Abstract
In 1960, Dr. Bayard Clarkson described a patient experiencing sporadic episodes of hypovolemia, hypotension, and edema. Plasma during the acute attack induced a “shock”-like syndrome when given systemically in rats. The unusual and enigmatic “Systemic Capillary Leak Syndrome” (SCLS) named for Dr. Clarkson is of unknown etiology and is characterized by transient, severe, reversible hemoconcentration and hypoalbuminemia due to leakage of fluids and macromolecules (up to 900 kDa) into tissues (1). Fewer than 150 cases of SCLS have been reported since 1960, but the nonspecific presenting symptoms and signs and high mortality rate may have resulted in under-recognition of this disorder. Given the substantial overlap of SCLS with other “shock” syndromes, including sepsis, anaphylaxis, and angioedema, clinicians should consider this diagnosis in patients with unexplained edema, increased hematocrit, and hypotension.
INTRODUCTION
The patient with the baffling constellation of signs and symptoms evaluated by Dr. Clarkson in 1960 exemplifies prototypical SCLS: an otherwise healthy 34 year-old woman with sudden onset low-grade fevers, progressively decreasing, and ultimately unmeasurable, blood pressure, massive swelling of the face, arms, and legs, and increasing hematocrit (>60 from a baseline of 35–40) (2). Now considered typical of SCLS, the unexplained shock and anasarca were quickly followed by a period of massive diuresis and diminution of peripheral edema. Dr. Clarkson’s patient eventually died of pulmonary edema and cardiac failure during this phase of an attack.
Dr. Clarkson’s studies raised the question of the cause of hypovolemic shock in SCLS. Rapid plasma clearance of T-1824 (Evans blue) dye and radiodinated albumin turnover rates suggested plasma extravasation followed by hemoconcentration and vascular collapse (3). Studies of thyroid, gonadal, and adrenal steroid function as well as tests of immune and metabolic function available at the time were unremarkable. However, an anomalous gamma globulin (“paraprotein”) was identified in the serum of the index patient. We now refer to this as monoclonal gammopathy of unknown significance (MGUS), usually IgG kappa, present in up to 82% of SCLS patients (4).
In this narrative review, we discuss the diagnosis of this extremely rare and often fatal disease, the up-to-date clinical management of an acute SCLS episode, and current empiric therapy to prevent attacks. In a few patients, treatment targeting the plasma cell population responsible for the monoclonal gammopathy, with or without a decrease in serum paraprotein, reduced leak symptoms. Newer therapies efficacious in MGUS-related syndromes and myeloma should also be explored for SCLS in clinical trials. Increased recognition of SCLS and an improved understanding of pathogenic mechanisms are vital to improving outcome.
METHODS
We performed Medline and Scopus searches of articles from 1960–2010 using the search terms “systemic capillary leak syndrome”, “idiopathic capillary leak syndrome”, “capillary leak”, “vascular leak”, and “vascular permeability”, retrieving articles in English, French, and Chinese. Given the extreme dearth of clinical cases, most references report findings from a single patient, and with the exception of the therapeutic experience from the Mayo Clinic, none included more than 3 patients. For this reason, we have made every effort to summarize trends from separate reports where similar procedures or tests were done (e.g. skin biopsies). However, conclusions from such studies should be interpreted with caution since in most cases there was considerable variability in disease severity, treatments and temporal association of sample collection to acute symptoms. Where possible, we compare and contrast published findings with our experience in evaluating and treating 25 well defined SCLS patients at Mayo Clinic and 16 patients seen at NIAID (some were seen at both institutions). The funding sources had no role in the design, analysis, or reporting of this study or in the decision to submit the manuscript for publication.
EPIDEMIOLOGY
100 cases of SCLS were reported in the world literature between 1960 and 2006, according to recent reviews (5–7). We identified an additional 26 published cases since 2006 (8–27). This apparent increase in incidence may be due to greater awareness and recognition of the disease. Although it has been described in children, the disease is sporadic and is diagnosed most often in previously healthy, middle-aged, Caucasian adults (median age ± S.D.: 45 ± 15 yrs; age range 5 months to 74 years). There is no geographical or gender preponderance (5). Based on 107 cases where information was available, 57% were male. SCLS has been described in a 5 month old infant (11) and in three children [aged 3 yrs. old (28) and 6 yrs. old (14, 29)] who presented with prototypical recurrent shock episodes. At the Mayo Clinic, we have seen a newborn that experienced an attack shortly after being birthed by a patient with SCLS but did not experience further SCLS episodes. Recently, a case of familial SCLS was reported (8); however, the clinical histories of affected relatives of the index patient were vague.
CAUSE AND PATHOPHYSIOLOGY
Histological studies
The molecular etiology of SCLS is unknown, and systematic research studies are limited due its rarity. Immune dysregulation may have a function in disease pathogenesis. Increased numbers of circulating CD25+ cells (30) and CD25+ T cells (14) were documented in two separate case reports, but no further immunophenotyping was performed. Skin biopsies in 4 out of 9 patients taken during acute SCLS episodes showed perivascular mononuclear infiltrates while the remainder was reported as normal by light microscopy (1, 31–36). However, one patient had a maculopapular rash after amoxicillin treatment that did not accompany subsequent episodes (36). 8 out of 10 muscle biopsies were normal, including those from 3 patients with cutaneous perivascular infiltrates (1, 32, 34–39). Although immunofluorescence staining for immunoglobulin (Ig) or complement was performed on a majority of the biopsy specimens, no Ig or complement deposits were seen in any of them.
Ultrastructural and functional studies have provided insight into endothelial dysfunction in SCLS. Electron photomicrographs of skeletal muscle endothelial cells from one SCLS patient suggested apoptosis (cell blebbing) without widening of intercellular gaps (40). Serum from other SCLS patients induced apoptosis of cultured microvascular endothelial cells derived from healthy donors (41). However, serum from patients with sepsis or pancreatitis induced apoptosis to a similar degree. When considered with the duration of capillary leak and its reversibility, these findings suggest that endothelial injury and apoptosis, rather than simply prolonged contraction/retraction, may cause the vascular leakage associated with SCLS. This distinction is critical, as many circulating components associated with anaphylaxis and shock typically induce endothelial shape change, retraction, and decreased intercellular connections rather than outright cell death (42).
Soluble mediators
No soluble factors have been associated specifically or typically with SCLS. Nonetheless, we cannot rule out a circulating factor that elicits attacks. Most serum analytes are within the normal range in SCLS patients, including complement and C1 esterase inhibitor levels (24), and no coagulation abnormalities, elevations in compounds known to induce capillary permeability [e.g. bradykinin, histamine (34), and prostaglandins], or endocrine dysfunction have been reported, with the isolated exception of increased C3a in one patient (1, 37, 43). In two recent studies, 3 patients had elevated serum IL-6 and TNFα, suggesting a systemic inflammatory response (14, 38). However, the role of these cytokines in SCLS pathogenesis is unclear as levels varied widely between patients, and no baseline levels were reported. Moreover, proinflammatory cytokines remained elevated long after capillary permeability resolved in 2 of the patients (14).
