Abstract
Purpose
To identify risk factors for chemotherapy-related nausea.
Methods
We examined risk factors for nausea in 1696 patients from three multicenter studies conducted from 1998 to 2004. All patients were beginning a chemotherapy regimen containing cisplatin, carboplatin, or doxorubicin. Nausea was assessed on a 1 – 7 scale four times a day for four days by diary
Results
1) Average nausea for breast cancer patients receiving doxorubicin (mean = 2.31) was significantly greater than for other patients receiving doxorubicin (mean 1.82), patients receiving cisplatin (mean 1.88) and patients receiving carboplatin (mean 1.45), Ps<0.01. 2) Mean nausea decreased steadily with age, P<0.0001. 3) Patients rating themselves more susceptible to nausea had significantly more nausea (adjusted mean = 2.51) than patients rating themselves less susceptible (adjusted mean = 1.92) and were 2.8 times more likely to experience severe nausea, Ps<0.0001. 4) Expected nausea was a significant predictor of average nausea, P=0.034, but not severe nausea, P=0.31. 5) No evidence that gender is a significant predictor of nausea in 299 patients with gender neutral cancers, P=0.35.
Conclusions
Specific patient characteristics, especially younger age and perceived susceptibility to nausea, can help clinicians in the early identification of patients who are more susceptible to treatment-related nausea.
Keywords: chemotherapy; nausea; risk factor; doxorubicin, expectancy
Background
Chemotherapy-related nausea is a widespread problem that causes extreme discomfort and seriously impairs patients’ quality of life (QOL). It negatively affects patients’ nutritional habits, abilities to work, and motivation to follow recommended treatment regimens.[1, 2] There is great variation in the frequency and severity of this type of nausea which cannot be explained by the pharmacological properties of the chemotherapeutic agents alone, and numerous risk factors for chemotherapy-induced nausea and vomiting (CINV) have been identified.[3, 4] The strongest of these are generally assumed to be younger age and female gender,[5–10] although others, including a history of low alcohol intake, experience of nausea and emesis during pregnancy, impaired QOL, greater anxiety, previous experience with chemotherapy, and susceptibility to motion sickness, have all shown significant correlations with subsequent CINV.[3, 8, 11–14] Many studies have also reported significant correlations between patients’ expectancies for nausea and the subsequent occurrence of nausea.[15–21] Although these expectancies may largely represent a patient’s acknowledgement of his/her own propensity to develop nausea based on past experience (e.g. nausea during pregnancy or susceptibility to motion sickness), they are also likely influenced by what the patient is told to expect from the range of information they receive from clinicians, other patients, family, friends, and the world at large.
In the analyses that follow, from a combined sample of three large multicenter studies, we examine risk factors, including nausea expectancies and perceived susceptibility to nausea, as predictors of chemotherapy-induced nausea.
Methods
Patients
The patients were participants in three multicenter clinical trials conducted from January, 1998, through June, 2004, at seventeen geographically diverse member sites of the University of Rochester Cancer Center Community Clinical Oncology Program. All patients were chemotherapy-naïve, 18 or older and were about to begin a first cancer treatment regimen containing cisplatin, carboplatin, or doxorubicin. Patients receiving concurrent radiotherapy or interferon, or with clinical evidence of bowel obstruction or symptomatic brain metastases, were excluded. All patients received a 5-HT3 receptor antagonist prior to chemotherapy. Dexamethasone or other corticosteroids were allowed, as were all ancillary treatments as appropriate for control of symptoms caused by the cancer or its treatment. The three studies, described briefly below with demographic and clinical details provided in Table 1, were all randomized clinical trials testing interventions to reduce CINV. The primary results of each study have been published elsewhere. [13, 22, 23] Written informed consent was obtained from each participant prior to data collection.
Table 1.
Demographic and Treatment Details.
