literature that anthocyanins have chemopreventive properties (19).
Materials and methods
Apparatus
HPLC was carried out with Waters Associates (Milford, MA) systems equipped with a model 996 photodiode array detector or a model 440 UV–vis detector. LC-ESI-MS was carried out with a Thermo Finnigan LCQ Deca instrument (Thermo Finnigan LC/MS Division, San Jose, CA) interfaced with a Waters Alliance 2690 multisolvent delivery system and equipped with an SPD-10 A UV detector (Shimadzu Scientific Instruments, Columbia, MD).
UV spectra were determined on a Beckman DU 7400 photodiode array spectrophotometer. NMR spectra were recorded in CF3CO2D:CD3OD/1:19 or in CF3CO2D:D2O using a 600 MHz Varian UNITY Inova equipped with a BioSelect triple resonance H/C/N probe.
Chemicals
BPDE was from Eagle-Picher Industries, Chemsyn Science Laboratories (Lenexa, KS). Cyanidin-3-O-glucoside, cyanidin chloride and cyanidin 3-O-rutinoside were obtained from Indofine (Sommerville, NJ) and Extrasynthese (Cenay Cedex, France). Solvents were reagent grade.
Freeze-dried black raspberries
Black raspberries (Rubus occidentalis) of the Jewel variety were obtained from the Stokes Fruit Farm in Wilmington, OH. They were grown conventionally on the same plot of the berry field as berries obtained in the previous years, picked mechanically at about the same degree of ripeness, washed and frozen at −20°C within an hour of the time of picking. The berries were then shipped frozen to Van Drunen Farms in Momence, IL where they were kept frozen until freeze-dried in a Virtis sublimator under vacuum at an initial temperature of −40°C. The freeze-dried berries (pulp and seeds) were then ground in a pulverizer to 40 mesh and the berry powder shipped frozen to The Ohio State University where it was stored frozen until shipped to The Cancer Center, University of Minnesota.
Soxhlet extraction of freeze-dried black raspberries
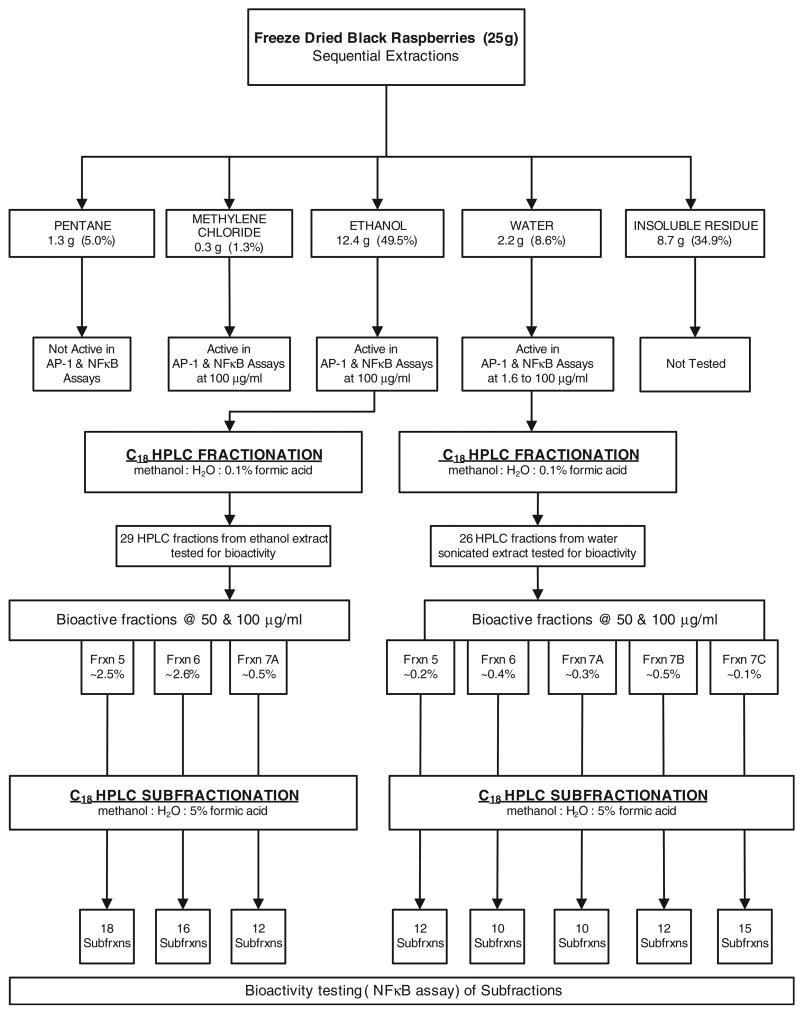
The approach is summarized in Figure 1. Freeze-dried black raspberries (25 g) were placed in a Soxhlet extraction thimble and extracted with 400 ml of pentane for 16 h. Then a fresh 400 ml of pentane was added and the extraction was repeated for 16 h. This procedure was repeated with the same volumes of methylene chloride and absolute ethanol. The residue was removed from the thimble and sonicated three times with 100 ml portions of water, 30 min each time. The extracts from each solvent extraction were combined and the solvents were removed by rotary evaporation, except in the case of water, which was removed by lyophilization. Each of the residues was dissolved in 0.1% DMSO and tested in the AP-1 and NFκB assays.
Fig. 1.
Summary of fractionation scheme used to identify constituents of freeze-dried black raspberries which could decrease BPDE-induced NFκB or AP-1 activity. Percent figures represent percentage by weight of the freeze-dried black raspberries.
Solvent extractions by stirring
Freeze-dried black raspberries (25 g) were placed in a 1 1 Erlenmeyer flask and sequentially extracted with stirring at room temperature with three 400 ml portions each of pentane, methylene chloride, absolute ethanol and water. An explosion-proof mechanical stirrer (Caframo, Wiarton, Ontario, Canada) was used. Each extraction was carried out for 16 h.
HPLC fractionation
Fractionation of the ethanol and water extracts was carried out by HPLC. Two semi-preparative Luna C18(2) 250 mm by 10 mm columns (5 μ particle size) (Phenomenex, Torrance, CA) in series were used. Solvents were A (water with 0.1% formic acid) and B (methanol with 0.1% formic acid) at a flow rate of 3 ml/min. The gradient was 5–16% B in 11 min, held for 10 min, increased to 50% B in 34 min, held for 10 min, increased to 80% B in 10 min and held for 20 min at 80% B. Detection was by UV at 254, 280 or 510 nm. Approximately 100 mg ethanol or water extract was injected on the HPLC columns. Multiple injections were carried out to obtain adequate material for testing. The ethanol extract was brought to a concentration of 300 mg/ml in water and each injection contained 100 mg extract. The water fraction was brought to 200 mg/ml in water and 100 mg injections were made.