Recently, 2 patients with SCLS were reported to have high levels of plasma vascular endothelial growth factor (VEGF) during an acute, severe episode, which decreased with symptom resolution (12). We have also found high baseline plasma VEGF in several SCLS patients (unpublished data). VEGF may contribute to endothelial permeability in several disorders including sepsis (44), ovarian hyperstimulation syndrome (45), and the MGUS-related disorder, POEMS syndrome (polyneuropathy, organomegaly, endocrinopathy, monoclonal gammopathy, and skin changes). There is considerable overlap between POEMS and SCLS in that POEMS patients often have evidence of chronic vascular leak such as peripheral edema, visceral effusions, papilledema, and polycythemia (46). VEGF is also increased in plasma cells of myeloma patients (47). However, we point out that the source of VEGF and its function in the pathogenesis in SCLS is not known.
Role of the monoclonal paraprotein
Whereas approximately 79–82% of SCLS patients have MGUS (5, 7), it is present in only 3% of the general population over 50 and is usually asymptomatic. MGUS, in contrast to multiple myeloma, is defined by a serum monoclonal Ig < 3 g/dL, the presence of <10% bone marrow plasma cells, and the absence of end-organ damage such as lytic bone lesions or renal failure. The risk of progression to myeloma is ~1%/yr (48), and the incidence is similar in patients with SCLS and in patients with MGUS. SCLS has occasionally been diagnosed in the setting of multiple myeloma (49–52), amyloidosis (49), and plasma cell leukemia (51).
Whether the monoclonal paraproteins present in SCLS contribute directly to disease pathogenesis is unclear. Purified monoclonal paraprotein from 5 SCLS patients failed to bind cultured endothelial cells or induce any cytotoxicity (12, 52–53). Although the amount of monoclonal Ig produced in MGUS is usually small, indicating few clonal plasma cells, it may produce a toxic monoclonal paraprotein that causes pathology either by aggregating and depositing in tissue, such as in light chain amyloidosis, or by binding to autologous common antigens (i.e., acting as an autoantibody) as in cold agglutinin disease, mixed cryoglobulinemia, vonWillebrand syndrome, paraproteinemic neuropathy, and Schnitzler syndrome (54-57).
In SCLS, we speculate that the paraprotein could bind and inhibit a factor crucial for endothelial barrier function. A classical example of this mechanism is acquired angioedema, which has been diagnosed in the setting of MGUS and other lymphoproliferative disorders (58). An autoantibody against the inhibitor of the first component of complement (C1-INH) leads to C1-INH inactivation, complement pathway activation, and overproduction of permeability factors such as bradykinin. Antibodies reactive with a putative endothelial protective factor could render SCLS patients more susceptible to inflammation-induced vascular injury.
SYMPTOMS AND SIGNS
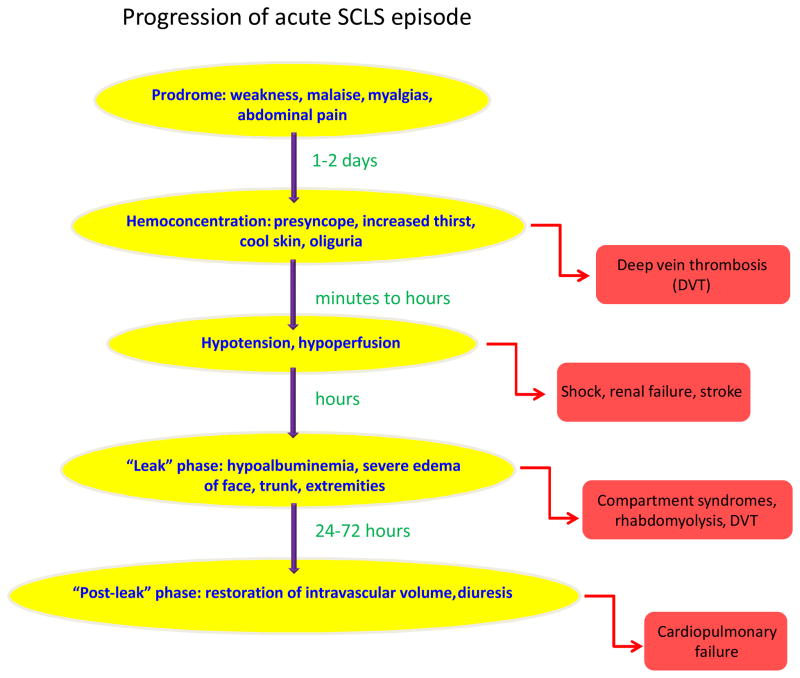
In patients with the classical acute form of SCLS, the rapid development of shock and edema due to plasma extravasation (up to 70% of total plasma volume) follows a typical prodrome of generalized weakness, fatigue, myalgias, and occasionally fevers, vomiting, abdominal pain, and diarrhea (4, 11, 37). We term this the “leak” phase (Figure 1). The hypotension usually lasts for several days accompanied by massive “third-spacing” of fluids (depicted in Figure 2). The symptoms reverse almost as quickly as they arise, with massive fluid mobilization from tissues into circulation and diuresis. We term this the “post-leak” phase. If patients are to die from SCLS, it is during the post-capillary leak phase due to cardiovascular overload secondary to the aftereffects of overzealous fluid resuscitation. The severity and frequency of episodes is similar in individual patients but varies widely from patient to patient. The pattern of attacks is usually stereotyped in each patient. Occasionally the pattern of attacks and duration of the capillary leak change over time.
Figure 1. Progression of a classical acute SCLS episode.
Typical prodromal symptoms lasting hours to days precede the rapid development of hemoconcentration and hypovolemia. Hypoalbuminemia and edema due to fluid and protein extravasation characterize the “leak phase”, which typically lasts several days. The “post-leak phase” is comprised of restoration of capillary barrier function with recruitment of fluids into the intravascular space and diuresis. Complications associated with each phase are listed to the right.
DIAGNOSIS
SCLS may be difficult to recognize and diagnose upon initial presentation. A characteristic triad of hypotension, hemoconcentration, and hypoalbuminemia in the absence of secondary causes of shock is typical of SCLS (59). Fluid resuscitation is typically followed by dramatic, rapidly developing, generalized edema of the face, trunk, and extremities (Figure 2). Although there is no pathognomonic marker for SCLS, it should be considered in any patient presenting to an emergency room or doctor’s office with acute, severe hypotension without obvious cardiac dysfunction particularly if it worsens after aggressive intravenous fluid resuscitation and/or vasopressor support and is accompanied by an elevated hematocrit (Hct). This singular combination of signs reflects the profoundly reduced intravascular volume and endothelial barrier dysfunction unique to SCLS. In addition to the complete blood count, measurement of serum albumin during the episode is important to confirm protein leakage from the intravascular compartment. The initial evaluation should also include routine blood and urine cultures to exclude sepsis, serum tryptase to rule out anaphylaxis, and a serum immunofixation electrophoresis to determine whether a paraprotein is present. However, we emphasize that while a monoclonal gammopathy, usually IgG kappa, supports the diagnosis of SCLS, it cannot be considered a primary criterion since it is not uniformly present (5, 7). A monoclonal protein was present in 19 out of 25 (76%) of classic SCLS cases followed at the Mayo Clinic.