| Study 1 | Study 2 | Study 3 | ||
|---|---|---|---|---|
| (N=325) | (N=700) | (N=671) | ||
| 1/98 – 9/00 | 11/99 – 7/01 | 6/01 – 6/04 | ||
| Age: | Mean (s.d.) | 57.6 (12.7) | 52.0 (11.0) | 53.0 (11.2) |
| Range | 28 – 91 | 23 – 84 | 25 – 90 | |
| Sex: | Male | 87 | 56 | 38 |
| Female | 238 | 644 | 633 | |
| Ethnicity: | White | 272 | 620 | 591 |
| Black | 22 | 35 | 57 | |
| Hispanic | 10 | 11 | 11 | |
| Other | 10 | 26 | 10 | |
| Missing data | 11 | 8 | 2 | |
| Education: | ||||
| ≥ 4 Years College | 101 | 236 | 180 | |
| < 4 Years College | 87 | 206 | 217 | |
| High School Graduate | 98 | 212 | 223 | |
| Non High School Graduate | 39 | 41 | 51 | |
| Missing Data | ---- | 5 | ---- | |
| Cancer site: | ||||
| Alimentary Tract | 13 (4.0%) | 6 (0.9%) | ---- | |
| Breast | 144 (44.3%) | 592 (84.6%) | 602 (89.7%) | |
| Genitourinary | 19 (5.8%) | 5 (0.7%) | 1 (0.1%) | |
| Gynecologic | 29 (8.9%) | 4 (0.6%) | 1 (0.1%) | |
| Head and Neck | 10 (3.1%) | 3 (0.4%) | ---- | |
| Hematologic | 26 (8.0%) | 68 (9.7%) | 64 (9.5%) | |
| Lung | 72 (22.2%) | 15 (2.1%) | ---- | |
| Other or missing | 12 (3.7%) | 7 (1.0%) | 3 (0.4%) | |
| Medications: | ||||
| Adriamycin | 171 (52.6%) | 665 (95.0%) | All | |
| Carboplatin | 106 (32.6%) | ---- | ---- | |
| Cisplatin | 48 (14.8%) | 35 (5.0%) | ||
| 5-HT3 receptor antagonist | All | All | All | |
In Study I, 360 patients were enrolled in a study testing the effect of an informational expectancy manipulation on CINV. All patients received ondansetron with dexamenthasone on the day of the treatment. Analyses of the 325 (73% female) evaluable patients showed that the expectancy manipulation reduced patients’ reported expectations for the development of nausea but did not reduce occurrence of nausea.[22]
In Study II, 739 patients were randomly assigned to 1) acupressure bands, 2) an acustimulation band, or 3) a no band control condition as an adjunct to standard antiemetics for the relief of CINV. Of these, 700 (92%female) provided evaluable data and patients in the acupressure condition experienced less nausea on the day of treatment compared to controls, p < .05. However, there were no significant differences in delayed nausea or vomiting among the three treatment conditions.[23]
In Study III, 671 (94% female) patients receiving doxorubicin-based chemotherapy were enrolled in a three-arm study that detected no difference in the ability of a serotonin receptor antagonist vs. prochlorperazine (given regularly three times daily or taken only as needed for symptoms) to control the severity of delayed nausea following their first infusion of chemotherapy.[13]
Measures
Demographic data and detailed information about diagnosis, chemotherapy agents, and antiemetics were provided by self-report questionnaires or abstracted from medical records by study personnel.
Nausea and emesis were measured in each study by a four-day patient report diary developed for this purpose by Burish[24] and Carey.[25] Each day was divided into four segments (morning, afternoon, evening, night) and patients reported the severity of nausea and number of vomiting episodes for the latter three periods on the day of treatment and for all four periods on the three following days (15 total reporting times). Severity of nausea was assessed on a 7-point rating scale, anchored at one end by 1 = “Not at all nauseated" and at the other end by 7 = "Extremely nauseated." The description “Moderately nauseated” was centered on the scale below the 4. Patients were given the questionnaires to complete at home.
Expectancies and perceived susceptibility for nausea were measured in Studies II and III but not in Study I. Nausea expectancies were assessed with the question "What do you think your level of nausea will be at its worst after this treatment?” The possible responses were "very mild or none at all," "mild," "moderate," "severe," “very severe,” and "intolerable," which were coded 1 – 6, respectively. Perceived susceptibility to nausea was assessed with the question, “In general, do you think you are more susceptible to nausea than your friends and family are?” Possible responses were “more” (scored as 3), “about the same” (scored as 2), and “less" (scored as 1). Patients in Studies II and III were also asked if they had nausea during pregnancy (coded 1 = yes, 0 = no or not applicable) and whether or not they were susceptible to motion sickness (coded 1 = yes, 0 = no).
Statistical Analyses
Combined data from all three studies (1696 total patients) were used to examine the degree of nausea in breast cancer patients compared with other patient groups, the effect of gender on nausea, and the relationship between severe nausea and vomiting. All other analyses used the combined dataset of Studies II and III (1371 total patients) only because Study I did not include the necessary questionnaire regarding expectancy and susceptibility to nausea.
The four-day patient report diary produced three separate measures of CINV: Average Nausea, Severe Nausea, and Vomiting. Each measure represents a different aspect of CINV. Average Nausea was the mean severity of nausea reported at each time point in the four days following treatment. Patients were said to have experienced Severe Nausea if they rated their nausea a 6 or 7 at least once. Vomiting was whether the patient reported vomiting once or more in the four days after their infusion. These three measures of CINV comprised the outcome measures throughout the following analyses and are consistent with measures used in our prior research on chemotherapy-related nausea. [18]
To investigate differences in average nausea between breast cancer patients receiving doxorubicin and other patients receiving various chemotherapeutic agents, patients in all three studies were classified into the following groups: breast cancer patients receiving doxorubicin, other patients receiving doxorubicin, patients receiving cisplatin, and patients receiving carboplatin. ANOVA, controlling for treatment arms from the three studies, followed by pairwise comparisons of least squares means (i.e. group means adjusted for treatment arms in the different studies) using the Tukey-Kramer procedure to control the Type I error rate was used to assess differences in average nausea between these groups. The proportion of patients experiencing severe nausea in these groups was similarly compared using logistic regression followed by comparisons of odds ratios.