A total of 30 fractions were collected from the ethanol extracts and 26 from the water fraction. The solvents were removed at reduced pressure on a Speed-Vac (ThermoSavant, Holbrook, NY) and the dried residues were shipped in dry ice to NYU. They were then dissolved in 0.1% dimethyl sulfoxide (DMSO) and immediately tested in the AP-1 and NFκB assays.
HPLC subfractionation
The same columns were used as in the fractionation described above. Solvents were A (water with 5% formic acid) and B (methanol with 5% formic acid). The flow rate was 3 ml/min. The gradient was 15–30% B in 120 min, then 5 min to 70% B, then held for 20 min. Detection was the same as in the above fractionation. The samples were made to a concentration of 10–50 mg/ml in 90:10 water:methanol containing 0.5% formic acid. Then, 25 mg of ethanol fractions 5–7A or water fractions 5–7C were injected.
Subfractions were collected, the solvents removed at reduced pressure and the dried residues shipped as above, dissolved in 0.1% DMSO and tested in the AP-1 and NFκB assays.
Cell-culture conditions and reagents
Mouse epidermal cell line JB6 Clone 41 (Cl 41) was transfected stably with either an AP-1-luciferase reporter (P+1-1) or a NFκB-luciferase reporter (Cl 41 NFκB mass1) (20–22). Cl 41, P+1-1 and Cl 41 NFκB mass1 were cultured in Eagle's Minimal Essential Medium supplemented with 5% fetal bovine serum (FBS), 2 mM l-glutamine and 25 μg of gentamicin/ml. Eagle's MEM was purchased from Calbiochem (San Diego, CA) and l-glutamine, gentamicin and FBS from Life Technologies, (Rockville, MD). The cells were cultured at 37°C in a humidified atmosphere of 5% CO2 in air. The cultures were dissociated with trypsin and transferred to new 75 cm2 culture flasks (Fisher, Pittsburgh, PA) from one to three times per week. The substrate for the luciferase assay was purchased from Promega (Madison, WI). BPDE was dissolved in DMSO at a stock concentration of 2 mM.
NFκB and AP-1 assays
These were carried out essentially as described previously (23–25). A confluent monolayer of P+1-1 or Cl 41 NFκB mass1 cells was trypsinized and 8 × 103 viable cells suspended in 100 μl of MEM supplemented with 5% FBS were added to each well of 96-well plates. Plates were incubated at 37°C in a humidified atmosphere of 5% CO2 in air. After the cell density reached 80–90%, the culture medium was replaced with an equal volume of MEM supplemented with 0.1% FBS and 2 mM l-glutamine. After 12 h, the cells were treated with different fractions of freeze-dried black raspberries or compounds as indicated. Cells were then exposed to BPDE at a final concentration of 2 μM. BPDE concentrations of 1–2 μM have been shown previously to produce maximum induction of NFκB and AP-1 activity in this system (26). The cells were extracted with lysis buffer (Promega) at 12 h after exposure to BPDE and the luciferase activity was determined using a luminometer (Wallac 1420 Victor 2 multilabel counter system) after the addition of 50 μl of lysis buffer for 30 min at 4°C. The time point of 12 h was determined based on previous time course results for BPDE exposure (26). The results are expressed as AP-1 or NFκB activity relative to control medium containing DMSO (0.1% v/v) only, which was used to dissolve the fractions (relative AP-1 or NFκB activity). DMSO was used because the fractions were insoluble in water alone.
Analysis of freeze-dried black raspberries for cyanidin glycosides
Freeze-dried black raspberries (1 g) were sonicated in 40 ml methanol containing 1% HCl for 2 h. After sonication, the extracts were centrifuged for 10 min. One milliliter of the supernatant was diluted with 4 ml of 80:20:1 water: methanol: formic acid. This was filtered through a 0.45 μm nylon filter and analyzed by HPLC with UV detection (280 and 520 nm). Two Phenomenex Luna C18(2) 250 × 4.6 mm columns were connected in series with a guard column. Solvent A was water with 5% formic acid and Solvent B was methanol with 5% formic acid. The gradient profile was 0–5 min at 20% B; then from 5 to 65 min 20–28% B; then from 65 to 85 min 28–80% B; then from 85 to 90 min at 80% B.
Results
The fractionation scheme is illustrated in Figure 1. The freezedried black raspberries were sequentially extracted with pentane, methylene chloride, ethanol and water. The resulting extracts were tested for inhibition of BPDE-induced NFκB and AP-1 activity. The results are illustrated in Figure 2A and B. Potential chemopreventive activity is indicated by a decrease in the expression of NFκB or AP-1 relative to DMSO solvent control, denoted by extract = 0, after treatment with BPDE. The pentane extract showed no decrease in either assay. The methylene chloride extract had intermediate activity in the NFκB assay and was inactive in the AP-1 assay. The ethanol and water extracts were significantly more active than the pentane or methylene chloride extracts in both assays (P < 0.05, t-test). The insoluble residue was not tested. Similar results were obtained in assays which tested extracts prepared by simply stirring the freeze-dried black raspberries with these solvents at room temperature (data not shown). Since the ethanol extract comprised nearly 50% by weight of the original material and the water extract had the highest activity, further fractionation of these extracts by HPLC was pursued.
Fig. 2.
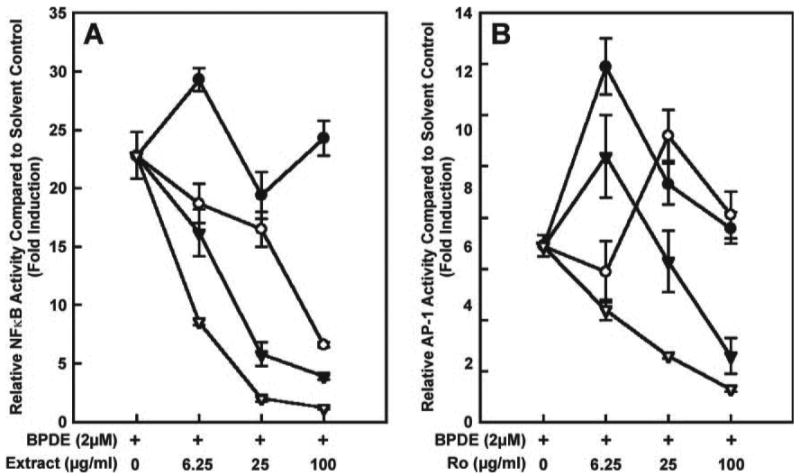
Ability of extracts of freeze-dried black raspberries to decrease BPDE (2 μM)-induced NFκB (A) and AP-1 (B) activity. Data are expressed as activity relative to the solvent control (0.1% DMSO, used to dissolve the extracts). The positive control is BPDE (2 μM, first point on x-axis). Closed circles, pentane extract; open circles, methylene chloride extract; closed triangles, ethanol extract; open triangles, water extract. Each point is the mean ± SD of three determinations.