Although visceral fluid extravasation as evidenced by pleural and pericardial effusions is not typical of classical acute SCLS episodes, a chest radiograph, electrocardiogram, and echocardiogram may exclude a primary cardiogenic cause of hypotension and peripheral edema. As the degree and duration of hypotension and edema may vary widely between SCLS patients, we propose a severity scale to aid in diagnosis of patients with recurrent “swelling” episodes suggestive of SCLS (Table 1). Grade 1 attacks are characterized by hypotension responding to oral hydration while Grade 2 episodes require IV fluids but no hospitalization. Grade 3 episodes are life threatening and require hospitalization and ICU management, and Grade 4 attacks are fatal. Grades 1–2 may be further sub-categorized into (A) Hgb increase ≤ 3 g/dL (e.g. 15 to 18) and albumin decrease ≤ 0.5 g/dL (e.g. 3.7 to 3.2) and (B) Hgb increase ≥ 3 g/dL (e.g. 15 to 19) or albumin decrease ≥ 0.5 g/dL e.g. 3.7 to 3.1). This scale may also determine prognosis and response to therapy in patients with a known diagnosis of SCLS.
Table 1.
SCLS Severity Scale
| Grade | Defining clinical course | Hemoconcentration | Hypoalbuminemia |
|---|---|---|---|
| 1 | Oral fluid resuscitation | ||
| a | Hgb ↑ ≤ 3 g/dL | Albumin ↓ ≤ 0.5 g/dL | |
| b | Hgb ↑ ≥ 3 g/dL | Albumin ↓ ≥ 0.5 g/dL | |
| 2 | IV fluids, no hospitalization | ||
| 3 | IV fluids, ICU monitoring | ||
| 4 | Fatal |
DIFFERENTIAL DIAGNOSIS
There are distinguishing features that should alert the clinician to the possibility of SCLS in patients presenting with hypotension and edema (Table 2). Evidence of underlying infection/sepsis, anaphylactic triggers, or other cause(s) of capillary leak is conspicuously absent. Elevated Hct (often > 60) and leukocytosis are often more severe than in sepsis or simple dehydration and can be mistaken for polycythemia vera (38). Unlike anaphylaxis, which usually resolves after administration of subcutaneous epinephrine, the hypotension of SCLS can be resistant even to very high doses of vasopressors. In our experience, urticaria, focal angioedema (e.g. perioral), and stridor due to laryngospasm, which often accompany anaphylaxis, are usually not observed in acute SCLS. Although flushing may be present at the onset of SCLS episodes and is a common symptom of mastocytosis or neuroendocrine tumors (e.g. carcinoid and pheochromocytoma), progression to severe hypotension and anasarca is not characteristic of the latter disorders. Although patients with the inferior vena cava (IVC) syndrome due to IVC thrombosis may present with rapidly developing hypotension and extremity edema, it is not typically associated with elevated Hct or low serum albumin.
Table 2.
Differential diagnosis of idiopathic systemic capillary leak syndrome
| Disorder | Similarities to SCLS | Distinguishing features |
|---|---|---|
| Nephrotic syndrome | Edema, hypoalbuminemia | Non-cyclical edema, proteinuria |
| Protein-losing enteropathy | Edema, hypoalbuminemia | Non-cyclical edema, diarrhea |
| Inferior vena cava syndrome | Lower extremity edema, hypotension | Progressive, irreversible hypotension, normal albumin |
| Idiopathic anaphylaxis | Acute hypotension, flushing | Normal albumin, hives, laryngeal edema more common, elevated serum tryptase |
| Hereditary angioedema | Acute cyclical edema | Normotension, visceral effusions, decreased C1 esterase inhibitor levels or function |
| Carcinoid | Flushing, hypotension | Absence of edema, elevated 5HT and 5HT metabolites |
| Pheochromocytoma | Flushing, hypotension | Labile hypertension, elevated norepinephrine, epinephrine metabolites |
| Mastocytosis | Flushing, hypotension | Urticaria pigmentosa, elevated serum tryptase |
| Polycythemia vera | Severe leukocytosis, erythrocytosis, thrombocytosis | Normotension, normal albumin, absence of edema |
| Gleich syndrome | Cyclical edema | Urticaria, eosinophilia, elevated IgM, normal albumin |
| Pancreatitis | Hypotension, hypoalbuminemia | Severe abdominal pain, nausea, vomiting; elevated amylase |
| Sepsis | Hypotension, edema | Normal albumin, acute respiratory distress syndrome (ARDS) |
| Ovarian hyperstimulation syndrome | Hypotension, hypovolemia, edema | History of ovulation induction, visceral effusions |
| Toxic shock syndrome | Hypotension | Normal albumin, renal failure, desquamatory rash, mucosal hyperemia, delirium |
A chronic form of SCLS has been described in several patients, which often presents a diagnostic challenge. These individuals have persistent (non-cyclical), progressive, generalized edema rather than acute attacks, and they may have visceral (pleural and pericardial) effusions (12, 60–61). Hypotension and hemoconcentration with hypoalbuminemia may be difficult to demonstrate in such patients, and a monoclonal paraprotein may not be present. A multitude of other disorders associated with chronic extremity edema, including Gleich syndrome, venous stasis, protein-losing enteropathy, and nephrotic syndrome, should be considered in these patients and can usually be excluded by careful history and routine laboratory studies.
TREATMENT
Acute management of a severe SCLS episode
All treatment strategies are based on observational data rather than controlled trials because of disease infrequency. We have had occasional success thwarting or minimizing the severity of the capillary leak with oral steroids upon early patient recognition of signs and symptoms of hypovolemia. However, in our experience steroids do not prevent the attack from progressing in most patients and may be deleterious to patients experiencing more frequent attacks. Oral electrolyte-containing fluids may reduce the severity of the attack if taken early before intravenous fluids can be administered. Patients presenting with signs and symptoms of a severe attack should be treated immediately in an intensive care setting. We have found that vasopressors and judicious fluid and colloid boluses using central venous pressure monitoring to prevent shock may reduce post-leak phase sequelae such as compartment syndrome or renal failure. Rapidly-infused boluses of colloid (25% albumin) rather than continuous crystalloid infusion are preferable to maintain hemodynamic stability. Boluses of 10% pentastarch (mol wt. 264 kD) were used in two patients with refractory hypotension during the leak phase unresponsive to aggressive crystalloid replacement and inotropic agents with some success (15).
Achieving normotension and normal central venous pressure can be considered overzealous in acute SCLS. Mild lactic acidosis, elevated creatinine phosphokinase (CPK) and/or liver transaminases, and oliguria are to be expected and may be tolerated to a certain extent as long as hemodynamic and metabolic parameters do not deteriorate and urine output and urine electrolytes are strictly monitored for signs of acute tubular necrosis. Deep venous thrombosis (DVT) may occur due to severe hemoconcentration leading to increased viscosity and stasis due to hypovolemia and compartment syndrome. Chronic anticoagulation should be considered in patients with DVT during episodes (Figure 1). Finally, consultation with an orthopedist is recommended to monitor intracompartmental pressure and consider fasciotomy and muscle debridement as clinically indicated by the risk of muscle necrosis (62). Risk rises as compartment pressures exceed mean arterial pressure (MAP), but sufficient MAP is needed to prevent underperfusion of muscles. Acute cardiac arrest with a serum K+ of 7.8 mmol/L occurred after fasciotomy during an SCLS attack. Hyperkalemia was due to acute release of K+ from muscles after re-perfusion (16).