A subset of the data (N=299 from all three studies) contained patients with tumor sites that are not gender-specific. Here, multiple linear regression analysis was used to assess whether gender affected average nausea after controlling for age, education, tumor site, chemotherapy type, and study arm.
A regression analysis was conducted on the largest homogenous patient group, i.e., female breast cancer patients receiving doxorubicin, to examine the role of age, expectancy, and perceived susceptibility on average nausea (N=1178). Patients from Study I were excluded from the analysis because susceptibility and expectancy information was not available. Age was classified into five groups: less than 40, 40–50, 50–60, 60–70, and greater than 70 years of age. For this analysis, the six-point expectancy measure was collapsed into a 1–4 scale because only 22 women responded with a 5 (very severe) or a 6 (intolerable) on the expectancy question. The responses of these 22 patients were included in the 4th group, which was coded as severe. Multiple least squares regression was run with age, perceived susceptibility and expectancy as independent variables, as well as treatment arm and education (to control for these factors). A similarly structured logistic regression analysis was used to examine the effect of age, expectancy and perceived susceptibility on occurrence of severe nausea.
Finally, Fisher’s Exact Test was used to determine whether vomiting was related to the occurrence of severe nausea. This was followed by determining the correlation between the occurrence of vomiting and severe nausea so as to estimate the strength of this relationship. All analyses were conducted with SAS Version 9.2 and results were considered significant if p<0.05, two-tailed. Given that this was a secondary analysis, adjustment for multiple comparisons was not performed.
Results
There was a total of 1696 patients in the dataset combined from all three studies. Table 1 shows patient characteristics for each study separately. Overall, 29% of these 1696 patients reported having severe nausea at some point during the 4-day period and 18% of the patients had no nausea. Mean nausea for all patients on the 1–7 scale was 2.18.
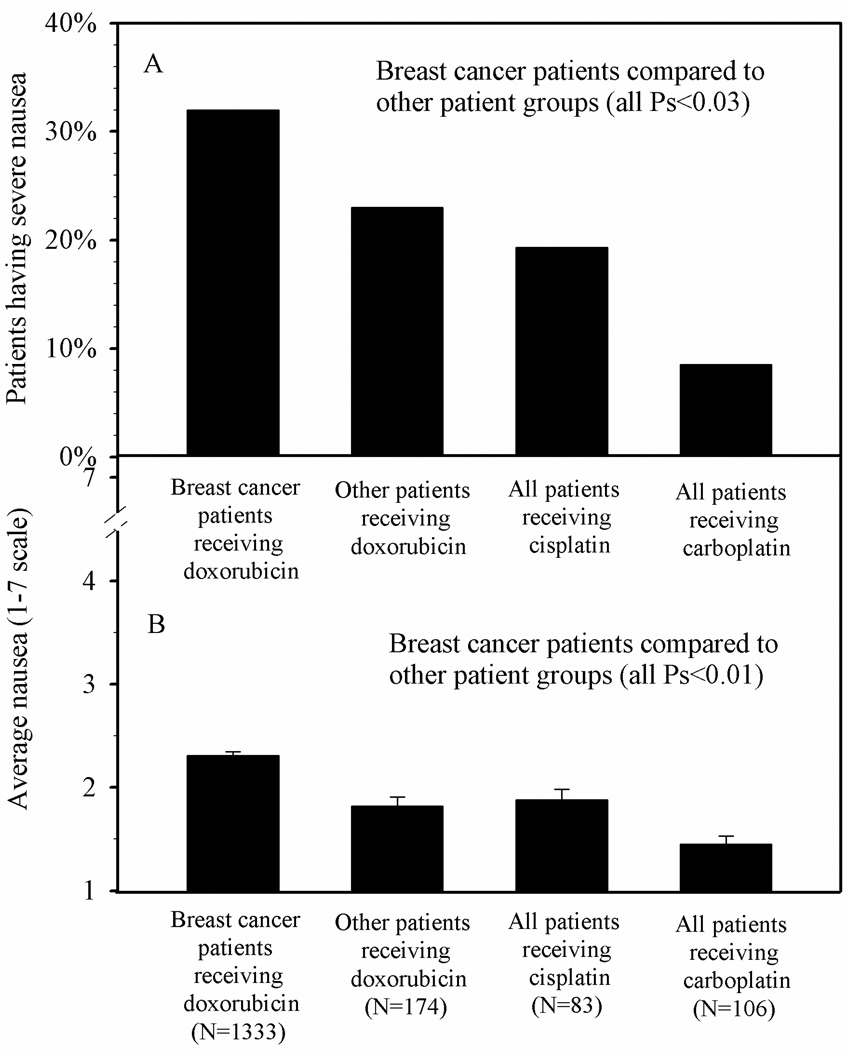
Average nausea for breast cancer patients receiving doxorubicin was greater than for other patients receiving doxorubicin, patients receiving cisplatin and patients receiving carboplatin (all Ps<0.01). In addition, non-breast cancer patients receiving doxorubicin and patients receiving cisplatin reported higher average nausea compared with those receiving carboplatin (both Ps<0.01). Similar patterns were seen for the percentage of patients experiencing severe nausea (all Ps<0.03). See Figure 1 and Table 2.