HPLC chromatograms of the ethanol and water extracts are illustrated in Figure 3A and B. Nearly 90% by weight of the material in the ethanol extract eluted in the first 15 min of the chromatogram (fractions 1A-E) and had limited UV absorbance at 254 nm. Material in the water extract was more evenly distributed, with only ∼20% by weight eluting in the first 15 min. Both fractions produced complex chromatograms with baseline rises indicating multiple unresolved components. Each of the HPLC fractions indicated in the chromatograms—29 from the ethanol extract and 26 from the water extract—was tested for inhibition of BPDE-induced NFκB and AP-1 activity (Figure 1). The results are summarized in Table I. The highlighted fractions showed >50% inhibition in both assays, usually at both concentrations tested. Based on their activities, on the amounts of material present and on the presence of a major UV absorbing peak at 43.1 min (ethanol extract, Figure 3A) and 43.6 min (water extract, Figure 3B), HPLC fractions 5–7A of the ethanol extract and 5–7C of the water extract were chosen for further study. The results suggested that the same constituents of both extracts might be responsible for the activity in these fractions. Fractions 5–7A of the ethanol extract comprised 5.6% by weight of the original freeze-dried black raspberries while fractions 5–7C of the water extract comprised 1.5% (Figure 1).
Fig. 3.
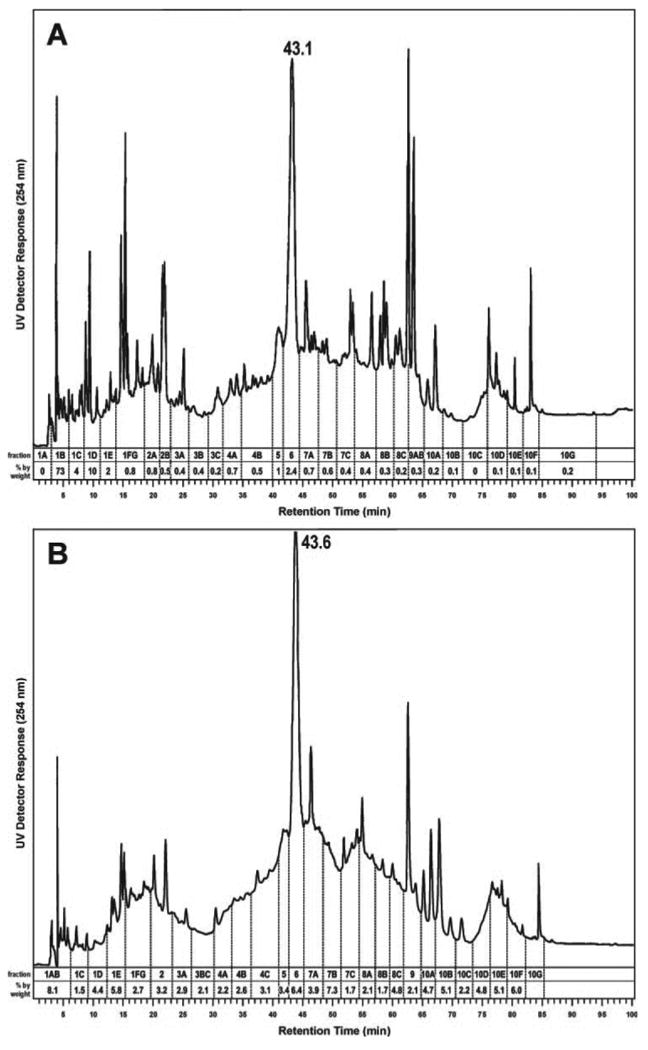
Chromatograms obtained upon HPLC analysis of (A) the ethanol extract and (B) the water extract of freeze-dried black raspberries.
Table I. Inhibition of BPDE-induced NFκB and AP-1 activity by fractions of the ethanol and water extracts of freeze-dried black raspberriesa,b.
| Ethanol fractions | Water fractions | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| HPLC fraction no. | NFκB activity | AP-1 activity | HPLC fraction no. | NFκB activity | AP-1 Activity | ||||
| Concentration of fraction in 0.1% DMSO (μg/ml) | Concentration of fraction in 0.1% DMSO (μg/ml) | ||||||||
| 50 | 10 | 50 | 10 | 50 | 10 | 50 | 10 | ||
| % Inhibition relative to BPDE (2 μM) and no added fraction | % Inhibition relative to BPDE (2 μM) and no added fraction | ||||||||
| 1a | −19.1 | −1.2 | NTc | NT | 1a and 1b | 14.5 | 9.3 | −4.0 | 1.0 |
| 1b | 2.9 | 8.3 | NT | NT | |||||
| 1c | 17.6 | 6.0 | 5.0 | 3.6 | 1c | 32.7 | 11.2 | 30.0 | −20.0 |
| 1d | −44.1 | 40.5 | 24.8 | −19.3 | 1d | 53.6 | 45.8 | 0.0 | −4.0 |
| 1e | 26.5 | 46.4 | 0.0 | −9.6 | 1e | 75.5 | 65.4 | 56.0 | 6.0 |
| 1f and 1g | 67.6 | 31.0 | 25.7 | −3.6 | 1f and 1g | 81.8 | 50.5 | 76.0 | 42.0 |
| 2a | 63.2 | 81.0 | 25.7 | 9.6 | 2 | 73.6 | 55.1 | 48.0 | 6.0 |
| 2b | 45.6 | 77.4 | 26.7 | −26.5 | |||||
| 3a | 52.9 | 36.9 | 18.8 | −2.4 | 3a | 65.5 | 44.9 | 48.0 | 25.0 |
| 3b | 45.6 | −15.5 | 32.7 | −20.5 | 3b and 3c | 72.7 | 67.3 | 89.0 | 50.0 |
| 3c | 70.6 | 64.3 | 57.4 | 0.0 | |||||
| 4a | 42.6 | 60.7 | 5.9 | −30.1 | 4a | 81.8 | 51.4 | 68.0 | 35.0 |
| 4b | 95.6 | 60.7 | 77.2 | 26.5 | 4b | 80.0 | 29.9 | 76.0 | 45.0 |
| 4c | 75.5 | 48.6 | 79.0 | 41.0 | |||||
| 5 | 100.0 | 83.3 | 96.0 | 51.8 | 5 | 93.6 | 57.9 | 92.0 | 63.0 |
| 6 | 100.0 | 52.4 | 91.1 | 53.0 | 6 | 99.1 | 50.5 | 93.0 | 72.0 |