Preventive/maintenance therapies
The pharmacological treatment of SCLS remains largely empiric (Table 3). Compounds that increase intracellular cAMP such as β-adrenergic agonists (terbutaline) and phosphodiesterase inhibitors (theophylline), which prevent cAMP degradation, have been used successfully for a number of years at the Mayo clinic and elsewhere to reduce the severity and frequency of SCLS attacks (59, 63–64). We recommend that patients newly diagnosed with SCLS be started on the regimen of theophylline and terbutaline. The dose of theophylline must be individualized on the basis of peak serum theophylline concentration, but doses ranging between 400–1600 mg/day in adults <60 years old and 10–36 mg/kg/day in children 1–9 years old are usually necessary to achieve peak serum concentrations between 10–20 mg/dL. Terbutaline should be given in at a total dose of 20–25 mg/day in divided doses as tolerated. A recent report demonstrated efficacy of intravenous theophylline for an acute SCLS episode when serum levels of 20 to 25 mg/dL were achieved (14). We have found that serum theophylline levels decrease over time, necessitating regularly monitoring to ensure that they are in the therapeutic range. The primary limitations of this therapy are side effects such as tremor, anxiety/irritability, insomnia, and palpitations. Moreover, cells undergo desensitization to β-agonists after prolonged exposure.
Table 3.
Treatment strategies for SCLS
| Therapy | Putative mechanism(s) | Efficacy in SCLS? | Reference(s) |
|---|---|---|---|
| Endothelial signal transduction | |||
| theophylline + terbutaline | phosphodiesterase inhibition,β-receptor stimulation; ↑ endothelial cAMP | Yes, acute and maintenance therapy | (59, 63) |
| epoprostenol | prostacyclin analogue; ↑ endothelial cAMP, vascular smooth muscle relaxation | Yes, in acute setting | (32) |
| HMG-coA reductase inhibitors (statins) | ↓ Rho prenylation | ND | |
| Dasatinib, imatinib | ↓Src or other tyrosine kinase activity | ND | |
| Bevacizumab | ↓VEGF activity | No (chronic form) | (14) |
| Immune modulation | |||
| Corticosteroids | anti-inflammatory | ± (chronic form of SCLS) | (10, 60–61) |
| Infliximab | ↓TNF activity | Yes, in acute setting | (14) |
| IMiDs (lenalidomide, thalidomide) | ↓plasma cell clone, anti-inflammatory mechanisms | Yes (thalidomide) | (67) |
| Plasmapheresis | ↓circulating monoclonal paraprotein | ? temporary | (53) |
| IVIG | Anti-inflammatory; anti-idiotypic mechanisms | Yes, maintenance therapy | (7, 9) |
| Hematological intervention | |||
| Autologous peripheral blood cell transplantation (PBST) | ↓plasma cell clone | ND | |
| Rituximab | ↓ B cells | ND | |
| Melphalan/prednisone | ↓plasma cell clone | Yes, in setting of myeloma, plasma cell leukemia | (49, 51) |
| Bortezomib | ↓plasma cell clone | ND | |
| Anakinra | ↓plasma cell clone | ND |
ND=no data; mAb=monoclonal antibody; IVIG=Intravenous immunoglobulin; cAMP=cyclic adenosine monophosphate
Although other maintenance therapies have been tried sporadically with varying degrees of effectiveness, we emphasize that all of the compounds discussed below remain unproven. The Anti-VEGF mAb bevacizumab has been used successfully in POEMS where VEGF appears to play a pathogenic role (65). Anti-VEGF therapy was used in one patient with the chronic form of SCLS, who had generalized edema, visceral effusions, IgA MGUS, and high plasma VEGF levels without success (12). However, neither hemoconcentration and hypoalbuminemia nor post-treatment VEGF levels were documented in this patient, making the diagnosis of SCLS and the treatment efficacy uncertain. Bevacizumab has not been used for acute, hypotensive SCLS episodes. Dowden et al. recently reported rapid improvement of a single patient with acute SCLS treated with infliximab, the humanized anti-TNFα monoclonal antibody (14).
Drugs that target an abnormal plasma cell population or its secreted products may be considered for the treatment of SCLS. Two SCLS patients with MGUS that evolved into myeloma had complete regression of their vascular leak symptoms after combination chemotherapy for myeloma (4). Immune modulatory drugs (IMiDs) such as thalidomide and lenalidomide are toxic to malignant plasma cells and have been used successfully to treat POEMS (66). Thalidomide was used in one patient with atypical SCLS accompanied by macular edema, who had improvement in visual symptoms and ophthalmological findings with treatment (67). Anakinra, an IL-1 receptor antagonist (IL-1RA) that blocks the biological activity of IL-1, has induced remission in patients with Schnitzler syndrome (68–69). Anakinra has also been used successfully in patients with “smoldering” and “indolent” multiple myeloma, which are precursors to active disease, but has not been explored for the treatment of SCLS (70).
Intravenous immunoglobulin (IVIG) has been used successfully for years in the treatment of autoantibody-mediated or what may be considered “anti-idiotypic” diseases. For instance, patients with MGUS and acquired von Willebrand disease respond very well to IVIG (56), suggesting that it could be effective in SCLS. Lambert et al. recently demonstrated efficacy of IVIG in thwarting acute SCLS episodes in 3 patients (9). Gousseff and Amoura report success in using IVIG as a prophylactic therapy for SCLS, but the details of their study have not yet been published (7). Although several of our patients recently treated with IVIG have noted some reductions in SCLS symptoms, not all cases reported in the literature have demonstrated a response (14, 25). At this point, the long-term effectiveness of IVIG for the treatment of SCLS is unknown.
PROGNOSIS
The prognosis of SCLS is uncertain, but Dhir et al. estimated the current 5-year survival rate to be 70% (6). Other studies have placed the overall 10-year mortality of SCLS at 25–34% (5, 7). In our experience at Mayo Clinic, the median survival of patients (counting only SCLS-related deaths) followed over a 30 year time period was approximately 15 years. As shown in Figure 1, complications may include compartment syndromes (62), renal failure from hypoperfusion-induced acute tubular necrosis or myoglobinuria secondary to rhabdomyolysis (62), and venous and arterial thrombosis including pulmonary embolism (71). In our estimation, most patients who survive a severe SCLS attack treated appropriately do not typically exhibit residual end-organ damage. Nonetheless, chronic renal failure and long-term sensorimotor deficits do occur as a result of ischemia and compartment syndromes.