Figure 1.
Observed incidence of severe nausea (proportion) and average nausea (mean + SE), following first treatment for breast cancer patients receiving doxorubicin-based treatments compared to other patients
Table 2.
Effects of treatment group on average nausea and incidence of severe nausea
| Average Nausea (1–7 scale) |
Severe Nausea (rating of 6 or 7) | ||||||
|---|---|---|---|---|---|---|---|
| Observed proportion with severe nausea |
Odds ratio relative to breast cancer patients receiving doxorubicin |
||||||
| Treatment group | Observed Mean |
Std. Error |
N | Estimate | LCBa | UCBa | |
| Breast cancer patients receiving doxorubicin | 2.31 | 0.04 | 1333 | 32.0% | N/A | N/A | N/A |
| Other patients receiving doxorubicin | 1.82 | 0.09 | 174 | 23.0% | 0.63 | 0.42 | 0.91 |
| All patients receiving cisplatin | 1.88 | 0.10 | 83 | 19.3% | 0.46 | 0.25 | 0.82 |
| All patients receiving carboplatin | 1.45 | 0.08 | 106 | 8.5% | 0.17 | 0.08 | 0.37 |
Lower and upper 95% confidence bounds.
For N=299 patients having non-gender-related cancers (hematological (52.8%), lung (29.1%), other (18.1%), we ran a regression to ascertain whether gender makes a difference in average nausea after controlling for tumor site, chemotherapy type, education and study arm. A gender-by-tumor type interaction term was included in the model to account for any dependencies of the gender effect on tumor type. Gender proved to be non-significant (P = 0.35 for the main gender effect and P = 0.57 for the gender-by-tumor type interaction).
For female breast cancer patients, regression analysis revealed that perceived susceptibility, age, and expectancy were important risk factors for average nausea. See Table 3. The risk factor with the largest effect according to the F statistic (F=19.8, P<0.0001) was susceptibility. Patients who said they were more susceptible to nausea than their friends and family had greater average nausea. The effect of age (F=16.9, P<0.0001) was nearly as large, with older patients reporting less nausea. The effect of expectancy was smaller, but still significant (F=2.9, P=0.034) with patients expecting more severe nausea reporting greater nausea.
Table 3.
Risk factors for incidence of average nausea in female breast cancer patients receiving doxorubicin
| Least Squares Meana | ||||
|---|---|---|---|---|
| Risk Factor | Estimate | LCBb | UCBb | |
| Perceived Susceptibility (F=19.8, P<0.0001) | ||||
| Less | 1.92 | 1.55 | 2.28 | |
| About the same | 2.13 | 1.76 | 2.51 | |
| More | 2.51 | 2.13 | 2.89 | |
| Age (Years) (F=1.70, P<0.0001) | ||||
| < 40 | 2.70 | 2.29 | 3.11 | |
| 40–49 | 2.48 | 2.11 | 2.85 | |
| 50–59 | 2.12 | 1.75 | 2.49 | |
| 60–69 | 1.80 | 1.42 | 2.17 | |
| 70+ | 1.84 | 1.39 | 2.28 | |
| Expectancy (F=2.9, P=0.034) | ||||
| None or very mild | 2.02 | 1.62 | 2.41 | |
| Mild | 2.11 | 1.74 | 2.48 | |
| Moderate | 2.26 | 1.90 | 2.63 | |
| Severe | 2.36 | 1.95 | 2.76 | |
Note: Results are from regression analysis after adjustment for education and study arm.
Least squares means are the means for given factor levels after adjustment for the other factors.
Lower and upper 95% confidence bounds.
In the similarly structured logistic regression equation examining the influence of age, perceived susceptibility, and expectancy on the report of severe nausea, perceived susceptibility and age were significant (both Ps<0.0001), but expectancy was not (P=0.31). See Table 4. The odds of reporting severe nausea for patients who said they were more susceptible to nausea than their friends and family were 2.85 times than those who said they were less susceptible. The effect of age was similar to that reported for average nausea, with a decrease of severe nausea with increasing age. For example, the odds of reporting severe nausea for patients aged 70 or more versus those less than 40 years of age was 0.33.
Table 4.