| 7a | 94.7 | 63.3 | 97.0 | 68.7 | 7a | 83.6 | 22.4 | 94.0 | 61.0 |
| 7b | 57.9 | 43.3 | 34.7 | 1.2 | 7b | 87.3 | 57.9 | 82.0 | 51.0 |
| 7c | 30.3 | 17.8 | 11.8 | −13.3 | 7c | 97.3 | 72.0 | 88.5 | 55.1 |
| 8a | 100.0 | 76.7 | 91.4 | 65.1 | 8a | 97.3 | 70.7 | 82.7 | 24.5 |
| 8b | 67.1 | 34.4 | 24.7 | −19.3 | 8b | 98.2 | 66.7 | 73.1 | 10.2 |
| 8c | 27.6 | 51.1 | 26.9 | 6.0 | 8c | 64.5 | 28.0 | −48.1 | −63.3 |
| 9a | 26.3 | 34.4 | 41.9 | −6.0 | 9 | 91.8 | 78.7 | 57.7 | −10.2 |
| 10a | 50.0 | 17.8 | 50.5 | 9.6 | 10a | 63.6 | 25.3 | −117 | −143 |
| 10b | 86.8 | 65.6 | 77.4 | 44.6 | 10b | 40.0 | 33.3 | −140 | −131 |
| 10c | 100.0 | 72.2 | 81.7 | 73.5 | 10c | 70.0 | 30.7 | −50.0 | −85.7 |
| 10d | 14.5 | 16.7 | −6.5 | −34.9 | 10d | 61.8 | −5.3 | −137 | −139 |
| 10e | −5.3 | 41.1 | 8.6 | −2.4 | 10e | 56.4 | −6.7 | −129 | −116 |
| 10f | 5.3 | 12.2 | 65.6 | 30.1 | 10f | 51.8 | −34.7 | −148 | −155 |
| 10g | 15.8 | 34.4 | −21.5 | −31.3 | |||||
See Materials and methods for details of NFκB and AP-1 assays.
Highlighted fractions had >50% inhibitory activity for at least one concentration in both assays.
NT, not tested.
These fractions were then subfractionated by HPLC (HPLC subfractionation, Figure 1). Figure 4A illustrates the results obtained upon HPLC subfractionation of ethanol fraction 5. The 18 subfractions A–R were tested for inhibition of BPDE-induced NFκB activity. As shown in the inset of Figure 4A, most subfractions had some activity, but the most active were subfractions M, N and O which corresponded to the three large UV absorbing peaks and ∼27% by weight of the fraction. Similarly, Figure 4B and C show the results from fractions 6 and 7A, respectively. As was the case with fraction 5, the greatest NFκB repressing activity was found in the subfractions corresponding to the large UV absorbing peaks eluting at ∼63–76 min, e.g. J, JJ, K and L in Figure 4B and J in Figure 4C. Essentially similar HPLC traces and results were obtained upon testing of the subfractions of the water extract. An example is shown in Figure 4D which demonstrates that the greatest NFκB suppressing activity was found in subfractions F and G of fraction 6. These subfractions corresponded to large UV absorbing peaks of retention time 55–71 min (Figure 4D).
Fig. 4.
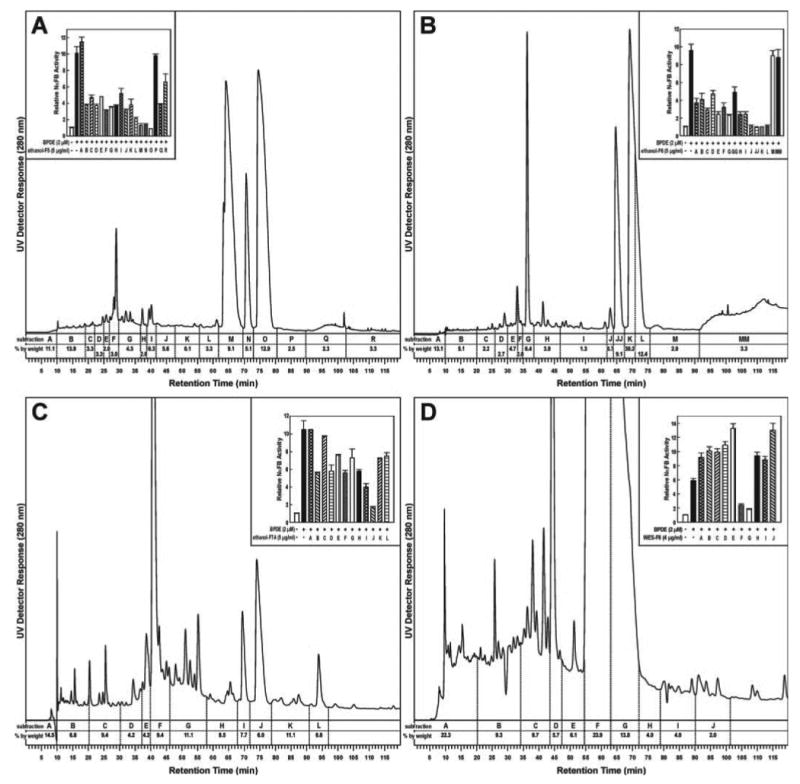
Chromatogram obtained upon HPLC analysis of (A) fraction 5 of the ethanol extract of freeze-dried black raspberries (see Figure 1); (B) fraction 6 of the ethanol extract: (C) fraction 7A of the ethanol extract; and (D) fraction 6 of the water extract. Subfractions were tested for their ability to decrease BPDE-induced NFκB activity and the results are shown in the insets as activity relative to control (0.1% DMSO) used to dissolve the extracts. Each bar represents the mean ± SD of three determinations.
Active constituents of the subfractions were identified by their spectral properties, in comparison with standards when available and by reference to previous studies on the constituents of black raspberries and related berries. The three major components of the active subfractions were thus identified as cyanidin-3-O-glucoside, cyanidin 3-O-(2G-xylosylrutinoside) and cyanidin 3-O-rutinoside, respectively (Figure 5).
Fig. 5.