AREAS OF UNCERTAINTY
Since the pathophysiology of SCLS is unknown and there are no validated criteria for diagnosis, we stress the importance of demonstrating hypotension, hemoconcentration and hypoalbuminemia in patients with recurrent episodes of edema to establish the diagnosis. It is unclear whether or not patients with chronic SCLS, who often lack cyclical leak/post leak phases, frank hypotensive episodes, and a serum monoclonal paraprotein, have a distinct pathophysiologic basis for their symptoms. In either case, secondary causes of edema must be rigorously excluded until unique diagnostic indicators of SCLS are discovered.
We also emphasize the need for prospective therapeutic studies for SCLS outside of the time-honored regimen of theophylline and terbutaline. Investigation of newer evidence-based therapeutic modalities may be warranted given the fatal nature of SCLS and the persistent difficulty in treating acute, grave attacks. Targeting other endothelial signal transduction pathways may be possible for SCLS. For example, statins may ameliorate vascular permeability (72–75) and could diminish symptoms in patients with chronic SCLS or reduce severity of acute flare-ups. Src and abl tyrosine kinases mediate permeability pathways induced by growth factors such as VEGF (76–77), and kinase inhibitors such as dasatinib or imatinib, which have been used safely for the treatment of leukemias and solid tumors (78–79), may stabilize endothelial barrier function while preserving angiogenesis (80).
If the plasma cell clone mediates disease pathology by producing a toxic paraprotein (a link yet to be established), more aggressive, potentially curative, hematological intervention might be considered for SCLS. In POEMS, high dose chemotherapy followed by autologous peripheral blood stem cell transplantation (auto PBST) has been successful (81–82). A single patient with SCLS and plasma cell leukemia saw complete resolution of capillary leak symptoms after auto PBST even when the leukemia relapsed (51). Thus, given the similarity of SCLS to POEMS on many fronts, we propose that auto PBST be considered for the treatment of patients with frequent, life-threatening SCLS episodes refractory to standard therapy.
Since little progress has been made in treatment of SCLS, we feel that it is time for this disease to be reexamined, particularly because it shares many phenotypic similarities with other “shock” syndromes such as sepsis, anaphylaxis, and angioedema. The endothelial dysfunction seen in SCLS as also typifies a diverse group of disorders including diabetic retinopathy (83), lupus nephritis (84), and infection with malaria and Ebola/Marburg viruses (85–86). Clarification of the unique features of SCLS will further our understanding of both the immune system and endothelial cell biology.
A succinct description of the signs, symptoms, and treatment of SCLS for both patients and physicians can be found on the National Organization for Rare Disorders (NORD) website (www.rarediseases.org). Additional information and a patient networking community are available on the RareShare website (http://www.rareshare.org/communities/systemic-capillary-leak-syndrome) and the Mayo Clinic Website (http://www.mayoclinic.org/systemic-capillary-leak-syndrome/).
References
- 1.Atkinson JP, Waldmann TA, Stein SF, et al. Systemic capillary leak syndrome and monoclonal IgG gammopathy; studies in a sixth patient and a review of the literature. Medicine (Baltimore) 1977;56(3):225–39. doi: 10.1097/00005792-197705000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Clarkson B, Thompson D, Horwith M, Luckey EH. Cyclical edema and shock due to increased capillary permeability. Am J Med. 1960;29:193–216. doi: 10.1016/0002-9343(60)90018-8. [DOI] [PubMed] [Google Scholar]
- 3.Noble RP, Gregersen MI, Porter PM, Buckman A. Blood Volume in Clinical Shock. I. Mixing Time and Disappearance Rate of T-1824 in Normal Subjects and in Patients in Shock; Determination of Plasma Volume in Man from 10-Minute Sample. J Clin Invest. 1946;25(2):158–71. doi: 10.1172/JCI101695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Amoura Z, Papo T, Ninet J, et al. Systemic capillary leak syndrome: report on 13 patients with special focus on course and treatment. Am J Med. 1997;103(6):514–9. doi: 10.1016/s0002-9343(97)00272-6. [DOI] [PubMed] [Google Scholar]
- 5.Kawabe S, Saeki T, Yamazaki H, Nagai M, Aoyagi R, Miyamura S. Systemic capillary leak syndrome. Intern Med. 2002;41(3):211–5. doi: 10.2169/internalmedicine.41.211. [DOI] [PubMed] [Google Scholar]
- 6.Dhir V, Arya V, Malav IC, Suryanarayanan BS, Gupta R, Dey AB. Idiopathic systemic capillary leak syndrome (SCLS): case report and systematic review of cases reported in the last 16 years. Intern Med. 2007;46(12):899–904. doi: 10.2169/internalmedicine.46.6129. [DOI] [PubMed] [Google Scholar]
- 7.Gousseff M, Amoura Z. Idiopathic capillary leak syndrome. Rev Med Interne. 2009;30(9):754–68. doi: 10.1016/j.revmed.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 8.Sion-Sarid R, Lerman-Sagie T, Blumkin L, Ben-Ami D, Cohen I, Houri S. Neurologic Involvement in a Child With Systemic Capillary Leak Syndrome. Pediatrics. 2010 doi: 10.1542/peds.2009-1691. [DOI] [PubMed] [Google Scholar]
- 9.Lambert M, Launay D, Hachulla E, et al. High-dose intravenous immunoglobulins dramatically reverse systemic capillary leak syndrome. Crit Care Med. 2008;36(7):2184–7. doi: 10.1097/CCM.0b013e31817d7c71. [DOI] [PubMed] [Google Scholar]
- 10.Keller K, Hauge E, Stengaard-Pedersen K. Chronic systemic capillary leak syndrome: a case responding to prednisolone treatment. Scand J Rheumatol. 2009;38(5):400–1. doi: 10.1080/03009740903161467. [DOI] [PubMed] [Google Scholar]