Risk factors for incidence of severe nausea in female breast cancer patients receiving doxorubicin
| Risk Factor | Odds Ratioa | |||
|---|---|---|---|---|
| Estimate | LCBb | UCBb | ||
| Perceived Susceptibility (Chisq=36.66, P<0.0001) |
||||
| More vs. Less | 2.85 | 4.00 | 2.03 | |
| More vs. About the same | 1.87 | 2.64 | 1.32 | |
| Age (Years) (Chisq=47.26, P<0.0001) |
||||
| 70+ vs. <40 | 0.33 | 0.65 | 0.17 | |
| 70+ vs. 40–49 | 0.56 | 1.03 | 0.30 | |
| 70+ vs. 50–59 | 1.07 | 1.97 | 0.58 | |
| 70+ vs. 60–69 | 1.45 | 2.87 | 0.74 | |
| Expectancy (Chisq=3.6, P=0.31) |
||||
| Severe vs. None or very mild | 1.35 | 2.29 | 0.79 | |
| Severe vs. Mild | 1.25 | 1.99 | 0.79 | |
| Severe vs. Moderate | 0.98 | 1.47 | 0.65 | |
Note: Results are from logistic regression analysis after adjustment for education and study arm.
Odds of severe nausea for the reference group divided by the odds of severe nausea for the other groups. Values less than 1.0 indicate a lower propensity to report severe nausea. For example, the odds of experiencing severe nausea for patients 70 years old or more are 0.33 times the odds of severe nausea for young patients less than 40 years old.
Upper and lower 95% confidence bounds.
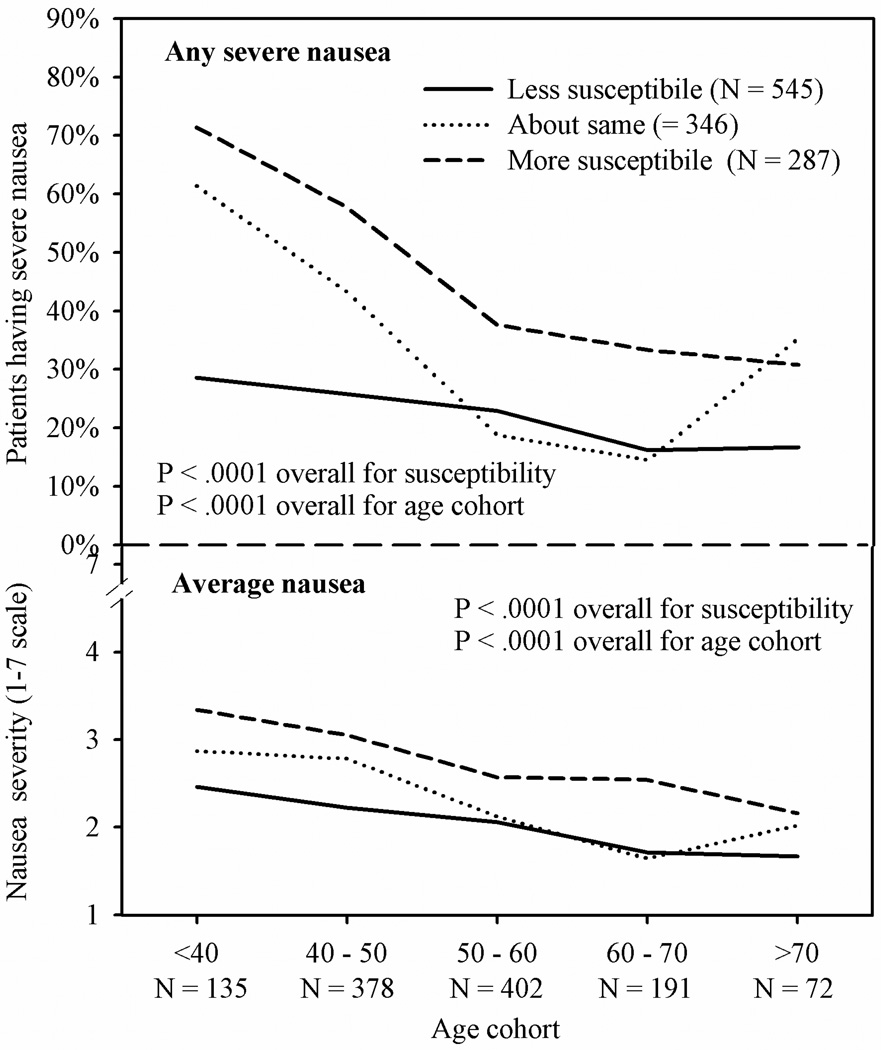
When age and susceptibility are considered together, the observed average nausea ranged from a mean of 3.34 in the youngest cohort of patients who classified themselves as more susceptible to nausea to a mean of 1.66 in patients in the oldest cohort who classified themselves as less susceptible to nausea. Observed incidences of severe nausea in these two groups were 71% and 17%, respectively. See Figure 2.
Figure 2.
Observed incidence of severe nausea (proportion) and average nausea (mean) in 1178 breast cancer patients following first treatment by age cohort and perceived susceptibility of nausea compared to friends and family members
Fisher’s Exact Test revealed a significant relationship between occurrence of severe nausea and vomiting (p<0.001). In all, 393 patients reported both severe nausea and vomiting, 434 patients reported vomiting but not severe nausea, 97 patients reported severe nausea with no vomiting, and 768 patients didn’t report severe nausea or vomiting. The tetrachoric correlation between occurrence of severe nausea and occurrence of vomiting was large, 0.63.