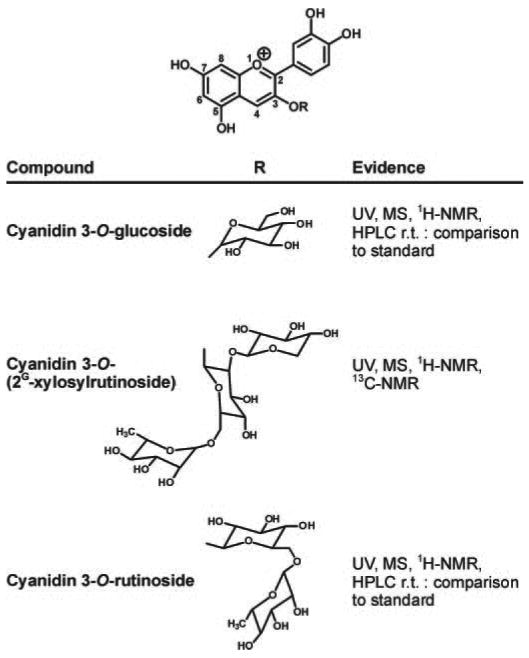
Structures of cyanidins and summary of evidence for their identification in bioactive fractions of freeze-dried black raspberries. Spectroscopic data were consistent with previously reported data (28).
The UV spectrum of subfraction M of ethanol fraction 5 (Figure 4A) is shown in trace 1 of Figure 6. This spectrum is characteristic of anthocyanins (27). The UV spectrum of standard cyanidin-3-O-glucoside is shown in trace 2 of Figure 6. The positive ion ESI-MS of the main peak of subfraction M showed a base peak of m/z 449 and a prominent fragment at m/z 287 corresponding to M+ and the aglycone of cyanidin-3-O-glucoside, respectively. A leading shoulder on the main peak of subfraction M had a base peak of m/z 581. The MS of the main peak of subfraction M was essentially identical to that of cyanidin-3-O-glucoside standard. The 1H-NMR spectrum of subfraction M was consistent with cyanidin-3-O-glucoside and had all resonances seen in the 1H-NMR spectrum of the standard. Co-injection of subfraction M with cyanidin-3-O-glucoside standard on HPLC demonstrated that they co-eluted. These results confirm the identity of the major peak in subfraction M as cyanidin-3-O-glucoside. Similar data were obtained for subfraction J of ethanol fraction 6 (Figure 4B), establishing its identity as cyanidin-3-O-glucoside.
Fig. 6.
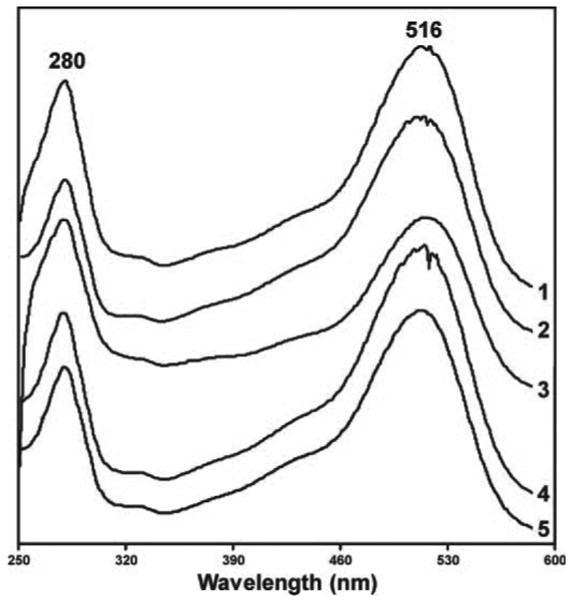
UV spectra of bioactive constituents and standards: 1, subfraction M of ethanol fraction 5; 2, standard cyanidin-3-O-glucoside; 3, subfraction JJ of ethanol fraction 6; 4, subfraction K of ethanol fraction 6; 5, standard cyanidin 3-O-rutinoside.
The UV spectrum of subfraction JJ of ethanol fraction 6 (Figure 4B) is shown in trace 3 of Figure 6. It was similar to that of cyanidin-3-O-glucoside. The positive ion ESI-MS of subfraction JJ had a base peak at m/z 727, corresponding to M+ of cyanidin 3-O-(2G-xylosylrutinoside) and an aglycone fragment at m/z 287. Subfraction N of ethanol fraction 5 (Figure 4A) had similar spectral properties. MS/MS of m/z 727 of subfraction JJ gave fragments at m/z 581 [M – rhamnose + H]+ (relative intensity, 7) and m/z 287 (26). There was also a small ion (relative intensity 0.2) at m/z 433. The 1H-NMR and 13C-NMR spectra of subfraction JJ were similar to those reported for cyanidin 3-O-(2G-xylosylrutinoside) (28). Heteronuclear correlation spectroscopy experiments were carried out to confirm the position of connectivity of the sugar moieties to the cyanidin. Three anomeric proton signals, between 4.45 and 5.40 p.p.m., were clearly distinguished from other sugar-based proton signals (between 2.95 and 4.00 p.p.m.) in the 1H-NMR spectrum. In the HMBC spectrum, it was clear that a single upfield cyanidin resonance (146 p.p.m.) displayed a cross-peak to an anomeric proton (5.39 p.p.m.). Based on the chemical shift values, this correlation was attributed to the attachment of the glucoside at the 3-position of cyanidin. There were no heteronuclear multiple bond correlation (HMBC) cross-peaks between rhamnosyl or xylosyl anomeric protons (4.55 and 4.70 p.p.m., respectively) and any of the cyanidin peaks. Additional HMBC correlations were consistent with the connectivity of the xylosyl and rhamnosyl moieties to the glucoside.
The UV spectrum of subfraction K of ethanol fraction 6 (Figure 4B) is shown in trace 4 of Figure 6, along with the spectrum of standard cyanidin 3-O-rutinoside (trace 5). The positive ion ESI-MS of subfraction K had a base peak at m/z 595 and an aglycone fragment at m/z 287, and was similar to that of standard cyanidin 3-O-rutinoside. The 1H-NMR of subfraction K was also similar to that of standard cyanidin 3-O-rutinoside. HPLC co-injection of subfraction K and the standard resulted in co-elution. These results demonstrate that subfraction K of ethanol fraction 6 is cyanidin 3-O-rutinoside. Subfraction L of ethanol fraction 6 (Figure 4B), subfraction O of ethanol fraction 5 (Figure 4A) and subfraction J of ethanol fraction 7A (Figure 4C) were similarly shown to consist mainly of cyanidin 3-O-rutinoside.
Using the identified cyanidin 3-O-(2G-xylosylrutinoside), and standard cyanidin 3-O-rutinoside and cyanidin-3-O-glucoside, the identities of the active subfractions of fractions 5–7C of the water extract were similarly established as these three cyanidin glycosides in comparison with UV spectra and HPLC co-injection.