- 11.Onal H, Aktuglu-Zeybek C, Altun G, Ozyilmaz I, Alhaj S, Aydin A. Capillary leak syndrome in a 5-month-old infant associated with intractable diarrhoea. Ann Trop Paediatr. 2007;27(1):81–6. doi: 10.1179/146532807X170556. [DOI] [PubMed] [Google Scholar]
- 12.Lesterhuis WJ, Rennings AJ, Leenders WP, et al. Vascular endothelial growth factor in systemic capillary leak syndrome. Am J Med. 2009;122(6):e5–7. doi: 10.1016/j.amjmed.2009.01.020. [DOI] [PubMed] [Google Scholar]
- 13.De Martino M, Sasso L, Pirozzi F, Bonaduce D. Systemic capillary leak syndrome or Clarkson’s disease: a case report. Intern Emerg Med. 2009;4(4):357–8. doi: 10.1007/s11739-009-0257-0. [DOI] [PubMed] [Google Scholar]
- 14.Dowden AM, Rullo OJ, Aziz N, Fasano MB, Chatila T, Ballas ZK. Idiopathic systemic capillary leak syndrome: Novel therapy for acute attacks. J Allergy Clin Immunol. 2009 doi: 10.1016/j.jaci.2009.06.043. [DOI] [PubMed] [Google Scholar]
- 15.Lee YS, Kim SY, Kwon CW, et al. Two cases of systemic capillary leak syndrome that were treated with pentastarch. Korean J Intern Med. 2007;22(2):130–2. doi: 10.3904/kjim.2007.22.2.130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Perry J, Balasubramanian S, Imray C. Systemic capillary leak syndrome resulting in compartment syndrome and the requirement for a surgical airway. Anaesthesia. 2009;64(6):679–82. doi: 10.1111/j.1365-2044.2009.05891.x. [DOI] [PubMed] [Google Scholar]
- 17.Solomon S, Pefanis A, Papaetis GS, Ginos C, Kythreotis P, Achimastos A. A patient with recurrent episodes of abdominal pain and hypovolemic shock. Am J Emerg Med. 2009;27(6):755, e5–7. doi: 10.1016/j.ajem.2008.10.010. [DOI] [PubMed] [Google Scholar]
- 18.Ingegnoli F, Sciascera A, D’Ingianna E, Fantini F. Systemic sclerosis, capillary leak syndrome and nasopharyngeal carcinoma: an unusual association or paraneoplastic manifestations? Rheumatology (Oxford) 2009;48(2):201–2. doi: 10.1093/rheumatology/ken422. [DOI] [PubMed] [Google Scholar]
- 19.Kjaergaard KD, Rickers H. Systemic capillary leak syndrome. Ugeskr Laeger. 2008;170(41):3251. [PubMed] [Google Scholar]
- 20.Dams K, Meersseman W, Verbeken E, Knockaert DC. A 59-year-old man with shock, polycythemia, and an underlying paraproteinemia. Chest. 2007;132(4):1393–6. doi: 10.1378/chest.07-0456. [DOI] [PubMed] [Google Scholar]
- 21.Matsumura M, Kakuchi Y, Hamano R, et al. Systemic capillary leak syndrome associated with compartment syndrome. Intern Med. 2007;46(18):1585–7. doi: 10.2169/internalmedicine.46.0254. [DOI] [PubMed] [Google Scholar]
- 22.Mosadik A, Kettani A, Faroudy M, Sbihi A. Idiopathic capillary leak syndrome: an exceptional aetiology for shock. Ann Fr Anesth Reanim. 2007;26(7–8):715–6. doi: 10.1016/j.annfar.2007.03.026. [DOI] [PubMed] [Google Scholar]
- 23.Bonadies N, Baud P, Peter HJ, Buergi U, Mueller BU. A case report of Clarkson’s disease: If you don’t know it, you’ll miss it. Eur J Intern Med. 2006;17(5):363–5. doi: 10.1016/j.ejim.2005.12.011. [DOI] [PubMed] [Google Scholar]
- 24.Sanghavi R, Aneman A, Parr M, Dunlop L, Champion D. Systemic capillary leak syndrome associated with compartment syndrome and rhabdomyolysis. Anaesth Intensive Care. 2006;34(3):388–91. doi: 10.1177/0310057X0603400308. [DOI] [PubMed] [Google Scholar]
- 25.Hollenberg J, Frykman J, Lundberg LG, Forsberg S. A case report of systemic capillary leak syndrome (Clarkson’s disease) Acta Anaesthesiol Scand. 2010 doi: 10.1111/j.1399-6576.2010.02214.x. [DOI] [PubMed] [Google Scholar]
- 26.Lions C, Wey PF, Puidupin M, Soubirou JL, Escarment J. Haemodynamic management of Clarkson’s disease: interest of the echocardiographic follow-up. Ann Fr Anesth Reanim. 2007;26(12):1079–81. doi: 10.1016/j.annfar.2007.09.017. [DOI] [PubMed] [Google Scholar]
- 27.Debourdeau P, Bory P, Zammit C, Pavic M, Colle B. Recurrent unexplained lipothymia: do not forget the capillary leak syndrome. Rev Med Interne. 2007;28(10):711–3. doi: 10.1016/j.revmed.2007.01.020. [DOI] [PubMed] [Google Scholar]
- 28.Foeldvari I, Waida E, Junker AK. Systemic capillary leak syndrome in a child. J Pediatr. 1995;127(5):739–41. doi: 10.1016/s0022-3476(95)70163-x. [DOI] [PubMed] [Google Scholar]
- 29.Karatzios C, Gauvin F, Egerszegi EP, et al. Systemic capillary leak syndrome presenting as recurrent shock. Pediatr Crit Care Med. 2006;7(4):377–9. doi: 10.1097/01.PCC.0000227120.61949.FB. [DOI] [PubMed] [Google Scholar]
- 30.Cicardi M, Gardinali M, Bisiani G, Rosti A, Allavena P, Agostoni A. The systemic capillary leak syndrome: appearance of interleukin-2-receptor-positive cells during attacks. Ann Intern Med. 1990;113(6):475–7. doi: 10.7326/0003-4819-113-6-475. [DOI] [PubMed] [Google Scholar]
- 31.Cicardi M, Berti E, Caputo V, Radice F, Gardinali M, Agostoni A. Idiopathic capillary leak syndrome: evidence of CD8-positive lymphocytes surrounding damaged endothelial cells. J Allergy Clin Immunol. 1997;99(3):417–9. doi: 10.1016/s0091-6749(97)70061-7. [DOI] [PubMed] [Google Scholar]
- 32.Fellows IW, Powell RJ, Toghill PJ, Williams TJ, Cohen GF. Epoprostenol in systemic capillary leak syndrome. Lancet. 1988;2(8620):1143. doi: 10.1016/s0140-6736(88)90563-6. [DOI] [PubMed] [Google Scholar]
- 33.Weinbren I. Spontaneous Periodic Oedema. A New Syndrome. Lancet. 1963;2(7307):544–6. doi: 10.1016/s0140-6736(63)92642-4. [DOI] [PubMed] [Google Scholar]
- 34.Kao NL, Richmond GW, Luskin AT. Systemic capillary leak syndrome. Chest. 1993;104(5):1637–8. doi: 10.1378/chest.104.5.1637c. [DOI] [PubMed] [Google Scholar]
- 35.Horwith M, Hagstrom JW, Riggins RC, Luckey EH. Hypovolemic shock and edema due to increased capillary permeability. Jama. 1967;200(2):101–4. [PubMed] [Google Scholar]
- 36.Fardet L, Kerob D, Rybojad M, et al. Idiopathic systemic capillary leak syndrome: cutaneous involvement can be misleading. Dermatology. 2004;209(4):291–5. doi: 10.1159/000080851. [DOI] [PubMed] [Google Scholar]