Discussion
Our analyses regarding predictors of chemotherapy-related nausea from the combined sample of 1696 patients following their first treatment yielded six findings of interest: 1) strong evidence that breast cancer patients experience significantly greater nausea than other patients, 2) confirmation of prior reports that younger patients experience greater nausea than older patients, 3) a new finding that perceived susceptibility to nausea is a significant predictor of actual nausea, 4) evidence that expectancy is a predictor of nausea, 5) the incidence of any severe nausea and any vomiting have a strong relationship, and 6) contrary to many prior reports, gender is not a significant predictor of nausea. Each of these findings is discussed below.
In the present analyses, we compared average nausea and incidence of severe nausea in breast cancer patients receiving doxorubicin to three other groups of patients. One of these groups was comprised of other patients receiving doxorubicin, typically those with hematologic cancers, and they would likely have received a lower dose of doxorubicin than the breast cancer patients. A second group was comprised of patients receiving cisplatin. The third group of patients received carboplatin. Our analyses showed that breast cancer patients receiving doxorubicin had significantly greater average nausea and a higher incidence of severe nausea than patients in the other three groups. Thirty-two percent of the breast cancer patients experienced severe nausea compared to less than 20% of patients receiving cisplatin and less than 10% of patients receiving carboplatin. Virtually all of the breast cancer patients in our study received not only doxorubicin but also cyclophosphamide, and it is possible that it is this particular combination of drugs that caused the high level of nausea observed in these patients rather than the doxorubicin alone.
Our finding that age is a significant predictor of nausea is not surprising; it has been reported many times previously.[3, 5, 6, 8–10, 26] In the present analyses, because of our large sample size, we were able to divide age into five categories (<40, 40–49, 50–59, 60–69, ≥ 70) rather than the dichotomous category of younger or older than 50 years that is often reported in the literature.[6, 27] Mean nausea decreased with age category, with patients in the oldest category having average nausea that was one point less on the 7-point scale than patients in the youngest age category. Incidence of severe nausea ranged from over 50% of patients in the youngest cohort to less than half that in each of the two oldest cohorts. These analyses were done only in female breast cancer patients receiving doxorubicin-based treatments as a method of controlling for cancer and type of treatment; therefore, we are not sure if this will generalize to men and other cancer treatments.
The simple question of whether patients believed that they were more susceptible to nausea than their friends or family was a substantial determinant of average nausea in our sample. We examined the predictive role of susceptibility only in female breast cancer patients receiving doxorubicin-based treatments, our largest homogenous patient group. Taking into account age, expectancy and education, patients rating themselves more susceptible to nausea had significantly greater average nausea than patients rating themselves less susceptible. They were also nearly three times as likely to experience severe nausea. Patients who rated their nausea susceptibility about the same as friends and family members fell close to midpoint between the other two patient groups in both average nausea and incidence of severe nausea.
In a similar vein, and in the same patient sample, nausea expectancy was a significant predictor of average nausea, even after controlling for age and perceived susceptibility. Individuals expecting nausea, whether because they consider themselves to be very susceptible to nausea or for any other reason, may be more likely to interpret vague or ambiguous sensations as nauseating than an individual not expecting the symptom.[28] The idea that perceptions are influenced by expectations in this way is a well established principle in the study of cognition.[29]
We report on one analysis regarding vomiting, that is, the relationship between severe nausea and vomiting. Far more patients reported vomiting (827) than severe nausea (490) and fewer than half (48%) of those reporting at least one incidence of emesis also reported having severe nausea. On the other hand, of the 490 patients reporting severe nausea, 80% reported at least one episode of emesis. These findings suggest that the occurrence of vomiting is a strong contributing factor to the report of severe nausea but that the two symptoms are clearly different because over half of the patients reporting emesis do not report having severe nausea.
We did not find a significant relationship between gender and nausea. We conducted these analyses on 299 (male = 155) patients with gender neutral cancers, i.e., excluding patients with breast, genitourinary, or gynecological cancers. Regression analysis controlling for age, education, chemotherapy type, and study arm showed that gender did not significantly predict average nausea or incidence of severe nausea in these 299 patients. Our findings are contrary to much of the current literature regarding the relationship between gender and nausea in cancer patients, and we speculate that this contradiction is due to our using a sample of patients with only gender neutral cancers in our analyses. Considering our previously discussed finding that breast cancer patients receiving doxorubicin have significantly more nausea than other patients, we speculate that prior reports showing an effect of gender on nausea that included breast cancer patients in their analyses are in error because they did not adequately control for the significantly higher level of nausea in breast cancer patients receiving doxorubicin. Further research on the effect of gender on nausea will be needed to determine if this hypothesis is correct.