Levels of the identified compounds in freeze-dried black raspberries were determined by extraction with acidic methanol followed by HPLC analysis. A representative chromatogram is illustrated in Figure 7. The indicated peaks were identified by their UV spectra and by co-injection with standards or the materials identified above. The isolated amounts (in mg/g dry weight) of each were: cyanidin-3-O-glucoside (2.1 mg/g), cyanidin 3-O-(2G-xylosylrutinoside) (12.1 mg/g) and cyanidin 3-O-rutinoside (20.5 mg/g).
Fig. 7.

Chromatogram obtained upon HPLC analysis of an acidic methanol extract of freeze-dried black raspberries with UV detection at 280 nm.
Standard cyanidin-3-O-glucoside and cyanidin chloride were good inhibitors of BPDE-induced NFκB activity (Figure 8). The lowest concentrations tested, 2.6 μM cyanidin-3-O-glucoside and 3.9 μM cyanidin chloride, inhibited NFκB activity by ∼60%.
Fig. 8.
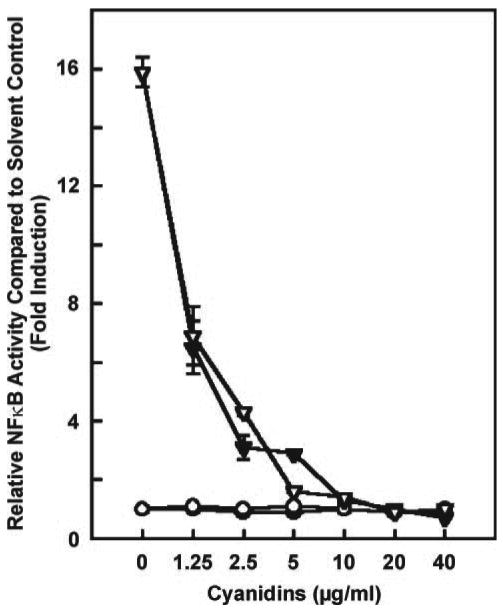
Ability of standard cyanidin-3-O-glucoside (open triangles) and cyanidin chloride (closed triangles) to decrease 2 μM BPDE-induced NFκB activity. The positive control is 2 μM BPDE with no cyanidin added (first point on x-axis). The cyanidins themselves, without BPDE, had no effect: cyanidin-3-O-glucoside (open circles) and cyanidin chloride (closed circles). Data are expressed as NFκB activity relative to control (0.1% DMSO) used to dissolve the extracts. Each point is the mean ± SD of three determinations.
Discussion
The results of this study demonstrate that anthocyanins in freeze-dried black raspberries are bioactive compounds with potential chemopreventive activity. Consistently, subfractions enriched in three anthocyanins—cyanidin-3-O-glucoside, cyanidin 3-O-(2G-xylosylrutinoside), and cyanidin 3-O-rutinoside—inhibited NFκB and AP-1 activity induced by BPDE. These anthocyanins were positively identified by their UV, MS and NMR spectral properties as well as their HPLC retention times which, in two cases, matched those of available standards. A standard of cyanidin 3-O-(2G-xylosylrutinoside) was not available, but its spectral properties were entirely consistent with the structure and with published data (28). The three anthocyanins comprised ∼3.4% of the freeze-dried black raspberries.
The identification of these three compounds is consistent with the data in the literature. Earlier studies summarized by Macheix et al. (29) had identified the same anthocyanins in black raspberries. More recent work using HPLC analysis also identified these compounds as major anthocyanins in black raspberries (30). However, Wu and Prior (31) recently postulated an alternate structure for cyanidin 3-O-(2G-xylosylrutinoside) in black raspberries. Based on the presence of an m/z 433 peak in the MS/MS, they proposed that the correct structure is cyanidin-3-sambubioside-5-rhamnoside. Our data are not consistent with this proposal because we did not see a significant m/z 433 peak and, more importantly, our HMBC data clearly demonstrated that the trisaccharide was attached to the cyanidin ring through the glucosyl moiety, with no connectivity between the cyanidin ring and the rhamnosyl moiety. In previous studies, two earlier eluting and relatively minor components were reported to be cyanidin 3-O-sophoroside and cyanidin 3-O-(2G–glucosylrutinoside) (30). Cyanidin 3-O-sambubioside has also been reported in earlier studies and there is some evidence of its presence here, as the leading shoulder on Peak M of Figure 4A, which had an M+ of m/z 581.
Our results are consistent with a previous study which demonstrated that a methanol extract of freeze-dried black raspberries inhibited AP-1 and NFκB activity in the assay system used here (14). Limited further fractionation of the methanol extract, by solvent partitioning and column chromatography, was also carried out in that study and the greatest activity was found in the most polar chromatographic fraction, in agreement with the data presented here. However, detailed fractionation and constituent identification were not pursued in the previous study.
Our results also agree with those of a recent study which investigated the effects of anthocyanidins (the aglycones of anthocyanins) on cell transformation and AP-1 activity (32). Cyanidin chloride (5–20 μM) and two other anthocyanidins having ortho-dihydroxy groups—delphinidin and petunidin—inhibited 12-O-tetradecanoylphorbol-13-acetate (TPA)-induced JB6 cell transformation and AP-1 activation. The inhibitory effects of anthocyanidins on AP-1 activation may be due in part to their ability to scavenge superoxide radicals or block mitogen-activated protein kinase pathways (32,33). However, the concentrations tested in these in vitro experiments are considerably higher than the levels of anthocyanins reached in humans. Stoner et al. (34) have recently shown that consumption of 45 g of freeze-dried black raspberries resulted in plasma concentrations of anthocyanins ranging from 0.004 to 0.038 μM. Bioavailability of anthocyanins is generally low (35).
Collectively, then, the results reported here showing that cyanidin glycosides in freeze-dried black raspberries inhibit BPDE-induced NFκB and AP-1 activity are fully consistent with previous reports demonstrating that: (i) fractions of freeze-dried black raspberries have such activity (14); (ii) cyanidin glycosides are constituents of freeze-dried black raspberries (29); and (iii) cyanidin chloride inhibits AP-1 activity (32).
A strength of this study was our approach to active constituent identification, which was based on an unbiased, bioassay-directed fractionation of freeze-dried black raspberries, and yielded consistent and convincing results using relatively small amounts of material. For example, the pentane extract, which would not contain polar constituents such as anthocyanidins, was inactive. However, our fractionation scheme was somewhat redundant in that the active constituents of the ethanol and water extracts were mostly the same, although the ethanol extract was more enriched in early eluting polar material, most likely fructose, glucose and other sugars (36). In retrospect, sequential extractions with pentane and an ethanol–water mixture might have been more efficient.