- 37.Jacox RF, Waterhouse C, Tobin R. Periodic disease associated with muscle destruction. Am J Med. 1973;55(1):105–10. doi: 10.1016/0002-9343(73)90156-3. [DOI] [PubMed] [Google Scholar]
- 38.Doubek M, Brychtova Y, Tomiska M, Mayer J. Idiopathic systemic capillary leak syndrome misdiagnosed and treated as polycythemia vera. Acta Haematol. 2005;113(2):150–1. doi: 10.1159/000083455. [DOI] [PubMed] [Google Scholar]
- 39.Navarro C, Garcia-Bragado F, Lima J, Fernandez JM. Muscle biopsy findings in systemic capillary leak syndrome. Hum Pathol. 1990;21(3):297–301. doi: 10.1016/0046-8177(90)90230-3. [DOI] [PubMed] [Google Scholar]
- 40.Johansson BR, Lofdahl CG. Ultrastructure of the microvessels in skeletal muscle in a case of systemic capillary leak syndrome. Acta Med Scand. 1979;206(5):413–6. doi: 10.1111/j.0954-6820.1979.tb13537.x. [DOI] [PubMed] [Google Scholar]
- 41.Assaly R, Olson D, Hammersley J, et al. Initial evidence of endothelial cell apoptosis as a mechanism of systemic capillary leak syndrome. Chest. 2001;120(4):1301–8. doi: 10.1378/chest.120.4.1301. [DOI] [PubMed] [Google Scholar]
- 42.van Hinsbergh V, van Nieuw Amerongen G. Intracellular signalling involved in modulating human endothelial barrier function. J Anat. 2002;200(5):525. doi: 10.1046/j.1469-7580.2002.00060.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chihara R, Nakamoto H, Arima H, et al. Systemic capillary leak syndrome. Intern Med. 2002;41(11):953–6. doi: 10.2169/internalmedicine.41.953. [DOI] [PubMed] [Google Scholar]
- 44.Ebihara I, Hirayama K, Kaneko S, et al. Vascular endothelial growth factor and soluble fms-like tyrosine kinase-1 in septic shock patients treated with direct hemoperfusion with a polymyxin B-immobilized fiber column. Ther Apher Dial. 2008;12(4):285–91. doi: 10.1111/j.1744-9987.2008.00589.x. [DOI] [PubMed] [Google Scholar]
- 45.Albert C, Garrido N, Mercader A, et al. The role of endothelial cells in the pathogenesis of ovarian hyperstimulation syndrome. Mol Hum Reprod. 2002;8(5):409–18. doi: 10.1093/molehr/8.5.409. [DOI] [PubMed] [Google Scholar]
- 46.Dispenzieri A, Moreno-Aspitia A, Suarez GA, et al. Peripheral blood stem cell transplantation in 16 patients with POEMS syndrome, and a review of the literature. Blood. 2004;104(10):3400–7. doi: 10.1182/blood-2004-05-2046. [DOI] [PubMed] [Google Scholar]
- 47.Kumar S, Witzig TE, Timm M, et al. Expression of VEGF and its receptors by myeloma cells. Leukemia. 2003;17(10):2025–31. doi: 10.1038/sj.leu.2403084. [DOI] [PubMed] [Google Scholar]
- 48.Rajkumar SV, Dispenzieri A, Kyle RA. Monoclonal gammopathy of undetermined significance, Waldenstrom macroglobulinemia, AL amyloidosis, and related plasma cell disorders: diagnosis and treatment. Mayo Clin Proc. 2006;81(5):693–703. doi: 10.4065/81.5.693. [DOI] [PubMed] [Google Scholar]
- 49.Vigneau C, Haymann JP, Khoury N, Sraer JD, Rondeau E. An unusual evolution of the systemic capillary leak syndrome. Nephrol Dial Transplant. 2002;17(3):492–4. doi: 10.1093/ndt/17.3.492. [DOI] [PubMed] [Google Scholar]
- 50.Hiraoka E, Matsushima Y, Inomoto-Naribayashi Y, et al. Systemic capillary leak syndrome associated with multiple myeloma of IgG kappa type. Intern Med. 1995;34(12):1220–4. doi: 10.2169/internalmedicine.34.1220. [DOI] [PubMed] [Google Scholar]
- 51.Ghosh K, Madkaikar M, Iyer Y, Pathare A, Jijina F, Mohanty D. Systemic capillary leak syndrome preceding plasma cell leukaemia. Acta Haematol. 2001;106(3):118–21. doi: 10.1159/000046600. [DOI] [PubMed] [Google Scholar]
- 52.Beermann W, Horstrup KA, Will R. Systemic capillary leak syndrome. Am J Med. 1998;105(6):554. doi: 10.1016/s0002-9343(98)00308-8. [DOI] [PubMed] [Google Scholar]
- 53.Zhang W, Ewan PW, Lachmann PJ. The paraproteins in systemic capillary leak syndrome. Clin Exp Immunol. 1993;93(3):424–9. doi: 10.1111/j.1365-2249.1993.tb08195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Zivkovic SA, Lacomis D, Lentzsch S. Paraproteinemic neuropathy. Leuk Lymphoma. 2009:1–12. doi: 10.1080/10428190903111922. [DOI] [PubMed] [Google Scholar]
- 55.Merlini G, Stone MJ. Dangerous small B-cell clones. Blood. 2006;108(8):2520–30. doi: 10.1182/blood-2006-03-001164. [DOI] [PubMed] [Google Scholar]
- 56.Michiels JJ, Berneman Z, Gadisseur A, et al. Immune-mediated etiology of acquired von Willebrand syndrome in systemic lupus erythematosus and in benign monoclonal gammopathy: therapeutic implications. Semin Thromb Hemost. 2006;32(6):577–88. doi: 10.1055/s-2006-949663. [DOI] [PubMed] [Google Scholar]
- 57.Rizzi R, Curci P, Rinaldi E, et al. Schnitzler’s syndrome: monoclonal gammopathy associated with chronic urticaria. Acta Haematol. 2008;120(1):1–4. doi: 10.1159/000143499. [DOI] [PubMed] [Google Scholar]
- 58.Cugno M, Castelli R, Cicardi M. Angioedema due to acquired C1-inhibitor deficiency: a bridging condition between autoimmunity and lymphoproliferation. Autoimmun Rev. 2008;8(2):156–9. doi: 10.1016/j.autrev.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 59.Tahirkheli NK, Greipp PR. Treatment of the systemic capillary leak syndrome with terbutaline and theophylline. A case series. Ann Intern Med. 1999;130(11):905–9. doi: 10.7326/0003-4819-130-11-199906010-00015. [DOI] [PubMed] [Google Scholar]
- 60.Airaghi L, Montori D, Santambrogio L, Miadonna A, Tedeschi A. Chronic systemic capillary leak syndrome. Report of a case and review of the literature. J Intern Med. 2000;247(6):731–5. doi: 10.1046/j.1365-2796.2000.00693.x. [DOI] [PubMed] [Google Scholar]
- 61.Stirling CM, Boulton-Jones JM, Simpson K. Progressive oedema in a 30-year-old. Lancet. 1998;352(9126):450. doi: 10.1016/s0140-6736(98)05348-3. [DOI] [PubMed] [Google Scholar]
- 62.Dolberg-Stolik OC, Putterman C, Rubinow A, Rivkind AI, Sprung CL. Idiopathic capillary leak syndrome complicated by massive rhabdomyolysis. Chest. 1993;104(1):123–6. doi: 10.1378/chest.104.1.123. [DOI] [PubMed] [Google Scholar]