Conclusion
Our findings confirm that age is a major risk factor for the development of chemotherapy-related nausea, with younger patients having significantly more nausea than older patients. They also provide strong evidence that breast cancer patients have more nausea than patients in other diagnostic groups. We also found that a simple question regarding susceptibility to nausea was a strong predictor of subsequent nausea and suggest this assessment be added to the antiemetic treatment guidelines as a risk factor. Studies examining the benefit to patient QOL from modifying antiemetic regimens based upon risk factors are warranted.
Acknowledgments
This work was supported by [grant number 1R25-CA102618-01A1] from the National Cancer Institute.
Reference List
- 1.Klastersky J, Schimpff SC, Senn HJ. Supportive Care in Cancer. 2nd ed. New York: Marcel Deckker; 1999. [Google Scholar]
- 2.Osoba D, Zee B, Warr D, Latreille J, Kaizer L, Pater J. Effect of postchemotherapy nausea and vomiting on health-related quality of life. The Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. Support Care Cancer. 1997;5:307–313. doi: 10.1007/s005200050078. [DOI] [PubMed] [Google Scholar]
- 3.Morrow GR, Roscoe JA, Hickok JT. Nausea and Vomiting. In: Holland JC, editor. Psychooncology. New York: Oxford University Press; 1998. pp. 476–484. [Google Scholar]
- 4.Hickok JT, Roscoe JA, Morrow GR, King DK, Atkins JN, Fitch TR. Nausea and emesis remain significant problems of chemotherapy despite prophylaxis with 5-hydroxytryptamine-3 antiemetics: A University of Rochester James P. Wilmot Cancer Center Community Clinical Oncology Program Study of 360 cancer patients treated in the community. Cancer. 2003;97:2880–2886. doi: 10.1002/cncr.11408. [DOI] [PubMed] [Google Scholar]
- 5.ASHP Therapeutic Guidelines on the Pharmacologic Management of Nausea and Vomiting in Adult and Pediatric Patients Receiving Chemotherapy or Radiation Therapy or Undergoing Surgery. Am J Health Syst Pharm. 1999;56:729–764. doi: 10.1093/ajhp/56.8.729. [DOI] [PubMed] [Google Scholar]
- 6.Grunberg SM. Chemotherapy-induced nausea and vomiting: prevention, detection, and treatment--how are we doing? The Journal of Supportive Oncology. 2004;2:1–10. [PubMed] [Google Scholar]
- 7.Hesketh PJ, Grunberg SM, Herrstedt J, de WR, Gralla RJ, Carides AD, Taylor A, Evans JK, Horgan KJ. Combined data from two phase III trials of the NK1 antagonist aprepitant plus a 5HT 3 antagonist and a corticosteroid for prevention of chemotherapy-induced nausea and vomiting: Effect of gender on treatment response. Support Care Cancer. 2006;14:354–360. doi: 10.1007/s00520-005-0914-4. [DOI] [PubMed] [Google Scholar]
- 8.Jordan K, Sippel C, Schmoll HJ. Guidelines for antiemetic treatment of chemotherapy-induced nausea and vomiting: past, present, and future recommendations. Oncologist. 2007;12:1143–1150. doi: 10.1634/theoncologist.12-9-1143. [DOI] [PubMed] [Google Scholar]
- 9.Herrstedt J, Dombernowsky P. Anti-emetic therapy in cancer chemotherapy: Current status. Basic & Clinical Pharmacology & Toxicology. 2007;101:143–150. doi: 10.1111/j.1742-7843.2007.00122.x. [DOI] [PubMed] [Google Scholar]
- 10.Gralla RJ, Osoba D, Kris MG, Kirkbride P, Hesketh PJ, Chinnery LW, Clark-Snow R, Gill DP, Groshen S, Grunberg S, Koeller JM, Morrow GR, Perez EA, Silber JH, Pfister DG. Recommendations for the use of antiemetics: evidence-based, clinical practice guidelines. American Society of Clinical Oncology. J Clin Oncol. 1999;17:2971–2994. doi: 10.1200/JCO.1999.17.9.2971. [DOI] [PubMed] [Google Scholar]
- 11.Gralla RJ, Itri LM, Pisko SE, Squillante AE, Kelson DP, Braun DW, Bordin LA, Braun TJ. Antiemetic efficacy of high-dose Metoclopramide: Randomized trials with placebo and Prochlorperazine in patients with chemotherapy-induced nausea and vomiting. N Engl J Med. 1981;305:905–909. doi: 10.1056/NEJM198110153051601. [DOI] [PubMed] [Google Scholar]
- 12.National Comprehensive Cancer Network. NCCN Clinical Practice Guidelinesin Oncology: Antiemesis, V.2.2009. [Accessed February 3, 2009];2008 Available at http://www.nccn.org/professionals/physician_gls/PDF/antiemesis.pdf.