A potential limitation of this study is the bioassay system used. While cyanidin glycosides were bioactive constituents of freeze-dried black raspberries based on the inhibition of BPDE-induced NFκB and AP-1 activity, it is unclear whether these results will translate to inhibition of NMBA-induced carcinogenesis in the rat esophagus. There is presently no evidence that NFκB is involved in esophageal tumor induction by NMBA in the rat. Recently, however, we have shown that AP-1 (c-Jun) is upregulated in NMBA-induced rat esophageal tumors, and its expression at both the m-RNA and protein levels is downregulated by dietary freeze-dried black raspberries (37). The most straightforward way to test the chemopreventive activity of the cyanidin glycosides identified here is to assess their activity in the same rat esophageal tumor model used for freeze-dried black raspberries. We are currently carrying out large scale fractionations to provide the material for these assays. If the cyanidin glycosides are effective chemopreventive agents in the rat esophageal tumor model, they would then be candidates for further efficacy testing in clinical studies of individuals at high risk for esophageal cancer, although we recognize that the NMBA model itself may be limited with respect to human esophageal cancer.
In summary, we have identified cyanidin-3-O-glucoside, cyanidin 3-O-(2G-xylosylrutinoside) and cyanidin 3-O-rutinoside as constituents of freeze-dried black raspberries that inhibit BPDE-induced NFκB and AP-1 activity. These results suggest that these same constituents might be responsible for the chemopreventive activity of freeze-dried black raspberries against chemically induced tumors in rodent models.
Acknowledgments
This study was supported by grants CA-96130, CA-103180 and ES-012451 from the National Institutes of Health. We thank Bob Carlson for outstanding editorial assistance.
Abbreviations
- AP-1
activator protein 1
- BPDE
benzo[a]pyrene-7,8-diol-9,10-epoxide
- DMSO
dimethyl sulfoxide
- NFκB
nuclear factor kappa B
- NMBA
N-nitrosomethylbenzylamine
Footnotes
Conflict of Interest Statement: None declared.
References
- 1.International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention. Vol. 8. IARC; Lyon: 2003. Fruit and Vegetables; pp. 63–67. [Google Scholar]
- 2.International Agency for Research on Cancer. IARC Handbooks of Cancer Prevention. Vol. 8. IARC; Lyon: 2003. Fruit and Vegetables; pp. 316–323. [Google Scholar]
- 3.World Cancer Research Fund and American Institute for Cancer Research. Food, Nutrition and the Prevention of Cancer: A Global Perspective. American Institute for Cancer Research; Washington, DC: 1997. pp. 118–29. [DOI] [PubMed] [Google Scholar]
- 4.Jemal A, Murray T, Ward E, Samuels A, Tiwari RC, Ghafoor A, Feuer EJ, Thun MJ. Cancer statistics, 2005. CA Cancer J Clin. 2005;55:10–30. doi: 10.3322/canjclin.55.1.10. [DOI] [PubMed] [Google Scholar]
- 5.Stoner GD, Gupta A. Etiology and chemoprevention of esophageal squamous cell carcinoma. Carcinogenesis. 2001;22:1737–1746. doi: 10.1093/carcin/22.11.1737. [DOI] [PubMed] [Google Scholar]
- 6.Carlton PS, Gopalakrishnan R, Gupta A, Liston BW, Habib S, Morse MA, Stoner GD. Piroxicam is an ineffective inhibitor of N-nitrosomethylbenzylamine-induced tumorigenesis in the rat esophagus. Cancer Res. 2002;62:4376–4382. [PubMed] [Google Scholar]
- 7.Liston BW, Nines R, Carlton PS, Gupta A, Aziz R, Frankel W, Stoner GD. Perillyl alcohol as a chemopreventive agent in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Cancer Res. 2003;63:2399–2403. [PubMed] [Google Scholar]
- 8.Stoner GD, Qin H, Chen T, Carlton PS, Rose ME, Aziz RM, Dixit R. The effects of L-748706, a selective cyclooxygenase-2 inhibitor, on N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis. Carcinogenesis. 2005;26:1590–1595. doi: 10.1093/carcin/bgi111. [DOI] [PubMed] [Google Scholar]
- 9.Kresty LA, Morse MA, Morgan C, Carlton PS, Lu J, Gupta A, Blackwood M, Stoner GD. Chemoprevention of esophageal tumorigenesis by dietary administration of lyophilized black raspberries. Cancer Res. 2001;61:6112–6119. [PubMed] [Google Scholar]
- 10.Casto BC, Kresty LA, Kraly CL, Pearl DK, Knobloch TJ, Schut HA, Stoner GD, Mallery SR, Weghorst CM. Chemoprevention of oral cancer by black raspberries. Anticancer Res. 2002;22:4005–4015. [PubMed] [Google Scholar]
- 11.Harris GK, Gupta A, Nines RG, Kresty LA, Habib SG, Frankel WL, LaPerle K, Gallaher DD, Schwartz SJ, Stoner GD. Effects of lyophilized black raspberries on azoxymethane-induced colon cancer and 8-hydroxy-2′-deoxyguanosine levels in the Fischer 344 rat. Nutr Cancer. 2001;40:125–133. doi: 10.1207/S15327914NC402_8. [DOI] [PubMed] [Google Scholar]
- 12.Daniel EM, Stoner GD. The effects of ellagic acid and 13-cis-retinoic acid on N-nitrosobenzylmethylamine-induced esophageal tumorigenesis in rats. Cancer Lett. 1991;56:117–124. doi: 10.1016/0304-3835(91)90085-v. [DOI] [PubMed] [Google Scholar]
- 13.Daniel EM, Krupnick AS, Heur YH, Blinzler JA, Nims RW, Stoner GD. Extraction, stability, and quantitation of ellagic acid in various fruits and nuts. J Food Compos Anal. 1989;2:338–349. [Google Scholar]
- 14.Huang C, Huang Y, Li J, Hu W, Aziz R, Tang MS, Sun N, Cassady J, Stoner GD. Inhibition of benzo(a)pyrene diol-epoxide-induced transactivation of activated protein 1 and nuclear factor kappaB by black raspberry extracts. Cancer Res. 2002;62:6857–6863. [PubMed] [Google Scholar]
- 15.Greten FR, Karin M. The IKK/NF-κB activation pathway—a target for prevention and treatment of cancer. Cancer Lett. 2004;206:193–199. doi: 10.1016/j.canlet.2003.08.029. [DOI] [PubMed] [Google Scholar]