- 63.Droder RM, Kyle RA, Greipp PR. Control of systemic capillary leak syndrome with aminophylline and terbutaline. Am J Med. 1992;92(5):523–6. doi: 10.1016/0002-9343(92)90749-2. [DOI] [PubMed] [Google Scholar]
- 64.Doorenbos CJ, van Es A, Valentijn RM, van Es LA. Systemic capillary leak syndrome. Preventive treatment with terbutaline. Neth J Med. 1988;32(3–4):178–84. [PubMed] [Google Scholar]
- 65.Koga H, Tokunaga Y, Hisamoto T, et al. Ratio of serum vascular endothelial growth factor to platelet count correlates with disease activity in a patient with POEMS syndrome. Eur J Intern Med. 2002;13(1):70–74. doi: 10.1016/s0953-6205(01)00199-6. [DOI] [PubMed] [Google Scholar]
- 66.Kuwabara S, Misawa S, Kanai K, et al. Thalidomide reduces serum VEGF levels and improves peripheral neuropathy in POEMS syndrome. J Neurol Neurosurg Psychiatry. 2008;79(11):1255–7. doi: 10.1136/jnnp.2008.150177. [DOI] [PubMed] [Google Scholar]
- 67.Staak JO, Glossmann JP, Esser JM, Diehl V, Mietz H, Josting A. Thalidomide for systemic capillary leak syndrome. Am J Med. 2003;115(4):332–4. doi: 10.1016/s0002-9343(03)00349-8. [DOI] [PubMed] [Google Scholar]
- 68.Dybowski F, Sepp N, Bergerhausen HJ, Braun J. Successful use of anakinra to treat refractory Schnitzler’s syndrome. Clin Exp Rheumatol. 2008;26(2):354–7. [PubMed] [Google Scholar]
- 69.Crouch R, Akhras V, Sarkany R. Schnitzler’s syndrome: successful treatment with anakinra. Australas J Dermatol. 2007;48(3):178–81. doi: 10.1111/j.1440-0960.2007.00375.x. [DOI] [PubMed] [Google Scholar]
- 70.Lust JA, Lacy MQ, Zeldenrust SR, et al. Induction of a chronic disease state in patients with smoldering or indolent multiple myeloma by targeting interleukin 1{beta}-induced interleukin 6 production and the myeloma proliferative component. Mayo Clin Proc. 2009;84(2):114–22. doi: 10.4065/84.2.114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Teelucksingh S, Padfield PL, Edwards CR. Systemic capillary leak syndrome. Q J Med. 1990;75(277):515–24. [PubMed] [Google Scholar]
- 72.Zeng L, Xu H, Chew TL, et al. HMG CoA reductase inhibition modulates VEGF-induced endothelial cell hyperpermeability by preventing RhoA activation and myosin regulatory light chain phosphorylation. Faseb J. 2005;19(13):1845–7. doi: 10.1096/fj.05-4240fje. [DOI] [PubMed] [Google Scholar]
- 73.Li J, Wang JJ, Chen D, et al. Systemic administration of HMG-CoA reductase inhibitor protects the blood-retinal barrier and ameliorates retinal inflammation in type 2 diabetes. Exp Eye Res. 2009;89(1):71–8. doi: 10.1016/j.exer.2009.02.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Jacobson JR, Barnard JW, Grigoryev DN, Ma SF, Tuder RM, Garcia JG. Simvastatin attenuates vascular leak and inflammation in murine inflammatory lung injury. Am J Physiol Lung Cell Mol Physiol. 2005;288(6):L1026–32. doi: 10.1152/ajplung.00354.2004. [DOI] [PubMed] [Google Scholar]
- 75.Dell’Omo G, Bandinelli S, Penno G, Pedrinelli R, Mariani M. Simvastatin, capillary permeability, and acetylcholine-mediated vasomotion in atherosclerotic, hypercholesterolemic men. Clin Pharmacol Ther. 2000;68(4):427–34. doi: 10.1067/mcp.2000.109787. [DOI] [PubMed] [Google Scholar]
- 76.Gavard J, Gutkind JS. VEGF controls endothelial-cell permeability by promoting the beta-arrestin-dependent endocytosis of VE-cadherin. Nat Cell Biol. 2006;8(11):1223–34. doi: 10.1038/ncb1486. [DOI] [PubMed] [Google Scholar]
- 77.Huang X, Wu D, Jin H, Stupack D, Wang JY. Induction of cell retraction by the combined actions of Abl-CrkII and Rho-ROCK1 signaling. J Cell Biol. 2008;183(4):711–23. doi: 10.1083/jcb.200801192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Jensen BM, Beaven MA, Iwaki S, Metcalfe DD, Gilfillan AM. Concurrent inhibition of kit- and FcepsilonRI-mediated signaling: coordinated suppression of mast cell activation. J Pharmacol Exp Ther. 2008;324(1):128–38. doi: 10.1124/jpet.107.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Brunton VG, Frame MC. Src and focal adhesion kinase as therapeutic targets in cancer. Curr Opin Pharmacol. 2008;8(4):427–32. doi: 10.1016/j.coph.2008.06.012. [DOI] [PubMed] [Google Scholar]
- 80.Eliceiri BP, Paul R, Schwartzberg PL, Hood JD, Leng J, Cheresh DA. Selective requirement for Src kinases during VEGF-induced angiogenesis and vascular permeability. Mol Cell. 1999;4(6):915–24. doi: 10.1016/s1097-2765(00)80221-x. [DOI] [PubMed] [Google Scholar]
- 81.Gherardi RK, Belec L, Soubrier M, et al. Overproduction of proinflammatory cytokines imbalanced by their antagonists in POEMS syndrome. Blood. 1996;87(4):1458–65. [PubMed] [Google Scholar]
- 82.Soubrier M, Sauron C, Souweine B, et al. Growth factors and proinflammatory cytokines in the renal involvement of POEMS syndrome. Am J Kidney Dis. 1999;34(4):633–8. doi: 10.1016/S0272-6386(99)70386-0. [DOI] [PubMed] [Google Scholar]
- 83.Ferrara N, Gerber HP, LeCouter J. The biology of VEGF and its receptors. Nat Med. 2003;9(6):669–76. doi: 10.1038/nm0603-669. [DOI] [PubMed] [Google Scholar]
- 84.Kumpers P, David S, Haubitz M, et al. The Tie2 receptor antagonist Angiopoietin-2 facilitates vascular inflammation in Systemic Lupus Erythematosus. Ann Rheum Dis. 2008 doi: 10.1136/ard.2008.094664. [DOI] [PubMed] [Google Scholar]
- 85.Yang ZY, Duckers HJ, Sullivan NJ, Sanchez A, Nabel EG, Nabel GJ. Identification of the Ebola virus glycoprotein as the main viral determinant of vascular cell cytotoxicity and injury. Nat Med. 2000;6(8):886–9. doi: 10.1038/78645. [DOI] [PubMed] [Google Scholar]
- 86.Wahl-Jensen VM, Afanasieva TA, Seebach J, Stroher U, Feldmann H, Schnittler HJ. Effects of Ebola virus glycoproteins on endothelial cell activation and barrier function. J Virol. 2005;79(16):10442–50. doi: 10.1128/JVI.79.16.10442-10450.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]