- 13.Hickok JT, Roscoe JA, Morrow GR, Bole CW, Zhao H, Hoelzer KL, Dakhil SR, Moore T, Fitch TR. 5-hydroxytryptamine-receptor antagonists versus prochlorperazine for control of delayed nausea caused by doxorubicin: a URCC CCOP randomised controlled trial. Lancet Oncol. 2005;6:765–772. doi: 10.1016/S1470-2045(05)70325-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Osoba D, Zee B, Pater J, Warr D, Latreille J, Kaizer L. Determinants of postchemotherapy nausea and vomiting in patients with cancer. Quality of Life and Symptom Control Committees of the National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol. 1997;15:116–123. doi: 10.1200/JCO.1997.15.1.116. [DOI] [PubMed] [Google Scholar]
- 15.Montgomery GH. Pre-infusion expectations predict post-treatment nausea during repeated adjuvant chemotherapy infusions for breast cancer. British Journal of Health Psychology. 2000;5:105–119. [Google Scholar]
- 16.Rhodes VA, Watson PM, McDaniel RW, Hanson BM, Johnson MH. Expectation and occurrence of postchemotherapy side effects: Nausea and vomiting. Cancer Pract. 1995;3:247–253. [PubMed] [Google Scholar]
- 17.Roscoe JA, Hickok JT, Morrow GR. Patient expectations as predictor of chemotherapy-induced nausea. Annals of Behavioral Medicine. 2000;22:121–126. doi: 10.1007/BF02895775. [DOI] [PubMed] [Google Scholar]
- 18.Roscoe JA, Bushunow P, Morrow GR, Hickok JT, Kuebler PJ, Jacobs A, Banerjee TK. Patient expectation is a strong predictor of severe nausea after chemotherapy: A University of Rochester Community Clinical Oncology Program study of patients with breast carcinoma. Cancer. 2004;101:2701–2708. doi: 10.1002/cncr.20718. [DOI] [PubMed] [Google Scholar]
- 19.Olver IN, Taylor AE, Whitford HS. Relationships between patients' pre-treatment expectations of toxicities and post chemotherapy experiences. Psychooncology. 2005;14:25–33. doi: 10.1002/pon.804. [DOI] [PubMed] [Google Scholar]
- 20.Roscoe JA, Jean-Pierre P, Shelke AR, Kaufman ME, Bole C, Morrow GR. The role of patients' response expectancies in side effect development and control. Current Problems in Cancer. 2006;30:40–98. doi: 10.1016/j.currproblcancer.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 21.Colagiuri B, Roscoe JA, Morrow GR, Atkins JN, Giguere JK, Colman LK. How do patient expectancies, quality of life, and postchemotherapy nausea interrelate? Cancer. 2008;113:654–661. doi: 10.1002/cncr.23594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shelke AR, Roscoe JA, Morrow GR, Colman LK, Banerjee TK, Kirshner JJ. Effect of a nausea expectancy manipulation on chemotherapy-induced nausea: A University of Rochester Cancer Center Community Clinical Oncology Program study. J Pain Symptom Manage. 2008;35:381–387. doi: 10.1016/j.jpainsymman.2007.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Roscoe JA, Morrow GR, Hickok JT, Bushunow PW, Pierce HI, Flynn PJ, Kirshner JJ, Moore DF, Jr., Atkins JN. The efficacy of acupressure and acustimulation wrist bands for the relief of chemotherapy-induced nausea and vomiting: A URCC CCOP multicenter study. J Pain Symptom Manage. 2003;26:731–742. doi: 10.1016/s0885-3924(03)00254-9. [DOI] [PubMed] [Google Scholar]
- 24.Burish TG, Carey MP, Krozely MG, Greco FA. Conditioned side effects induced by cancer chemotherapy: Prevention through behavioral treatment. J Consult Clin Psychol. 1987;55:42–48. doi: 10.1037//0022-006x.55.1.42. [DOI] [PubMed] [Google Scholar]
- 25.Carey MP, Burish TG. Etiology and treatment of the psychological side effects associated with cancer chemotherapy: A critical review and discussion. Psychol Bull. 1988;104:307–325. doi: 10.1037/0033-2909.104.3.307. [DOI] [PubMed] [Google Scholar]
- 26.Chemotherapy-induced nausea and vomiting (CINV): Prevention, detection, and treatment--how are we doing? ONS News. 2004;19:61–62. [PubMed] [Google Scholar]
- 27.Schwartzberg LS. Chemotherapy-induced nausea and vomiting: clinician and patient perspectives. The Journal of Supportive Oncology. 2007;5:5–12. [PubMed] [Google Scholar]
- 28.Barsky AJ, Saintfort R, Rogers MP, Borus JF. Nonspecific medication side effects and the nocebo phenomenon. JAMA. 2002;287:622–627. doi: 10.1001/jama.287.5.622. [DOI] [PubMed] [Google Scholar]
- 29.Blakeslee S. Techniques to manage the mind - How hypnosis is gaining respect. [Accessed February 3, 2009];Magazine for Hypnosis and Hypnotherapy. 2005 at http://hypnos.co.uk/hypnomag/hypnosisnews/hypnosisrespect.htm. [Google Scholar]