- 16.Young MR, Yang HS, Colburn NH. Promising molecular targets for cancer prevention: AP-1, NF-κB and Pdcd4. Trends Mol Med. 2003;9:36–41. doi: 10.1016/s1471-4914(02)00009-6. [DOI] [PubMed] [Google Scholar]
- 17.Afaq F, Saleem M, Krueger CG, Reed JD, Mukhtar H. Anthocyanin- and hydrolyzable tannin-rich pomegranate fruit extract modulates MAPK and NF-κB pathways and inhibits skin tumorigenesis in CD-1 mice. Int J Cancer. 2005;113:423–433. doi: 10.1002/ijc.20587. [DOI] [PubMed] [Google Scholar]
- 18.Wang SY, Feng R, Bowman L, Penhallegon R, Ding M, Lu Y. Antioxidant activity in lingonberries (Vaccinium vitis-idaea L.) and its inhibitory effect on activator protein-1, nuclear factor-κB, and mitogen-activated protein kinases activation. J Agric Food Chem. 2005;53:3156–3166. doi: 10.1021/jf048379m. [DOI] [PubMed] [Google Scholar]
- 19.Cooke D, Steward WP, Gescher AJ, Marczylo T. Anthocyans from fruits and vegetables- does bright colour signal cancer chemopreventive activity? Eur J Cancer. 2005;41:1931–1940. doi: 10.1016/j.ejca.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 20.Li J, Chen H, Tang MS, Shi X, Amin S, Desai D, Costa M, Huang C. PI-3K and Akt are mediators of AP-1 induction by 5-methylchrysene-1,2-diol-3,4-epoxide (5-MCDE) in mouse epidermal Cl41 cells. J Cell Biol. 2004;165:77–86. doi: 10.1083/jcb.200401004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li J, Tang MS, Liu B, Shi X, Huang C. A critical role of PI-3K/Akt/JNKs pathway in benzo[a]pyrene diol-epoxide (BPDE)-induced AP-1 transactivation in mouse epidermal Cl41 cells. Oncogene. 2004;23:3932–3944. doi: 10.1038/sj.onc.1207501. [DOI] [PubMed] [Google Scholar]
- 22.Li J, Chen H, Ke Q, Feng Z, Tang MS, Liu B, Amin S, Costa M, Huang C. Differential effects of polycyclic aromatic hydrocarbons on transactivation of AP-1 and NF-kappaB in mouse epidermal cl41 cells. Mol Carcinog. 2004;40:104–115. doi: 10.1002/mc.20020. [DOI] [PubMed] [Google Scholar]
- 23.Huang C, Ma WY, Ryan CA, Dong Z. Proteinase inhibitors I and II from potatoes specifically block UV-induced activator protein-1 activation through a pathway that is independent of extracellular signal-regulated kinases, c-Jun N-terminal kinases, and P38 kinase. Proc Natl Acad Sci USA. 1997;94:11957–11962. doi: 10.1073/pnas.94.22.11957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Huang C, Ma WY, Hecht SS, Dong Z. Inositol hexaphosphate inhibits cell transformation and activator protein-1 activation by targeting phosphatidylinositol-3′ kinase. Cancer Res. 1997;57:2873–2878. [PubMed] [Google Scholar]
- 25.Huang C, Ma WY, Dong Z. Requirement for phosphatidylinositol 3-kinase in epidermal growth factor-induced AP-1 transactivation and transformation in JB6 P+ cells. Mol Cell Biol. 1996;16:6427–6435. doi: 10.1128/mcb.16.11.6427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li J, Chen H, Ke Q, Feng Z, Tang MS, Liu B, Amin S, Costa M, Huang C. Differential effects of polycyclic aromatic hydrocarbons on transactivation of AP-1 and NF-κB in mouse epidermal cl41 cells. Mol Carcinog. 2004;40:104–115. doi: 10.1002/mc.20020. [DOI] [PubMed] [Google Scholar]
- 27.Merken HM, Beecher GR. Measurement of food flavonoids by high-performance liquid chromatography: a review. J Agric Food Chem. 2000;48:577–599. doi: 10.1021/jf990872o. [DOI] [PubMed] [Google Scholar]
- 28.Cabrita L, Froystein NA, Andersen OM. Anthocyanin trisaccharides in blue berries of Vaccinium padifolium. Food Chem. 2000;69:33–36. [Google Scholar]
- 29.Macheix J, Fleuriet A, Billot J. Fruit Phenolics. CRC Press; Boca Raton, FL: 1990. pp. 117–118. [Google Scholar]
- 30.McGhie TK, Ainge GD, Barnett LE, Cooney JM, Jensen DJ. Anthocyanin glycosides from berry fruit are absorbed and excreted unmetabolized by both humans and rats. J Agric Food Chem. 2003;51:4539–4548. doi: 10.1021/jf026206w. [DOI] [PubMed] [Google Scholar]
- 31.Wu X, Prior RL. Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: fruits and berries. J Agric Food Chem. 2005;53:2589–2599. doi: 10.1021/jf048068b. [DOI] [PubMed] [Google Scholar]
- 32.Hou DX, Kai K, Li JJ, Lin S, Terahara N, Wakamatsu M, Fujii M, Young MR, Colburn N. Anthocyanidins inhibit activator protein 1 activity and cell transformation: structure-activity relationship and molecular mechanisms. Carcinogenesis. 2004;25:29–36. doi: 10.1093/carcin/bgg184. [DOI] [PubMed] [Google Scholar]
- 33.Hou DX, Fujii M, Terahara N, Yoshimoto M. Molecular mechanisms behind the chemopreventive effects of anthocyanidins. J Biomed Biotechnol. 2004;2004:321–325. doi: 10.1155/S1110724304403040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stoner GD, Sardo C, Apseloff G, et al. Pharmacokinetics of anthocyanins and ellagic acid in healthy volunteers fed freeze-dried black raspberries daily for 7 days. J Clin Pharmacol. 2005;45:1153–1164. doi: 10.1177/0091270005279636. [DOI] [PubMed] [Google Scholar]
- 35.Manach C, Williamson G, Morand C, Scalbert A, Remesy C. Bioavailability and bioefficacy of polyphenols in humans I Review of 97 bioavailability studies. Am J Clin Nutr. 2005;81:230S–242S. doi: 10.1093/ajcn/81.1.230S. [DOI] [PubMed] [Google Scholar]
- 36.Wrolstad RE, Culbertson JD, Nagaki DA, Madero CF. Sugars and nonvolatile acids of blackberries. J Agric Food Chem. 1980;28:553–558. doi: 10.1021/jf60229a038. [DOI] [PubMed] [Google Scholar]
- 37.Chen T, Hwang H, Rose ME, et al. Chemopreventive properties of black raspberries in N-nitrosomethylbenzylamine-induced rat esophageal tumorigenesis:down regulation of cyclooxygenase-2, inducible nitric oxide synthase, and c-Jun. Cancer Res. 2006;66:2853–2859. doi: 10.1158/0008-5472.CAN-05-3279. [DOI] [PMC free article] [PubMed] [Google Scholar]